Abstract
Swimming advisories due to excessive Escherichia coli concentrations are common at 63rd Street Beach, Chicago, Ill. An intensive study was undertaken to characterize the source and fate of E. coli in beach water and sand at the beach. From April through September 2000, water and sand samples were collected daily or twice daily at two depths on three consecutive days per week (water samples, n = 1,747; sand samples, n = 858); hydrometeorological conditions and bird and bather distributions were also recorded. E. coli concentrations in sand and water were significantly correlated, with the highest concentration being found in foreshore sand, followed by those in submerged sediment and water of increasing depth. Gull contributions to E. coli densities in sand and water were most apparent on the day following gull activity in a given area. E. coli recolonized newly placed foreshore sand within 2 weeks. Analysis of variance, correlation, cluster analyses, concentration gradients, temporal-spatial distribution, demographic patterns, and DNA fingerprinting suggest that E. coli may be able to sustain population density in temperate beach sand during summer months without external inputs. This research presents evidence that foreshore beach sand (i) plays a major role in bacterial lake water quality, (ii) is an important non-point source of E. coli to lake water rather than a net sink, (iii) may be environmentally, and perhaps hygienically, problematic, and (iv) is possibly capable of supporting an autochthonous, high density of indicator bacteria for sustained periods, independent of lake, human, or animal input.
Along the coasts of the Great Lakes, many communities have adopted beach monitoring programs to protect visitors from potentially harmful microbes in accordance with the requirements of the U.S. Environmental Protection Agency (EPA) (7). Increased coastal development and increased recreational use of beaches have resulted in greater threats of water contamination and the associated public health hazard. While indicator bacteria may be present in the swimming water, sediment and sand along the water's edge may also be a significant source of microbes (53).
In the United States, freshwater beaches are routinely analyzed for Escherichia coli because it is an indicator of sewage contamination and thus the possible presence of human pathogens (bacteria, protozoa, and viruses) (3, 22, 29). Potential sources may include sewage overflows (34), leaking septic systems, and birds occupying the beach (28, 30, 46, 50). Rainfall and onshore winds can cause a dramatic increase in concentration and can exacerbate the contamination problem (15, 56). Bacterial counts in water eventually fall to levels considered to be safe for swimming; however, many indicators remain in the sediment and adjacent beach sand (17, 36) and can impact beach water quality at a later time.
Numerous studies have shown that beach sand can harbor significant levels of indicator bacteria (32, 46), which are often present in higher concentrations than in the water column (10, 21, 34, 43). Bacteria present in the sand include E. coli, Streptococcus spp., Campylobacter spp., and Pseudomonas spp. (30, 31). Due to the higher organic content of detritus and silts associated with the sand, these bacteria may be sustained in this moist environment for a long time (10, 13, 16, 37, 51). The mechanism by which these bacteria initially populate the beach sand is poorly understood. More specifically, it is unknown whether occurrence is passive (e.g., via animal feces, deposition on shore, or concentration by sand) or active (e.g., growing during suitable conditions or recovery of previously nonculturable cells).
Beach visitors tend to spend a majority of their time in contact with the sand, and there is increased public concern about associated contamination (41, 47). Due to the potential for higher densities of pathogens, prolonged contact with contaminated beach sand may conceivably be a greater threat to beach visitors than water exposure (32, 47). Children are particularly at risk because they spend a great deal more time playing and digging in the wet sand, and the elderly and infirm are more susceptible to illnesses associated with contamination. Bacteria harbored in the sand may persist longer than in the water because they adhere to sediment particles, unlike free bacteria in the water.
Historically, 63rd Street Beach in Chicago, Ill., has had elevated levels of E. coli in its swimming water. When E. coli levels exceed the U.S. EPA recommended limit of 235 CFU/100 ml, the beach is closed to swimming, resulting in a loss of recreational opportunities for hundreds of visitors. Many visitors, however, remain at the beach to enjoy activities on the shore, including playing in the sand, where E. coli and perhaps pathogens may also be present.
The purpose of the present research was to examine the concentration and interaction of E. coli in sand and beach water of a selected beach along southern Lake Michigan. Concentrations of E. coli, the direction of E. coli movement between sand and water, and the influences of sand, water, bird presence, and hydrometeorology were examined over 6 months. Potential inputs, relationships, and concentration gradients were explored. The question of whether concentrations of E. coli in sand were ultimately controlling beach water quality at 63rd Street Beach was examined.
MATERIALS AND METHODS
Study area.
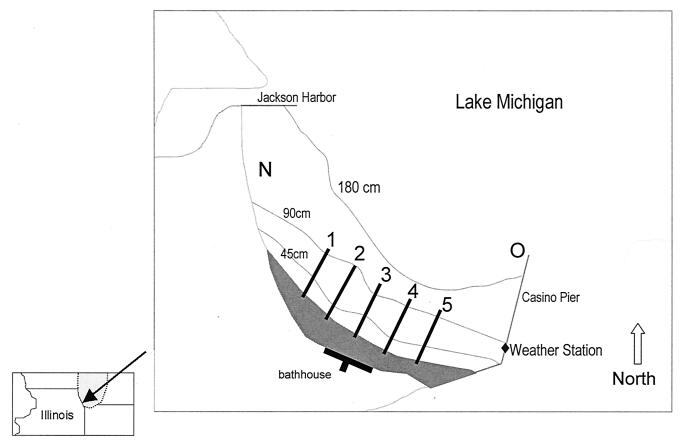
The study area was located on the southwest shore of Lake Michigan at 63rd Street Beach (Fig. 1). The beach is approximately 12 km south of downtown Chicago, within the Jackson Park area. On the north end of the beach, stone revetment extends along the shore, ending at Jackson Harbor and an associated breakwater. The southern end of the beach ends at Casino Pier, a doglegged breakwater that partially encloses the beach basin. During the recreation season, Memorial Day through Labor Day, the Chicago Park District rakes the beach daily using a beach-grooming machine. Five transects were established 100 m apart perpendicular to the shore. At each transect, sediment samples were recovered onshore 1 m from the furthest extent of the waves (foreshore sand) and from the point at which the overlying water was 45 cm deep (submerged sand). Water samples were collected from above the underlying sand at depths of 45 and 90 cm for each transect. An additional water-sampling site was located at the end of Casino Pier (referred to as offshore).
FIG. 1.
63rd Street Beach, with five sampling transects identified. Sampling sites were 1 m onshore and in water 45 and 90 cm deep. The shaded area indicates beach sand; contour lines indicate selected depths. N, north revetment sampling sight; O, offshore sampling site.
Sample collection. (i) Water and sand.
Between April and September 2000, water and sediment samples were aseptically collected on three consecutive days per week, usually Tuesday through Thursday, along each of the five transects. Sediment samples were collected at 7:00 a.m. each sampling day by pushing a 2.3- by 30-cm slotted recovery probe (AMS, American Falls, Idaho) containing a sterile butyrate liner at least 20 cm into the sediment at two sites (foreshore and submerged) along the transect. Upon extraction, any overlying water was carefully decanted and the liner was capped and removed from the probe. A sixth point was selected for sediment sampling each morning in the center of the onshore area occupied by the highest concentration of gulls (ring-billed gulls, Larus delawarensis, and herring gulls, Larus argentatus).
Water samples were collected at 7:00 a.m. and 1:00 p.m. each sampling day along the five transects at 45- and 90-cm depths. A sterile polyethylene bag was dipped below the water surface to collect water. During morning sampling, an additional water sample was collected off the north revetment and offshore.
After collection, all samples were kept at 4°C and taken to the laboratory for analysis. All samples were analyzed within 3 h of collection.
(ii) Daily observations.
At the time of each morning and afternoon sampling, weather parameters, including air and water temperatures, wind direction, and wind speed, were recorded. Along each transect, wave height was measured. Also, at each of the five transects, the gulls onshore and bathers in the lake were counted. Gull feces were counted at each transect by using triplicate 0.5-m2 quadrats randomly determined within the first 5 m of shore.
A weather station was installed onsite. Insolation, temperature, wind direction and speed, rainfall, and relative humidity were continuously logged. These weather data were supplemented by digital information gathered by a southern Lake Michigan offshore buoy maintained by the National Oceanic and Atmospheric Administration. From this weather station, data for wind speed, wind direction, wind gust, wave height, pressure, air temperature, and water temperature were collected hourly.
Sample analyses.
For sediment samples, total sample volume was calculated by measuring sediment core length (nearest 1 cm). The liner was then emptied, and contents were rinsed into a sterile 250-ml polypropylene bottle by using 100 ml of sterile phosphate-buffered diluent water (pH 6.8) (4). All sample bottles were shaken simultaneously for 5 min at 210 rpm on an Eberbach rotary platform shaker. The suspended particles were allowed to settle for a few minutes before sample volumes were removed by pipette. Recovery of E. coli in seeded sand was estimated to be 80, 91, and 96% in sequential washings. Testing was conducted according to standard methods (4). Samples were tested by the membrane filtration method (mTEC agar; Difco Laboratories, Detroit, Mich.) at three volumes (0.1, 1.0, and 4.0 ml). Results were reported as CFU/100 ml. Water samples were also analyzed at three volumes (1, 10, and 50 ml) by using the method described above.
Sand replacement.
In preparation for the swimming season, surface foreshore sand at 63rd Street Beach was removed from the shoreline to about 10 m inland to a depth of 10 to 15 cm along 400 m of shoreline. Clean, E. coli-free sand from North Avenue and Montrose upland areas was used as a replacement on 24 and 25 May 2000. Sand samples were collected from the trucks for E. coli analysis prior to sand placement on the beach and were confirmed to be free of E. coli.
Statistical analyses.
Statistical analyses were performed using SPSS, version 10.0, and SYSTAT, version 9, software (SPSS, Inc., Chicago, Ill.). E. coli data were log10 transformed to meet parametric assumptions of equality of variances and normal distribution. These assumptions were confirmed using the Kolmogorov-Smirnov test for normality and Levene's test for equality of variance coupled with examination of P-P plots. Hydrometeorological data were not transformed. Pearson correlation analysis was used for comparing E. coli levels in sand and water at different sites. Bather and bird data could not be adequately adjusted, and thus nonparametric analysis was used. Significance was set at a P value of 0.05 unless otherwise stated. The lack of temporal autocorrelation between sampling days or weeks for E. coli allowed for time-independent analysis of variance. Samples collected at the same time or day were paired.
RESULTS
Interaction of sand and water.
E. coli counts were higher in the foreshore and submerged sand than in beach water, with concentrations in foreshore sand typically being several orders of magnitude higher than in water (Table 1). Approximately 27% of the water samples collected in the swimming area exceeded the U.S. EPA recommended level of 235 CFU/100 ml for swimmable water. If we equate cubic centimeters of whole fresh sand to milliliters of beach water (admittedly, these are not equivalent due to diluent differences), 95% of the foreshore sand and 76% of the submerged sand collected had E. coli counts in excess of the U.S. EPA recommendation for swimming. The U.S. EPA criterion comparisons are based on the assumption that the number of CFU/100 cm3 of sand is roughly equivalent to the number of CFU/100 ml of water, which is likely to be too conservative. While dry weights were not routinely taken in this study, we estimated from other experiments with similar sand that 63rd Street Beach sand contained roughly 20% (wt/wt) water content. This unpacked dry beach sand has a specific gravity near that of water (0.95), and thus equivalent aqueous loading of bacteria would have approximately five times the values reported here. Presumably, sand biofilms play an important role in bacterial retention and support (40), and thus even the expression of CFU per unit of interstitial water is not entirely accurate. The proper expression of E. coli counts in saturated beach sand remains an unresolved and important issue.
TABLE 1.
E. coli counts in sand and water at 63rd Street Beach
| Sample source | Mean count (CFU/100 ml) ± SD | Median | Geometric mean |
|---|---|---|---|
| Foreshore sand | 1.1 × 104 ± 8.5 × 102 | 4.8 × 103 | 4.0 × 103 |
| Submerged sand | 2.6 × 103 ± 7.6 × 102 | 7.6 × 102 | 7.2 × 102 |
| Water (all) | 1.1 × 102 ± 1.3 × 102 | 6.0 × 101 | 4.3 × 101 |
| Water (45 cm) | 1.6 × 102 ± 1.8 × 102 | 8.4 × 101 | 6.2 × 101 |
| Water (90 cm) | 7.5 × 101 ± 9.3 × 101 | 3.6 × 101 | 2.6 × 101 |
| Offshore water | 3.4 × 101 ± 4.4 × 101 | 1.0 × 101 | 1.2 × 101 |
Mean levels of E. coli in foreshore sand among the five transects (Table 1) were significantly different (P = 0.004). Foreshore transects 1 and 2 had higher counts of E. coli. In submerged sand, mean E. coli counts were not significantly different among transects (P = 0.604). E. coli counts in foreshore and submerged sand were significantly correlated when same-day samples were compared (r = 0.717, P < 0.01, n = 78).
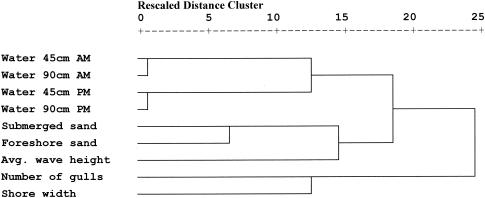
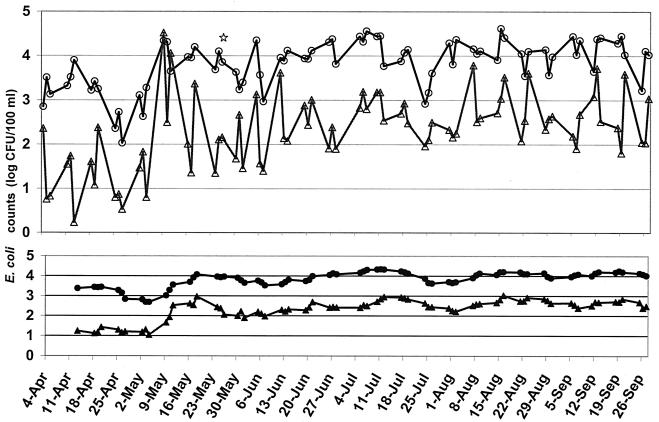
Most beach-monitoring programs are based on the presumed relationship between E. coli counts in water on the sampling day and those on the next day (reporting day). There was very little relationship between E. coli counts on the day of sampling and those on the reporting day (r2 = 0.17, n = 57). However, even with the difference in E. coli counts, there were consistent correlations between E. coli counts in foreshore and submerged sand and all combinations of water (samples from the mornings, the afternoons, and at depths of 45 and 90 cm) (Fig. 2). A recognizable pattern of increasing and decreasing counts in sand and water was apparent over time (Fig. 3). Additionally, E. coli counts were significantly correlated with all water samples taken at points distant from the beach (i.e., offshore and north revetment).
FIG. 2.
Hierarchical cluster analysis of distance for E. coli concentrations in sand and water and selected factors associated with E. coli concentrations. Connected vertical lines designate joined cases. The dendrogram rescales the actual distances to numbers between 0 and 25, preserving the ratio of the distances between steps (SPSS, Inc.).
FIG. 3.
Daily mean log E. coli counts in sand and water over the sampling period. The lower portion shows the 3-day moving average. Circles, foreshore sand; triangles, 45-cm-deep water. The star indicates when sand was replaced along 400 m of the shore to a depth of 10 to 15 cm and inland 10 m.
E. coli and physical factors.
E. coli counts in both foreshore and submerged sand were correlated with both air and water temperatures. The mean daily air temperature ranged from 1.35 to 23.11°C during the sampling season and was correlated with E. coli counts in foreshore sand (r = 0.593, P < 0.001) and submerged sand (r = 0.401, P < 0.001). The mean daily water temperature ranged from 4.3 to 22.0°C and was significantly correlated with E. coli counts in foreshore sand (r = 0.592, P < 0.001) and submerged sand (r = 0.396, P < 0.001). Throughout the sampling season, there was a gradual increase in E. coli counts in sand and water. This has been observed in a previous study (56) and has been attributed to higher survival and perhaps growth rates in warmer temperatures. Concentrations of E. coli in water were also correlated with air and water temperatures (air temperature, r = 0.327 and P = 0.018; water temperature, r = 0.333 and P = 0.017).
In regression analysis, wind speed was also a predictor for E. coli counts in sand, and wave height and wind direction, in addition to temperature factors, were useful for predicting E. coli concentration in sand. Many of the implicated physical factors are directly related to wind effects and seasonal characteristics. Throughout the sampling period, wind direction was from the south (between 90 and 270°) on 70% of the sampling days. Southerly winds elevate water stage along the breakwater of Jackson Harbor; combined with the prevailing nearshore current (from north to south), the confluence of physical factors presumably traps water in the beach basin.
Lagged interaction.
To evaluate the temporal influence of the foreshore sand-water interaction, correlations between the daily means of E. coli concentrations in water (for samples from both 45 and 90 cm) and foreshore E. coli concentrations for the same day were inspected and lagged one day. Since the beach was sampled on Tuesday through Thursday, two comparisons were appropriate for lagged samples (only samples from 2 days per week were analyzed for unlagged samples to keep degrees of freedom constant). The hypothesis was that a significant correlation between E. coli in sand and the previous day’s water concentration would indicate that concentrations in sand were mostly influenced by water E. coli content, while a significant correlation between E. coli in sand and the next day’s water concentration would suggest that water was predominantly influenced by sand E. coli content. Further, direct correlations between sand and water on the same day or during the same week would describe the daily or weekly simultaneous relationship with no indication of the direction of influence.
Concentrations of E. coli in foreshore sand and submerged sand were correlated with mean E. coli concentrations in water on the same day (foreshore, r = 0.625 and P < 0.001; submerged, r = 0.375 and P = 0.002). Additionally, concentrations in foreshore and submerged sand were correlated only with concentrations in morning water samples the next day (foreshore, r = 0.470 and P < 0.001; submerged, r = 0.348 and P = 0.011) but not later that afternoon (foreshore, P = 0.180; submerged, P = 0.163). Interestingly, E. coli counts in foreshore sand were also correlated with mean overall E. coli counts in water for the previous day (r = 0.520, P < 0.001).
Potential sources.
The impact of gull density, a potential constant source of E. coli at the beach, on counts in sand and water was examined. The numbers of birds in morning and afternoon observations were correlated (r = 0.128, P = 0.041), and there was no significant difference in either morning or afternoon bird density among the five transects. The number of birds present was not correlated with the concentration of E. coli in the sand or water when compared on a daily basis. When the number of gulls was lagged one day, i.e., the gull count on one day was compared with E. coli concentrations in the sand and water the next day, the significance level in a Spearman correlation analysis increased for most categories of counts (Table 2); only E. coli counts in the submerged sand were not correlated with the lagged number of gulls.
TABLE 2.
Spearman correlation between the number of gulls on the beach and the combined E. coli counts
| Sample source | Correlation between E. coli counts and no. of gulls
|
|||||
|---|---|---|---|---|---|---|
| Unlagged
|
Lagged 1 daya
|
|||||
| Spearman's rho value | P value | nc | Spearman's rho value | P value | n | |
| Foreshore sand | 0.216 | 0.133 | 50 | 0.369b | 0.008 | 50 |
| Submerged sand | 0.005 | 0.972 | 50 | 0.202 | 0.159 | 50 |
| Water (a.m.) | 0.248 | 0.083 | 50 | 0.468b | 0.001 | 50 |
| Water (p.m.) | 0.032 | 0.838 | 44 | 0.352b | 0.019 | 44 |
| Water (45 cm) | 0.147 | 0.310 | 50 | 0.483b | 0.001 | 50 |
| Water (90 cm) | 0.223 | 0.119 | 50 | 0.371b | 0.018 | 50 |
“Lagged 1 day” means that a comparison was made between the number of gulls on a particular day and the E. coli counts on the following day.
Significant correlation.
n, number of comparisons.
The sand sample in the flock of gulls (gull sand) was obviously impacted by gulls, with dense feces, feathers, and refuse being present. It should be noted that foreshore sand was sampled 1 m from shore, and samples within the gull aggregation were often collected further inland. The concentrations of E. coli in foreshore sand and gull sand were essentially the same (log means ± standard deviations [SD], 4.02 ± 0.75 versus 3.99 ± 1.13), and a paired t test revealed a strong correlation (r = 0.414, P < 0.0001) but no significant difference (P = 0.556). The E. coli density in gull sand was also correlated with those in submerged sand and water at both depths (P < 0.01).
Sand replacement.
Following the sand replacement action, foreshore sand was quickly recolonized to earlier E. coli levels within a 2-week period. Examination of the foreshore sand for E. coli 2 weeks before and 2 weeks after sand placement activity showed that E. coli numbers were significantly higher preceding sand addition (2.6 ± 0.14 versus 1.9 ± 0.15 log CFU/100 ml) even though the air temperature was cooler and there were no significant storms during these observations. The numbers of E. coli in sand remained steady for 2 weeks but increased thereafter. This was not unlike observations, albeit over a shorter time scale and with faster increase, seen in the laboratory growth experiments (data not shown).
DISCUSSION
E. coli distribution on the beach.
Some important features emerge regarding the general distribution of E. coli at 63rd Street Beach: (i) E. coli concentrations in foreshore sand greatly exceed those in water and, to a lesser degree, submerged sand; and (ii) E. coli concentrations are higher in shallow water than in deeper water. A revealing result was the relative equilibrium of E. coli density in sand compared with that in water. Fluctuations were noticeable; E. coli counts in sand exhibited a rise-and-fall pattern but never decreased to zero. While the variation in water is about twice that in sand (the coefficients of variation were 282 for sand and 446 for water at 45 cm), the two media were correlated, even with data lagging, indicating a continued flux between sand and water. We argue that this flux has bidirectional components, but the net movement of E. coli is presumably from the foreshore lakeward, driven by swash and suspension. Byappanahalli et al. (11) investigated a related mechanism where shorelines act as reservoirs of E. coli, which enters the aquatic system through marginal erosion.
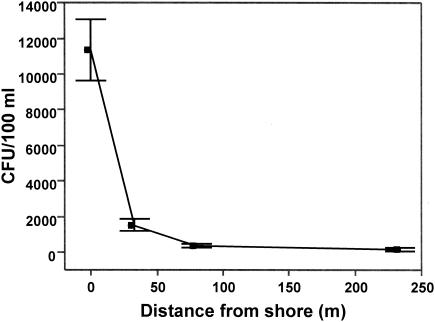
The spatial decrease in E. coli counts from foreshore sand to water with increasing depth was noteworthy (Fig. 4). Counts in foreshore sand were much higher than counts in water, a pattern seen in other studies (30, 44, 46). In water, E. coli counts were consistently higher in samples from a depth of 45 cm than in those from 90 cm, with counts decreasing in samples collected further from shore, a pattern that has also been shown in other lakes (50). However, when the foreshore sand and water are considered alone, the pattern becomes more dramatic, with counts in sand (mean, 11,000 CFU/100 ml) decreasing to minimal levels in offshore water, 700 m from shore (mean, 34 CFU/100 ml). Widely accepted paradigms of spatial dispersal patterns dictate that most passive organisms (8) and aqueous contamination (14) follow an exponential concentration gradient.
FIG. 4.
E. coli counts in sand and water at 63rd Street Beach by distance from shore. Error bars indicate ± 1 standard error of the mean.
This model suggests that foreshore sand is a source of E. coli, and the net loss from the system is due to aqueous dilution, dispersal, death, or sediment deposition within increasingly deeper water. It suggests that beach sand is a reservoir or source of E. coli and is less subject to daily external influences, a result that is consistent with ambient mathematical models for prediction of E. coli counts in water (G. Olyphant and R. Whitman, submitted for publication).
In a 1996 study at two Indiana beaches on Lake Michigan (data not shown), Whitman found that E. coli counts in sand were not significantly different at 1-m intervals from shore to 5 m onto the beach but that E. coli counts in the sand were consistently significantly higher than in the beach water (P < 0.05). In an examination of sand at 5-m intervals from shore to 40 m onto the beach in 2001 and 2002, Whitman (unpublished data) again found no significant difference between sand samples. This provides further evidence that the water is not continually concentrating E. coli in the sand. Given the high counts in sand compared with those in water, it is highly unlikely that sand would concentrate counts so effectively—essentially acting as an E. coli sink—without any reciprocal movement from sand to water. Further, the steady equilibrium in water and sand E. coli counts over time is not consistent with such a theory and is more consistent with traditional models of an independent population.
Sand colonization.
When sampling began in early April, E. coli counts in sand were already elevated, an occurrence unlikely to be the result of early-season multiplication, given the high counts (April mean, 2,277 CFU/100 ml) and early-season temperatures (April mean, 4.9°C). Research has shown that fecal indicators can persist in sand throughout the year with little variation in counts (44); survival in cold temperatures is typically high for indicator bacteria such as E. coli (54), which provides an early spring source of E. coli to the nearshore water. With the onset of warm temperatures, E. coli counts increased dramatically between April and May, the period with the greatest increase in average air and water temperatures over the sampling period. Populations remained relatively stable in sand thereafter. If beach sand were continually concentrating E. coli from water, then there should be periods of progressively increasing density over time followed by population density-independent declines due to episodic resource loss (e.g., food depletion, storms, and desiccation), but such a pattern was not observed.
During this study, there was circumstantial evidence that E. coli could increase in density rapidly under certain conditions. The increase in E. coli counts following sand replacement showed that E. coli populations could quickly reestablish to densities seen before replacement within a 2-week period. It is not certain whether the increase was in situ growth (as opposed to deposition by gulls or concentration from lake water), but similar increases have been noted elsewhere (20).
Potential sources. (i) Gulls.
Shorebirds have often been cited as a direct source of E. coli in beach sand and water (39, 46, 50), and other wildlife can also contribute to E. coli counts. The abundance of gulls at 63rd Street Beach (daily mean number, 216) could be considered a significant source of E. coli given the high density of indicator bacteria present in bird feces (39). It was believed that local flocks would elevate E. coli concentration in the immediate area of congregation; however, E. coli concentrations outside (foreshore sand) and inside (gull sand) the flock were remarkably similar. While this does not discount the contribution of gulls because of their movements over the beach through time, it points out that supplemental or internally generated sources may be important in the maintenance of the unusually stable foreshore E. coli populations.
Ting et al. (W. T. E. Ting, C. C. Tseng, D. S. Johnson, L. Dominguez, J. Vander Hoogt, M. Saluta, and R. L. Whitman, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. Q-305, 2000), using randomly amplified polymorphic DNA-PCR analysis of sand, water, and gull feces, suggested that both sand and water environments exerted selection pressure on the original bird E. coli. Haack et al. (S. Haack, L. Reynolds, J. Underwood, M. Wolcott, and R. Whitman, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., abstr. Q-375, 2001) analyzed 160 E. coli isolates from 63rd Street Beach using repetitive sequence-based PCR DNA fingerprinting and API-Vitek phenotypic responses and concluded that there was strong strain similarity between gulls and sand and water isolates. Using pulsed-field gel electrophoresis with Salmonella enterica serovar Typhimurium isolates, an even stronger genomic correlation was found between gulls and sand and water isolates from 63rd Street Beach (M. Wolcott, S. Haack, L. Reynolds, B. Berlowski, H. Gutzman, and R. Whitman, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., abstr. Q-379, 2001). When these E. coli or S. enterica serovar Typhimurium isolates were challenged with 14 antibiotics, over 93% showed susceptibility, suggesting possible nonhuman sources of contamination (23). Kinzelman et al. (submitted for publication), working at another southwestern Lake Michigan beach, found that genomic homogeneity was greater in foreshore sand than in water or submerged sand, a finding also supported by Davis and Gordon (18). This may support the suggestion that foreshore sand provides selective pressure on certain genotypes (52) and, if so, may support in situ multiplication of certain strains of E. coli in foreshore sand.
While these previous studies show that E. coli and S. enterica serovar Typhimurium originating from local gull populations are also present in the sand and water at 63rd Street Beach, dispersal of these bacteria is hard to characterize. Bacteria isolated from lake water may have originated from foreshore sand suspension (38) or direct gull defecation. E. coli density in sand correlated best with 1-day-lagged gull counts, suggesting that mobilization of gull feces from daytime resting areas to foreshore sand and water is delayed; alternately, nighttime roosting in the water or nearby areas could impact counts on the following day. Other research on fecal coliform counts in gulls showed that direct defecation into the lake water would certainly have an effect on water quality but indicated that the impact of feces high on the beach, although likely, was harder to implicate (2).
(ii) Other sources.
Bathing activities have been considered to be a potential source of E. coli through the shedding of indicator bacteria, resuspension of contaminated sediments, or transport of bacteria from the sand to the water (44, 48). Surveys at 63rd Street Beach, however, found that there was no correlation between the number of bathers and bacterial counts in the sand or water.
Although E. coli is capable of surviving in groundwater for several weeks (35, 54), groundwater is not likely to be a source for the beach unless it has been heavily and directly contaminated. At 63rd Street Beach, vibracores that extended 1 m below the water table (piezomentric surface) were collected along the shore. E. coli counts in the sand decreased to zero at approximately 30 cm below the water table, but counts above the water table were similar to counts in foreshore sand (data not shown). If groundwater carried E. coli into the beach, then counts would be high above and below the water table. Piezometers and seepage meters at the beach also failed to show significant E. coli input from groundwater.
Influencing factors.
Throughout the study season, there was a mean increase in E. coli counts in sand and water and a sustained relationship between these two media, but there were large fluctuations in overall counts, some of which could be attributed to hydrometeorological conditions. The situation of 63rd Street Beach is such that prevailing southerly winds directly force water onshore, which may increase E. coli concentration in the water (45). This action delivers nutrients to the E. coli population in the sand and washes sandborne bacteria into water (24, 56), a characteristic that has also been linked to tidal action (9, 20, 49). The lack of periodicity in counts could be related to the relative lack of tidal action in the Great Lakes compared with marine waters.
Relative increases in E. coli concentrations resulted from storm events and associated wind, but E. coli counts in sand subsequently decreased to prestorm levels; they did not decrease to zero (Fig. 3). In similar fashion, concentrations in water spiked in response to storm-related stirring of sediments and then decreased to ambient levels, typically much lower than those observed in foreshore and submerged sand. E. coli counts in water were more variable than in sand and were more responsive to hydrometeorological changes.
Moist sand in temperate regions provides a presumably suitable environment for many microflora; the presence of moisture, protection from lethal sunlight (26), a large surface area for biofilms, buffered temperatures, microhabitat cover from predation (17, 50), a steady supply of organic material by lake wash, and continual but gentle groundwater circulation that supplies nutrients (algae, plankton, and debris) and exchanges gases all make sand a viable, albeit suboptimal, environment for sustenance of enteric bacteria. Further, the rate of E. coli die-off is perhaps lower in sand and sediment than in water (17). The density and persistence of E. coli in water are much less, presumably because it is a harsher and less suitable habitat than sand (36). Factors that impact E. coli densities in sand include desiccation, UV exposure, erosion (storms) and deposition, and nutrients.
Potential for growth.
Much research has been conducted in tropical areas and evidence has been presented showing that indicator bacteria such as E. coli and enterococci can persist and thrive in natural environments (soil, water, and plants) (12, 25, 27). In addition to tropical areas, this phenomenon has been recognized in Australia (6), Germany (42), New Zealand (5), and Florida (20). Given the importance of an effective indicator for monitoring beaches for sewage contamination, determining whether or not E. coli and other indicator bacteria are growing in the sand is significant to environmental science and our understanding of the ecology of these important species.
At 63rd Street Beach, the Chicago Park District attempted to remove E. coli contamination by replacing existing marginal sand with many truckloads of E. coli-free sand, but within 2 weeks, E. coli counts were similar to those collected before sand removal. Presumably, the relatively biologically unexploited replacement sand may have provided a niche suitable for rapid colonization and, potentially, growth of E. coli. Natural levels of E. coli can increase dramatically in foreshore sand when lake plankton, an organic source commonly available in the sand (1, 19), are added (55). Further potential evidence for growth is the increase in E. coli numbers associated with beach grooming (36), a tilling process that aerates, remoistens, and turns over the sand, providing bacteria with protection from desiccation and irradiation (36).
Hardina and Fujioka (33) cite warm temperature and higher nutrient concentrations as factors favoring the multiplication of E. coli in tropical soil and not water. Most conditions outlined for E. coli growth in tropical soil are met in the temperate United States during summer, and differences in thermal seasonality are ecologically limiting and quantitatively distinctive only during the cooler months. With the growing acceptance of evidence from tropical soil environments, it is possible that the E. coli community at 63rd Street Beach may be naturally occurring and multiplying. It is not possible to control for all of the potential inputs in an open system such as a beach, but we have eliminated the possibility of some (i.e., newly placed sand) and identified the minimal impact of others (i.e., resident gulls and water seeding the sand). More research into the environmental requirements and potential for in situ growth is necessary before E. coli multiplication in temperate environments can be confirmed, but this study provides initial data supporting that hypothesis.
In summary, we postulate that the E. coli population in the lake at 63rd Street Beach is primarily derived from beach sand, some of which originates from gull feces. DNA fingerprinting, concentration gradients, temporal population equilibrium, correlation, cluster analysis, and spatial distribution characteristics suggest that E. coli may sustain itself passively, by persistence, or actively, by sporadic multiplication, in foreshore sand during the swimming season. There is some evidence that nearshore deposition plays a localized supplemental role by contributing some E. coli organisms to the system. The findings presented here emphasize that beach sand (i) plays a major role in bacterial lake water quality, (ii) is an important source of indicator bacteria to the water rather than a net sink, (iii) may be environmentally, and perhaps hygienically, problematic, and (iv) is possibly capable of supporting an autochthonous, high density of indicator bacteria for sustained periods, independent of lake, human, or animal input.
Acknowledgments
We thank Muruleedhara Byappanahalli, Douglas Wilcox, and Eric Garza, U.S. Geological Survey, for their critical review of the manuscript. Dawn Shively helped review the manuscript and prepare figures. We acknowledge Maria Goodrich, Yvette Shiu, Jessie Stenftenagel, Justin LaPlante, and Valerie Price for collecting samples; Tom Horvath and Ellen Oberdick assisted with data organization and analysis; and Charles Bowling and Ellen Flanagan provided laboratory support. We thank Naren Prasad, Christine Wolski, Marcia Jimenez, and Pamela Thomas from the City of Chicago for their support throughout this project. Alfred Dufour, U.S. EPA, was a source of encouragement and creative conversation.
This study was funded through an interagency agreement between the U.S. Geological Survey and the City of Chicago in cooperation with the Chicago Park District.
Footnotes
Contribution 1252 of the U.S. Geological Survey Great Lakes Science Center.
REFERENCES
- 1.Adachi, K., K. Kimoto, and J. Higano. 1998. Primary productivity of sandy shores, p. 137-148. In W. H. Howell, B. J. Keller, P. K. Park, J. P. McVey, K. Takayanagi, and Y. Uekita (ed.), Nutrition and technical development of aquaculture. National Research Institute of Fisheries Engineering, Ibaraki, Japan.
- 2.Alderisio, K. A., and N. DeLuca. 1999. Seasonal enumeration of fecal coliform bacteria from the feces of ring-billed gulls (Larus delawarensis) and Canada geese (Branta canadensis). Appl. Environ. Microbiol. 65:5628-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhajiar, B. J., S. L. Stramer, D. O. Cliver, and J. M. Harkin. 1988. Transport modelling of biological tracers from septic systems. Water Res. 22:907-915. [Google Scholar]
- 4.American Public Health Association. 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, D.C.
- 5.Anderson, S. A., S. J. Turner, and G. D. Lewis. 1997. Enterococci in the New Zealand environment: implications for water quality monitoring. Water Sci. Technol. 35:325-331. [Google Scholar]
- 6.Ashbolt, N. J., M. R. Dorsch, P. T. Cox, and B. Banens. 1997. Blooming E. coli, what do they mean? In D. Kay and C. Fricker (ed.), Coliforms and E. coli, problem or solution? The Royal Society of Chemistry, Cambridge, United Kingdom.
- 7.Beaches Environmental Assessment and Coastal Health Act. 2000. Stat. 870-877, Public Law 106-284, vol. 33, United States Code 1251, p. 114.
- 8.Begon, M., J. L. Harper, and C. R. Townsend. 1986. Ecology: individuals, populations, and communities. Sinauer Associates, Inc., Sunderland, Mass.
- 9.Boehm, A. B., S. B. Grant, J. H. Kim, S. L. Mowbray, C. D. McGee, C. D. Clark, D. M. Foley, and D. E. Wellman. 2002. Decadal and shorter period variability of surf zone water quality at Huntington Beach, California. Environ. Sci. Technol. 36:3885-3892. [DOI] [PubMed] [Google Scholar]
- 10.Burton, G. A., Jr., D. Gunnison, and G. R. Lanza. 1987. Survival of pathogenic bacteria in various freshwater sediments. Appl. Environ. Microbiol. 53:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byappanahalli, M., M. Fowler, D. Shively, and R. Whitman. 2003. Ubiquity and persistence of Escherichia coli in a midwestern stream. Appl. Environ. Microbiol. 69:4549-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byappanahalli, M. N. 2000. Assessing the persistence and multiplication of fecal indicator bacteria in Hawaii soil environment. Ph.D. thesis. University of Hawaii at Manoa, Honolulu.
- 13.Chan, K.-Y., S. H. Wong, and C. Y. Mak. 1979. Effects of bottom sediments on the survival of Enterobacter aerogenes in seawater. Mar. Pollut. Bull. 10:205-210. [Google Scholar]
- 14.Coughtrey, P. J., M. H. Martin, and M. H. Unsworth (ed.). 1987. Pollutant transport and fate in ecosystems. Blackwell Scientific Publications, Oxford, United Kingdom.
- 15.Crowther, J., D. Kay, and M. D. Wyer. 2001. Relationships between microbial water quality and environmental conditions in coastal recreational waters: the Fylde coast, UK. Water Res. 35:4029-4038. [DOI] [PubMed] [Google Scholar]
- 16.Darakas, E. 2002. E. coli kinetics: effect of temperature on the maintenance and respectively the decay phase. Environ. Monit. Assess. 78:101-110. [DOI] [PubMed] [Google Scholar]
- 17.Davies, C. M., J. A. H. Long, M. Donald, and N. J. Ashbolt. 1995. Survival of fecal microorganisms in marine and freshwater sediments. Appl. Environ. Microbiol. 61:1888-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis, S. A., and D. M. Gordon. 2002. The influence of host dynamics on the clonal composition of Escherichia coli populations. Environ. Microbiol. 4:306-313. [DOI] [PubMed] [Google Scholar]
- 19.DeLancey, L. B. 1989. Trophic relationship in the surf zone during the summer at Folly Beach, South Carolina. J. Coastal Res. 5:477-488. [Google Scholar]
- 20.Desmarais, T. R., H. M. Solo-Gabriele, and C. J. Palmer. 2002. Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Appl. Environ. Microbiol. 68:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle, J. D., B. Tunnicliff, R. Kramer, R. Kuehl, and S. K. Brickler. 1992. Instability of fecal coliform populations in waters and bottom sediments at recreational beaches in Arizona. Water Res. 26:979-988. [Google Scholar]
- 22.Dufour, A. P. 1984. Health effects criteria for fresh recreational waters. EPA-600/1-84-004. U.S. Environmental Protection Agency, Research Triangle Park, N.C.
- 23.Fogarty, L. R., S. K. Haack, M. J. Wolcott, and R. L. Whitman. 2003. Abundance and characteristics of the recreational water quality indicator bacteria Escherichia coli and enterococci in gull faeces. J. Appl. Microbiol. 94:865-878. [DOI] [PubMed] [Google Scholar]
- 24.Francy, D., and R. Darner. 2000. Comparison of methods for determining Escherichia coli concentrations in recreational waters. Water Res. 34:2770-2778. [Google Scholar]
- 25.Fujioka, R. S., and M. N. Byappanahalli. 2001. Microbial ecology controls the establishment of fecal bacteria in tropical soil environment, p. 273-283. In K. H. T. Matsuo, S. Takizawa, and H. Satoh (ed.), Advances in water and wastewater treatment technology: molecular technology, nutrient removal, sludge reduction and environmental health. Elsevier, Amsterdam, The Netherlands.
- 26.Fujioka, R. S., H. H. Hashimoto, E. B. Siwak, and R. H. F. Young. 1981. Effect of sunlight on survival of indicator bacteria in seawater. Appl. Environ. Microbiol. 41:690-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujioka, R. S., C. Sian-Denton, M. Borja, J. Castro, and K. Morphew. 1999. Soil: the environmental source of Escherichia coli and enterococci in Guam's streams. J. Appl. Microbiol. Symp. Suppl. 85:83S-89S. [DOI] [PubMed] [Google Scholar]
- 28.Fujioka, R. S., K. Tenno, and S. Kansako. 1988. Naturally occurring fecal coliforms and fecal streptococci in Hawaii's freshwater streams. Toxic. Assess. 3:613-630. [Google Scholar]
- 29.Geldreich, E. E., and N. A. Clarke. 1966. Bacterial pollution indicators in the intestinal tract of freshwater fish. Appl. Microbiol. 14:429-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghinsberg, R. C., L. Bar Dov, Y. Sheinberg, and Y. Nitzan. 1994. Monitoring of selected bacteria and fungi in sand and seawater along the Tel Aviv coast. Microbios 77:29-40. [PubMed] [Google Scholar]
- 31.Ghinsberg, R. C., V. Drasinover, Y. Sheinberg, and Y. Nitzan. 1995. Seasonal distribution of Aeromonas hydrophila and Vibrio species in Mediterranean coastal water and beaches: a possible health hazard. Biomed. Lett. 51:151-159. [Google Scholar]
- 32.Ghinsberg, R. C., P. Leibowitz, H. Witkin, A. Mates, Y. Seinberg, D. L. Bar, Y. Nitzan, and M. Rogol. 1994. Monitoring of selected bacteria and fungi in sand and seawater along the Tel Aviv coast. MAP Tech. Rep. Ser. 87:65-81. [Google Scholar]
- 33.Hardina, C. M., and R. S. Fujioka. 1991. Soil: the environmental source of Escherichia coli and enterococci in Hawaii's streams. Environ. Toxicol. Water Qual. 6:185-195. [Google Scholar]
- 34.Irvine, K. N., and G. W. Pettibone. 1993. Dynamics of indicator bacteria populations in sediment and river water near a combined sewer outfall. Environ. Technol. 14:531-542. [Google Scholar]
- 35.Keswick, B. H., C. P. Gerba, S. L. Secro, and I. Cech. 1982. Survival of enteric viruses and indicator bacteria in groundwater. J. Environ. Sci. Health 17:903-912. [Google Scholar]
- 36.Kinzelman, J., R. L. Whitman, E. K. Jackson, M. N. Byappanahalli, and R. C. Bagley. Evaluation of beach grooming techniques on Escherichia coli density in foreshore sands at North Beach, Racine, WI. Lake Reservoir Manag., in press.
- 37.LaLiberte, P., and D. J. Grimes. 1982. Survival of Escherichia coli in lake bottom sediment. Appl. Environ. Microbiol. 43:623-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LeFevre, N. M., and G. D. Lewis. 2003. The role of resuspension in enterococci distribution in water at an urban beach. Water Sci. Technol. 47:205-210. [PubMed] [Google Scholar]
- 39.Levesque, B., P. Brousseau, F. Bernier, E. Dewailly, and J. Joly. 2000. Study of the bacterial content of ring-billed gull droppings in relation to recreational water quality. Water Res. 34:1089-1096. [Google Scholar]
- 40.Lock, M. A. 1993. Attached microbial communities in rivers, p. 518. In T. E. Ford (ed.), Aquatic microbiology: an ecological approach. Blackwell Scientific Publications, Oxford, United Kingdom.
- 41.Mendes, B., M. J. Nascimento, and J. S. Oliveira. 1993. Preliminary characterization and proposal of microbiological quality standard of sand beaches. Water Sci. Technol. 27:453-456. [Google Scholar]
- 42.Muller, T., A. Ulrich, E. M. Ott, and M. Muller. 2001. Identification of plant-associated enterococci. J. Appl. Microbiol. 91:268-278. [DOI] [PubMed] [Google Scholar]
- 43.Obiri-Danso, K., and K. Jones. 1999. Distribution and seasonality of microbial indicators and thermophilic campylobacters in two freshwater bathing sites on the River Lune in northwest England. J. Appl. Microbiol. 87:822-832. [DOI] [PubMed] [Google Scholar]
- 44.Obiri-Danso, K., and K. Jones. 1999. The effect of a new sewage treatment plant on faecal indicator numbers, campylobacters and bathing water compliance in Morecambe Bay. J. Appl. Microbiol. 86:603-614. [DOI] [PubMed] [Google Scholar]
- 45.Obiri-Danso, K., and K. Jones. 2000. Intertidal sediments as reservoirs for hippurate negative campylobacters, salmonellae and faecal indicators in three EU recognised bathing waters in north west England. Water Res. 34:519-527. [Google Scholar]
- 46.Oshiro, R., and R. Fujioka. 1995. Sand, soil, and pigeon droppings: sources of indicator bacteria in the waters of Hanauma Bay, Oahu, Hawaii. Water Sci. Technol. 31:251-254. [Google Scholar]
- 47.Papadakis, J. A., A. Mavridou, S. C. Richardson, M. Lampiri, and U. Marcelou. 1997. Bather-related microbial and yeast populations in sand and seawater. Water Res. 31:799-804. [Google Scholar]
- 48.Sherry, J. P. 1986. Temporal distribution of faecal pollution indicators and opportunistic pathogens at a Lake Ontario bathing beach. J. Great Lakes Res. 12:154-160. [Google Scholar]
- 49.Solo-Gabriele, H. M., M. A. Wolfert, T. R. Desmarais, and C. J. Palmer. 2000. Sources of Escherichia coli in a coastal subtropical environment. Appl. Environ. Microbiol. 66:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Standridge, J. H., J. J. Delfino, L. B. Kleppe, and R. Butler. 1979. Effect of waterfowl (Anas platyrhynchos) on indicator bacteria populations in a recreational lake in Madison, Wisconsin. Appl. Environ. Microbiol. 38:547-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Struck, P. H. 1988. The relationship between sediment and fecal coliform levels in Puget Sound Estuary. J. Environ. Health 50:403-407. [Google Scholar]
- 52.Topp, E., M. Welsh, Y.-C. Tien, A. Dang, G. Lazarovits, K. Conn, and H. Zhu. 2003. Strain-dependent variability in growth and survival of Escherichia coli in agricultural soil. FEMS Microbiol. Ecol. 44:303-308. [DOI] [PubMed] [Google Scholar]
- 53.U.S. Environmental Protection Agency. 1999. EPA action plan for beaches and recreational waters: reducing exposures to waterborne pathogens. EPA/600/R-98/079. Office of Research and Development and Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 54.Van Donsel, D. J., E. E. Geldreich, and N. A. Clarke. 1967. Seasonal variations in survival of indicator bacteria in soil and their contribution to storm-water pollution. Appl. Microbiol. 15:1362-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitman, R. L., M. C. Andrzejewski, K. J. Kennedy, and T. A. Sobat. 1994. Composition, spatial-temporal distribution and environmental factors influencing the interstitial beach meiofauna of southern Lake Michigan. Verh. Int. Verein. Limnol. 25:1389-1397. [Google Scholar]
- 56.Whitman, R. L., M. B. Nevers, and P. J. Gerovac. 1999. Interaction of ambient conditions and fecal coliform bacteria in southern Lake Michigan waters: monitoring program implications. Nat. Areas J. 19:166-171. [Google Scholar]