Abstract
A history of dieting is common in individuals suffering from eating disorders for which depression and mood disturbances are also comorbid. We investigated the effect of a history of caloric restriction (HCR) in rats that involved cyclic food restriction and refeeding with varying levels of access to palatable food (PF) on: 1) responses to the SSRI, fluoxetine; 2) monoamine levels in brain regions central to the control of feeding, reward, and mood regulation; and 3) behavioral tests of anxiety and depression. HCR coupled with intermittent but not daily access to PF exaggerated rats’ anorectic response to fluoxetine (p<0.05); was associated with a significant 71% and 58% reduction of 5-HT and dopamine, respectively, in the medial prefrontal cortex; and induced behaviors consistent with models of depression. HCR, irrespective of access to PF, abolished the strong association between 5-HT and dopamine turnover in the nucleus accumbens in control rats (r =0.71 vs. -0.06, p<0.01). Access to PF, irrespective of HCR, reduced hypothalamic dopamine. Together, these findings suggest that a history of frequent food restriction-induced weight fluctuation imposes neurochemical changes that negatively impact feeding and mood regulation.
Keywords: Caloric restriction, palatable food, high fat, bulimia, weight cycling, mood regulation, anxiety, negative affect, rats, animal model, 5-HT, SSRI
1. Introduction
The physiological and psychological consequences of caloric restriction and intake of highly palatable “junk” food (PF) have become of increasing interest to basic and clinical researchers working to understand abnormal eating behavior and addiction. Recent work with an animal model of stress-induced binge-eating has led to initial discoveries of the mechanisms that underlie binge-eating including 5-HT and opioid dysregulation (Boggiano, et al., 2005; Placidi, et al., 2004). Besides the availability of PF, this rat binge-eating model is based on a history of caloric restriction (HCR) with recovery of body weight and food intake to that maintained by never-restricted rats, which is more typical of human dieting that commonly antecedes eating disorders. Binge-eating in this model was ultimately evoked by stress (induced by footshock) but we later learned that it is a history of reduced caloric intake with PF and not a history of stress that is important in changing the animals’ physiology to respond by binge-eating (Hagan, et al., 2002). Similarly, we and others have found that the coupling of caloric restriction with PF elicits profound hyperphagia or binge-eating under sated conditions (Bello, et al., 2006; Hagan and Moss, 1997) and provokes withdrawal-like symptoms in rats (Colantuoni, et al., 2002). This raises the hypothesis that despite recovery of body weight and food intake to that matching controls’, a history of caloric restriction is capable of inducing neurochemical changes or adaptations that increase susceptibility to binge-eating and to possibly other conditions common in eating disorders such as substance abuse and depression.
A prime neurochemical candidate to be affected by HCR is serotonin (5-HT). This monoamine is integral to the regulation of feeding and mood. Hypothalamic elevations of 5-HT promote satiety and so reduce rate and amount of food intake (De Vry and Schreiber, 2000; Leibowitz and Alexander, 1998; Simansky, 1996). With respect to mood regulation, dysregulated or reduced 5-HT activity contributes to mood disturbances, particularly depression (Jimerson, et al., 1990; Ressler and Nemeroff, 2000; Wurtman and Wurtman, 1995). Clinical studies reveal associations between caloric restriction and 5-HT disruption (Anderson, et al., 1990; Cowen, et al., 1996; Goodwin, et al., 1987; Walsh, et al., 1995), especially in individuals with eating disorders that typically have HCR and suffer mood disturbances such as anxiety (Godart, et al., 2004; Kaye, et al., 2004; Keel, et al., 2005; Sassaroli, et al., 2005) and depression (Keel, et al., 2005; Mora-Giral, et al., 2004; Patton, et al., 1997; Stice, et al., 2004). Despite these clinical observations, possible associations between altered 5-HT function and caloric restriction have not been studied in animal models of eating or emotional disorders. Similarly, little is know about the physiological mechanisms that may link dieting behavior to emotional disorders in humans, physiology that could be uncovered with an adequate animal model.
Therefore, in this study, we subjected female rats to a history of caloric restriction, controlling for frequency of PF intake. Changes in 5-HT function, feeding, and mood were implicated from feeding responses to SSRI treatment, direct assay of 5-HT and dopamine levels in the hypothalamus, nucleus accumbens, and medial prefrontal cortex (mPFC), and from behavioral tests of anxiety and depression.
2. Materials and Methods
2.1. Subjects
Female Sprague-Dawley rats (N=80; 90 days old; Harlan: Indianapolis, IN) were acclimated to individual woodchip-bedded cages under a 12/12 hour light-dark schedule (lights out at 1200) with ad lib chow and water for two weeks prior to all experiments. For the first two experiments, 48 rats weight-matched into one of three diet conditions: Chow only, Intermittent PF, or Daily PF. Within each diet condition, the rats were again weight-matched into one of two further groups: a HCR or no-HCR group making a total of six groups (n = 8/group). The remaining 32 rats were used for the third experiment, also weight-matched into HCR and no-HCR conditions. During the HCR protocol, rats were given ad lib water at all times (except where noted). Food intake was measured daily, taking care to retrieve all visible food from the bedding to correct for spillage, with fresh food given immediately prior to lights out. Body weights were recorded on at least three occasions during each cycle of the HCR protocol (first day of restriction, first day of refeeding and last/”test” day of each cycle). The Institutional Animal Care and Use Committee at the University of Alabama at Birmingham approved all procedures.
2.2. Diets
Regular rat chow (Harlan-Teklad, Indianapolis, IN) and Double-Stuf Oreo cookies (Nabisco, Hanover, NJ) as the PF were used. The chow contains 3.5% fat kcals, 70% carbohydrate kcals, 17% protein kcals, 9.5% moisture, and yields 3.74 kcals/gram. The Oreo cookies contain 43% fat kcals, 57% carbohydrate kcals, 0.02% protein kcals, and yields 4.83 kcals/gram. Both diets were accessible to rats through their cage tops, and the Oreos were provided as whole cookies.
2.3. History of Cyclic Restriction (HCR) Protocol
The protocol involved cycling rats through days of restricted and ad lib chow with and without PF during refeeding, as outlined in Table 1. Each cycle lasted 12 days and rats were cycled for a total of 12 cycles. PF was given right after the restriction phase to simulate the way humans typically “break” a diet (with PF). If body weight and 24hr food intake of the HCR rats did not recover to their no-HCR cohort’s level by “Test Day” (day 12), an extra day was added to the ad lib refeeding portion of the cycle (to days 8–11). After a 24 hr reading of food intake from Day 12, the rats were started through another cycle starting with Day 1.
Table 1.
Protocols for a history of caloric restriction (HCR) with various diet conditions used in Experiment 1 and 2. Only the Intermittent PF condition was used in Experiment 3.
| Diet Groups | Restriction Subgroups | Days 1–5“Refeeding” | Days 6–7“Refeeding” | Days 8–11“Refeeding” | Day 12“Test Day” |
|---|---|---|---|---|---|
| Chow Only | No-HCR | Ad lib chow | Ad lib chow | ||
| HCR | *66% chow | ||||
| Intermittent PF | No-HCR | Ad lib chow | Ad lib chow + ad lib PF | Ad lib chow | Ad lib chow + ad lib PF |
| HCR | *66% chow | ||||
| Daily PF | No-HCR | Ad lib Chow + Ad lib PF | Ad lib chow + ad lib PF | ||
| HCR | *66% Chow + Ad lib PF | ||||
66% calculated from the mean total calories consumed by the entire group (N=80) prior to the start of cycling; Daily PF/HCR group received the same amount of kcals as the 66% amount given to the Chow Only and Intermittent PF HCR groups, but half of the kcals came from chow and half from PF.
2.4. Data analysis
Food intake, body weight, neurochemical levels, and behaviors on the anxiety and depression tests were analyzed by within- and between-group analyses of variance (ANOVAs) and Bonferroni post-hoc tests, or independent group t-tests if only two groups were included in the analysis. Pearson’s r correlation coefficient was used to assess associations between monoamine levels and metabolite/monoamine ratios for turnover measures, and to measure the inter-rater reliability scores. When group differences in hypothalamic levels of monoamines were evident, linear regression was performed to determine whether total chow, PF intake, or total protein, carbohydrate or fat intake predicted these levels of monoamines. The alpha level was set at p < 0.05 (two-tailed), except as noted.
2.5. Euthanasia
Food was removed from rats approximately 1 hour prior to unanesthetized guillotine decapitation prior to lights out (IACUC approved). All rats were euthanized on the last day of the 12th cycle.
2.6. Experimental Procedures
2.6.1. Experiment 1. Effect of HCR and Frequency of PF Access on the Anorectic Effect of Fluoxetine
Fluoxetine hydrochloride (Sigma, St. Louis, MO) was dissolved in physiological saline (control solution) and administered i.p. in a 3 mg/kg dose. A multiple dose study in same sex and age rats revealed that this was the minimum dose required to significantly decrease intake of the same PF (Placidi, et al., 2004). The rats were cycled according to group assignment through the HCR protocol (Table 1) and were administered saline or fluoxetine on Day 12 of cycle 4 and with the alternate treatment at the end of cycle 5. Prior to the injection, food was removed for 1hr. Immediately after the injection, preweighed chow or chow and PF (according to assigned diet condition) were presented (at lights out) and food intake recorded at 1, 2, 4, and 24 hrs post injection.
A second fluoxetine experiment was conducted during cycles 6 & 7, this time using trigger-induced food intake. The rats were administered saline and fluoxetine as above but this time the injection was given one hour following a “trigger” of chow (8.2 kcals) for Chow Only groups, or chow and PF (8.2 kcals total) for Intermittent and Daily PF groups. After this trigger, which was meant to stimulate appetite, all food was withheld for one hour after lights out. Intake was again recorded at 1, 2, 4, and 24 hrs post injection.
2.6.2. Experiment 2. The Effect of HCR and Frequency of PF Access on Monoamine Levels in the Hypothalamus, Nucleus Accumbens, and Medial Prefrontal Cortex
The same rats used for Exp. 1 were used here. After decapitation, brains were extracted and an anterior section of the hypothalamus, the nucleus accumbens, and mPFC were dissected using methods adopted from Heffner et al. (Heffner, et al., 1980) and with guidance from the Rat Atlas of Paxinos & Watson (Paxinos and Watson, 1998). The hypothalamus was dissected from a section taken from just anterior to the optic chiasm to 2 mm posterior of that first slice. A square region was cut from the ventral-medial segment of this section (app. 2 mm off each side of the 3rd ventricle), encompassing the paraventricular nucleus (PVN), the anterior hypothalamus, the anterior portion of the lateral hypothalamus, and the medial preoptic region. A cut made 2 mm in front of the anterior hypothalamus slice provided the slice from which bilateral nucleus accumbens were isolated using 2 mm punches around each anterior commissure. The mPFC was taken from a medial 2 mm punch from the slice extending 2 mm anterior to the anterior-most slice of the nucleus accumbens. Tissue was immediately weighed, submerged in a 0.05 N perchloric acid solution containing 10 ng/200 μl of dihydroxybenzylamine (DHBA: the internal standard), and homogenized and centrifuged to obtain supernatant. 5-HT, dopamine, and the metabolites 5HIAA and DOPAC, were measured using high performance liquid chromatography (HPLC) with a C-18 reverse-phase column (Varian Inc., Lake Forest, CA) with electrochemical detection (4-channel CoulArray EC detector; ESA, Inc., MA; potentials set at –100, 150, 250, 450 mV). The mobile phase contained 20% methanol, 32.5 mg/L of sodium dodecyl sulfate, 0.1 mM disodium ethylenediamine tetraacetate (EDTA), 30 mM citric acid, and 60 mM monobasic sodium phosphate, and adjusted with phosphoric acid to pH 2.8. Data were integrated using CoulArray® for Windows®32 software (ESA, Inc., MA), and then corrected for tissue weight.
2.6.3. Experiment 3. Effect of Intermittent PF with and without HCR on Measures of Anxiety and Depression
A new group of N = 32 rats of the same sex and age used in the previous experiments were cycled through the Intermittent PF protocol used in Exp. 1 & 2 (see Table 1, Intermittent PF diet condition, N = 16 HCR, N = 16 no-HCR). Rats were maintained on chow during each of the behavioral tests.
Anxiety Tests
Rats in the no-HCR and HCR groups were weight-matched and assigned for testing in counterbalanced order with either the elevated maze (N= 16) or the open field test (N=16), both validated measures of anxiety in rodents (Pellow, et al., 1985; Prut and Belzung, 2003). The tests were conducted during the light in a room adjacent to the home colony room (same light and temperature conditions), approximately 2 hrs prior to lights out over a 2-day period at the end of the 7th cycle. For the elevated maze test, a simplified “V” version of the plus maze was used to measure rodent activity in an open versus closed space (Placidi, et al., 2004). Discrete measures of activity for each rat were recorded for 5 minutes by a DVD recorder positioned to view both arms of the maze and rated by two assistants blind to group conditions. For the open field test, a 61 x 61 x 61 cm clear polycarbonate box with a 36-square grid was used. Each rat was placed in the same corner of the open field, with its head facing in the direction of the centermost four squares of the entire arena. Activity was then recorded for 5 minutes using a DVD recorder positioned above the arena. Two raters blind to group conditions recorded the number of line crosses, number of entries into the center (i.e. any square not bordered by a wall) and duration spent in the center and in the periphery.
Depression Tests
The forced swim, sucrose solution, and novelty interaction tests were used as models of depression. The forced swim test is a commonly used validated test of depression in rodents (Cryan, et al., 2005; Rygula, et al., 2005). This test was conducted in N = 8 Intermittent PF/HCR and N = 8 Intermittent PF/no-HCR weight-matched rats counterbalanced for the type of anxiety test in which they previously served. The swim test was conducted on the “Test Day” (Table 1) of the 8th cycle, during daylight in a room adjacent to their home colony room. A clear glass cylinder (height: 43 cm, diameter: 20 cm) was filled with 25 ± 1°C water to a depth of 30 cm, matching conditions previously validated (Cryan et al., 2005; Rygula et al., 2005). Rats were placed one at a time in the water for 15 minutes as a standard pretest on the day prior to the actual test. They were placed in the water again 24 hours later for a recorded 5-minute test. Activity during each test phase was DVD-recorded and blind-rated for latency to become inactive, time spent inactive, struggling/climbing, or swimming/diving.
The sucrose solution test assesses anhedonia, a characteristic of depression. Anhedonia is interpreted from reduced intake of a dilute sucrose solution with repeated access to it (Bekris, et al., 2005; Grippo, et al., 2006). Weight-matched (N = 8 Intermittent PF/HCR and N = 8 Intermittent PF/no-HCR) rats were selected prior to any cycling and used for these tests throughout cycling. These rats also served in anxiety and depression tests during cycling but care was taken to ensure that an equal number of “sucrose test” rats served in each of the anxiety and depression tests. Prior to any cycling, two baseline sucrose tests were conducted to determine the amount of a dilute (1%) sucrose solution the animals ingested. A second bottle containing only water was also available and its intake also measured. Since there were no statistical differences in sucrose solution and water intake within and between rats, the average of the two tests was taken as the baseline score for sucrose and water intake. The rats were again given the 1% sucrose solution and water in a second bottle during the last refeeding day of the 1st, 2nd, 5th, and 10th cycle. Hence, HCR rats were fully recovered from restriction and were back to normal body weight when these tests were conducted. On the test days, food and water was removed 90 min prior to lights out and at lights off pre-measured amounts of chow, water, and sucrose solution, were provided (water and sucrose in identical bottles). Care was taken to ensure there was no leakage from either bottle. Food and liquid intakes were recorded at 1, 4, and 24 hr intervals after presentation. Sucrose was firstly placed in the location usually held by the rats’ water bottles, while their more familiar water bottle was moved adjacent to the sucrose bottle. Then at each intake interval, the water and sucrose bottles were switched to ensure the rats did not drink more from one bottle due to location familiarity.
The novelty interaction test is based on decreased interaction with a novel object as indicative of anhedonia (Bevins and Bardo, 1999). Rats that did not serve in the sucrose test served in this test which was conducted only once on the last day of refeeding on the 5th cycle. Water was provided in two identical bottles, a familiar bottle present in their cage for one week, and a clean, new bottle. Procedure for placement of the bottles, switching their location, and measurement of intake was the same as that described for the sucrose test.
3. Results
3.1. Experiment 1. Effect of HCR and Frequency of PF Access on the Anorectic Effect of Fluoxetine
Under the non-“triggered” saline conditions, the HCR and no-HCR Intermittent PF group consumed approximately twice the kcals of the Chow Only and Daily PF groups (see means below, 1H: F(2,45) = 9.393, p < 0.001; 2H: F(2,45) = 12.066, p < 0.001). While there was no interaction between drug, diet group, and HCR condition, there was an interaction between diet group and drug at 1 and 2 hours post injection (1H: Chow Only saline = 7.67 ± 0.70, fluoxetine = 5.54 ± 0.51 kcals; Intermittent PF saline = 13.45 ± 1.82, fluoxetine = 7.01 ± 0.92 kcals; Daily PF saline = 6.43 ± 0.83, fluoxetine 4.61 ± 0.79 kcals; F(2,42) = 3.53, p < 0.05; 2H: Chow Only saline = 12.79 ± 1.13, fluoxetine = 10.59 ± 1.24 kcals; Intermittent PF saline = 21.57 ± 1.98, fluoxetine = 12.70 ± 0.95 kcals; Daily PF saline = 12.66 ± 1.14, fluoxetine = 6.85 ± 0.97 kcals; F(2,42) = 4.613, p < 0.05) such that the anorectic effect of fluoxetine was greater in the Intermittent PF group regardless of restriction history.
When a food trigger was provided, the Intermittent PF/HCR group overate in comparison to the Intermittent PF/no-HCR group (see means below; saline bars Fig. 1; t(13) = 1.944, p(1-tailed) < 0.05), even though these groups had consumed the same amount without the trigger. When fluoxetine was administered 1 hour after a food trigger, the greatest total kcal reductions (taken as a percentage of saline-induced intake) were in the Chow/no-HCR and Intermittent PF/HCR groups (Chow Only: no-HCR = 74%, HCR = 32%; Intermittent PF: no-HCR = 46%, HCR = 62%; Daily PF: no-HCR = 46%, HCR = 31%), but these reductions did not obtain statistical significance. Analysis of the raw data revealed a marginal drug x diet group x HCR interaction at 1 hour post injection for intake of PF (F(1, 27) = 4.023, p = 0.055), such that the anorectic effect of fluoxetine was more potent in the Intermittent PF/HCR group, compared to the other groups (Intermittent PF/no-HCR saline = 10.97 ± 2.61, fluoxetine = 6.14 ± 1.84 kcals; Intermittent PF/HCR saline = 15.94 ± 1.84, fluoxetine = 4.53 ± 1.18 kcals; Daily PF/no-HCR saline = 5.80 ± 1.44, fluoxetine = 3.02 ± 1.41 kcals; Daily PF/HCR saline = 4.59 ± 1.29, fluoxetine = 3.44 ± 1.81 kcals: Fig. 1).
Fig. 1.
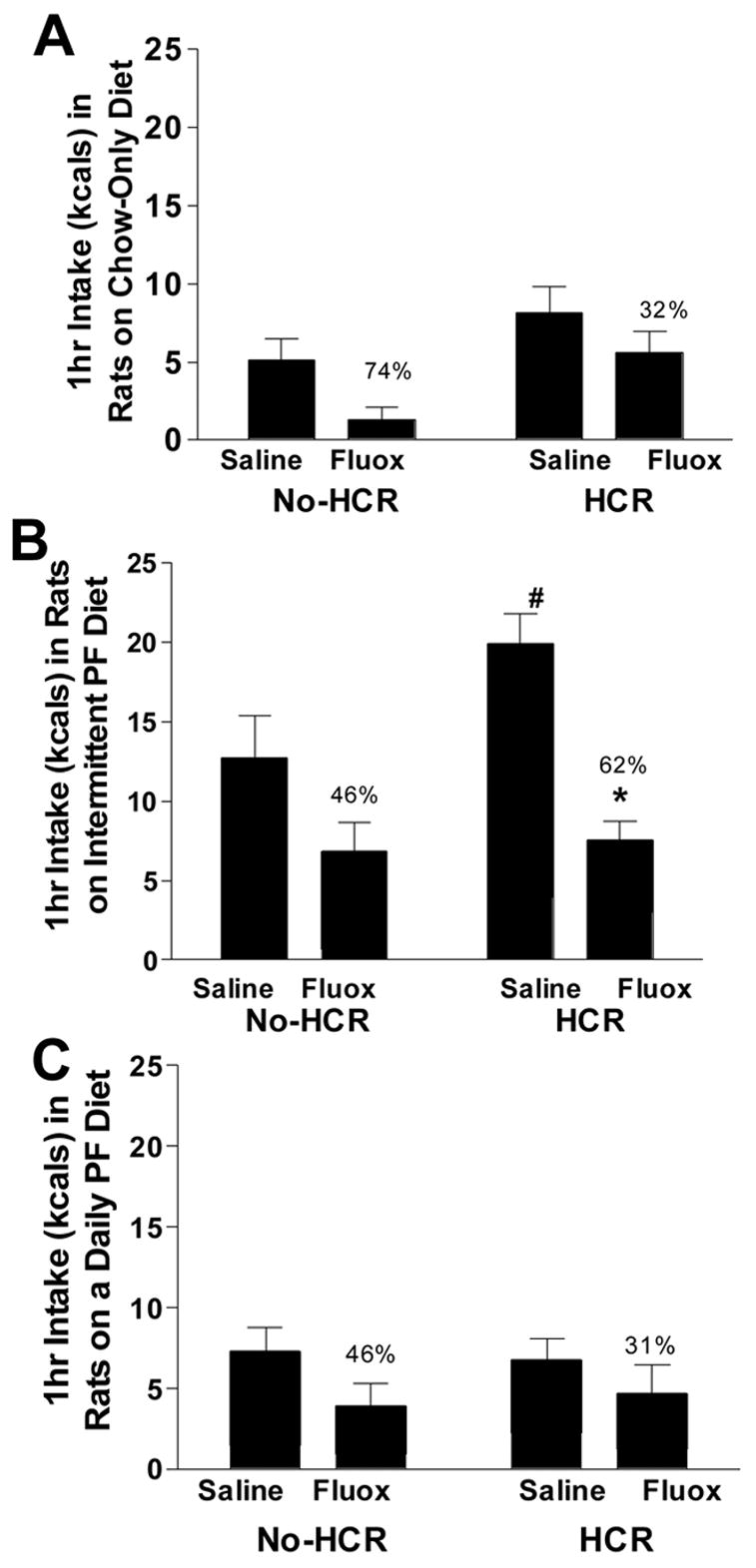
The effect of fluoxetine (i.p. 3mg/kg) on trigger-induced 1-hour intake of rats on a Chow Only diet (A), Intermittent palatable food (PF) diet (B), and Daily PF diet (C) with and without a history of caloric restriction (HCR). * Drug x diet group x HCR condition, p = 0.055; # Intermittent PF/HCR ate more following trigger vs. Intermittent PF/no-HCR group, p < 0.05. Percents represent the reduction in intake per total amount of kcals eaten.
The effect of fluoxetine to decrease intake in all diet conditions was apparent at 2 and 4 hrs post injection. At 2hrs, Chow Only rats treated with saline ate 6.83 ± 1.31, and with fluoxetine ate 4.53 ± 1.03 kcals. The Intermittent PF group treated with saline ate 7.64 ± 1.45, and with fluoxetine ate 6.59 ± 1.25 kcals. The Daily PF group treated with saline ate 4.54 ± 1.12, versus 2.01 ± 0.51 kcals with fluoxetine; main drug effect: F(1,41) = 35.06, p < 0.001. At 4hrs, the Chow Only group treated with saline consumed 14.68 ± 1.68 vs.11.29 ± 1.17 kcals with fluoxetine. The Intermittent PF group treated with saline ate 30.64 ± 1.76 vs. 21.48 ± 2.26 kcals with fluoxetine. The Daily PF group treated with saline consumed 19.97 ± 1.92, and with fluoxetine, consumed 14.13 ± 2.31 kcals; main effect of drug: F(1,41) = 23.91, p < 0.001). By 24 hrs there was no sign of a drug effect.
3.2. Experiment 2. The Effect of HCR and Frequency of PF Access on Monoamine Levels in the Hypothalamus, Nucleus Accumbens, and Medial Prefrontal Cortex
At the end of Exp. 1, body weights of the Daily PF group were greater than those of the Chow Only and Intermittent PF groups (Chow Only: no-HCR = 273 ± 3.6g, HCR = 267 ± 6.5g; Intermittent PF: no-HCR = 271.6 ± 3.3g, HCR = 270.6 ± 5.2g; Daily PF: no-HCR = 306.5 ± 10.3g, HCR = 308.8 ± 20.3g; main effect of diet group F(2,44) = 9.284, p < 0.001). Body weights of no-HCR and HCR groups did not differ within each diet condition. During the 3rd through 12th cycles, each group consumed the same amount of kcals across each cycle, but diet composition varied by group depending on whether PF was available, and the amount of time it was available to them. As expected, the Intermittent PF and Daily PF groups consumed less chow than the Chow Only group, and the Intermittent PF group consumed less PF than the Daily PF group, across each 12-day cycle (e.g. Cycle 11: Chow Only = 656.20 ± 10.92 kcals; Intermittent PF: chow = 495.77 ± 19.01, PF = 192.56 ± 13.72 kcals; Daily PF: chow = 242.21 ± 19.67, PF = 437.35 ± 35.30 kcals; between groups comparisons of chow and PF p values < 0.001). Given the macronutrient composition of the two diets, the intakes of chow and PF resulted in significantly different protein, fat and carbohydrate intakes between the diet groups (e.g. Cycle 11 kcals from protein: Chow Only = 123.23 ± 2.05, Intermittent PF = 93.14 ± 3.57, Daily PF = 45.57 ± 3.69, F = 155.23, p < .001 all groups different from each other p values < 0.001; kcals from carbohydrate: Chow Only = 507.57 ± 8.45, Intermittent PF = 490.24 ± 10.29, Daily PF = 436.64 ± 14.34, F = 11.12, p < 0.001, Chow Only versus Daily PF p < 0.001, Chow Only versus Intermittent PF p = n.s., Intermittent PF versus Daily PF p < 0.01; kcals from fat: Chow Only = 25.40 ± 0.42, Intermittent PF = 101.99 ± 5.37, Daily PF = 197.43 ± 14.65, F = 90.73, p < 0.001, all groups different from each other p values < 0.001). One rat from the Intermittent PF/HCR group was not included in the post-mortem measures due to illness prior to euthanasia.
While there were no differences between 5-HT and 5HIAA as a function of HCR or diet group in the anterior portion of the hypothalamus (Table 2), there was a difference in dopamine and DOPAC levels in this region as a function of diet group (dopamine: F(2,35) = 4.322, p < 0.05; DOPAC: F(2,37) = 3.516, p < 0.05; Fig. 2A & B). The groups with access to PF (irrespective of no-HCR or HCR condition) had lower hypothalamic dopamine and DOPAC levels compared to the Chow Only groups and this difference achieved statistical significance for the Intermittent PF vs. Chow Only group. Linear regression using total intake from Cycle 11, as representative of the typical intake across an entire cycle, failed to find that total intake from chow or from PF predicted hypothalamic dopamine and DOPAC. Likewise, linear regression of Cycle 11’s total protein, total carbohydrate, and total fat intake showed that intake of each macronutrient failed to predict hypothalamic dopamine and DOPAC.
Table 2.
Levels of 5-HT, 5HIAA, dopamine (DA), DOPAC, and their turnover ratios in the anterior portion of the hypothalamus for rats maintained on chow only, intermittent PF, or daily PF, with and without a history of HCR. Data are mean ± SEM ng / mg.
| 5-HT | 5HIAA | DA | DOPAC | 5HIAA/ 5-HT | DOPAC/ DA | |
|---|---|---|---|---|---|---|
| Hypothala mus | ||||||
| Chow/ no-HCR (N) | 1.12±0.26 (8) | 0.28±0.09 (7) | 3.38±0.67 (7) | 0.33±0.05 (8) | 0.21±0.03 (7) | 0.11±0.01 (7) |
| Chow/HCR (N) | 1.13±0.11 (6) | 0.21±0.05 (5) | 3.59±0.70 (6) | 0.37±0.07 (6) | 0.20±0.04 (5) | 0.11±0.01 (6) |
| Int./ no-HCR (N) | 0.98±0.12 (5) | 0.21±0.06 (5) | 1.78±0.441 (5) | 0.19±0.061 (5) | 0.20±0.05 (5) | 0.10±0.01 (5) |
| Int./HCR (N) | 0.83±0.12 (7) | 0.14±0.05 (7) | 2.06±0.431 (7) | 0.21±0.041 (7) | 0.15±0.03 (7) | 0.11±0.01 (7) |
| Daily/ no-HCR (N) | 0.75±0.16 (6) | 0.14±0.05 (6) | 2.26±0.47 (6) | 0.21±0.06 (6) | 0.19±0.06 (6) | 0.09±0.01 (6) |
| Daily/HCR (N) | 0.80±0.12 (8) | 0.13±0.03 (7) | 2.23±0.61 (7) | 0.25±0.07 (8) | 0.17±0.04 (7) | 0.09±0.01 (7) |
Less DA and DOPAC in the Intermittent PF group compared to the Chow Only group.
Fig. 2.
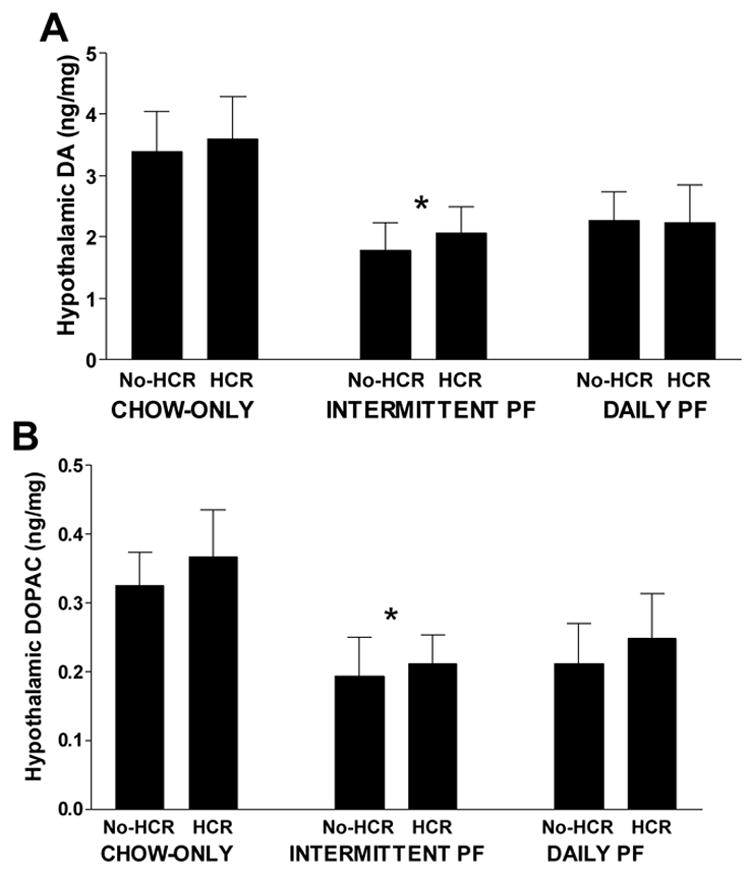
Levels of dopamine (A) and DOPAC (B) from an anterior section of the hypothalamus in rats fed a chow only diet, intermittent palatable food (PF) diet, and daily PF. *Pooled Intermittent PF rats with and without HCR vs. pooled Chow Only rats with and without HCR, p < 0.05.
In the nucleus accumbens, no differences in monoamine levels were detected among the groups. However, a strong positive association was found between 5-HT turnover and dopamine turnover in the accumbens in rats without HCR (r = 0.71, p < 0.01), regardless of diet condition (Figure 3A). This association was completely absent in rats with HCR across diet conditions (r = -0.061, ns; Fig. 3B).
Fig. 3.
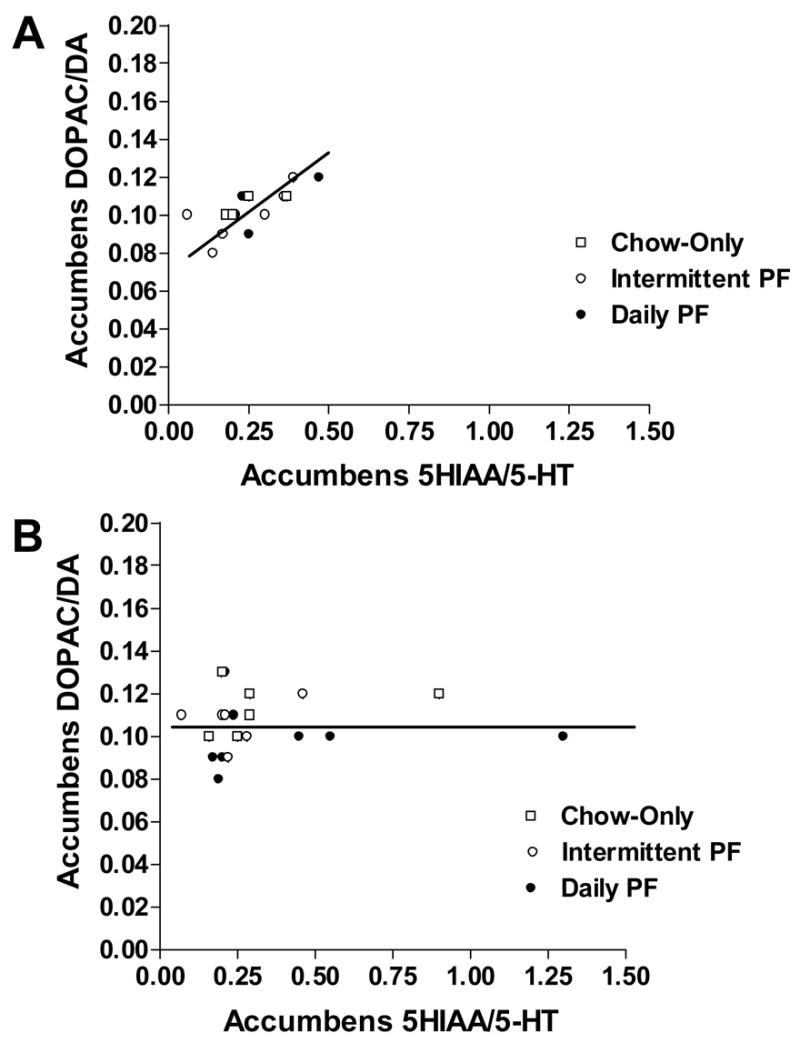
The effect of no-HCR (A) and HCR (B) across all diet conditions, on the relationship between 5-HT turnover and dopamine turnover in the nucleus accumbens. No-HCR: r = 0.71, p < 0.01 and HCR: r = -0.061, ns.
In the mPFC, no interaction between diet condition and HCR history, or main effects of each of these on monoamine or metabolite levels was detected. However, within the Intermittent PF group, HCR caused a decrease in 5-HT and dopamine levels compared to no-HCR (5-HT: t(10) = 3.376, p < 0.05; dopamine: t(10) = 2.575, p < 0.05; Fig. 4A & B).
Fig. 4.
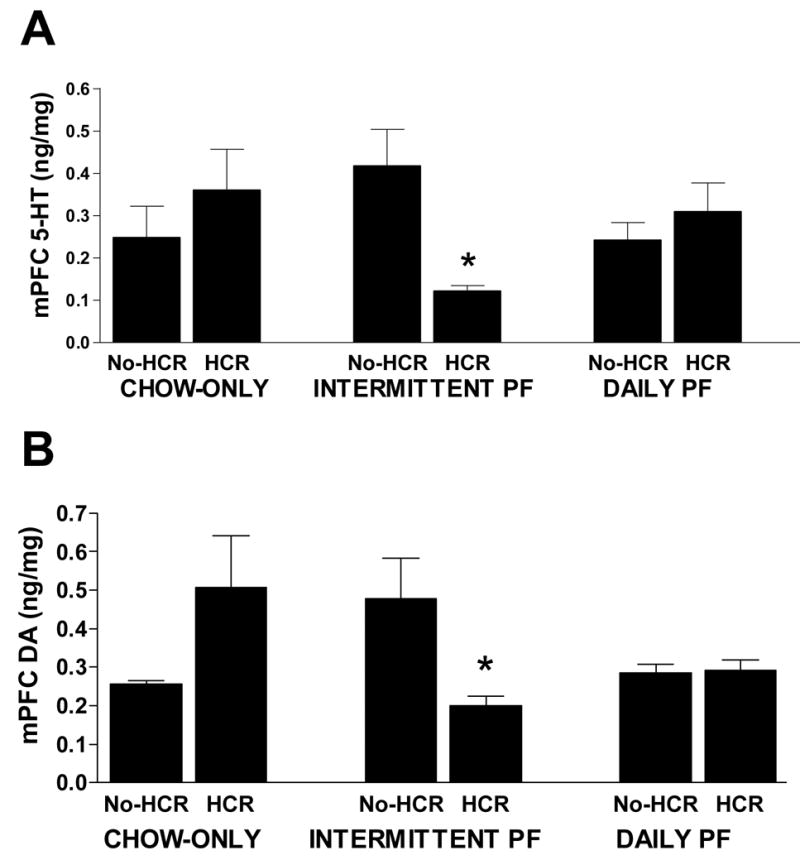
Levels of 5-HT (A) and dopamine (B) in the mPFC of rats fed a chow only diet, an intermittent palatable food (PF) diet, and a daily PF diet, with and without a history of caloric restriction (HCR). * Intermittent PF/HCR vs. Intermittent PF/no-HCR, p < 0.05. HCR had no significant effect on Chow Only and Daily PF groups.
3.3. Experiment 3. Effect of Intermittent PF with and without HCR on Measures of Anxiety and Depression
Anxiety Tests
For the elevated maze test, the inter-rater reliability coefficients were high (0.92 – 0.97) across the various measures. Rater scores were averaged and revealed that while rats clearly preferred spending time in the closed vs. the open arm (222 vs. 78 ± 12 seconds, respectively), there were no significant differences between the groups. There was a non-significant trend for the HCR group to have fewer closed arm entries (no-HCR = 3.06 ± 0.45, HCR = 2.31 ± 0.31), but no differences in head dipping, peeping out, or rearing, were noted.
For the open field test, the inter-rater reliability coefficients were high (0.89 – 0.93) for the measures and again, no differences in the average of the raters’ scores were found between the HCR groups on any of the open field parameters. All rats preferred to be in the periphery rather than the center (286 vs. 14 ± 2 seconds, respectively).
Depression Tests
Rats from the no-HCR and HCR groups weighed the same at the time of forced swim testing. The inter-rater reliability for latency to become inactive was 0.96, and for total time inactive was 0.78. The rats spent very little time swimming (on average < 5 seconds) and so this was added to the climbing/struggling time to give an overall “active/struggling” score, for which the inter-rater reliability was 0.91. The averaged rater scores revealed that the HCR rats became inactive more quickly than no-HCR rats (t(14) = 2.109, p(1-tailed) < 0.05; Fig 5A), and spent more total time inactive than no-HCR rats (t(14) = 1.868, p(1-tailed) < 0.05; Fig. 5B), consistent with depression.
Fig. 5.
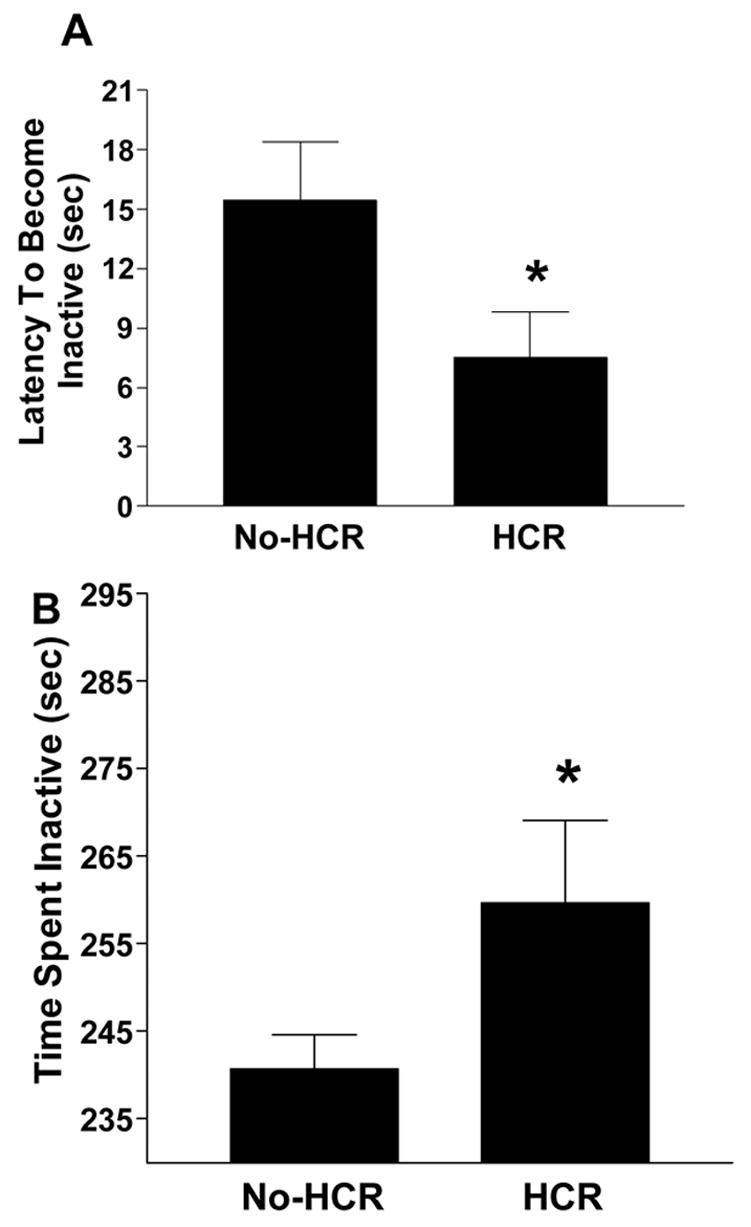
The effect of a history of caloric restriction (HCR) on (A) latency to become inactive and (B) total time inactive during the forced swim test. * Intermittent PF/HCR vs. Intermittent PF/no-HCR (only diet condition tested), p < 0.05.
For the sucrose test, after the 1st cycle, there was a persistent trend with repeated availability of sucrose solution for rats with HCR to consume less sucrose than rats in the no-HCR group. A statistical difference was found in the 10th cycle when HCR rats drank less sucrose over 4 hours than the no-HCR rats (1H: no-HCR = 13.24 ± 1.56, HCR = 10.90 ± 1.31 mls; 4H: no-HCR = 42.24 ± 3.88; HCR = 31.86 ± 3.73 mls; t(14) = 1.93, p < 0.05; 24H: no-HCR = 107.26 ± 9.15, HCR = 95.23 ± 12.72 mls). Water intake was the same in both groups.
In the novelty interaction test there was a significant group x bottle interaction effect at the 1st hour such that rats without HCR drank more from the novel bottle and less from the familiar water bottle, while HCR rats drank more from the familiar bottle and less from the novel bottle (1H: F(1,14) = 13.036, p < 0.01; Fig. 6). By 2 hours this interaction was no longer evident, with the HCR group drinking the same amount from each bottle, and by 24 hours, both groups were drinking more from the novel bottle.
Fig. 6.
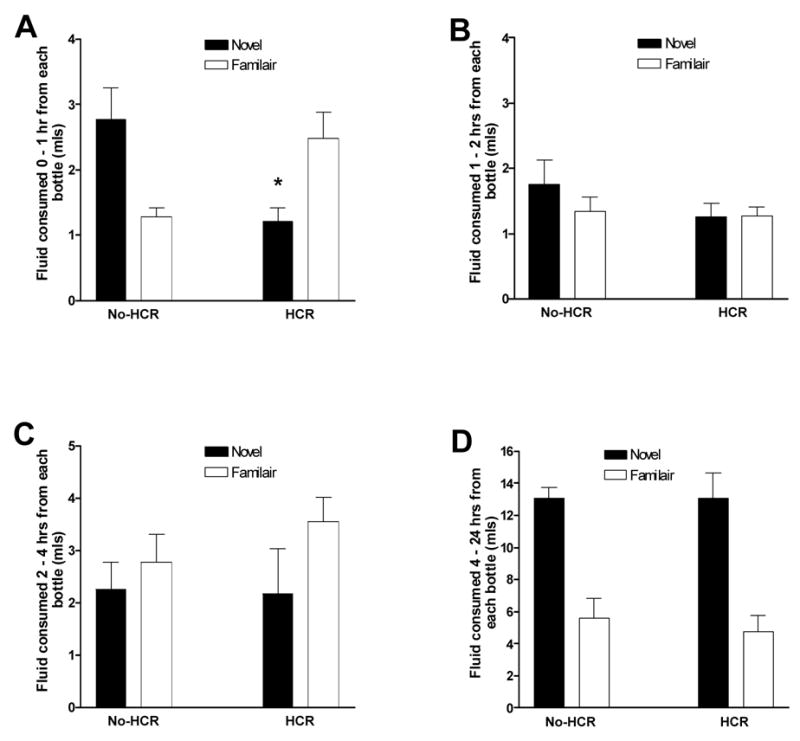
The effect of a history of caloric restriction (HCR) on incremental water intake from a familiar- versus a novel-positioned bottle, at hour 0–1 (A), hour 1–2 (B), hour 2–4 (C), and hour 4–24 hours (D). *Significant bottle by HCR condition interaction, p < 0.05.
4. Discussion
The goal of this study was to determine whether a history of caloric restriction in rats would yield pharmacological, neurochemical, and behavioral evidence consistent with altered monoamine control of feeding and mood regulation. Our results confirmed that a history of cyclic caloric restriction with intermittent PF (to mimic human dieting patterns) imposed such changes. Briefly, access to PF alone caused a reduction of dopamine and DOPAC in an anterior section of the hypothalamus, and HCR alone disrupted the normal relationship between 5-HT and dopamine turnover in the nucleus accumbens. The combination of HCR with intermittent PF resulted in an exaggerated anorectic effect of fluoxetine following a food trigger, reduced 5-HT and dopamine levels in the mPFC, and was associated with behavioral evidence of depression. The overarching significance of these findings is that the changes were observed in animals that were sated (not food-deprived) and at normal body weight (neither emaciated nor obese). Because stressed rats with HCR + Intermittent PF binge-eat (Hagan, et al., 2002), the persistent neurochemical changes found here may describe part of the mechanism that renders organisms susceptible to binge-eating. These physiological changes may also help explain the comorbidity of depression among individuals with eating disorders and warn of possible depression-favoring changes that may occur among dieters.
Just the presence of PF, whether intermittently or daily, decreased dopamine and its metabolite in an anterior section of the hypothalamus. While we cannot rule out the possibility that inadequate protein intake may have resulted in reduced dopamine and DOPAC in the hypothalamus, the data presented does not support this hypothesis. Firstly, protein (and likewise, carbohydrate and fat) intake failed to predict hypothalamic levels of dopamine or DOPAC; secondly, despite the fact that the Daily PF group consumed less than half the amount of protein consumed by the Intermittent PF group, both groups had the same amount of hypothalamic dopamine and DOPAC; and thirdly, there was not a consistent reduction in dopamine or DOPAC in the other brain areas assayed (nucleus accumbens and mPFC), in the Intermittent PF and Daily PF groups,.
Little is known about the function of dopamine in this hypothalamic section. However, lateral hypothalamic dopamine is present in this region and has been implicated in body weight set point (Boyle and Keesey, 1975), meal size regulation (Yang, et al., 1997), and PF-induced reward (Park and Carr, 1998). Given that animals fed PF consume larger meals than those without PF (Warwick, et al., 2003), and that lateral hypothalamic dopamine is released in proportion to the amount of food consumed during a meal (Meguid, et al., 1995), lower dopamine there may provide a mechanism by which animals fed PF are able to consume larger meals. Lateral hypothalamic neurons are also efferent to the nucleus accumbens and known to affect accumbens opioid and dopamine signaling for reward (Will, et al., 2003). Therefore, although we found no change in nucleus accumbens dopamine levels in these rats, altered hypothalamic dopamine levels may render these rats susceptible to altered reward signaling from PF. To our knowledge, this is the first time a reduction of hypothalamic dopamine has been associated with intake of PF. Further exploration of dopamine function in discrete hypothalamic regions, particularly the lateral hypothalamus, of rats with access to PF may be warranted by our findings.
Unlike PF, a history of cyclic caloric restriction and refeeding (HCR), meant to simulate human dieting, did result in changes within the nucleus accumbens. Specifically, there is a strong positive relationship between dopamine and 5-HT turnover in control rats that is completely absent in rats with HCR. Importantly, this is the second time we have observed this relationship between monoamine turnovers in rats with and without HCR (Hagan, et al., 2004). We now can confirm that the previously observed effect was not uniquely influenced by intermittent access to PF, and in fact, was not influenced by PF at all, since it was also observed in HCR rats fed only chow or daily PF and chow. There is a growing body of evidence to suggest functional interactions between 5-HT and dopamine in the nucleus accumbens (Bowers, et al., 2000; De Deurwaerdere, et al., 2004; Kuroki, et al., 2003; Liegeois, et al., 2002; Yan and Yan, 2001). It is possible, therefore, that the 5-HT/dopamine interaction is reflected by the strong correlation in turnover ratios present in rats without HCR. If so, the fact that rats with HCR lack this correlation suggests that 5-HT may not be able to influence dopamine activity in the nucleus accumbens as it normally would. Interestingly, the interaction between 5-HT and dopamine in the accumbens is compromised in animal models of depression, but is recovered after antidepressant treatment (Zangen, et al., 2001). This is relevant to our findings in that, like these rat models of depression, our rats exposed to HCR and intermittent PF exhibited behavioral evidence consistent with depression.
We were particularly interested in changes within rats exposed to the combination of HCR and intermittent PF because these are conditions most characteristic of human dieting. The SSRI fluoxetine was used as pharmacological probe of 5-HT regulation in these rats. We found differential responses only after the rats were triggered to overeat by having food withheld for an hour following access to just a small amount of food. It is possible that a differential fluoxetine effect was not seen under non-triggered conditions because this test was conducted on an earlier cycle than that involving the food trigger. However, we have seen in the past that intake behavior in this model is remarkably consistent across cycles, and so it is unlikely that duration of cycling impacted the results. It should be noted that overeating following a food trigger is a significant behavioral finding. That is, a history of restriction with access to intermittent PF but not the absence of these factors, nor each of these factors alone, renders rats susceptible to overeating following a small food trigger. The power of the combination of these factors to induce overeating is supported by other studies showing that restriction followed by PF causes subsequent overeating of PF, superceding that induced by intermittent access to PF alone (Bello, et al., 2006; Hagan and Moss, 1997). This has potential treatment implications for human binge-eating. Regarding the effect of fluoxetine, a previous study in our lab found its anorectic effect to be most potent in Intermittent PF/HCR rats following stress (Placidi, et al., 2004). In this study we did not stress the rats and found that proportionally speaking, fluoxetine was most potent in reducing the Chow Only/no-HCR group and in the Intermittent PF/HCR, but did not statistically differ from all other groups. If we only consider its effect on PF intake, fluoxetine had a greater anorectic effect on the PF intake of Intermittent PF/HCR rats. HCR may have reduced central levels of 5HT as has been shown with food restriction (Krieger, et al., 1980; Stanley, et al., 1989) to induce receptor upregulation as a possible mechanism for greater anorectic effects from SSRI treatment. Given that the proportional fluoxetine-induced reduction was the same for Chow Only/no-HCR rats, an alternative explanation that fluoxetine is having a rate-limiting effect, appears plausible. However, the Intermittent PF/no-HCR group consumed a greater overall amount of kcals than did the Chow Only/no-HCR group and so fluoxetine’s effect is unlikely due to differing baseline intakes.
More direct evidence of monoamine dysregulation was observed in assays of the mPFC. Rats with Intermittent PF/HCR had less 5-HT and dopamine here compared to their no-HCR counterparts. Reduced 5-HT and dopamine in the mPFC is associated with behaviors indicative of negative affect and/or mood disorders. For example, 5-HT depletion in this region reduces exploratory behavior (Lipska, et al., 1992) and lowers consumption of sucrose-ethanol solutions in rats (Deckel, et al., 1997), both behaviors consistent with these models of depression. Dopaminergic depletion in this region has been found to impair cognitive performance (Clinton, et al., 2006), and similarly, depletion of 5-HT in the mPFC also results in perseverative responding to a stimulus, suggesting a type of cognitive inflexibility similar to behavior seen in humans with obsessive-compulsive disorder (Clarke, et al., 2004). These effects are important to consider in the present rat model because people suffering from eating disorders, some of which involve HCR and intermittent access to PF, present symptoms of impaired concentration and depression and are more likely than the healthy, non-disordered population to suffer from obsessive-compulsive disorder and a rigid style of thinking (Kaye, et al., 2004; Speranza, et al., 2001).
Indeed, rats with HCR in this study exhibited behaviors consistent with models of depression. Specifically, they were less active in the forced swim test, a gold-standard measure of depression in rodents (Cryan, et al., 2005). They also consumed less of a dilute palatable sucrose solution with repeated access to it; a pattern interpreted to reflect anhedonia (Bekris, et al., 2005; Grippo, et al., 2006). We obtained modestly significant results with this measure, but the strength of the effect may have been compromised by the fact that the results rely on the ingestion of a palatable substance in rats with prior access to PF. To the best of our knowledge, this is the first time this test has been used under these circumstances. The finding that these Intermittent PF/HCR rats also drank less from a novel water bottle compared to rats without HCR lends further support of anhedonia. Rats exhibit a conditioned place preference for locations in which they have repeatedly been exposed to novel objects (Bevins and Bardo, 1999), suggesting that interaction with novel objects is rewarding.
This result, however, could also reflect neophobia and this appears quite plausible given that after the first hour of avoidance of the novel bottle, the HCR group began drinking as much from the novel as the familiar bottle with time. Since neophobia is more a trait of anxiety than of depression, we cannot completely rule out the possibility that HCR did not also induce anxiety. Failure to observe differences from no-HCR rats in the elevated maze and open-field test, however, suggests depression, over anxiety, as the more salient consequence of HCR. Anxiety is often exhibited in humans with a history of dieting and/or eating disorders (Sassaroli et al., 2005; Godart et al., 2004; Kaye et al., 2004; Keel et al., 2005), and a limitation in this study is that the specific open field arena and elevated maze used for these experiments have not previously been validated. Therefore, we cannot rule out the possibility that the measures used here were not sensitive enough to unmask differences in anxiety-related behavior due to a history of restriction. Nevertheless, the decreased monoamine levels in the mPFC and the consistent results of the behavioral tests of depression together, suggest that HCR with intermittent access to PF can negatively impact affect.
In regard to the influence that ingestion of the PF might have had on the neurochemical and behavioral results, an important caveat to keep in mind is that it may not have been palatability per se, but the macronutrients in this food that affected the results. It will be interesting in future studies to determine the extent to which high-fat, high-sugar, and the addition of protein alone or in combination, modify the outcomes of this study. However, if it is palatability and not macronutrient composition that interacts with HCR to elicit the findings described, they should be replicated with any highly palatable substance.
In conclusion, this study provides the first evidence of behavioral and neurochemical changes induced by a history of caloric restriction. Our findings, which point to impaired regulation of feeding, reward, and mood, are particularly important given that all the tests were conducted at a time when food intake and body weight had normalized in the rats with a history of restriction. This warns that cyclic caloric restriction combined with intermittent access to PF can induce behavioral and neurochemical changes that persist despite recovery of normal body weight and food intake. These factors also yielded behavioral and neurochemical markers of depression, a common symptom among restrictive dieters and those with eating disorders. Importantly, since these markers were observed in rats, the comorbid symptoms in depression that plague these individuals may not be due to a cognitive response to the consequences of their maladaptive eating behavior per se, so much as to a direct effect of their dieting and eating practices on brain function.
Acknowledgments
Supported by NIH grant DK066007 (MMB). We would also like to thank Drs. Alan Randich, and Jim Cox of the UAB Department of Psychology, and Dr. Tim Nagy of the UAB Nutrition Sciences Department for their helpful advice and input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson IM, Parry-Billings M, Newsholme EA, Fairburn CG, Cowen PJ. Dieting reduces plasma tryptophan and alters brain 5-HT function in women. Psychol Med. 1990;20:785–791. doi: 10.1017/s0033291700036473. [DOI] [PubMed] [Google Scholar]
- Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 2005;161:45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Bello NT, Guarda AS, Hyun J, Moran TH. Reduced caloric availability with intermittent access to sweetened fat leads to binge-type feeding in rats. Society for the Study of Ingestive Behavior Abstracts. 2006:x00023;182. [Google Scholar]
- Bevins RA, Bardo MT. Conditioned increase in place preference by access to novel objects: antagonism by MK-801. Behav Brain Res. 1999;99:53–60. doi: 10.1016/s0166-4328(98)00069-2. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci. 2005;119:1207–1214. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Henry MB, Thielen RJ, McBride WJ. Serotonin 5-HT(2) receptor stimulation of dopamine release in the posterior but not anterior nucleus accumbens of the rat. J Neurochem. 2000;75:1625–1633. doi: 10.1046/j.1471-4159.2000.0751625.x. [DOI] [PubMed] [Google Scholar]
- Boyle PC, Keesey RE. Chronically reduced body weight in rats sustaining lesions of the lateral hypothalamus and maintained on palatable diets and drinking solutions. J Comp Physiol Psychol. 1975;88:218–223. doi: 10.1037/h0076187. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Sucharski IL, Finlay JM. Desipramine attenuates working memory impairments induced by partial loss of catecholamines in the rat medial prefrontal cortex. Psychopharmacology (Berl) 2006;183:404–412. doi: 10.1007/s00213-005-0221-2. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Cowen PJ, Clifford EM, Walsh AE, Williams C, Fairburn CG. Moderate dieting causes 5-HT2C receptor supersensitivity. Psychol Med. 1996;26:1155–1159. doi: 10.1017/s003329170003587x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdere P, Navailles S, Berg KA, Clarke WP, Spampinato U. Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci. 2004;24:3235–3241. doi: 10.1523/JNEUROSCI.0112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vry J, Schreiber R. Effects of selected serotonin 5-HT(1) and 5-HT(2) receptor agonists on feeding behavior: possible mechanisms of action. Neurosci Biobehav Rev. 2000;24:341–353. doi: 10.1016/s0149-7634(99)00083-4. [DOI] [PubMed] [Google Scholar]
- Deckel AW, Shoemaker WJ, Arky L. Effects of 5,7-dihydroxytryptamine lesions of the prefrontal cortex on consumption of sucrose-ethanol solutions: relationship to prefrontal monoamines. Alcohol Clin Exp Res. 1997;21:631–636. [PubMed] [Google Scholar]
- Godart NT, Perdereau F, Curt F, Lang F, Venisse JL, Halfon O, Bizouard P, Loas G, Corcos M, Jeammet P, Flament MF. Predictive factors of social disability in anorexic and bulimic patients. Eat Weight Disord. 2004;9:249–257. doi: 10.1007/BF03325078. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Fairburn CG, Cowen PJ. Dieting changes serotonergic function in women, not men: implications for the aetiology of anorexia nervosa? Psychol Med. 1987;17:839–842. doi: 10.1017/s0033291700000635. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006;59:309–316. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disord. 1997;22:411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge-eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Placidi RJ, Viana JB, Oswald KD. Dieting and stress combine synergistically to produce an animal model of binge-eating. Annual Meeting of the American Psychiatry Association; 2004;May 4; New York, NY. [Google Scholar]
- Heffner TG, Hartman JA, Seiden LS. A rapid method for the regional dissection of the rat brain. Pharmacol Biochem Behav. 1980;13:453–456. doi: 10.1016/0091-3057(80)90254-3. [DOI] [PubMed] [Google Scholar]
- Jimerson DC, Lesem MD, Kaye WH, Hegg AP, Brewerton TD. Eating disorders and depression: is there a serotonin connection? Biol Psychiatry. 1990;28:443–454. doi: 10.1016/0006-3223(90)90412-u. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161:2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- Keel PK, Klump KL, Miller KB, McGue M, Iacono WG. Shared transmission of eating disorders and anxiety disorders. Int J Eat Disord. 2005;38:99–105. doi: 10.1002/eat.20168. [DOI] [PubMed] [Google Scholar]
- Krieger DT, Crowley WR, O'Donohue TL, Jacobowitz DM. Effects of food restriction on the periodicity of corticosteroids in plasma and on monoamine concentrations in discrete brain nuclei. Brain Res. 1980;188:167–174. doi: 10.1016/0006-8993(80)90565-x. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Meltzer HY, Ichikawa J. 5-HT 2A receptor stimulation by DOI, a 5-HT 2A/2C receptor agonist, potentiates amphetamine-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Brain Res. 2003;972:216–221. doi: 10.1016/s0006-8993(03)02516-2. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Alexander JT. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol Psychiatry. 1998;44:851–864. doi: 10.1016/s0006-3223(98)00186-3. [DOI] [PubMed] [Google Scholar]
- Liegeois JF, Ichikawa J, Meltzer HY. 5-HT(2A) receptor antagonism potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and inhibits that in the nucleus accumbens in a dose-dependent manner. Brain Res. 2002;947:157–165. doi: 10.1016/s0006-8993(02)02620-3. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Arya A, Weinberger DR. Serotonin depletion causes long-term reduction of exploration in the rat. Pharmacol Biochem Behav. 1992;43:1247–1252. doi: 10.1016/0091-3057(92)90510-m. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Yang ZJ, Koseki M. Eating induced rise in LHA-dopamine correlates with meal size in normal and bulbectomized rats. Brain Res Bull. 1995;36:487–490. doi: 10.1016/0361-9230(95)92128-3. [DOI] [PubMed] [Google Scholar]
- Mora-Giral M, Raich-Escursell RM, Segues CV, Torras-Claraso J, Huon G. Bulimia symptoms and risk factors in university students. Eat Weight Disord. 2004;9:163–169. doi: 10.1007/BF03325062. [DOI] [PubMed] [Google Scholar]
- Park TH, Carr KD. Neuroanatomical patterns of fos-like immunoreactivity induced by a palatable meal and meal-paired environment in saline- and naltrexone-treated rats. Brain Res. 1998;805:169–180. doi: 10.1016/s0006-8993(98)00719-7. [DOI] [PubMed] [Google Scholar]
- Patton GC, Carlin JB, Shao Q, Hibbert ME, Rosier M, Selzer R, Bowes G. Adolescent dieting: healthy weight control or borderline eating disorder? J Child Psychol Psychiatry. 1997;38:299–306. doi: 10.1111/j.1469-7610.1997.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. California: Academic Press; 1998. [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Placidi RJ, Chandler PC, Oswald KD, Maldonado C, Wauford PK, Boggiano MM. Stress and hunger alter the anorectic efficacy of fluoxetine on the binge-eating of rats with a history of caloric restriction. Int J Eat Disord. 2004;36:328–341. doi: 10.1002/eat.20044. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sassaroli S, Bertelli S, Decoppi M, Crosina M, Milos G, Ruggiero GM. Worry and eating disorders: a psychopathological association. Eat Behav. 2005;6:301–307. doi: 10.1016/j.eatbeh.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73:37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- Speranza M, Corcos M, Godart N, Loas G, Guilbaud O, Jeammet P, Flament M. Obsessive compulsive disorders in eating disorders. Eat Behav. 2001;2:193–207. doi: 10.1016/s1471-0153(01)00035-6. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Schwartz DH, Hernandez L, Leibowitz SF, Hoebel BG. Patterns of extracellular 5-hydroxyindoleacetic acid (5-HIAA) in the paraventricular hypothalamus (PVN): relation to circadian rhythm and deprivation-induced eating behavior. Pharmacol Biochem Behav. 1989;33:257–260. doi: 10.1016/0091-3057(89)90459-0. [DOI] [PubMed] [Google Scholar]
- Stice E, Burton EM, Shaw H. Prospective relations between bulimic pathology, depression, and substance abuse: unpacking comorbidity in adolescent girls. J Consult Clin Psychol. 2004;72:62–71. doi: 10.1037/0022-006X.72.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh AE, Oldman AD, Franklin M, Fairburn CG, Cowen PJ. Dieting decreases plasma tryptophan and increases the prolactin response to d-fenfluramine in women but not men. J Affect Disord. 1995;33:89–97. doi: 10.1016/0165-0327(94)00078-n. [DOI] [PubMed] [Google Scholar]
- Warwick ZS, Synowski SJ, Rice KD, Smart AB. Independent effects of diet palatability and fat content on bout size and daily intake in rats. Physiol Behav. 2003;80:253–258. doi: 10.1016/j.physbeh.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ, Wurtman JJ. Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res. 1995;3:477S–480S. doi: 10.1002/j.1550-8528.1995.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Yan QS, Yan SE. Activation of 5-HT(1B/1D) receptors in the mesolimbic dopamine system increases dopamine release from the nucleus accumbens: a microdialysis study. Eur J Pharmacol. 2001;418:55–64. doi: 10.1016/s0014-2999(01)00913-x. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Meguid MM, Chai JK, Chen C, Oler A. Bilateral hypothalamic dopamine infusion in male Zucker rat suppresses feeding due to reduced meal size. Pharmacol Biochem Behav. 1997;58:631–635. doi: 10.1016/s0091-3057(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Zangen A, Nakash R, Overstreet DH, Yadid G. Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology (Berl) 2001;155:434–439. doi: 10.1007/s002130100746. [DOI] [PubMed] [Google Scholar]


