Abstract
We investigated whether changes in memory or static balance in chronic alcoholics, occurring with abstinence or relapse, are associated with changes in lateral and fourth ventricular volume. Alcoholics meeting DSM-IV criteria for Alcohol Dependence (n = 15) and non-alcoholic controls (n = 26) were examined twice at a mean interval of two years with standard Wechsler Abbreviated Scale of Intelligence (WASI), Wechsler Memory Scale—Revised (WMS-R) tests, an ataxia battery, and structural MRI. At study entry, alcoholics had been abstinent on average for over 4 months and achieved lower scores than controls on WASI General IQ Index, WMS-R General Memory Index, and the ataxia battery. The ten alcoholics who maintained sobriety at retest did not differ at study entry in socio-demographic measures, alcohol use, or WASI and WMS-R summary scores from the five relapsers. At follow-up, abstainers improved more than controls on the WMS-R General Memory Index. Ataxia tended to improve in abstainers relative to controls. Associations were observed between memory and lateral ventricular volume change and between ataxia and fourth ventricular volume change in alcoholics but not in the controls. Both memory and ataxia can improve with sustained sobriety, and brain-behavior associations suggest selective brain structural substrates for the changes observed.
Keywords: MRI, ventricles, recovery, ataxia, brain-behavior association
1. Introduction
Chronic excessive alcohol use can impair a range of cognitive and motor functions (Tarter and Ryan, 1983; Parsons, 1987b; Fein et al., 1990; Nixon, 1993; Sullivan et al., 2000d), but recovery of some of these functions can occur with sobriety (Brandt et al., 1983; Parsons, 1987a; Lishman, 1990; Bates et al., 2005, Rourke, 1999 #14594). Cognitive functions most likely to recover, patient characteristics most likely to predict recovery, and time course for such recovery have important implications for treatment (Goldman, 1995; Nixon et al., 1998; Bates et al., 2002). When memory tasks require active strategies for encoding, semantic organization, and retrieval of learned material, they probably draw on frontal executive systems (e.g., Incisa della Rocchetta and Milner, 1993; Fletcher et al., 1998; Savage et al., 2001) and medial temporal systems required for formation of new memories (for review see, Gabrieli, 1998). Deficits in such strategic memory tasks can occur with chronic alcoholism (Brandt et al., 1983; Rourke and Grant, 1999; Munro et al., 2000; Sullivan et al., 2000d; Sullivan et al., 2002; Fama et al., 2004); (for reviews see, Parsons, 1987b; Riege, 1987; Oscar-Berman and Marinkovic, 2003) but can also recover with abstinence (Parsons, 1987a; Fein et al., 1990; Fein et al., 2006). Memory tasks that do not require strategic search through encoded material but instead rely on selection of a correct item from a given set, such as word recognition, are typically spared in uncomplicated alcoholism (Salmon et al., 1986; Oscar-Berman and Pulaski, 1997; Sullivan et al., 1997; Sullivan et al., 2000d).
There is no consensus on the nature, extent, or course of recovery of memory processes requiring strategic search. One study (Bates et al., 2005) found a clinically significant, moderate effect size for change in memory in a large sample of alcoholics retested 6 weeks after entering treatment, whereas another (Mann et al., 1999) found no evidence for treatment-related improvements in memory tests after a comparable interval in a sample of 49 alcoholic men even though improvement occurred in other functional domains. Cross-sectional studies have shown that alcoholics sober for several months (Sullivan et al., 2000d; Sullivan et al., 2002; Meyerhoff, 2005; Rosenbloom et al., 2005), one year (Hochla et al., 1982; Parsons et al., 1990; Munro et al., 2000; Rosenbloom et al., 2004), or as long as seven years (Brandt et al., 1983) may still show memory deficits relative to non-alcoholic controls. However, other cross-sectional studies have shown that performance on memory tests is related to length of abstinence (Joyce and Robbins, 1993; Oscar-Berman et al., 2004), and that alcoholics sober for more than 4 years are undistinguishable from controls on memory testing (Grant et al., 1984; Reed et al., 1992; Oscar-Berman et al., 2004; Fein et al., 2006). Furthermore, a longitudinal study showed memory improvement relative to baseline after 4 years of abstinence (Rosenbloom et al., 2004).
In addition to demonstrating fragility in strategic memory processes of the type served by frontal and medial temporal lobe brain systems, alcoholics commonly exhibit impaired static posture, owing to disruption of selective cerebellar systems (Gilman et al., 1990; Sullivan et al., 2000a; Sullivan et al., 2006). Although partial functional recovery of balance can occur with sobriety (Diener et al., 1984), it may take years to manifest (Rosenbloom et al., 2004).
Structural brain volume deficits in chronic alcoholism include reduction in cortical gray and white matter volume and enlargement of lateral ventricles. Some of these abnormalities reverse with abstinence (Ron et al., 1982; Carlen et al., 1984; Shear et al., 1994; Pfefferbaum et al., 1995; O'Neill et al., 2001; Gazdzinski et al., 2005; Meyerhoff, 2005). Observed associations between longitudinally detected change in brain structure and in memory with abstinence (Sullivan et al., 2000c) suggest that these gross morphological brain changes may have functional significance. Demonstration of such relationships is not always forthcoming, although association between improvement in cognitive performance and MRI-detected levels of spectroscopic measures of tissue integrity have recently been reported (Durazzo et al., 2006).
Given the relevance of functional recovery in the human alcoholic condition, the challenge of retaining alcoholics in longitudinal study, and the relative dearth of clinical reports on this issue, repeated measures findings based even on small samples can contribute to our knowledge of the scope and limits of human neural plasticity following years of excessive drinking. Indeed, we recently demonstrated the value of longitudinal over cross-sectional analysis to detect small brain changes in small subject samples (Rohlfing et al., 2006). Here we report on change over 2 years in performance on standardized tests of memory, intelligence, and ataxia and relate observed changes to morphometrically measured change in the lateral ventricles and the fourth ventricle. Greater volume of the lateral ventricles, which impinge on a number of cortical regions and subcortical structures, has been associated with less volume of cortical gray and white matter as well as of basal ganglia in alcoholics (cf, Symonds et al., 1999). The fourth ventricle, located anterior and adjacent to the cerebellum, is not typically reported to be greater in alcoholics than controls (cf, Sullivan et al., 2000b) but associations between fourth ventricle and cerebellar vermis volumes have been reported in fragile-X patients (Aylward and Reiss, 1991), supporting use of volume change in this structure as a surrogate for change in the volume of the cerebellar vermis.
2. Methods
2.1 Subjects
Presented in this report is a subset of alcoholics (12 men, 14 women) and controls (8 men, 7 women) from a previously described sample (Rosenbloom et al., 2005; Pfefferbaum et al., 2006a; Pfefferbaum et al., 2006b) who returned for follow-up testing on average after 22 ± 6 months. The sample included 5 non-caucasian participants (4 controls and 1 alcoholic). Other group characteristics are summarized in Table 1. The study was approved by the Institutional Review Boards at Stanford University School of Medicine and SRI International. All subjects gave written informed consent after the nature of the study and procedures were fully explained to them and received a modest stipend for their participation. Subjects were then evaluated using a formal psychiatric interview (Structured Clinical Interview for the DSM-IV, SCID) (First et al., 1998), a semi-structured assessment of lifetime alcohol consumption (Skinner, 1982; Skinner and Sheu, 1982; Pfefferbaum et al., 1992), and a variety of instruments to assess medical history, family history of alcoholism (positive if subject identified any primary relative with “problem drinking”), socio-economic status (SES) (Hollingshead and Redlich, 1958), handedness (Crovitz and Zener, 1962), and smoking history (Heatherton et al., 1991). Alcoholics, initially tested after periods of sobriety ranging from 3 weeks to 2 years (median = 6 weeks), met DSM-IV criteria for Alcohol Dependence. Controls did not meet criteria for any lifetime or current Axis I diagnosis and had never drunk more than 4 (men) or 3 (women) standard drinks a day over an extended period (one month). No subject had a history of schizophrenia or bipolar disorder, significant liver disease, major medical or neurological illness, head injury involving loss of consciousness for more than 30 minutes, or current (in past 3 months) illicit drug use. All but 3 of the alcoholic patients had a lifetime diagnosis of either Depression, Anxiety, or Substance use disorders and 8 were currently taking anti-depressants. However, only 3 of them currently met criteria for an Axis I disorder.
Table 1.
Baseline Characteristics of Controls, Abstaining and Relapsing Alcoholics
| Controls (n = 26) | Alcoholic vs Control | All Alcoholics(n=15) | Abstainers(n = 10) | Abstainer vs Relapser | Relapsers (n=5) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | t-test or χ2*p-value | Mean | (SD) | Mean | (SD) | Mann-Whitney-U or χ2 p-value | Mean | (SD) | |
| Demographics and Cognitive Status | ||||||||||
| Age | 52.8 | (10.6) | ns | 48.8 | (8.6) | 47.3 | 8.2 | ns | 51.8 | (9.7) |
| Body Mass Index | 26.2 | (5.3) | ns | 28.9 | (4.8) | 30.4 | 5.3 | U = 11.0, p=.09 | 26.0 | (1.1) |
| Ethnicity (percent non-caucasian) | 15% | ns | 7% | 10% | ns | 0% | ||||
| Handedness (Crovitz) | 18.2 | (10.1) | t=1.8, p=.08 | 25.3 | (15.3) | 22.3 | (12.0) | ns | 31.4 | (20.5) |
| Years of Education | 16.0 | (2.3) | t=2.3, p=.03 | 14.1 | (2.9) | 13.9 | (3.3) | ns | 14.6 | (2.2) |
| Lifetime Socioeconomic Status | 22.4 | (8.6) | ns | 26.8 | (12.4) | 26.6 | (14.1) | ns | 27.2 | (9.6) |
| Lifetime Alcohol Consumption (kg) | 56.6 | (64.4) | t=7.6, p=<.0001 | 760.7 | (471.6) | 732.8 | (562.8) | ns | 816.4 | (244.7) |
| Percent who Currently Smoke | 0% | – | χ2=16.1, p=<.0001 | 60% | – | 50% | – | ns | 80% | – |
| Percent Female | 54% | – | ns | 47% | – | 40% | – | ns | 60% | – |
| Premorbid Intelligence (NART) | 113.9 | (5.8) | ns | 111.6 | (8.7) | 110.2 | (9.0) | ns | 114.4 | (8.1) |
| Dementia Rating Scale Total | 141.3 | (2.0) | t=2.5, p=.02 | 139.1 | (3.5) | 138.9 | (4.2) | ns | 139.6 | (1.5) |
| Mini Mental Status Exam Total | 28.2 | (1.1) | ns | 28.1 | (1.2) | 27.9 | (1.4) | ns | 28.4 | (0.9) |
| Amnesia Index (Full IQ - General Memory Index) | (1.3) | (10.7) | ns | (0.7) | (12.7) | 1.5 | (13.7) | ns | −5.0 | (10.5) |
| Clinical Status | ||||||||||
| Global Assessment of Function (SCID) | -- | -- | 63 | (14.2) | 57.9 | (10.8) | U =6.0, p=.05 | 75.0 | (15.8) | |
| Length of Illness | -- | -- | 15.4 | (9.9) | 13.5 | (8.3) | ns | 20.5 | (13.0) | |
| Days Sober Before Test | -- | -- | 131 | (188.8) | 65.6 | (59.0) | U=9.5, p=.057 | 262.6 | (290.7) | |
| Family History Alcoholism | 34% | – | χ2=3.2, p=.07 | 65% | – | 70% | – | ns | 50% | – |
| Retest Interval (years) | 1.80 | (0.40) | ns | 1.93 | (0.77) | 1.85 | (0.74) | ns | 2.10 | (0.88) |
| WMS-R | ||||||||||
| Verbal Memory Index | 115.4 | (12.3) | ns | 108.8 | (17.5) | 103.6 | (14.1) | U=10.0, p=.07 | 119.2 | (20.6) |
| Visual Memory Index | 118.3 | (2.1) | t=1.7, p=.05 | 112.3 | (11.2) | 109.4 | (10.9) | ns | 118.0 | (10.4) |
| General Memory Index | 119.5 | (12.5) | t=2.0, p=.03 | 110.1 | (17.2) | 105.6 | (15.3) | U=10.5, p=.08 | 119.0 | (18.8) |
| Attention & Concentration | 110.4 | (13.4) | ns | 104.5 | (17.5) | 106.0 | (21.4) | ns | 101.6 | (4.4) |
| Delayed Memory Index | 120.8 | (16.0) | ns | 116.9 | (15.2) | 112.8 | (11.6) | ns | 125.2 | (19.5) |
| WASI | ||||||||||
| Verbal Intelligence Quotient | 114.5 | (12.0) | ns | 107.5 | (14.6) | 103.8 | (15.1) | ns | 110.3 | (10.7) |
| Performance Intelligence Quotient | 118.3 | (13.7) | t=2.1, p=.02 | 109.5 | (11.3) | 109.3 | (11.8) | ns | 109.7 | (6.6) |
| Full Scale Intelligence Quotient | 118.7 | (13.6) | t=2.1, p=.02 | 109.4 | (13.2) | 107.2 | (13.4) | ns | 111.4 | (8.9) |
| Balance and Gait | ||||||||||
| Eyes open composite | 59.8 | (8.3) | t=2.68, p=.005 | 51.4 | (11.7) | 51.8 | (12.7) | ns | 50.6 | (11.0) |
| Eyes closed composite | 26.3 | (18.6) | t=2.93, p=.003 | 11.5 | (7.5) | 10.9 | (8.9) | ns | 12.7 | (1.9) |
| Brain Measures | ||||||||||
| Fourth Ventricle (Z-score) | 0.74 | (0.2) | ns | 0.79 | (0.2) | 0.81 | (0.23) | ns | 0.74 | (0.2) |
| Lateral Ventricle (Z-score). | –0.145 | (1.1) | ns | 0.34 | (1.9) | 0.25 | (1.7) | ns | 0.51 | (2.2) |
p-values listed for Alcohol vs. Control comparisons for WMS, WASI, Balance, and Brain measures are 1-tailed. All others are 2-tailed.
At follow-up, the semi-structured alcohol consumption interview was re-administered to document quantity and frequency of drinking since last assessment and length of current sobriety. Ten alcoholics were classified as Abstainers and five were classified as Relapsers based on self-report. The Abstainers had been sober on average for almost 2 years and included two who reported one or two drinks on a single occasion, and one person who had been sober for 2.5 years after a significant lapse (75 drinks in a week)]. The Relapsers had been sober on average for 80 days at time of test (ranging from 2 to 181 days) and had drunk quantities ranging from 11 to over 3,000 drinks.
2.2 Neuropsychological Assessments
Tests at baseline and follow-up included the National Adult Reading Test (NART) (Nelson, 1982), Mini Mental State Exam (MMSE) (Folstein et al., 1975), Dementia Rating Scale (Mattis, 1988), Wechsler Memory Scale-Revised, (WMS-R) (Wechsler, 1987), Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999) and Walk-a-Line ataxia battery (Fregly et al., 1972). The ataxia battery consists of three tasks, each performed first with eyes-open and then eyes-closed: stand heel-to-toe with arms folded across the chest for 60-seconds; walk heel-to-toe for 10 steps; and stand on one foot at a time for 30 seconds. Each condition was repeated twice, unless a perfect performance was demonstrated on the initial trial, in which case a subject received a perfect score. Two composite scores, reflecting performance with eyes-open and eyes-closed were computed for this analysis (Sullivan et al., 2000c).
2.3 Brain Imaging
Each subject underwent MRI brain imaging at baseline and follow-up, each within a week of neuropsychological testing. SPoiled Gradient Recalled (SPGR) echo volumetric acquisitions of 94 slices, each 2 mm thick with no inter-slice spacing, were collected in the coronal plane (TR = 25 ms; TE = 5 ms, flip angle = 30 degrees, matrix = 256 × 192, and reformatted to pixel size 0.9375 × 0.9375 mm). A detailed description of procedures used to measure volume of brain regions at baseline and to calculate volumetric change over follow-up is provided elsewhere (Pfefferbaum et al., 2006c; Rohlfing et al., 2006). Analysis of baseline scans employed atlas-based parcellation in which each individual scan was aligned to a template brain mask on which regions had been identified. Measures of lateral ventricular volume were corrected for normal variations associated with supratentorial cranial volume (head size) and age from a larger sample of healthy adults (Pfefferbaum et al., 2006c). Thus values for ventricular volume used in this analysis took normal differences in age and head size into account.
Analysis of change for each individual employed a series of image registrations between baseline and follow-up scans. To avoid computational bias that could potentially favor either volume increase or decrease, the two images for each subject were registered in both directions. From the two resulting forward and backward coordinate transformations, an inverse-consistent map of local volume changes (Jacobian determinants) was computed at each pixel for each subject (Rohlfing et al., 2006). Change values for each pixel within two regions defined from a standard brain image template encompassing (1) the lateral ventricles and (2) the fourth ventricle were integrated to approximate volume change in each structure and converted to a number above or below 100 reflecting the percent of volume increase or reduction in the follow-up interval.
2.4 Statistical Analysis
Baseline measures for alcoholics and controls and future Abstainers and Relapsers were compared using t-tests. One-tailed significance levels were employed to identify hypothesized deficits in cognitive performance, gait and balance, and brain measures in alcoholics compared to controls with family-wise Bonferroni-correction as follows: for the five (verbal, visual, general, attention/concentration, delayed) WMS-R scales, P = 0.02; for the three WASI scales (verbal, performance, full), P = 0.03; for the two ataxia scales (eyes-open, eyes-closed), P = 0.05. Cognitive and ataxia recovery effects in the Abstainers were assessed with repeated measures ANOVAs for Group (Control, Abstainer) and Time. Group x Time interactions at the trend level were followed up with one-tailed paired t-tests given the overarching hypothesis that sobriety would result in improved scores. Repeated measures ANOVAs were limited to Abstainers and controls because drinking patterns in the small sample of Relapsers were highly variable with regard to quantity, frequency and recency.
To assess the relationship between behavior change and ventricular volume change (expressed as a number above or below 100, or percent over baseline) behavior change was also expressed as a percent of baseline score and converted to a number above or below 100. One-way ANOVA evaluated group effects (Control, Abstainer, Relapser) on lateral and fourth ventricular change. The relationship between percent change in behavior and brain was assessed with Spearman correlations because of small sample size and presence of statistical outliers. Pearson or Spearman correlations were used to test associations between subject descriptors and brain or behavioral change. Statistical analyses were conducted using Statview 4.5 (SAS Institute Inc. Cary, NC, USA).
3. Results
3.1 Baseline Demographic, Clinical, and Cognitive Characteristics
Table 1 summarizes the demographic, clinical, cognitive, and brain characteristics of controls (n = 26) and alcoholics (n = 15), and the subgroups of Abstaining (n = 10) and Relapsing (n = 5) alcoholics at their baseline test. In addition to means and standard deviations, Table 1 provides statistics for all group comparisons of demographic and study variables.
3.1.1 Controls vs. Alcoholics
Alcoholics were of similar age, body mass index, socioeconomic status, and premorbid intelligence as controls. The groups also comprised equivalent proportions of women and both were predominantly Caucasian. However, alcoholics had fewer years of education, had drunk more alcohol, and were more likely to be a current smoker and have a positive family history of alcoholism than controls. These alcoholics also achieved lower scores than controls on the Dementia Rating Scale, WASI Performance, and Full Scale Intelligence indices. Alcoholic’s scores on the WMS-R General and Visual Memory indices, though lower than controls, did not meet Bonferoni-corrected levels for significance (Table 1). Furthermore, alcoholics and controls did not differ in an amnesia index, that is, the difference between WASI General Intelligence and WMRS-R General Memory (IQ-GMI) (Oscar-Berman et al., 1993). Alcoholics performed worse on the ataxia battery than controls, particularly in the eyes-closed conditions. At baseline, the alcoholics had a 0.34 SD enlargement of the lateral ventricles compared with controls but the difference was not statistically significant. Fourth ventricle size did not differentiate groups at baseline.
3.1.2 Relapsers vs. Abstainers
These two subgroups of alcoholics were demographically and clinically similar at baseline, with the exception that Relapsers had a lower BMI and were rated higher on the Global Assessment of Functioning Scale than Abstainers. Both groups achieved similar scores on general cognitive indices, with the exception that the Relapsers tended to have better verbal and general memory scores than Abstainers. At baseline, the two alcoholic groups did not differ in ataxia scores or brain volumetric measures. However, the groups did differ in mean length of sobriety when they entered the study. Of the 10 Abstainers, 8 were first tested within 2 months of sobriety, while only 2 of the 5 Relapsers were first tested within 2 months of sobriety and 2 were tested when they had been sober for over 1 year. Because of these differences, we used baseline length of sobriety as a covariate in one-way ANCOVAs comparing Abstainers and Relapsers. This covariate eliminated group differences for Global Assessment of Function, Verbal Memory, and General Memory; however, BMI difference was sustained. The lack of group difference for ventricular volume or Eyes Closed Balance and Gait also endured when baseline sobriety was considered.
Although a past history of comorbid condition was not predictive of drinking outcome in this study (χ2 = 1.9, p = .17), those currently afflicted tended to be more likely to relapse (χ2 = 5.0, p = .08).
3.2 Change in Standardized Memory and Intelligence Scores
Repeated-measures ANOVAs for controls and abstaining alcoholics identified a Group x Time interaction for the WMS-R General Memory Index (F (1,33) = 5.61, p = 0.024) but not the WASI General IQ Index (F (1,33) = 0.02, ns) (Figure 2 upper panel). Of the other memory indices, only the Verbal Memory Index showed a significant Group x Time interaction (F (1,33) = 4.37, P = 0.4) (Figure 2, lower panel). Examination of each of the two tests that comprise the Verbal Memory Index, Logical Memory (Story Recall) and Verbal Paired Associates, revealed trends for an interaction for Logical Memory (F (1,33) = 3.25, P = 0.08) and Verbal Paired Associates (F (1,33) = 2.54, P = 0.12). Paired t-tests showed that differences between baseline and follow-up scores were not significant for any group on Verbal Paired Associates. By contrast, for the Logical Memory subtest, controls (t (24) = 2.2, P = 0.02, 1-tailed) and Abstainers (t (9) = 3.14, P = 0.005, 1-tailed) both improved over time (Figure 2, lower panel). In contrast to the WMS-R, there was no significant Group x Time interaction for WASI Verbal, Performance, or Full Scale IQ indices (all p’s were > .4, 1-tailed).
Figure 2.
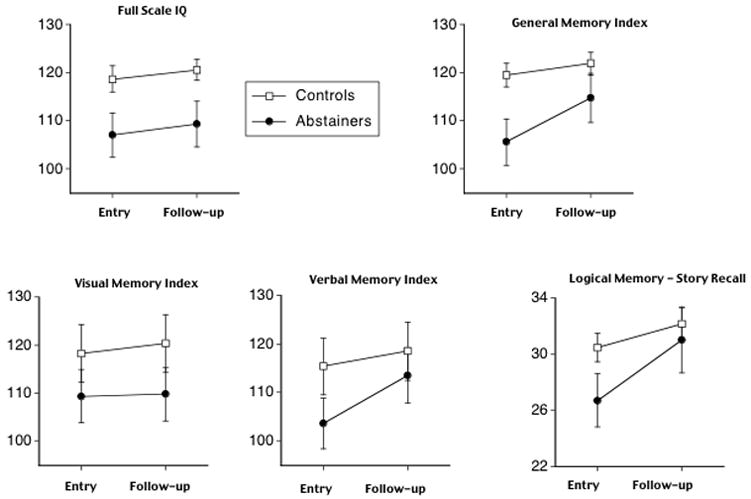
Control and Abstainer group means and standard errors for baseline and follow-up scores on Full Scale IQ from WASI and General Memory Index from WMS-R (upper row); Visual and Verbal Memory indices and Logical Memory from the WMS-R.
3.3 Change in Balance and Gait
Repeated-measures ANOVAs for controls and Abstainers identified a trend for a Group x Time interaction for Ataxia with eyes-closed (F (1,34) = 3.1, P = 0.09), but not with eyes-open (Figure 3). Paired t-tests between baseline and follow-up did not identify significant differences for either Abstainers or Controls.
Figure 3.
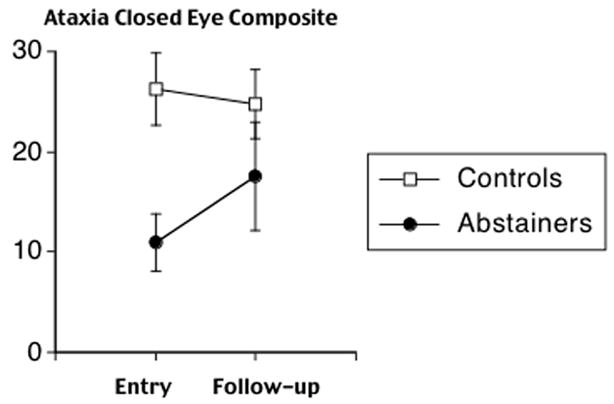
Control and Abstainer group means and standard errors for baseline and follow-up scores on composite measure of ataxia with eyes-closed.
3.4 Change in Brain Structure
One-way ANOVA for percent change in lateral ventricular volume between baseline and follow-up identified a group (Control, Abstainer, Relapser) effect (F (2,38) = 7.4, P = 0.002). Follow-up paired t-tests indicated significant differences between Abstainers and Relapsers (t = 2.7, P = 0.01, 1-tailed) and between Controls and Relapsers (t = 3.59, P = 0.0006, 1-tailed), but not between Controls and Abstainers. This effect reflected an increase of ~17% in volume for the Relapsers compared with increases of only 1% for Abstainers and 3% for Controls. Although change in fourth ventricles between baseline and follow-up showed the same profile of group differences as seen in the lateral ventricles the group effect was not significant (F (2,38) = 1.22, P = 0.3); Relapsers increased by ~2%, controls reduced by ~3%, and Abstainers reduced by ~3%.
3.5 Association between Brain and Behavior
Memory
Changes in lateral ventricular volume and the WMS-R General Memory Index were significantly associated across all alcoholics (Rho = − 0.63, p < 0.02), but not across controls (Rho = − 0.26, P = 0.19) (Figure 4) or in subgroups of Abstainers (Rho = − 0.53, p = 0.11) or Relapsers (Rho = − 0.10, P = 0.85) examined alone. By contrast, changes in fourth ventricular volume and the WMS-R General Memory Index were not significantly associated in either alcoholics (Rho = − 0.27, P = 0.3), or in controls (Rho = − 0.12, P = 0.55).
Figure 4.
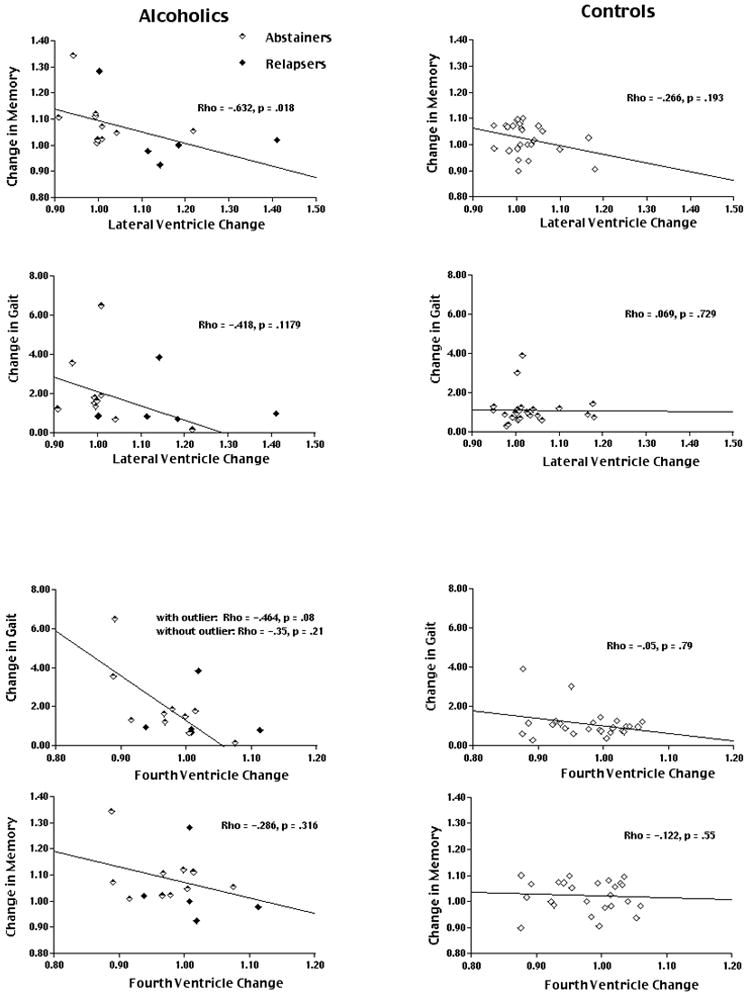
Plots of lateral ventricular change (upper 4 graphs) and fourth ventricular change (lower 4 graphs) relative to memory and gait change in alcoholics (Abstainers and Relapsers) (left column) and controls (right column). Correlations and significance levels are shown on each graph.
Ataxia
Changes in fourth ventricular volume and Closed Eye ataxia tended to be associated across all alcoholics (Rho = − 0.464, P = 0.08), among Abstainers alone (Rho = − 0.61, p = 0.07), but not in controls (Rho = − 0.05, P = 0.79) or Relapsers alone. Changes in lateral ventricular volume and Closed Eye ataxia were not significantly associated in either alcoholics (Rho = − 0.42, P = 0.12) or in controls (Rho = − 0.07, P = 0.73).
3.6 Other Contributors to Change in Verbal Memory, Ataxia, or Ventricular Volumes
We examined age, education, IQ, socioeconomic status, length of illness, global assessment of functioning, body mass index, lifetime alcohol, and length sober at baseline as predictors of percent change in memory, ataxia, lateral, or fourth ventricles in the alcoholics as a group. The only baseline variable that showed predictive power for either brain or behavioral change was length of sobriety. Greater length of sobriety at baseline was significantly associated with less memory improvement (Rho = − 0.558, p < 0.04) and more lateral ventricular (Rho = 0.538, p < 0.05) enlargement, but was not associated with gait or fourth ventricular change. Length of sobriety at baseline also showed a positive, though insignificant, association with memory performance at baseline (Rho = 0.474, p = 0.13), but was not related to baseline lateral ventricular volume (Rho = − 0.14, p = 0.6).
The amount of alcohol drunk in the follow-up interval and length of sobriety at follow-up testing were not related to change in memory or ataxia scores.
4. Discussion
This naturalistic study of the effects of self-reported sobriety or continued drinking over a mean retest interval of 22 months identified memory and gait and balance as components of our test battery for which significant improvement was detected in self-proclaimed abstaining alcoholics relative to controls. By contrast, self-proclaimed abstaining alcoholics did not manifest greater improvement relative to controls on standardized measures of intelligence. A modest increase in lateral ventricular volume in the abstaining alcoholics was comparable to that seen in the controls, whereas relapsing alcoholics showed significant greater lateral ventricular enlargement than either abstaining alcoholics or controls. While the changes in fourth ventricles did not differ between groups, the profile of change, with Relapsers showing increased volume relative to both controls and Abstainers, was similar to that observed for lateral ventricles.
This sample of previously treated alcohol dependent individuals is consistent with other samples in demonstrating considerable individual variation in vulnerability to the effects of alcoholism on cognitive performance. The fact that Relapsers manifest unimpaired memory at baseline compared with Abstainers suggests another dimension along which samples of people who have consumed large amounts of alcohol over their life differ: level of impairment ranges from notable to none. Lack of detected impairment may be more common in non-treatment seeking alcoholics than treatment-seeking ones (Fein and Landman, 2005). Further, studies with larger samples have shown that familial alcoholism, antisocial personality, and physical well-being, all perhaps phenotypes for a more basic genetic trait, as well as age and education, can all predict level of neuropsychological deficits (Grant et al., 1984; Tarter and Edwards, 1986; Parsons, 1987b; Bates et al., 2002; Bates et al., 2005). Our study, however, does not have the power (cf, Bates et al., 2004) to determine whether such variables contributed to the Relapsers’ initial superior performance.
Most (8 of 10) of the Abstainers were initially tested within only 2 months of sobriety, whereas most (3 of 5) of the Relapsers were tested after more than 6 months of sobriety. Across the entire sample, alcoholics who had been sober longer at study entry tended to perform better on the memory tests, and this higher baseline may have also contributed to lack of change among the Relapsers. Sobriety at baseline, however, was not associated with baseline ventricular volume, but did predict subsequent change – longer sober showed more enlargement, an effect that may have been driven by one participant, sober for over 2 years at study entry, who relapsed during the follow-up period.
While the 5 Relapsers were high performers at study entry, 4 of them showed reduction on the General Memory Index at retesting. This reduction in memory performance among the Relapsers is difficult to interpret, especially as they differed widely in length of sobriety at first assessment, were retested at varying intervals and had consumed highly variable amounts of alcohol between assessments. The extent to which lower memory performance of the 10 Abstainers at study entry was due to innate differences from the Relapsers or recency of testing since attaining sobriety is unknown. However, 9 of the Abstainers showed memory improvement at retest that was on average greater than that seen in controls. This suggests that sobriety enhanced the normal practice effect on self-cuing and active retrieval needed to perform various memory tasks, an effect particularly evident for Story Recall.
The amount and direction of change in overall memory performance as indexed by the WMS-R across all alcoholic subjects was related to amount of change in the lateral ventricular volume. Our measure of lateral ventricular volume might be taken as an approximation of the integrity of adjacent structures and regions invoked in the WMS-R battery (cf, Symonds et al., 1999). These include frontal lobe systems, used for strategic executive function, such as self-cueing and semantic organization (Incisa della Rocchetta and Milner, 1993; Fletcher et al., 1998; Savage et al., 2001), and medial temporal lobe structures used for nonstrategic declarative memory (Zola-Morgan and Squire, 1992; Corkin et al., 1997). Importantly, associations were not present between change in memory and change in the fourth ventricle, a CSF-filled space adjacent to the cerebellum.
The alcoholics in this study (both Relapsers and Abstainers) also showed evidence for improvement in ataxia over time relative to the controls. However, improvement in ataxia was modestly associated with decreases in volume of the fourth ventricle, specifically in Abstainers, but showed no association with change in volume of the lateral ventricles. The proximity of the fourth ventricle to the cerebellum makes it a proxy measure for this complex structure (cf, Symonds et al., 1999), the integrity of which is associated with static balance and gait (Sullivan et al., 2000a; Sullivan et al., 2006). The lack of association between change in either behavioral measure and either ventricular volume among the controls may reflect a limited range of age-related change occurring over one year among controls compared to greater recovery, or exacerbation-related change occurring among the alcoholics.
This report is limited by its small sample size, varying lengths of sobriety at study entry, and reliance on self-reported estimates of drinking that varied across subjects in quantity, recency, and duration. Despite these limitations, this naturalistic study provides support for the concept that fluctuations in the volumes of CSF-filled space may provide a proxy measure for structural brain changes associated with cognitive and motor processes that are amenable to recovery with sobriety. The results need to be evaluated in a larger sample, matched on length of sobriety at baseline, and with expanded assessments of regional brain change.
Figure 1.
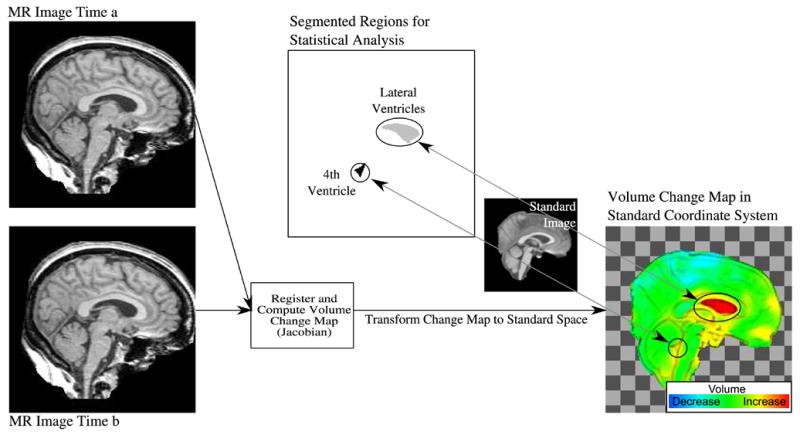
Illustration of process for generating volume change maps and for computing structure-specific volume change values. The MR image from time b is registered nonrigidly to the image from time a. The map of local volume changes (Jacobian determinants of the deformation field) is transformed into the standard image coordinate space for inter-subject analysis. Regions of interest were defined in standard space for the lateral ventricles and the 4th ventricle. Integrating the local volume changes in each subject over each of the two regions of interest yielded the structure-specific relative volume changes used for statistical group comparisons and regression modeling.
Acknowledgments
We would like to thank and acknowledge Carla Raassi and Marya Schulte for the invaluable help they provided for this project. A partial report of this work was presented at the 2005 Annual Meeting of the Research Society on Alcoholism. This work was supported by NIH grants AA12388, AA05965, AA10723.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Aylward EH, Reiss A. Area and volume measurement of posterior fossa structures in MRI. Journal of Psychiatric Research. 1991;25:159–168. doi: 10.1016/0022-3956(91)90020-b. [DOI] [PubMed] [Google Scholar]
- Bates ME, Barry D, Labouvie EW, Fals-Stewart W, Voelbel G, Buckman JF. Risk factors and neuropsychological recovery in clients with alcohol use disorders who were exposed to different treatments. Journal of Consulting and Clinical Psychology. 2004;72:1073–1080. doi: 10.1037/0022-006X.72.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Experimental and Clinical Psychopharmacology. 2002;10:193–212. doi: 10.1037//1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- Bates ME, Voelbel GT, Buckman JF, Labouvie EW, Barry D. Short-term neuropsychological recovery in clients with substance use disorders. Alcoholism: Clinical and Experimental Research. 2005;29:367–377. doi: 10.1097/01.alc.0000156131.88125.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Butters N, Ryan C, Bayog R. Cognitive loss and recovery in long-term alcohol abusers. Archives of General Psychiatry. 1983;40:435–442. doi: 10.1001/archpsyc.1983.01790040089012. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Wilkinson DA, Wortzman G, Holgate R. Partially reversible cerebral atrophy and functional improvement in recently abstinent alcoholics. Canadian Journal of Neurological Sciences. 1984;11:441–446. doi: 10.1017/s0317167100045972. [DOI] [PubMed] [Google Scholar]
- Corkin S, Amaral DG, Gonzalez RG, Johnson KA, Hyman BT. H M's medial temporal lobe lesion: Findings from magnetic resonance imaging. Journal of Neuroscience. 1997;17:3964–3979. doi: 10.1523/JNEUROSCI.17-10-03964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crovitz HF, Zener KA. Group test for assessing hand and eye dominance. American Journal of Psychology. 1962;75:271–276. [PubMed] [Google Scholar]
- Diener HC, Dichgans J, Bacher M, Guschlbauer Improvement in ataxia in alcoholic cerebellar atrophy through alcohol abstinence. Journal of Neurology. 1984;231:258–262. doi: 10.1007/BF00313662. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Rothlind JC, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: Preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcoholism: Clinical and Experimental Research. 2006;30:539–551. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: Contributions from explicit memory, executive function, and age. Alcoholism: Clinical and Experimental Research. 2004;28:1657–1665. doi: 10.1097/01.alc.0000145690.48510.da. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. Western Journal of Medicine. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B. Treated and treatment-naive alcoholics come from different populations. Alcohol. 2005;35:19–26. doi: 10.1016/j.alcohol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcoholism: Clinical and Experimental Research. 2006;30 doi: 10.1111/j.1530-0277.2006.00185.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1998. [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. I Encoding. Brain. 1998;121:1239–1248. doi: 10.1093/brain/121.7.1239. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fregly AR, Graybiel A, Smith MS. Walk on floor eyes closed (WOFEC): A new addition to an ataxia test battery. Aerospace Medicine. 1972;43:395–399. [PubMed] [Google Scholar]
- Gabrieli JDE. Cognitive neuroscience of human memory. Annual Review of Psychology. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug and Alcohol Dependence. 2005;78:263–273. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gilman S, Adams K, Koeppe RA, Berent S, Kluin KJ, Modell JG, Kroll P, Brunberg JA. Cerebellar and frontal hypometabolism in alcoholic cerebellar degeneration studied with Positron Emission Tomography. Annals of Neurology. 1990;28:775–785. doi: 10.1002/ana.410280608. [DOI] [PubMed] [Google Scholar]
- Goldman MS. Recovery of cognitive function in alcoholics: the relationship to treatment. Alcohol, Health & Research World. 1995;19:148–154. [PMC free article] [PubMed] [Google Scholar]
- Grant I, Adams KM, Reed R. Aging, abstinence and medical risk factors in the prediction of neuropsychologic deficit among long-term alcoholics. Archives of General Psychiatry. 1984;41:710–718. doi: 10.1001/archpsyc.1984.01790180080010. [DOI] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, Fagerstrom K. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of the Addictions. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hochla NA, Fabian MS, Parsons OA. Brain-age quotients in recently detoxified alcoholic, recovered alcoholic and nonalcoholic women. Journal of Clinical Psychology. 1982;38:207–212. doi: 10.1002/1097-4679(198201)38:1<207::aid-jclp2270380135>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Hollingshead A, Redlich F. Social Class and Mental Illness. John Wiley and Sons; New York: 1958. [Google Scholar]
- Incisa della Rocchetta A, Milner B. Strategic search and retrieval inhibition: the role of the frontal lobes. Neuropsychologia. 1993;31:503–524. doi: 10.1016/0028-3932(93)90049-6. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Robbins TW. Memory deficits in Korsakoff and non-Korsakoff alcoholics following alcohol withdrawal and the relationship to length of abstinence. Alcohol and Alcoholism Supplement. 1993;2:501–505. [PubMed] [Google Scholar]
- Lishman WA. Alcohol and the brain. British Journal of Psychiatry. 1990;156:635–644. doi: 10.1192/bjp.156.5.635. [DOI] [PubMed] [Google Scholar]
- Mann K, Gunther A, Stetter F, Ackermann K. Rapid recovery from cognitive deficits in abstinent alcoholics: A controlled test-retest study. Alcohol and Alcoholism. 1999;34:567–574. doi: 10.1093/alcalc/34.4.567. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale (DRS) Professional Manual. Psychological Assessment Resources, Inc; Odessa, FL: 1988. [Google Scholar]
- Meyerhoff DJ. Brain spectroscopic imaging, morphometry, and cognition in social drinkers and recovering alcoholics. Alcoholism: Clinical and Experimental Research. 2005;29:153–154. [Google Scholar]
- Munro CA, Saxton J, Butters MA. The neuropsychological consequences of abstinence among older alcoholics: a cross-sectional study. Alcoholism: Clinical and Experimental Research. 2000;24:1510–1516. [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART) Nelson Publishing Company; Windsor, Canada: 1982. [Google Scholar]
- Nixon SJ. Application of theoretical models to the study of alcohol-induced brain damage. In: Hunt W, Nixon SJ, editors. Alcohol Induced Brain Damage, NIAAA monograph. National Institute of Health; Rockville, MD: 1993. pp. 213–228. [Google Scholar]
- Nixon SJ, Tivis RD, Jenkins MR, Parsons OA. Effects of cues on memory in alcoholics and controls. Alcoholism: Clinical and Experimental Research. 1998;22:1065–1069. [PubMed] [Google Scholar]
- O'Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcoholism: Clinical and Experimental Research. 2001;25:1673–1682. [PubMed] [Google Scholar]
- Oscar-Berman M, Clancy JP, Weber DA. Discrepancies between IQ and memory scores in alcoholism and aging. The Clinical Neuropsychologist. 1993;7:281–296. [Google Scholar]
- Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcoholism: Clinical and Experimental Research. 2004;28:667–675. doi: 10.1097/01.alc.0000122761.09179.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcoholism and the brain: an overview. Alcohol Research and Health. 2003;27:125–133. [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Pulaski JL. Association learning and recognition memory in alcoholic Korsakoff patients. Neuropsychology. 1997;11:282–289. doi: 10.1037//0894-4105.11.2.282. [DOI] [PubMed] [Google Scholar]
- Parsons OA. Do neuropsychological deficits predict alcoholics treatment course and recovery? In: Parsons OA, Butters N, Nathan PE, editors. Neuropsychology of Alcoholism: Implications for Diagnosis and Treatment. Guilford Press; New York: 1987a. pp. 273–290. [Google Scholar]
- Parsons OA. Neuropsychological consequences of alcohol abuse: Many questions-some answers. In: Parsons OA, Butters N, Nathan PE, editors. Neuropsychology of Alcoholism. Guilford Press; New York: 1987b. pp. 153–175. [Google Scholar]
- Parsons OA, Schaeffer KW, Glenn SW. Does neuropsychological test performance predict resumption of drinking in posttreatment alcoholics? Addictive Behaviors. 1990;15:297–307. doi: 10.1016/0306-4603(90)90073-7. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of Aging. 2006a;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biological Psychiatry. 2006b;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Lane B, Ha CN, Rosenbloom MJ, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcoholism: Clinical and Experimental Research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, Sullivan EV. Contribution of alcoholism to brain dysmorphology in HIV infection: Effects on the ventricles and corpus callosum. Neuroimage. 2006c;33:239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Reed RJ, Grant I, Rourke SB. Long–term abstinent alcoholics have normal memory. Alcoholism: Clinical and Experimental Research. 1992;16:677–683. doi: 10.1111/j.1530-0277.1992.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Riege WH. Specificity of memory deficits in alcoholism. Recent Developments in Alcoholism. 1987;5:81–109. doi: 10.1007/978-1-4899-1684-6_4. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Sullivan EV, Pfefferbaum A. Deformation-based brain morphometry to track the course of alcoholism: Differences between intra-subject and inter-subject analysis. Psychiatry Research: NeuroImaging. 2006;146:157–170. doi: 10.1016/j.pscychresns.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ron MA, Acker RW, Shaw GK, Lishman WA. Computerized tomography of the brain in chronic alcoholism: A survey and follow-up study. Brain. 1982;105:497–514. doi: 10.1093/brain/105.3.497. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, O'Reilly A, Sassoon SA, Sullivan EV, Pfefferbaum A. Persistent cognitive deficits in community-treated alcoholic men and women volunteering for research: Limited contribution from psychiatric comorbidity. Journal of Studies on Alcohol. 2005;66:254–265. doi: 10.15288/jsa.2005.66.254. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology. 2004;18:589–597. doi: 10.1037/0894-4105.18.3.589. [DOI] [PubMed] [Google Scholar]
- Rourke SB, Grant I. The interactive effects of age and length of abstinence on the recovery of neuropsychological functioning in chronic male alcoholics: a 2-year follow-up study. Journal of the International Neuropsychological Society. 1999;5:234–246. doi: 10.1017/s1355617799533067. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Butters N, Schuckit M. Memory for temporal order and frequency of occurrence in detoxified alcoholics. Alcohol. 1986;3:323–329. doi: 10.1016/0741-8329(86)90009-1. [DOI] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Heckers S, Wagner AD, Schacter DL, Alpert NM, Fischman AJ, Rauch SL. Prefrontal regions supporting spontaneous and directed application of verbal learning strategies: evidence from PET. Brain. 2001;124:219–231. doi: 10.1093/brain/124.1.219. [DOI] [PubMed] [Google Scholar]
- Shear PK, Jernigan TL, Butters N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1994;18:172–176. doi: 10.1111/j.1530-0277.1994.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Skinner HA. Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Addiction Research Foundation; Toronto, Canada: 1982. [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices: The lifetime drinking history and the MAST. Journal of Studies on Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: Relation to ataxia. Neuropsychology. 2000a;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, Pfefferbaum A. Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Archives of General Psychiatry. 2000b;57:894–902. doi: 10.1001/archpsyc.57.9.894. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch, and stance on cerebellar vermian–related sway and tremor: A quantitative MRI and physiological study. Cerebral Cortex. 2006;16:1077–1086. doi: 10.1093/cercor/bhj048. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: Relationships to changes in brain structure. Neuropsychology. 2000c;14:178–188. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism: Clinical and Experimental Research. 2000d;24:611–621. [PubMed] [Google Scholar]
- Sullivan EV, Shear PK, Zipursky RB, Sagar HJ, Pfefferbaum A. Patterns of content, contextual, and working memory impairment in schizophrenia and nonamnesic alcoholism. Neuropsychology. 1997;11:195–206. doi: 10.1037//0894-4105.11.2.195. [DOI] [PubMed] [Google Scholar]
- Symonds LL, Archibald SL, Grant I, Zisook S, Jernigan TL. Does an increase in sulcal or ventricular fluid predict where brain tissue is lost? Journal of Neuroimaging. 1999;9:201–209. doi: 10.1111/jon199994201. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Edwards KL. Multifactorial etiology of neuropsychological impairment in alcoholics. Alcoholism: Clinical and Experimental Research. 1986;10:128–135. doi: 10.1111/j.1530-0277.1986.tb05059.x. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Ryan C. Recent Developments in Alcoholism. Vol. 1. 1983. Neuropsychology of alcoholism. Etiology, phenomenology, process, and outcome; pp. 449–469. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale – Revised. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Wechsler D. The Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; New York: 1999. [Google Scholar]
- Zola-Morgan S, Squire L. The components of the medial temporal lobe memory system. In: Squire LR, Butters N, editors. Neuropsychology of Memory. 2. The Guilford Press; New York: 1992. pp. 325–335. [Google Scholar]


