Abstract
Cell replacement therapy in Parkinson's disease depends on a reliable source of purified dopamine (DA) neurons (PDN) and the identification of factors relevant to their survival. Our goal was to genetically tag and purify by flow cytometry embryonic midbrain DA neurons from a transgenic mouse line carrying 11 kb of human tyrosine hydroxylase promoter driving expression of the enhanced green fluorescent protein (GFP) for studies in vivo and in vitro. A 99% purification of GFP+ cells was achieved. When transplanted into 6-hydroxydopamine-treated rat striatum, PDN survived, became well-integrated and produced recovery from amphetamine-induced motor behaviors. However, when grown in culture, PDN died within days of plating. No known growth factors prevented PDN death as did incubation with novel factors in glia/glial-conditioned media. We conclude that GFP-tagged DA neurons can be purified to homogeneity and can survive and function when grown with glial factors in vitro or after transplantation in vivo.
Introduction
Realizing the promise of cell replacement therapy in neuro-degenerative diseases like Parkinson's (PD) remains elusive. Although fetal human brain transplants have oftentimes provided enduring benefit in animal models and PD patients, results have also been inconsistent and unpredictable (Brundin et al., 2000). The challenge now is to ascertain why some grafts succeed while others do not. At issue is a lack of uniformity in source tissue, both in terms of the number and type of cells harvested for transplant and in their ability to thrive and integrate following transplantation.
One of the chief difficulties in regulating the number of DA neurons in the graft is the degree of cell heterogeneity in source tissue. Depending on the dissected piece of fetal midbrain, more than 95–99% of cells in the transplant are other than the DA neurons they are meant to replace. Transplantation of these unwanted cells has produced serious side effects (Freed et al., 2001), and in rare cases, even death (Folkerth and Durso, 1996). Moreover, such heterogeneity has made it impossible to regulate the number of DA neurons present in the graft, resulting either in too few cells to achieve efficacy or so many cells that patients develop debilitating dyskinesias (Freed et al., 2001; Trott et al., 2003).
Attempts to circumvent these issues by generating prospective DA neurons from a more uniform source, such as stem or progenitor cells, have been partially successful, as only a fraction of these cells respond to DA differentiation cues in vitro and in vivo (Bjorklund et al., 2002; Deacon et al., 1998; Iacovitti and Stull, 1997; Iacovitti et al., 2001; Kawasaki et al., 2000; Kim et al., 2002; Lee et al., 2000; Park et al., 2004; Schulz et al., 2003; Yang et al., 2002, 2003, 2004). Therefore, whether DA neurons are derived from stem, progenitor or fetal tissue, it is critical that methods be developed to purify and quantify them prior to transplantation.
A second major impediment in cell replacement therapy derives from the fact that more than half of all implanted cells die within several days of engraftment (Barker et al., 1996; Emgard et al., 1999; Sortwell et al., 2000). Thus, fundamental to the success of this approach is a better understanding of the factors (trophic agents, cytokines, etc.) which can improve the survival of transplanted cells. Studies have implicated any of a number of different growth factors in DA neuronal viability and neuroprotection, including members of the glial-derived neurotrophic factor (GDNF) (Grondin et al., 2003)/transforming growth factor-β (TGFβ)(Krieglstein et al., 1998; Roussa and Krieglstein, 2004), nerve growth factor (NGF) (Nagatsu et al., 2000) or other trophic and non-trophic agents (antioxidants, anti-apoptotics, etc.) (Fernandez-Espejo, 2004; Stull et al., 2002; Teismann and Schulz, 2004). However, the degree to which these factors act directly on DA neurons has until now been obfuscated by the heterogeneity of cell types present in vivo and in midbrain cultures.
In an effort to surmount these obstacles, in this study, we sought to devise new ways to isolate embryonic DA neurons as a pure population and identify those factors and conditions which best support their survival and growth in vitro and in vivo. To do so, we have taken advantage of the cloning of 11 kb human tyrosine hydroxylase gene promoter (hTH). This sequence has been shown to accurately target expression of the reporter green fluorescent protein (GFP) to DA neurons in transgenic mice (Kessler et al., 2003). Because GFP can be directly visualized in live fetal DA neurons, tagged cells can be purified via fluorescent activated cell sorting (FACS) and studied in culture and after transplantation into animal models of PD. Hopefully, these studies will serve as proof of principle that mouse DA neurons can indeed be FACS-purified and survive in vivo, making possible the application of these techniques for the purification of tagged human DA neurons (from embryonic, fetal or adult stem/progenitor cells) for the treatment of Parkinson's.
Results
Purification of fetal DA neurons by FACS
Previous studies from this laboratory have established that the hTH-GFP transgenic mouse faithfully expresses GFP in TH-positive DA neurons of the developing and adult mouse midbrain (Kessler et al., 2003). Based on the specificity of this expression, in this study, we developed a strategy to purify hTH-GFP tagged DA neurons for study in culture and after transplantation. Using a dissecting microscope equipped with fluorescent optics, the VM from E13–14 transgenic mouse embryos was isolated from surrounding non-fluorescent tissue. Despite the homogeneous green appearance of the segregated hTH-GFP midbrain (Fig. 1A), more than 50% of Bis+ nuclei were found in non-GFP+ cells (Fig. 1B) when cells were dissociated and plated in culture. Although this represents a significant enrichment over routine dissection methods (which produce, at best, 5% DA neurons from the VM; Stull et al., 2002), in order to achieve homogeneity, the GFP-fluorescent cell population was FACS sorted.
Fig. 1.

Dissection, FACS analysis and recovery of PDN. (A) Shown is the ventral midbrain regions microdissected from the brains of TH-GFP mice prior to transplantation. (B) Following dissociation of midbrain regions in panel A, GFP-positive cells (green) were plated in culture and counterstained for the general nuclear marker, Bis-benzimide (blue). Note that, despite the uniformly green appearance of microdissected tissue in panel A, the majority of cells with blue nuclei do not stain green for GFP in panel B. Because of the high level of GFP fluorescence, blue-green nuclei are not readily distinguished in the green GFP+ cells. (C) FACS analysis from a typical experiment of midbrain cells from TH-GFP mice. Shown in the left panel is the pool of healthy cells (circumscribed) which were passed through a Beckman Coulter Elite ESP sorter equipped with a 15 mW argon laser and a 100 μm nozzle tip (excitation wavelength of 488 nM). Shown in the right panel is the FACS analysis demonstrating several peaks in fluorescence, extending up to 4 logs in signal intensity.
Only the pool of healthy cells (Fig. 1C; circumscribed in left panel) were passed through the cell sorter. FACS analysis (Fig. 1C; right panel) revealed the presence of several populations of cells, including a large non-fluorescent cell peak (<1 log GFP), cells with low fluorescence (<10 log GFP; labeled D) and cells which were highly fluorescent (>10 log GFP; labeled B). The latter cell population, extending in signal intensity 3–4 logs above control (non-transgenic; data not shown), was collected and plated in tissue culture. For the experiment shown in Fig. 1C, the estimated yield at various steps in the procedure was as follows: 29 litters or 190 embryos homozygous for hTH-GFP were dissected, yielding 18 × 106 cells. Of these, 13.3 × 106 cells or 73.9% were deemed healthy and chosen for FACS analysis. Following sorting, the most fluorescent cells (labeled B) numbered 1.73 × 106 cells. Thus, the yield of “pure” highly fluorescent cells was approximately 10% of total dissected cells or 13% of sorted cells. Although other fractions containing GFP+ cells (peak D) might have increased the yield, those cells were intentionally forfeited in order to achieve greater cell purity (peak B). Purified DA neurons were then used in transplant or tissue culture paradigms.
To determine the actual levels of homogeneity attained with this procedure, FACS sorted cells from peak B were plated in a complete serum-supplemented media in culture. Thirty minutes after plating, PDN cultures were fixed and examined for endogenous GFP (Fig. 2A) and the general nuclear marker Bisbenzimide (Fig. 2B) and images merged (Fig. 2C). The overlap of markers within nearly all cells (97.3 ± 2%; n = 4 platings) indicated that there were almost no contaminating non-GFP+ cells present in the most pure FACS fraction (see arrow in Fig. 2C). When sister cultures were examined 1 day later, we found that virtually all cells that were GFP+ were also TH+ as evidenced by the number of yellow cells in the merged images (Fig. 2D) (98.7% ± 1% overlap; n = 4 platings). In addition to TH, other markers of a DA differentiated phenotype were also present in GFP+ cells, including AADC and Nurr 1 (Figs. 2E, F). These results demonstrate that FACS greatly enriches the population of fluorescent (TH+/GFP+) cells, making it possible to purify to near homogeneity DA midbrain neurons from transgenic hTH mice for study in vivo and in vitro. It is however important to bear in mind that this population contains DA neurons derived from both the ventral tegmental (A10) and substantia nigra (A9) midbrain regions, which are indistinguishable at this stage.
Fig. 2.

Localization of cellular signals in PDN at various times in vitro. Cells were examined for GFP (A), the general nuclear marker Bis-benzimide (B) and images merged (C) 30 min after plating. Note the overlap of green and blue markers within nearly all cells, indicating that only GFP+ cells had been collected by FACS. Arrows indicate the few Bis+ cells which are GFP− (97.3% ± 2% overlap; n = 4 platings). One day later, sister cultures of GFP+ cells were fixed and stained for TH and images merged (D) (98.7% ± 1% overlap; n = 4 platings). Note that all GFP+ cells stained positively for TH (yellow cells with green GFP+ nuclei and red TH+ processes); exceptions indicated by arrows. In addition, cells also stained for DA phenotypic markers AADC (E) and Nurr 1 (F).
Transplantation of PDN into Parkinsonian rats
We next examined whether PDN cells could survive and function after transplantation into a rat with a Parkinsonian lesion. Thus, freshly sorted PDN (n = 12) were stereotaxically implanted into the striata of rats with verified 6-OHDA lesions, and ipsilateral rotation was monitored for up to 12 weeks as an index of changes in motor behavior. Upon sacrifice, we found that transplanted PDN survived and became well-integrated into host tissue (Fig. 3C). Thus, in the example shown here where many PDN neurons (5201 ± 71) survived 12 weeks after implantation, engrafted PDN elaborated an extensive network of terminal processes (Fig. 3B) extending over a large portion of denervated striatum (Fig. 3A).
Fig. 3.
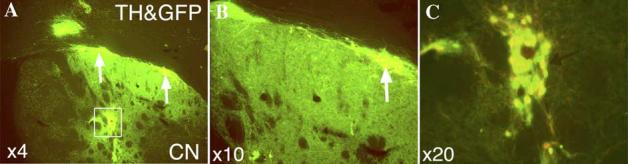
The co-localization of endogenous GFP fluorescence and TH immunocytochemistry in PDN 12 weeks after their transplantation into the striatum of 6-OHDA lesioned rats. (A) Low power view through one brain section of the graft showing PDN perikarya (rectangle, arrows) and the area of terminal plexus in the striatum. (B) Higher power views of the re-innervated striatum and PDN (arrow) in panel A. (C) Higher power view of the PDN shown in rectangle in panel A. Total survival of PDN in all brain sections through the graft = 5201 ± 71.
In addition, in PDN-engrafted rats, there was a significant change in motor behavior after amphetamine challenge consistent with an increase in the releasable pool of DA on the lesioned side when compared with control (lesion, no graft) rats. Thus, the ipsi-lateral amphetamine-induced rotation scores in control animals remained consistently high (10.6 ± 3.4 rotations/min) after repeated tests (2–3 tests) during the 12-week postoperative period (Fig. 4). In contrast, there was a significant decline in rotational scores (3.9 ± 1.7) in rats that received a PDN graft (Fig. 4). After sacrifice, animals were perfused, brains sectioned and immunocytochemically stained for TH. Surviving TH-immunoreactive neurons counted along the length of the graft ranged in number (560–5201 TH+ cells), representing <1% survival of the original cells implanted. In 3 additional cases, motor scores improved at 6 weeks but declined again by 12 weeks, at which time there was evidence of dying and dead PDN cells in the graft (data not shown), suggesting that transplanted PDN initially ameliorated motor deficits but ceased to do so once death had ensued.
Fig. 4.

Analysis of rotational behavior after transplantation of PDN into the 6-OHDA lesioned striatum. The ipsilateral rotational score after amphetamine challenge was tested at 3 and 6 weeks after 6-OHDA injection for all rats (n = 21). The rotation score for control rats (6-OHDA, no PDN; n = 12) remained at high levels (10.6 ± 3.4 turns/min) for the next 12 weeks. In contrast, in rats where PDN were transplanted into the central striatum (n = 9), there was a significant decrease in the number of rotations (3.9 ± 1.7 turns/min; **P < 0.01).
Viability of purified sorted DA neurons in long-term culture
In an effort to better define the ways in which we might improve the survival and enhance the growth of transplanted PDN, cell viability was studied in culture where individual parameters could be assessed after modification. Moreover, because FACS purification allowed DA neurons to be segregated from other cell types, it was possible to study for the first time the effects of individual cells and factors specifically on PDN. To do so, FACS sorted PDN, and for comparison, unsorted (mixed) transgenic VM cells were plated either in DM or SM media at a relatively high density that normally supports survival (1.5 × 105 cells/0.5 cm2). At various time intervals, GFP+ DA neurons (green) were stained with ethidium (red) using the Live/Dead Cell mediated cytotoxicity kit (Molecular Probes) to identify dying cells (Fig. 5). The vast majority of PDN did not stain red for ethidium 30 min after plating (Fig. 5A) nor at 1 day in vitro (Figs. 5B, D), suggesting that all cells were healthy early on regardless of the media into which they were seeded. However, by 3–5 days in vitro, most PDN grown on DM (Fig. 5C) or SM (Fig. 5F) had died in culture. In contrast, less pure fractions of FACS sorted cells (data not shown) or unsorted mixed VM cells (Figs. 5D and H) thrived in either media at comparable times, indicating that the death of PDN could not be attributed to damaging conditions prior to plating (i.e. dissociation or FACS sorting). Together, these findings raised the possibility that PDN may require for their long-term survival essential growth factors not found in DM, or even SM, but which are normally present in mixed cell cultures and the transplanted brain.
Fig. 5.
Time course of survival in cultures of PDN or unpurified (mixed) VM cells grown on various media. GFP+ (green) PDN cells (A–C, E–G) or VM cells (D, H) were stained with ethidium (red) from the Live/Dead Cell-mediated cytotoxicity kit in order to identify dying cells. Cultures were grown either on DM (A–D) or SM (E–H) for 30 min (A), 1 day (B, E), 3 days (C, F) or 5 days (D, H). Note that all cultures were re-fed fresh media at 2 –3 days.
Glia produce essential survival factors for PDN
Since glial cells are abundantly present in mixed cell cultures and since glia, particularly those derived from relevant brain regions (Rousselet et al., 1988), are thought to be a vital source of trophic factors for DA neurons in vivo (for review; Fernandez-Espejo, 2004; Teismann and Schulz, 2004), we next tested their ability to sustain PDN viability in culture. Therefore, PDN and mixed VM cells were grown on glia derived from a number of different brain areas and/or on media previously conditioned by them. We found that all glia, regardless of regional derivation, and all glial CM were capable of rescuing PDN from cell death in culture. In contrast, CM collected from neuron-only cultures did not prolong the survival of PDN beyond 3–5 days (Table 1). Thus, even at 10 days in vitro, PDN grown on glia or glial CM (diluted 1:1 in DM) (Fig. 6A; Table 1) continued to be healthy, eliciting complex networks of neuronal processes. Mixed VM cells, which already survived well in culture (see Fig. 5D), exhibited no marked increase in viability with the addition of GCM (Fig. 6C).
Table 1.
Effects of various growth substances and growth conditions on the percent survival of PDN in culture over time
| Treatment | 1 day | 2 days | 3 days | 5 days | 7 days | 10 days |
|---|---|---|---|---|---|---|
| Serum | 100 | 98 ± 7 | 43 ± 4* | 2 ± 8** | ||
| DM | 100 | 21 ± 8** | 7 ± 7** | |||
| Co-culture/conditioned media | ||||||
| Glial beds | 100 | 97 ± 3 | 98 ± 7 | 96 ± 6 | 98 ± 3 | 95 ± 7 |
| Glial CM | 100 | 99 ± 4 | 99 ± 2 | 97 ± 7 | 94 ± 4 | 78 ± 6* |
| Neuronal CM | 100 | 100 ± 3 | 48 ± 6** | 12 ± 3** | 2 ± 7** | |
| Growth factors | ||||||
| GDNF | 100 | 79 ± 9 | 32 ± 8* | 2 ± 7** | ||
| Neurturin | 100 | 100 ± 4 | 47 ± 7** | 4 ± 5** | ||
| Persephin | 100 | 5 ± 6** | ||||
| Artemin | 100 | 101 ± 3 | 12 ± 3** | 2 ± 5** | ||
| GDNF family | 100 | 103 ± 6 | 69 ± 6* | 2 ± 6** | ||
| TGFβ-3 | 100 | 9 ± 9** | ||||
| TGFβ-3 + GDNF |
100 | 49 ± 7** | 25 ± 5** | 3 ± 5** | ||
| TGFβ-3 + GDNF family |
100 | 50 ± 6* | 28 ± 4** | 6 ± 6** | ||
| TGFβ-3 + FGF8 + SHH |
100 | 76 ± 4* | 55 ± 8* | 49 ± 5** | 2 ± 5** | |
| BDNF | 100 | 96 ± 5 | 14 ± 7** | |||
| NGF | 100 | 99 ± 6 | 22 ± 7* | 8 ± 6** | ||
| NT3 | 100 | 3 ± 3** | ||||
| Neurotrophin family |
100 | 72 ± 2* | 8 ± 8** | 4 ± 6** | ||
| FGF2 | 100 | 3 ± 7** | ||||
| EGF | 100 | 9 ± 2** | ||||
| IGF1 | 100 | 6 ± 6** | ||||
| IL1/IL2/LIF | 100 | 44 ± 5** | 8 ± 4** | |||
| GDNF/BDNF/ bFGF |
100 | 53 ± 7** | 7 ± 4** | |||
| Depolarizing agents | ||||||
| KCl | 100 | 3 ± 9** | ||||
| DA | 100 | 99 ± 2 | 95 ± 75 | 26 ± 8* | 3 ± 6** | |
| Anti-apoptotics | ||||||
| Z-VAD-FMK | 100 | 1 ± 5** | ||||
| Z-DEVD-FMK | 100 | 4 ± 4** | ||||
| Antioxidants | ||||||
| Trolox | 100 | 29 ± 4** | 13 ± 8** | 11 ± 7** | 2 ± 7** | |
| Melatonin | 100 | 6 ± 4** | ||||
With the exception of PDN grown in serum containing (SM) media, all other growth factors were tested at 10 and 100 ng/ml in defined serum-free (DM) media (data from 100 ng/ml shown here). In co-culture experiments, PDN were plated onto glial or neuronal beds or on DM conditioned (CM) by them. All cultures were re-fed every 2 –3 days, and cell viability was assessed at various times in the live cultures (see Experimental methods). All data are expressed as percent of initial plating density (1 day). Values represent the mean ± SEM (n = 3 platings).
P < 0.01.
P < 0.001.
Fig. 6.

Survival of PDN or VM cells on glial factors. PDN (A, B) or mixed VM cells (C, D) were grown on strial glial CM (A, C) or on DM containing GDNF (100 ng/ml) (B, D). Note that the survival of GFP+ PDN was supported by glial CM but not GDNF. In contrast, TH+ VM cells, at the densities used, exhibited no marked change in survival over control with the addition of GCM or GDNF.
Glial-derived factors and other growth substances are not trophic for PDN
Since a number of glial factors, particularly GDNF working in concert with TGFβ-3, are thought to be critical for the survival of DA neurons in vivo and in vitro (Grondin et al., 2003; Krieglstein et al., 1998, 2002), we next tested their ability to substitute for glia or glial CM in supporting the viability of PDN cells in culture. Surprisingly, while individual members of the GDNF, TGFβ and NGF growth factor families and other relevant growth substances (such as FGF8 and SHH) could extend PDN survival an additional 1–2 days in culture, none mimicked the long-term trophic effects of glia or glial CM on PDN (Table 1; Figs. 6A, B). Even incubation with combined growth factors (i.e. GDNF, neurturin, artemin, persphin, TGFβ-3) provided little added benefit (Table 1). As expected, in mixed VM cell cultures (which already contain glia and glial factors), GDNF (Fig. 6D), BDNF or related family members did not substantially increase viability (although more DA neuronal aggregation was noted).
Because PDN are relatively immature cells which may not contain the necessary receptors/signaling molecules needed to mediate GDNF's effects, we next measured mRNA for the c-Ret receptor and compared it to adult dopaminergic tissues (Fig. 7A). Using real-time PCR, we found that c-Ret mRNA was expressed in E13 PDN. As expected, levels in PDN were lower than in adult (8 weeks) substantia nigra, though higher than in adult striatum (Fig. 7B). This finding suggests that, despite the lack of a GDNF effect on these cells, at least some of the necessary molecular machinery for GDNF is indeed present in PDN.
Fig. 7.

PCR-based analysis of c-Ret in PDN. (A) Gel electrophoresis of amplification products. Lane 1: no template control (NC), lane 2: positive control of β-actin (PC), lane 3: adult striatum (CN), lane 4: adult substantia nigra (SN), and lane 5: purified dopamine neurons (PDN). (B) Table comparing c-ret PCR products in PDN and other DA tissues by quantitative analysis (as described in Experimental methods and table legend).
Additionally, we also showed that treatment with other classes of substances implicated in neuronal survival, such as depolarizing agents, caspase inhibitors and antioxidants, only transiently prolonged the life of PDN cells in culture (Table 1). Thus, only soluble factors of unknown identity, produced in and secreted by glia, were able to sustain PDN in long-term culture.
Discussion
The findings of the present study demonstrate that it is indeed possible to FACS isolate a pure population of midbrain DA neurons from transgenic mice carrying an 11 kb construct of the 5′ flanking region of the human TH gene driving expression of an enhanced GFP reporter (Kessler et al., 2003). Whereas a similar paradigm using a rat TH-GFP promoter yielded a 60% enrichment of DA neurons (Sawamoto et al., 2001), in the current study, cells were purified essentially to homogeneity (99%+). This technical advance afforded us a unique opportunity to re-examine issues important in DA cell transplantation and to address them for the first time without the confounding presence of other cell types.
Importantly, we found that, despite the hazards posed by FAC sorting, the preponderance of PDN cells emerged from the process in good health. As evidence of this fact, in vivo, GFP+ PDN cells persisted for up to 12 weeks following their transplantation into the 6-OHDA rat striatum. Although the percentage of surviving PDN (<1%) in the graft was less than that seen previously with mixed midbrain cells (5%) (Barker et al., 1996; Kordower et al., 1995), in PDN grafts, perikarya became well-integrated into the host brain, giving rise to a large and complex plexus of DA terminals in the striatum. In contrast, DA neurons in VM grafts remained confined within the needle tract, usually lining up along the border of the transplant, where they extended processes for a limited distance into host tissue (Barker et al., 1996). Perhaps donor glia, which were present in mixed cell grafts, but absent in PDN grafts, contributed to the formation of scar tissue around the transplant. Without that mechanical and chemical barrier (Gates et al., 1996; Schwab, 1996; Schulz et al., 1998), possibly PDN were free to incorporate better into neighboring host brain and to elaborate an expanded arbor of processes.
It is noteworthy that, regardless of the differences in the appearance of these grafts, significant functional recovery from lesion-induced motor deficits was observed both in the present PDN study and in previous studies using mixed cell transplants (for review, Mokry, 1995). Unclear is whether the underlying mechanisms of recovery are the same or different in these two models. One possibility is that the behavioral benefits seen in mixed fetal cell grafts arise from DA produced and released primarily through non-synaptic mechanisms, as has been suggested previously (Reum et al., 2002; Zoli et al., 1998), while PDN grafts, by virtue of their ability to assimilate into the brain, may establish a more synaptic-like interaction with target tissue. However, until electron microscopic analysis and in vivo microdialysis studies can be completed, these remain mere speculations.
Although our in vivo studies made clear that the brain milieu was conducive to PDN survival, we nonetheless sought to identify factors/cells/conditions that would enhance their growth and function. By addressing this issue in PDN cultures, we could modify test parameters to study their individual and combinatorial effects on DA neurons in the absence of other cell types, something that had not before been possible. We were in fact surprised to find that PDN, which were generally healthy for the first day or two in culture, did not survive long-term, even in a serum containing media. Only when PDN were grown in co-culture with glial beds derived from any of a number of brain regions or incubated with media conditioned by them did cells survive and flourish indefinitely in vitro. Since CM generated from neurons derived from the same brain region did not produce factors trophic for PDN, it suggests that PDN may depend for their survival on the presence of a soluble paracrine factor(s), derived specifically from glial cells.
Importantly, factors like GDNF and the GDNF family members (neurturin, artemin, persephin) which are believed to be neurotrophic for DA neurons (for review; Fernandez-Espejo, 2004; Teismann and Schulz, 2004), when administered alone or in combination with other important DA cues (i.e. TGFβ-3, FGF8 and SHH), did not mimic the survival effect of glia or glial CM on PDN in culture. The mRNA for the GDNF receptor signaling kinase c-Ret was easily detected in PDN cells, albeit at lower levels than those found in the adult substantia nigra, suggesting that GDNF receptor signaling may indeed be possible in these immature cells.
The inability of GDNF to affect PDN survival was unforeseen given that many published studies using mixed VM cell cultures had reported improved viability with these reagents (Akerud et al., 1999; Burke et al., 1998; Clarkson et al., 1995; Horger et al., 1998; Krieglstein et al., 1998; Lin et al., 1993; Roussa and Krieglstein, 2004). While such discrepancies could result from alterations of PDN during the purification process, it is more likely due to other important dissimilarities in the various experimental models. For instance, the cause of cell death and the molecular interventions to prevent it may be quite distinct. Consistent with this explanation was the fact that PDN and VM cultures were seeded at healthy high densities (1.5 × 105 cells/0.5 cm2), while, in most published studies, mixed VM cells were plated at much lower densities (5.0 × 104 cells/cm2) that cause oxidative stress in cells. Possibly, in the latter model, test factors do not serve as neurotrophins but rather act to rescue cells from stress-induced death. Further suggestive of this possibility is the fact that other classes of substances, such as antioxidants, anti-apoptotics and depolarizing agents, will rescue DA neurons in low density mixed cell cultures (Iacovitti et al., 1999; Stull et al., 2002) but did not sustain PDN viability even in high density cultures in our studies. Alternatively, it is possible that the majority of DA neurons (VTA versus SN) present in PDN cultures is critically different from that in VM cultures.
Another important distinction that might explain differences in growth factor responsiveness in the two culture systems is the way in which these agents mediate their effects on DA neurons. In homogeneous cell cultures like PDN, growth factors act directly on the cells of interest. However, because of the heterogeneity of cell types in VM cultures, growth factor effects may involve primary interactions with other cells in the dish, which then may produce secondary, tertiary, etc. effects on DA neurons. In the latter case, factors like GDNF (and/or TGFβ-3) might be necessary but insufficient to support PDN survival on their own. Instead, these agents might exert their influence by altering the production of other critical reagents in other cultured cells, like glia. According to this proposition, GDNF would sustain DA neuron viability only when combined with other glial factors as occurs in mixed VM and PDN-glia/glial CM cultures. Moreover, since similar glial factor interactions likely exist in the brain, this interpretation is also consistent with in vivo studies demonstrating that GDNF can partially prevent developmental cell death in the substantia nigra (Connor et al., 2001; Jackson-Lewis et al., 2000; Kholodilov et al., 2004; Oo et al., 2003) and the death of DA neurons in models of PD (Bilang-Bleuel et al., 1997; Choi-Lundberg et al., 1998; Olsin, 1997).
Because we do not yet know the identity of this putative glial-derived factor(s), it is not clear whether it is a novel protein or merely a novel role for a known protein. Future experiments which substantially reduce GDNF via specific antibodies, small interfering RNAs or null mutation of the factor or its receptor (Cacalano et al., 1998; Enomoto et al., 1998; Moore et al., 1996; Sanchez et al., 1996) should allow us to dissociate known from novel glial factor effects. In addition, it will be important to characterize this factor's specificity of action. Of particular interest is whether this substance is trophic for DA neurons only, and if so, whether all classes of DA neurons are equally responsive. By crossing hTH-GFP mice with ptx3 mutant mice (aphakia), which contain ventral tegmental (A10) DA neurons but not substantia nigra (A9) neurons (Hwang et al., 2003), it should be possible to FACS purify A10 neurons for studies of their survival with this and other factors.
In summary, the findings of the present study suggest that it is indeed possible to isolate pure populations of transgenic mouse DA neurons for optimization of the number, survival and growth conditions in the transplanted Parkinsonian brain. Inevitably, however, it is human neurons that will be of greatest interest in this regard. Therefore, with the mouse PDN model as proof of principle, we are currently adapting this approach for the purification of human DA neurons generated from embryonic stem or progenitor cells. Using cells which have been lentivirally tagged with the hTH-GFP construct, it should be possible, following differentiation of stem/progenitor cells, to FACS purify human DA neurons, study the glial factors which best support their survival and determine the ideal number required for transplantation into rat and monkey models of disease. Hopefully, these studies will lay the foundation needed to generate consistently successful grafts in human patients with PD.
Experimental methods
Cell dissociation, FACS sorting and culture methods
Ventral mesencephalons (VM) from embryonic day (E) 13–14 transgenic (hTH-GFP) as described previously (Stull et al., 2002)or as indicated in the text. Cells were dissociated in 1 mg/ml papain (Roche) in HBSS-CMF, dissociated, filtered through 70 μm nylon mesh and either directly plated in culture (1.5 × 105 cells/0.5 cm2 density) or first sorted on a Beckman Coulter Elite ESP sorter equipped with a 15 mW argon laser and a 100 μm nozzle tip (excitation wavelength of 488 nM). After FACS, cells were pelleted at 1000 RPM (at room temperature), re-suspended in phosphate-buffered saline with added glucose (6 mg/ml) and transplanted into rats (see below) or plated into culture at a density of 1.5 × 105 cells/0.5 cm2. In vitro, cells were routinely maintained on serum-free defined media (DM) or media supplemented with 10% fetal bovine serum (SM) as described previously (Iacovitti et al., 1999; Stull et al., 2002). In some cases (see Table 1), DM was supplemented with added growth substances in the following concentrations: all growth factors at 10 and/or 100 ng/ml, including GDNF family members (GDNF, neurturin, artemin, persphin) TGFβ-3, the neurotrophin family members (NGF, brain derived neurotrophic factor [BDNF], neurotrophin 3 [NT3]); fibroblast growth factor (FGF) 2 and 8; sonic hedgehog (SHH); epidermal growth factor (EGF), cytokines interleukin (IL) 1 and 2 and leukemia growth factor (LIF); depolarizing agents 40 μM KCl, 20 μM DA, anti-apoptotic agents, including the caspase inhibitors Z-VAD-FMK and Z-DEVD-FMK at 100 μM, 250 μM trolox (water soluble vitamin E), 100 μM melatonin. All long-term cultures are re-fed with the indicated media every 2–3 days in culture.
Generation of co-cultures and conditioned media
Glial beds (predominantly astrocytes) were generated from postnatal day 1 rat using well-established methods (McCarthy, 1980). Glia were derived either from DA-associated sites (striatum, midbrain) or unrelated sites (cerebral cortex). Cultures were fed every 2–3 days by replacing 50% of media with fresh SM. Once cells reached confluence, either co-cultures were established (PDN were plated as above) or glial-conditioned media (GCM) was collected and frozen at −80°C. Prior to use in culture, GCM was diluted 1:3 vol:vol. To generate CM from cultures of neurons only, striatal neurons were isolated from E13 –14 rat embryonic ganglionic eminences as described previously (Du and Iacovitti, 1995) and CM generated as described above.
6-OHDA lesions
As described previously (Jin and Iacovitti, 1995), twenty-one Fischer 344 rats (Taconic) were made Parkinsonian for these studies. Briefly, rats were anesthetized with sodium pentobarbital (30 mg/kg i.p.), placed in a stereotaxic apparatus (Kopf Instruments) and a 26-gauge Hamilton syringe containing 6-OHDA (Sigma; 20 μg/ml in 4 μl PBS containing 0.2 mg/ml ascorbate) was lowered into the right median forebrain bundle (AP: −4.4 mm, ML: −1.2 mm, DV: −7.8 mm from bregma). The 6-OHDA solution was gradually injected at a rate of 1 μl/min. All lesions were verified 3 and 6 weeks later by assessment of rotational behavior in an automated rotometer system (Columbus Instruments) following amphetamine challenge (5 mg/kg, i.p.). Only rats with consistent and stable lesions (>10 ipsilateral turns/min on multiple tests) were used for transplantation studies.
Transplantation procedures
Animals with verified lesions were implanted with 10 μl of a suspension of fetal midbrain cells from transgenic mice (0.5–1 × 105 cells/1 μl) following FACS purification. Cells were deposited at two depths along the needle track (AP: +1.2 mm, ML: −2.7 mm, DV: −5.4 mm and −4.9 mm) in the hope of generating a continuous dorsal to ventral strand of transplanted cells in the striatum on the side ipsilateral to the 6-OHDA lesion as described previously (Yang et al., 2004). All transplant recipients received cyclosporin A (10 mg/kg IP) daily, beginning the day prior to transplantation.
Immunocytochemistry
Cultures were rinsed twice and then fixed with 4% paraformaldehyde (Du and Iacovitti, 1995). Likewise, in transplantation experiments, rats were perfused with 500 ml of cold (4°C) 4% periodate–lysine–paraformaldehyde. Brain sections were cut at 30 μm on a freezing microtome and processed for immunocytochemistry using immunofluorescence staining method described previously (Yang et al., 2002, 2003, 2004). We stained with polyclonal rabbit antibodies to TH (Pel-Freeze; 1:100), AADC (Protos Biotech, 1:100), Nurr 1 (Santa Cruz, 1:200) and secondary antibodies from Jackson Immunoresearch: donkey anti-rabbit-rhodamine (1:100). Cells were counterstained with the nuclear dye Bis-benzimide (Hoechst 33258; Molecular Probes, final conc. = 1 mg/ml). Endogenous GFP fluorescence was visualized with FITC fluorescence optics. In cultures, stained cells (TH, GFP, Bisbenzimide) were counted in 3–5 representative fields and expressed as a percent of total cells in 3–5 platings. In grafts, stained cells were counted along the entire length of the graft using a Nikon-Scanalytics Image System or a Zeiss LSM510 Confocal Image System.
Cell viability analysis in vitro
In growth factor experiments, the viability of PDN or VM cells was assessed in live cultures over a 10-day period in vitro. At 1, 2, 3, 5, 7, and 10 days, cultures were photographed for cell count analysis (3–5 representative fields). All photographs were analyzed by an independent experimenter for cell viability which was defined as those GFP+ cells possessing a large stellate perikarya bearing one or more processes (see Fig. 6A) compared to dead or dying cells with a small neurite-less round profile. In some cases, viability was further assessed using the Viability/Cytotoxicity Kit from Molecular Probes as described previously (Stull et al., 2002). In brief, cultures were rinsed twice with PBS and subsequently incubated for 30 min with 4 μM ethidium homodimer-1 (final concentration in PBS) which fluoresces red. Cells were immediately analyzed on a fluorescence microscope using rhodamine optics and 3–5 representative fields were photographed. Endogenous GFP expression in cells was simultaneously imaged, and photomicrographs were later analyzed for cell number.
RT-PCR analysis
Real time PCR was performed according to the manufacturer's instructions (Cell to cDNATM II from Ambion; SYBR Green PCR Master Mix and RT-PCR from Applied Biosystem) as modified for use in our laboratory (Suon et al., 2004). Briefly, cells plated at 1 × 105/dish were harvested, counted and incubated with lysis buffer (75°C for 10 min). DNase was added into each tube with 0.06 μg/μl of final concentration. Genomic DNA was digested by incubating cell lysate at 37°C for 45 –60 min. In the pilot experiment, the RT reaction was conducted by incubating 5–10 μl of cell lysate, 4 μl of dNTP mix, 2 μl of 16 μl random decamers and 5–10 μl of nuclease-free water (70°C for 3 min) followed by the addition of 2 μl of 10 × RT buffer, 1 μl of M-MLV RTase and 1 μl of RNase inhibitor supplied in the kit at 42°C for an hour. The PCR reaction was carried out for each target gene and the internal control gene, β-actin. Primer sequences (forward and reverse) were as follows: β-actin: (5′-TCACCCACACTGTGCCCATCTACGA-3′, 5′-CAGCGGAACCGCTCATTGCCAATG-3′) c-Ret: (5′-GCGCCCCGAGTGTGAGGAATGTGG-3′, 5′-GCTGATGCAATGGGCGGCTTGTGC-3′). 10 μl of PCR products was resolved on a 1% agarose gel by electrophoresis (at 100 V for 20 min). By comparing the intensity of each PCR band, a suitable cell number (2–5 × 105) for further cDNA preparation was chosen. For further analysis of the expression of genes, real time PCR was performed with the agents supplied in the ABI Prism7700 Sequence Detection System kit as follows: 50°C for 2 min, 95°C for 10 min, 95°C 15 s and 60°C 1 min, total 40 cycles. Serial titration was conducted with different concentrations (1 nM to 100 nM) for each pair of primers (available upon request) and different volumes of the template of each gene and of β-actin gene (1 nM to 50 nM). The best concentrations, which gave the same efficiency in the real time PCR for each target gene and internal control gene β-actin, were determined. Six duplicate samples were run in each real time PCR reaction in order to calculate mean value of CT for each amplified gene. The results were analyzed with the Sequence Detection System software 1.7 and computed according to Comparative CT Method for relative quantitation of gene expression (Applied Biosystems).
Statistical analysis
Data were statistically analyzed by one way analysis of variance. When P < 0.05, then the F test was followed by the two-tailed Student's t test to compare the statistical significance between control and experimental groups. Differences were considered significant only when the P value was less than 0.05.
Acknowledgments
This work was supported by NIH NS32519, NS43309 and NS48315.
References
- Akerud P, Alberch J, Eketjall S, Wagner J, Arenas E. Differential effects of glial cell line-derived neurotrophic factor and neurturin on developing and adult substantia nigra dopaminergic neurons. J. Neurochem. 1999;73:70–78. doi: 10.1046/j.1471-4159.1999.0730070.x. [DOI] [PubMed] [Google Scholar]
- Barker RA, Dunnett SB, Faissner A, Fawcett JW. The time course of loss of dopaminergic neurons and the gliotic reaction surrounding grafts of embryonic mesencephalon to the striatum. Exp. Neurol. 1996;141:79–93. doi: 10.1006/exnr.1996.0141. [DOI] [PubMed] [Google Scholar]
- Bilang-Bleuel A, Revah F, Colin P, Locquet I, Robert JJ, Mallet J, Horellou P. Intrastriatal injection of an adenoviral vector expressing glial-cell-line-derived neurotrophic factor prevents dopaminergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 1997;94:8818–8823. doi: 10.1073/pnas.94.16.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund LM, Sanchez-Pernaute R, Chung S, Anderson T, Chen IYC, McNaught KSP, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinsonian rat model. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P, Karlsson J, Emgard M, Schierle GS, Hansson O, Petersen A, Castilho RF. Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell. Transplant. 2000;9:179–195. doi: 10.1177/096368970000900205. [DOI] [PubMed] [Google Scholar]
- Burke RE, Antonelli M, Sulzer D. Glial cell line-derived neurotrophic growth factor inhibits apoptotic death of postnatal substantia nigra dopamine neurons in primary culture. J. Neurochem. 1998;71:517–525. doi: 10.1046/j.1471-4159.1998.71020517.x. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Schallert T, Crippens D, Davidson BL, Chang YN, Chiang YL, Qian J, Bardwaj L, Bohn MC. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Exp. Neurol. 1998;154:261–275. doi: 10.1006/exnr.1998.6887. [DOI] [PubMed] [Google Scholar]
- Clarkson ED, Zawada WM, Freed CR. GDNF reduces apoptosis in dopaminergic neurons in vitro. NeuroReport. 1995:145–149. [PubMed] [Google Scholar]
- Connor B, Kozlowski DA, Unnerstall JR, Elsworth JD, Tillerson JL, Schallert T, Bohn MC. Glial cell line-derived neurotrophic factor (GDNF) gene delivery protects dopaminergic terminals from degeneration. Exp. Neurol. 2001;169:83–95. doi: 10.1006/exnr.2001.7638. [DOI] [PubMed] [Google Scholar]
- Deacon T, Dinsmore J, Costantini LC, Ratliff J, Isacson O. Blastula-stage stem cells can differentiate into dopaminergic and serotonergic neurons after transplantation. Exp. Neurol. 1998;149:28–41. doi: 10.1006/exnr.1997.6674. [DOI] [PubMed] [Google Scholar]
- Du X, Iacovitti L. Synergy between growth factors and neurotransmitters required for catecholamine differentiation in brain neurons. J. Neurosci. 1995;15:5420–5427. doi: 10.1523/JNEUROSCI.15-07-05420.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emgard M, Karlsson J, Hansson O, Brundin P. Patterns of cell death and dopaminergic neuron survival in intrastriatal nigral grafts. Exp. Neurol. 1999;160:279–288. doi: 10.1006/exnr.1999.7198. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Jr., Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- Fernandez-Espejo E. Pathogenesis of Parkinson's disease: prospects of neuroprotective and restorative therapies. Mol. Neurobiol. 2004;29:15–30. doi: 10.1385/MN:29:1:15. [DOI] [PubMed] [Google Scholar]
- Folkerth RD, Durso R. Survival and proliferation of nonneural tissues, with obstruction of cerebral ventricles, in a parkinsonian patient treated with fetal allografts. Neurol. 1996;46:1219–1225. doi: 10.1212/wnl.46.5.1219. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield Culver HS, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N. Engl. J. Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Gates MA, Laywell ED, Fillmore H, Steindler DA. Astrocytes and extracellular matrix following intracerebral transplantation of embryonic ventral mesencephalon or lateral ganglionic eminence. Neuroscience. 1996;74:579–597. doi: 10.1016/0306-4522(96)00146-7. [DOI] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Ai Y, Gash DM, Gerhardt GA. Intracranial delivery of proteins and peptides as a therapy for neurodegenerative diseases. Prog. Drug Res. 2003;61:101–123. doi: 10.1007/978-3-0348-8049-7_4. [DOI] [PubMed] [Google Scholar]
- Horger BA, Nishimura MC, Armanini MP, Wang LC, Poulsen KT, Rosenblad C, Kirik D, Moffat B, Simmons L, Johnson E, Jr., Milbrandt J, Rosenthal A, Bjorklund A, Vandlen RA, Hynes MA, Phillips HS. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J. Neurosci. 1998;18:4929–4937. doi: 10.1523/JNEUROSCI.18-13-04929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DY, Ardayfio P, Kang UJ, Semina EV, Kim KS. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res. Mol. Brain Res. 2003;114:123–131. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- Iacovitti L, Stull ND. Induction of tyrosine hydroxylase in hNT neurons. NeuroReport. 1997;8:1471–1474. doi: 10.1097/00001756-199704140-00029. [DOI] [PubMed] [Google Scholar]
- Iacovitti L, Stull ND, Mishizen A. Neurotransmitters, KCl and antioxidants rescue striatal neurons from apoptotic cell death in culture. Brain Res. 1999;816:276–285. doi: 10.1016/s0006-8993(98)00955-x. [DOI] [PubMed] [Google Scholar]
- Iacovitti L, Stull ND, Jin H. Differentiation of human dopamine neurons from an embryonic carcinomal stem cell line. Brain Res. 2001;912:99–104. doi: 10.1016/s0006-8993(01)02723-8. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Vila M, Djaldetti R, Guegan C, Liberatore G, Liu J, O'Malley KL, Burke RE, Przedborski S. Developmental cell death in dopaminergic neurons of the substantia nigra of mice. J. Comp. Neurol. 2000;424:476–488. doi: 10.1002/1096-9861(20000828)424:3<476::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Jin BK, Iacovitti L. Dopamine differentiation factors produce partial motor recovery in 6-hydroxydopamine lesioned rats. Neurobiol. Dis. 1995;2:1–12. doi: 10.1006/nbdi.1995.0001. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Kessler MA, Yang M, Gollomp KL, Jin H, Iacovitti L. The human tyrosine hydroxylase promoter. Mol. Brain Res. 2003;112:8–23. doi: 10.1016/s0169-328x(02)00694-0. [DOI] [PubMed] [Google Scholar]
- Kholodilov N, Yarygina O, Oo TF, Zhang H, Sulzer D, Dauer W, Burke RE. Regulation of the development of mesencephalic dopaminergic systems by the selective expression of glial cell line-derived neurotrophic factor in their targets. J. Neurosci. 2004;24:3136–3146. doi: 10.1523/JNEUROSCI.4506-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Freeman TB, Snow BJ, Vingerhoets FJG, Mufson EJ, Sanberg PR, Hauser RA, Smith DA, Nauert GM, Perl DP, Olanow WC. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N. Engl. J. Med. 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- Krieglstein K, Henheik P, Farkas L, Jaszai J, Galter D, Krohn K, Unsicker K. Glial cell line-derived neurotrophic factor requires transforming growth factor-beta for exerting its full neurotrophic potential on peripheral and CNS neurons. J. Neurosci. 1998;18:9822–9834. doi: 10.1523/JNEUROSCI.18-23-09822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K, Strelau J, Schober A, Sullivan A, Unsicker K. TGF-beta and the regulation of neuron survival and death. J. Physiol. 2002;96:25–30. doi: 10.1016/s0928-4257(01)00077-8. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat. Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doharty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- McCarthy DK, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokry J. Experimental models and behavioural tests used in the study of Parkinson's disease. Physiol. Res. 1995;44:143–150. [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J. Neural Transm., Suppl. 2000;60:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Olsin L. The coming of age of the GDNF family and its receptors: gene delivery in a rat Parkinson model may have clinical implications. Trends Neurosci. 1997;20:277–279. doi: 10.1016/s0166-2236(97)01098-9. [DOI] [PubMed] [Google Scholar]
- Oo TF, Kholodilov N, Burke RE. Regulation of natural cell death in dopaminergic neurons of the substantia nigra by striatal glial cell line-derived neurotrophic factor in vivo. J. Neurosci. 2003;23:5141–5148. doi: 10.1523/JNEUROSCI.23-12-05141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee KS, Lee YJ, Shin HA, Cho HY, Wang KC, Kim YS, Lee HT, Chung KS, Kim EY, Lim J. Generation of dopaminergic neurons in vitro from human embryonic stem cells treated with neurotrophic factors. Neurosci. Lett. 2004;359:99–103. doi: 10.1016/j.neulet.2004.01.073. [DOI] [PubMed] [Google Scholar]
- Reum T, Olshausen F, Mazel T, Vorisek I, Morgenstern R, Sykova E. Diffusion parameters in the striatum of rats with 6-hydroxydopamine-induced lesions and with fetal mesencephalic grafts. J. Neurosci. Res. 2002;70:680–693. doi: 10.1002/jnr.10332. [DOI] [PubMed] [Google Scholar]
- Roussa E, Krieglstein K. Induction and specification of midbrain dopaminergic cells: focus on SHH, FGF8, and TGF-beta. Cell Tissue Res. 2004;318:23–33. doi: 10.1007/s00441-004-0916-4. [DOI] [PubMed] [Google Scholar]
- Rousselet A, Fetler L, Chamak B, Prochiantz A. Rat mesencephalic neurons in culture exhibit different morphological traits in the presence of media conditioned on mesencephalic or striatal astroglia. Dev. Biol. 1988;129:495–504. doi: 10.1016/0012-1606(88)90395-8. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Nakao N, Kobayashi K, Matsushita N, Takahashi H, Kakishita K, Yamamoto A, Yoshizaki T, Terashima T, Murakami F, Itakura T, Okano H. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz MK, Schnell L, Castro AJ, Schwab ME, Kartje GL. Cholinergic innervation of fetal neocortical transplants is increased after neutralization of myelin-associated neurite growth inhibitors. Exp. Neurol. 1998;149:390–397. doi: 10.1006/exnr.1997.6731. [DOI] [PubMed] [Google Scholar]
- Schulz TC, Palmarinin GM, Noggle SA, Weiler DA, Mitalipova MM, Condie BG. Directed neuronal differentiation of human embryonic stem cells. BMC Neurosci. 2003;4:27–40. doi: 10.1186/1471-2202-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME. Molecules inhibiting neurite growth: a minireview. Neurochem. Res. 1996;21:755–761. doi: 10.1007/BF02532297. [DOI] [PubMed] [Google Scholar]
- Sortwell CE, Daley BF, Pitzer MR, Collier TJ. Time course of apoptotic cell death within mesencephalic cell suspension grafts: implications for improving grafted dopamine neuron survival. Exp. Neurol. 2000;165:268–277. doi: 10.1006/exnr.2000.7476. [DOI] [PubMed] [Google Scholar]
- Stull ND, Polan DL, Iacovitti L. Antioxidants protect dopamine neurons and their terminals from toxin induced destruction in vivo and in vitro. Brain Res. 2002;931:181–185. doi: 10.1016/s0006-8993(02)02269-2. [DOI] [PubMed] [Google Scholar]
- Suon S, Jin H, Donaldson AE, Caterson EJ, Tuan RS, Deschennes G, Marshall C, Iacovitti L. Adult human bone marrow stem cells transiently differentiate in culture to express CNS proteins. Stem Cells Dev. 2004;13:625–635. doi: 10.1089/scd.2004.13.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann P, Schulz JB. Cellular pathology of Parkinson's disease: astrocytes, microglia and inflammation. Cell Tissue Res. 2004;318:149–161. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- Trott CT, Fahn S, Greene P, Dillon S, Winfield H, Winfield L, Kao R, Eidelberg D, Freed CR, Breeze RE, Stern Y. Cognition following bilateral implants of embryonic dopamine neurons in PD: a double blind study. Neurol. 2003;60:1938–1943. doi: 10.1212/01.wnl.0000070181.28651.3b. [DOI] [PubMed] [Google Scholar]
- Yang M, Snyder EY, Stull ND, Berk M, Iacovitti L. Neuronal stem cells spontaneously differentiate into dopaminergic neurons after transplantation into the intact or 6-hydroxydopamine lesioned rat. Exp. Neurol. 2002;177:50–60. doi: 10.1006/exnr.2002.7989. [DOI] [PubMed] [Google Scholar]
- Yang M, Donaldson AE, Jiang Y, Iacovitti L. Factors influencing the differentiation of dopaminergic traits in transplanted neural stem cells. Mol. Cell. Neurobiol. 2003;23:851–864. doi: 10.1023/A:1025017423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Donaldson AE, Marshall CE, Shen J, Iacovitti L. Studies on the differentiation of dopaminergic traits in human progenitor cells in vitro and in vivo. Cell. Transplant. 2004;113:535–547. doi: 10.3727/000000004783983729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Torri C, Ferrari R, Jansson A, Zini I, Fuxe K, Agnati LF. The emergence of the volume transmission concept. Brain Res. Rev. 1998;26:136–147. doi: 10.1016/s0165-0173(97)00048-9. [DOI] [PubMed] [Google Scholar]



