Introduction
Polyethylene glycols (PEGs) are widely used in biological and pharmaceutical analysis to stabilize proteins for separation, purification, storage, and delivery1 and they also are used as the precipitants in crystallization process for X-ray crystallography of proteins.2 However, the analysis protein and peptide samples contaminated with PEG using mass spectrometry either using electrospray ionization mass spectrometry (ESI-MS) or matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) is very challenging due to ion signal suppression and interference from the PEGs. Many off-line and on-line sample cleanup methods including LC column, capillary electrophoresis, membrane filtration, solid-phase extraction, dialysis and ion exchange cartridge, etc. have been implemented to remove salts, detergents, or other small molecules from complex biological samples before ESI and MALDI mass analysis.3–7 Unfortunately, samples with high concentration PEGs are still problematic. They can easily carry over to the sample fraction and frequently contaminate LC columns. Furthermore, sample purification procedures are time consuming and low throughput.
Very recently, a titanium dioxide (TiO2) microcolumn has been developed as an efficient method to enrich phosphorylated peptides.8–12 In this study, this same microcolumn is shown to assist rapid removal of high concentration PEG contaminants from protein and peptides samples.
Methods
Materials
Protein samples (cytochrome c and α-casein13) and all the solvents used were obtained from Sigma (St. Louis, MO). Trypsin was obtained from Promega (Madison, WI). The three wall-coated titanium dioxide micropipette tips (Trap’nTips™, TiO2) were obtained from New Objective (Woburn, MA).
Sample preparation
For the whole protein example, 2 μM α-casein or cytochrome c was mixed with 20 μM PEG 4000 before TiO2 cleanup. For the peptides example, 1 μg α-casein was digested with trypsin at 1:25 (w/w) in 100 mM ammonium bicarbonate (pH 8.0) overnight at 37°C and then 20 μM PEG 4000 was added into this digestion mixture before TiO2 cleanup.
TiO2 cleanup procedure
For the phosphorylated protein and peptides (α-casein and α-casein digest), the sample was dissolved in a 10 μl solution of 20 mg/ml 2, 5-dihydroxy-benzoic acid (DHB) with 80% acetonitrile, 0.1% TFA, and then was loaded onto the TiO2 tip which had been previously wetted by the solution of 80% acetonitrile, 0.1% TFA. After loading the sample, the TiO2 tip was washed by both 20 mg/ml DHB with 80% acetonitrile, 0.1% TFA (3 times) and 80% acetonitrile, 0.1% TFA solutions (7 times) and then the sample was eluted by 75 mM ammonium hydroxide (NH4OH, PH = 10.5) in water. For unphosphorylated proteins (cytochrome c), the sample was dissolved in 10 μl solution of 20 mg/ml DHB with 10% acetonitrile, 0.1% TFA, and then was loaded onto the TiO2 tip which had been previously wetted by the solution of 10% acetonitrile, 0.1% TFA. After loading the sample, the TiO2 tip was washed by both 20 mg/ml DHB with 10% acetonitrile, 0.1% TFA (3 times) and 10% acetonitrile, 0.1% TFA solutions (7 times) and then the sample was eluted by 80% acetonitrile, 0.1% TFA. All the protein and peptide samples after cleanup were dried under the speedvacuum and reconstituted in 50% acetonitrile, 1% formic acid.
FTMS instrumentation
A custom built qQq-FTMS with a nanospray source and 7T actively shielded magnet (Cryomagnetics Inc., Oak Ridge, TN) was utilized in this study.14, 15 A ~2–3 μl sample was loaded into a glass capillary tip pulled with a micropipette puller (Model P-97, Sutter Instruments Co., Novato, CA) to ~1 μm orifice diameter and a stainless steel wire was inserted into the distal end of the tip to form the electrical connection. For all the samples, around 1100 V spray voltage was used to form the spray.
Results and discussion
Recently, TiO2 microcolumns have been widely utilized in enrichment of phosphorylated peptides, and it was discovered that the addition of 2, 5-dihydroxy-benzoic acid (DHB) helps to efficiently displace nonphosphorylated peptides from the TiO2 while retaining the phosphorylated peptides.8–12 Here, this microcolumn was tested for efficient removal of high concentrations of PEG contaminants from protein and phosphorylated peptides samples. Figure 1 (left) shows the results of this study with whole phosphorylated protein. Figure 1A (left) shows the 14+ to 27+ charge states of α-casein. When 2 μM α-casein is mixed with 20 μM PEG 4000, the PEG charge state distributions are clear16, but the α-casein is almost unobservable (Figure 1B left). This is a classic example of the matrix effect, also known as the signal suppression effect and is common in electrospray mass spectrometry. After sample cleanup, as described above, the PEG 4000 peaks were completely eliminated (Figure 1C left) and the α-casein peaks are again observable. Furthermore, additional “chemical noise” baseline between m/z 1000–2000 is also suppressed. Based on the previous work with these columns8–12, most likely, both phosphorylated protein and PEG are bound to the TiO2 microcolumn, and then the PEG was washed off the column by 80% acetonitrile, 0.1% TFA with DHB due to the large molar excess of DHB which competes for adsorption to the surface of TiO2. The phosphorylated protein was then eluted by NH4OH (pH = 10.5).
Figure 1.
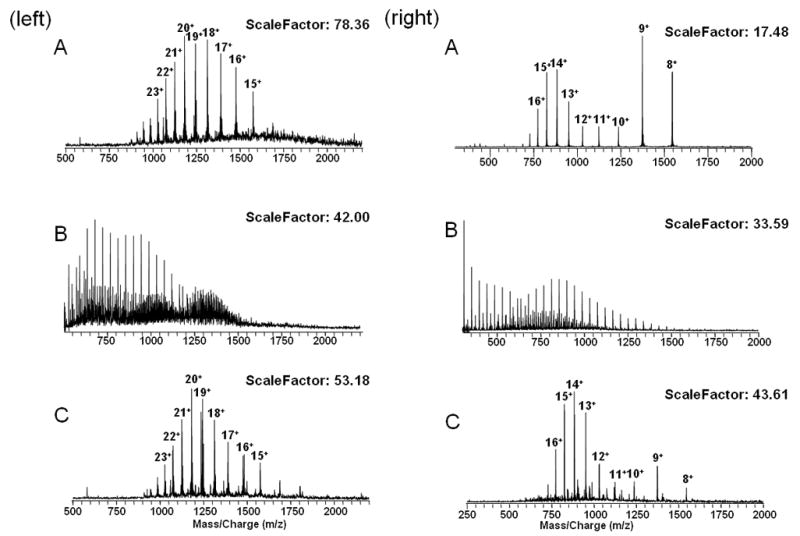
(left) A. ESI spectrum of 2 μM α-casein. B. ESI spectrum of 2 μM α-casein with 20 μM PEG 4000. C. ESI spectrum of 2 μM α-casein with 20 μM PEG 4000 after TiO2 cleanup. (right) A. ESI spectrum of 2 μM cytochrome c. B. ESI spectrum of 2 μM cytochrome c with 20 μM PEG 4000. C. ESI spectrum of 2 μM cytochrome c with 20 μM PEG 4000 after TiO2 cleanup. Scalefactor is inverse proportion to intensity, and baseline noise is constant so that signal/noise is a valid measure of relative intensity.
TiO2 can also separate high concentration PEG from unphosphorylated proteins. For example, Figure 1A (right) shows the 8+ to 18+ charge states of cytochrome c, Figure 1B (right) shows the spectrum of a mixture of 2 μM cytochrome c and 20 μM PEG 4000; the cytochrome c peaks are not observable. Cytochrome c charge state distributions are rescued after TiO2 cleanup, albeit not to the same extent as was observed with the phosphoprotein α-casein (Figure 1C right). The conditions used to wash the TiO2 microcolumn in this unphosphorylated protein are little different from that of phosphorylated protein. The solutions of 10% acetonitrile, 0.1% TFA with and without DHB were utilized to get rid of PEG and the protein was eluted by 80% acetonitrile, 0.1% TFA.
Figure 2 demonstrated that, as previously reported8–12, phosphopeptides are efficiently enriched by TiO2. The tryptic digest peptides of α-casein are observable in Figure 2A. While some compete effectively for signal against PEG 4000, most of the peaks are not observed, and phosphopeptides in particular are difficult to detect (Figure 2B). However, phosphopeptides are rescued by TiO2 microcolumn cleanup (Figure 2C).
Figure 2.
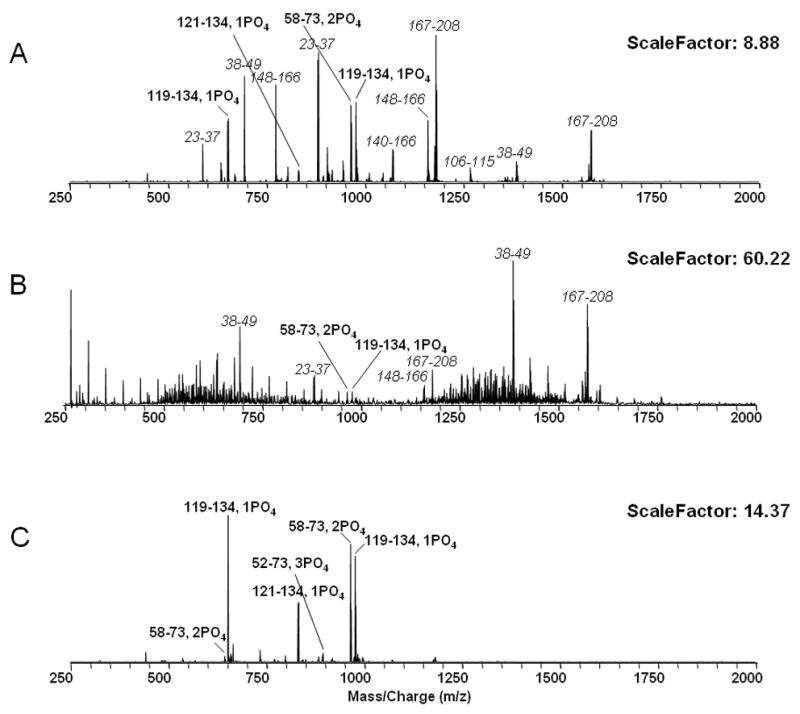
A. ESI spectrum of 1 μg α-casein tryptic digest. B. ESI spectrum of 1 μg α-casein tryptic digest with 20 μM PEG 4000. C. ESI spectrum of 1 μg α-casein tryptic digest with 20 μM PEG 4000 after TiO2 cleanup.
In summary, the method described here has demonstrated that the TiO2 microcolumn can rapidly remove PEG contaminants from protein and phosphorylated peptide samples to improve the characterization of the proteins and peptides by mass spectrometry.
Acknowledgments
This research was supported by NIH P41-RR10888, N01-HV28178, MDS Sciex, and the ACS Petroleum Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quellec P, Gref R, Dellacherie E, Sommer F, Tran MD, Alonso MJ. Protein encapsulation within poly(ethylene glycol)-coated nanospheres. II. Controlled release properties. J Biomed Mater Res. 1999;47:388–395. doi: 10.1002/(sici)1097-4636(19991205)47:3<388::aid-jbm14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Patel S, Cudney B, Mcpherson A. Polymeric Precipitants for the Crystallization of Macromolecules. Biochem Bioph Res Co. 1995;207:81–828. doi: 10.1006/bbrc.1995.1260. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Hofstadler SA, Bresson JA, Udseth HR, Tsukuda T, Smith RD, Synder AP. On-line dual microdialysis with ESI-MS for direct analysis of complex biological samples and microorganism lysates. Anal Chem. 1998;70:1797–1801. doi: 10.1021/ac971193k. [DOI] [PubMed] [Google Scholar]
- 4.Shen J, Buko A. Rapid identification of proteins in polyethylene glycol-containing samples using capillary electrophoresis electrospray mass spectrometry. Anal Biochem. 2002;311:80–83. doi: 10.1016/s0003-2697(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Orlando R. Solid-phase extraction/MALDI-MS: Extended ion-pairing surfaces for the on-target cleanup of protein samples. Anal Chem. 1999;71:4753–4757. doi: 10.1021/ac990328e. [DOI] [PubMed] [Google Scholar]
- 6.Puchades M, Westman A, Blennow K, Davidsson P. Removal of sodium dodecyl sulfate from protein samples prior to matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Sp. 1999;13:344–349. doi: 10.1002/(SICI)1097-0231(19990315)13:5<344::AID-RCM489>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 7.Xu N, Lin Y, Hofstadler SA, Matson D, Call CJ, Smith RD. A microfabricated dialysis device for sample cleanup in electrospray ionization mass spectrometry. Anal Chem. 1998;70:3553–3556. doi: 10.1021/ac980233x. [DOI] [PubMed] [Google Scholar]
- 8.Pinkse MW, Uitto PM, Hilhorst MJ, Ooms B, Heck AJ. Selective isolation at the femtomole level of phosphopeptides from proteolytic digests using 2D-NanoLC-ESI-MS/MS and titanium oxide precolumns. Anal Chem. 2004;76:3935–3943. doi: 10.1021/ac0498617. [DOI] [PubMed] [Google Scholar]
- 9.Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jorgensen TJ. Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol Cell Proteomics. 2005;4:873–886. doi: 10.1074/mcp.T500007-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Kweon HK, Hakansson K. Selective zirconium dioxide-based enrichment of phosphorylated peptides for mass spectrometric analysis. Anal Chem. 2006;78:1743–1749. doi: 10.1021/ac0522355. [DOI] [PubMed] [Google Scholar]
- 11.Sano A, Nakamura H. Titania as a chemo-affinity support for the column-switching HPLC analysis of phosphopeptides: Application to the characterization of phosphorylation sites in proteins by combination with protease digestion and electrospray ionization mass Spectrometry. Anal Sci. 2004;20:861–864. doi: 10.2116/analsci.20.861. [DOI] [PubMed] [Google Scholar]
- 12.Posewitz MC, Tempst P. Immobilized gallium(III) affinity chromatography of phosphopeptides. Anal Chem. 1999;71:2883–2892. doi: 10.1021/ac981409y. [DOI] [PubMed] [Google Scholar]
- 13.Collet C, Joseph R, Nicholas K. Molecular Characterization and Invitro Hormonal Requirements for Expression of 2 Casein Genes from a Marsupial. J Mol Endocrinol. 1992;8:13–20. doi: 10.1677/jme.0.0080013. [DOI] [PubMed] [Google Scholar]
- 14.Jebanathirajah JA, Pittman JL, Thomson BA, Budnik BA, Kaur P, Rape M, Kirschner M, Costello CE, O’Connor PB. Characterization of a new qQq-FTICR mass spectrometer for post-translational modification analysis and top-down tandem mass Spectrometry of whole proteins. J Am Mass Spectrom. 2005;16:1985–1999. doi: 10.1016/j.jasms.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor PB, Pittman JL, Thomson BA, Budnik BA, Cournoyer JC, Jebanathirajah J, Lin C, Moyer S, Zhao C. A new hybrid electrospray Fourier transform mass spectrometer: Design and performance characteristics. Rapid Commun Mass Spectrom. 2006;20:259–266. doi: 10.1002/rcm.2307. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor PB, McLafferty FW. Oligomer Characterization of 4-23 kDa Polymers by Electrospray Fourier Transform Mass Spectrometry. J Am Chem Soc. 1995;117:12826–12831. [Google Scholar]


