Abstract
The frequency of various types of unilateral spatial neglect and associated areas of neural dysfunction after left hemisphere stroke are not well characterized. Unilateral spatial neglect (USN) in distinct spatial reference frames have been identified after acute right, but not left hemisphere stroke. We studied 47 consecutive right handed patients within 48 hours of left hemisphere stroke to determine the frequency and distribution of types of right USN using cognitive testing and MRI imaging. The distribution of USN types was different from the previously reported distribution following acute right hemisphere stroke. In this left hemisphere stroke population, allocentric neglect was more frequent than egocentric neglect.
Keywords: spatial neglect, spatial attention, acute ischemic stroke, global processing, local processing, temporal cortex, parietal cortex
Unilateral spatial neglect (USN) following brain damage is often defined as the inability to attend or respond to space contralateral to the damage, not attributable to a primary sensory or motor deficit (Heilman, Watson, Valenstein, 1994). This definition does not capture the heterogeneity of neglect. Varieties of USN can be distinguished on the basis of sensory modality, regions of space affected, reference frame, or mode of output (Arguin & Bub, 1993; Cubelli, Nichelli, Bonito, De Tanti, Inzaghi, 1991; Haywood & Coltheart, 2000; Hillis & Caramazza, 1995a; Nichelli, Venneri, Pentore & Cubelli, 1993; Ota, Fujii, Suzuki, Fukatsu, & Yamadori, 2001; for review see Behrmann & Geng, 2002; Hillis, 2006; Halligan, Fink, Marshall, & Vallar, 2003; Vallar, Borrini, & Paulesu, 2003). USN can also present as a reading disturbance (“neglect dyslexia” -- the failure to read contralesional sides of words; Riddoch, 1991) or spelling disorder (“neglect dysgraphia” -- errors in spelling or letter formation on the contralesional side of words; Ellis, Flude & Young, 1987; Baxter & Warrington, 1983; Hillis & Caramazza, 1989, 1995b). Neglect can be revealed in many astounding ways, but also in many subtle ways. These heterogeneous presentations complicate the construction of cognitive models of spatial attention that might account for all types of USN.
In comparison to USN following right brain damage (RBD), USN following left brain damage (LBD) is less frequently reported, less often studied, and therefore inadequately characterized. Furthermore, symptoms from right hemispatial neglect are sometimes misattributed to naming or visual errors (Berndt, Haendiges, & Mitchum, 2005). Although right USN has attracted some attention (Albert, 1973; Stone et al., 1991; Stone, Halligan & Greenwood, 1993; Ogden, 1985; Beis et al., 2004; Hildebrandt, Schutze, Ebke, Brunner-Beeg, Eling, 2005), it is likely that right USN remains under-diagnosed or even misdiagnosed as aphasia with visual or lexical errors. These deficits are important because they might help reveal the spatial representations and operations normally involved in naming and reading.
When one says that a patient is neglecting the right side, it is important to clarify, “the right side of what?” (Hillis & Rapp, 1998). For example, patients with egocentric USN make errors on the contralesional space defined by the midline of the body, head or retina. Although one can also administer tests to distinguish between USN in body-centered, head-centered, or retinocentric coordinates, these types of neglect are generally characterized together as “egocentric” USN (Hillis, Rapp, Benzing & Caramazza, 1998). In contrast, patients with allocentric USN make errors on the contralesional side of individual items regardless of where the items appear with respect to the viewer. Two forms of allocentric USN have been described. The first, stimulus-centered USN, is characterized by errors on the contralesional side of the visual stimulus, irrespective of the location of the stimulus with respect to the viewer (Cubelli et al., 1991; Haywood & Coltheart, 2000; Hillis & Caramazza, 1991 & 1995a, Nichelli et al., 1993; Ota et al., 2001, Subbiah & Caramazza, 2000). To illustrate, patients with right stimulus-centered USN might read feet as “feel”1 when presented left to right, feet as “feet” when presented vertically, or feet as “meet” when mirror reversed, because the right side of the stimulus changes in each instance. Patients with stimulus-centered USN might also neglect only the contralesional parts of each separate visual stimulus while drawing a scene (e.g. Ogden scene). In a second form of allocentric USN, object-centered USN, the patient neglects one side of the canonical representation of each stimulus. The canonical representation refers to an abstract, 3-D representation of the object in the orientation in which it is usually viewed. For example, when reading the word feet a patient with right object-centered USN may read “feel” when the stimulus is presented left to right, mirror reversed, or vertically, because the letter “t” is always on the canonical “right” side of the word and is therefore neglected regardless of its location or modality of presentation. However, because few objects have canonical left and right sides (exceptions are maps, flags, and words) these forms of allocentric neglect are often difficult to distinguish. In this paper, stimulus- and object-centered neglect are combined under the term “allocentric” neglect (Hillis et al., 2005, Chatterjee, 1994; Vallar et al, 2003). Unless these levels of representation are acknowledged during the design and selection of stimuli, it is possible to overlook dissociations between types of USN.
These distinct spatial representations have been found to be associated with distinct anatomical regions in a study of patients with right hemisphere stroke (Hillis et al., 2005). In that study, imaging data from diffusion weighted imaging (DWI) and dynamic contrast perfusion weighted imaging (PWI) were used to determine regions of ischemia and/or hypoperfusion (the total area of neural dysfunction) associated with each type of USN. DWI shows areas of acute, dense ischemia or infarct, and is sensitive to stroke within minutes of brain injury. PWI shows the relative latency of arrival and clearance of blood flow to each voxel of the image. Areas of tissue with a time to peak arrival of contrast of > 4 seconds delay, relative to the homologous region in the contralesional hemisphere, are areas that may be getting enough blood to survive, but not enough to function correctly and can be considered “hypoperfused” (Barber, et al., 1998; Butcher et al., 2005; Hillis et al., 2001b). Therefore, tissue areas of dense ischemia and/or hypoperfusion can be considered dysfunctional. The associations between ischemia/hypoperfusion and deficits are likely to be the most reliable in the acute phase, when there has been insufficient time for cortical reorganization or rehabilitation (which result in other areas of the brain assuming function of the dysfunctional regions).
Most studies of spatial attention after LBD have been designed in a way that it is difficult to obtain information on any type of neglect other than egocentric neglect. Therefore, the frequency of occurrence of egocentric and allocentric neglect have not yet been reported accurately in the LBD population. To determine the frequency and character of right USN, which has been reported to occur in 0 to 76% (Beis et al., 2004) of patients after left hemisphere stroke, we administered neglect tests to 47 consecutive right handed patients with acute left hemisphere ischemic stroke within 48 hours of symptom onset who consented to the research. To determine the areas of the brain responsible for each type of USN, we identified areas of the structural infarct and/or hypoperfusion beyond the infarct that were associated with each type of neglect in 22 of the 47 patients with both DWI and PWI imaging. The distribution of the types of USN (allocentric versus egocentric) was compared to that of subjects with RBD in our earlier study (Hillis et al., 2005).
Methods
Subjects
A consecutive series of 47 patients were studied within 48 hours of onset of stroke symptoms. Exclusion criteria included the following: altered level of consciousness, left-handedness, <10th grade education (for reading tasks), ongoing sedation, insufficient data collection, or hemorrhage. Of the initial 73 patients, 47 were included in the final analysis; 12 were excluded because they were left handed, and 14 were excluded due to limited data collection. Limited data collection was due to: refusal to complete testing or interruption for clinical care. All patients or their closest relative (for patients with aphasia interfering with comprehension) provided informed consent for the study, using methods and consent forms approved by the Johns Hopkins Institutional Review Board. Age ranged from 23 to 90 years (mean = 59.2 ±15.9). Education ranged from 6 to 21 years (mean = 12.6 ± 3.4).
Imaging
Of the 47 patients, 22 had the following MRI sequences (the Johns Hopkins Acute Stroke Protocol) within 48 hours of onset of symptoms: axial T2, fluid-attenuated inversion recovery (FLAIR), gradient echo, diffusion-weighted images (DWI), apparent diffusion coefficient (ADC) maps, dynamic contrast perfusion-weighted images (PWI), and magnetic resonance angiogram of the circle of Willis. The reported analyses used DWI (after confirming the acuity of the lesion as dark on ADC maps) and PWI (co- registered to T2 to provide anatomical boundaries that are less visible on PWI). DWI and PWI scans were 5 mm in thickness and provided whole-brain coverage. Areas of hypoperfusion in PWI were determined with time-to-peak maps, using ImageJ (http://rsb.info.nih.gov/ij). Trained technicians examined 16 regions of interest (ROIs) for the presence or absence of hypoperfusion. Hypoperfusion was defined as > 4 sec mean delay in time-to-peak arrival of contrast across voxels in the region of interest (ROI) relative to the homologous region in the right hemisphere. This threshold was based on evidence that tissue with this degree of hypoperfusion is dysfunctional, although it may not be at risk for progressing to infarction, whereas a delay of < 2.5 sec is not associated with dysfunction (Hillis, Barker, Beauchamp, Gordon, Wityk, 2000; Hillis et al., 2001a). ROIs were defined manually using the Damasio and Damasio (1989) templates. The 16 ROI’s were chosen because they have been reported previously to be infarcted in patients with hemispatial neglect (Heilman, Watson, Valenstein, 1994 & 1997) or have been reported to show activation in functional imaging studies of spatial attention (for review, see Vallar et al., 2003).
PWI scans, co-registered with T2, have high spatial resolution, in that it is possible to analyze the degree of delay in the arrival of contrast in each voxel of the image. However, we did not use a voxel-based approach to structure-function mapping, because a large number of subjects both with and without hypoperfusion of each voxel are required to conduct such an analysis and still avoid identifying random association by applying Bonferroni correction for multiple comparisons. It is also recognized that defining ROIs by Brodmann’s areas has limitations (e.g., individual variability in the cytoarchitectural fields), but these limitations are not solved or ameliorated by using a voxel based approach. For these reasons, our use of Brodmann’s area landmarks should be considered approximate. Nevertheless, this method has high interjudge reliability, some theoretical rationale (separate cytoarchitectural fields likely have different functions), and some empirical support (because lesions involving specific Brodmann’s areas have been associated with hemispatial neglect). Although large areas of brain often showed small changes in perfusion (1–2 s delay relative to the homologous region in the intact hemisphere) on PWI, we used a threshold of delay that has been demonstrated previously to correspond to dysfunction.
Neglect Testing
Tests of hemispatial neglect included the following: copy a scene of two trees, a house, and a fence (Ogden, 1985); modification of the line cancellation test (Albert, 1973); line bisection, in which the page was presented 45° to the left and 45° to the right of the midsagittal plane and at the midsagittal plane of the viewer (25–30 cm from the trunk); clock copy; reading words; reading sentences; oral spelling; and a gap-detection test (Ota et al., 2001). In the gap-detection test, a page of 30 circles was presented: 10 circles had a gap on the left side, 10 had a gap on the right side, and 10 had no gap. Two forms of this test were presented: one with large circles and one with small circles.
Scoring of each test included percentage or error responses on each test (or deviation from midpoint in line bisection measured as percentage of line length). In copying a scene, each stroke in the scene to be copied was counted as a point; each omitted stroke was scored as one error, and each distorted or misplaced stroke was scored as one-half of an error. In the gap-detection test, there were three percentage-of-error scores: percentage of circles omitted (no response to the stimulus), percentage of left gaps missed, and percentage of right gaps missed, among the stimuli for which there was some response (an error consisted of incorrectly circling a circle with a gap). Norms on these tests were obtained from 57 normal subjects with no evidence of stroke or other neurological disorder on MRI, mean age 64 ± 11 years; mean 13.7 ± 3.5 years of education. Performance on these tests ranged from 0 ± 0% errors (clock copy) to 3.2 ± 3.4% error (line bisection) across tests, and no normal subjects made >10% errors on any of these tests. Therefore, neglect was defined as >10% errors (2 standard deviations from the highest mean error rate) on any task. In addition, each subject was evaluated with regard to the presence of egocentric USN, allocentric USN, or both forms of neglect.
Egocentric neglect was defined as:
significantly (p<0.05, by chi square) higher error rate when the stimulus page was presented to the right of the body compared to the mid-plane or the left of the body in line cancellation or line bisection; and/or
significantly (p<0.05, by chi square) higher error rate in oral reading of words in the right versus left column on a page; and/or
significantly (p<0.05, by chi square) more errors in marking circles on the right versus the left side of the page in the gap detection test; and/or
failing to copy one or more figures on the right half of the page, with no omissions on the left of the page.
No egocentric neglect was defined as no significant difference in number of errors for right versus left on any tasks.
Allocentric neglect was defined as >50% of errors on the right versus left of each stimulus on both sides of the viewer in:
reading words; and
copying figures; and
detecting gaps in circles: and/or
significantly (p<0.05) more errors on final letters than initial letters of words in spelling, recognition of words spelled aloud, or vertical reading
Criteria for no allocentric neglect was defined as ≤50% of errors on the right side of the stimuli for all tasks regardless of the side of presentation, no significant rise in error rate on the canonical right sides of objects.
Results
Distribution of USN (all participants)
Nine out of 47 (19.1%) patients tested had one or more form of neglect. The distribution of the neglect types in LBD patients was markedly different from the distribution previously reported for RBD. One patient had only egocentric neglect, six had only allocentric neglect, and two had both. (Fig. 1, 2, 3a). The range and median error rates across tests for each group are listed in Table 1.
Fig. 1.
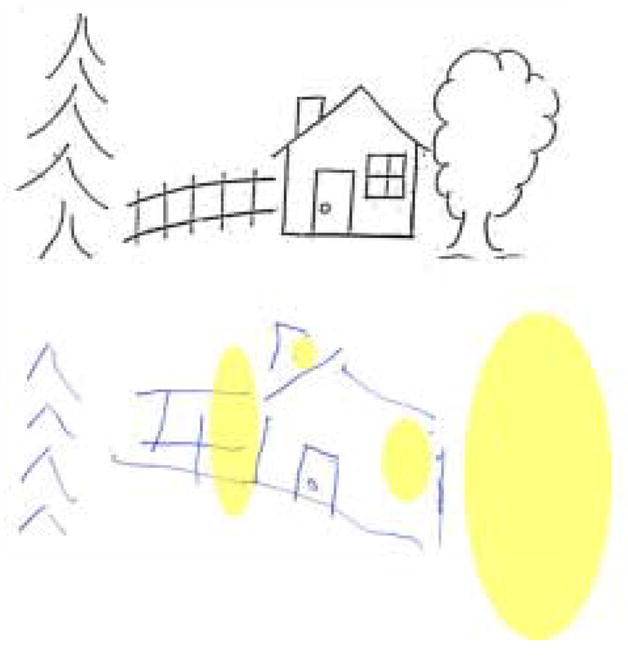
Copy of the Ogden scene by a patient with both right egocentric hemispatial neglect and allocentric spatial neglect. The ovals highlight the areas that are neglected.
Table 1.
The median error rate (and range) on neglect tests for patients with each type of neglect. Error rates can be high in the no neglect group if the errors were not neglect/neglect dyslexic in nature. Some tests were not performed (NT).
| Test | Egocentric Neglect Only (n=1) | Allocentric Neglect Only (n=6) | Egocentric + Allocentric Neglect (n= 2) | No Neglect (n=38) |
|---|---|---|---|---|
| Oral Reading | 37 | 20 (11–60) | 11 & 72 | 0 (0–100) |
| Writing | NA | 34 (9–53) | 25 & 37 | 14 (0–53) |
| Vertical reading | 48 | 33 (8–65) | 0 & 21 | 2 (0–39) |
| Copy Scene | 22 | 6 (0–22) | 47 & 50 | 3 (0–30) |
| Clock | 7 | 7 (0–80) | 80 & 88 | 7 (0–31) |
| Line Bisection | 3 | 5 (1–10) | 0 & 5 | 2 (0–8) |
| Line cancellation | 16 | 5 (0–24) | 13 & 19 | 0 (0–5) |
| Recognition of oral spelling | 100 | 45 (10–92) | 100 | 8 (0–93) |
| Stimulus Omission Right side of Gap Detection Page | 0 | 0 (0–20) | 100 & NA | 0 (0–7) |
Lesion analysis (subset of patient with adequate imaging data)
Five of the 22 patients with adequate imaging data (DWI and PWI) had neglect; two had both types of neglect, while three had allocentric neglect only. None of these patients had only egocentric USN. Therefore, the analysis of the affected (dysfunctional) Brodmann areas included 22 patients, of whom five had one or more forms of neglect. The Brodmann areas (BA) of infarct and/or hypoperfusion are listed in Table 2 for each group. The one patient in this study who had egocentric neglect alone did not have adequate imaging. Both patients with allocentric plus egocentric neglect had tissue dysfunction (imaging abnormality) in BA 37, 39, 40, and 44 (temporal and frontoparietal cortex). The three patients with allocentric neglect alone had tissue dysfunction in BA 18, 19, and/or 37 (occipitotemporal cortex). These results are consistent with the hypothesis from our previous study of patients with RBD that egocentric neglect is associated with dysfunction of more dorsal visual pathways (including BA 40 and 44), and allocentric neglect is more associated with ventral visual pathways (including BA 19 and 37). Scans from illustrative patients are shown in Figure 4. However, in the present study there were no significant associations between area of tissue dysfunction and neglect type by Fisher’s Exact tests after Bonferroni correction for multiple comparisons, since there were so few patients in each group.
Table 2.
Brodmann’s Areas that were affected (hypoperfused and/or infarcted) in each type of neglect
| BA | BA | BA | BA | BA | BA | BA | BA | BA | BA | BA | BA | BA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 10 | 18 | 19 | 21 | 22 | 37 | 39 | 40 | 44 | 45 | 46 | |
| Egocentric and Allocentric (N=2) | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 100 | 100 | 100 | 100 | 50 | 50 |
| Allocentric Only (N=3) | 0 | 0 | 0 | 67 | 67 | 0 | 0 | 67 | 0 | 0 | 0 | 0 | 0 |
| No Neglect (N=17) | 18 | 29 | 0 | 12 | 29 | 12 | 18 | 47 | 24 | 18 | 0 | 6 | 6 |
Fig. 4.
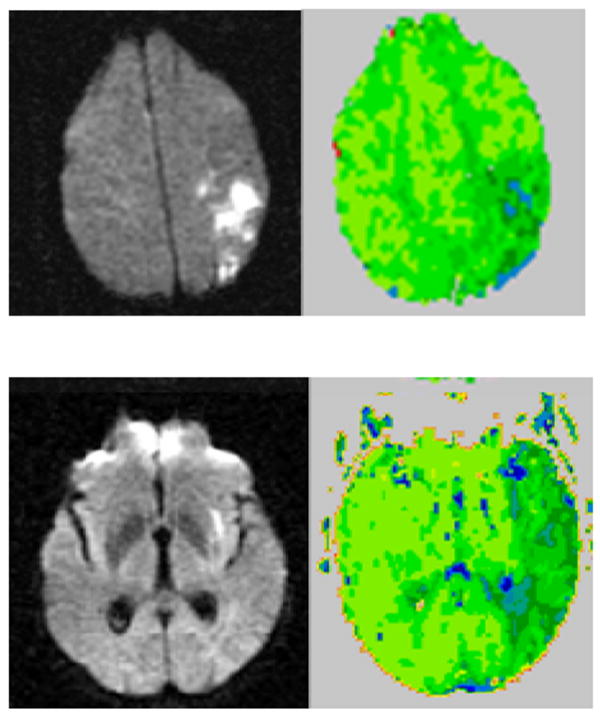
The top two images (DWI on the left and PWI on the right) are from a patient with right egocentric neglect, the bottom two images are from a patient with right allocentric neglect. Scans are in radiological convention (left hemisphere on right). Blue/darker green areas are hypoperfused.
The two patients with the combination of egocentric and allocentric neglect and adequate imaging had volumes of infarct and/or hypoperfusion (total dysfunctional tissue) of 40.6 and 306.1 cubic centimeters. For the three patients with imaging and allocentric neglect alone, the mean (± SD) volume was 14.4 ± 17.8 cubic centimeters; median was 8.8cc. The mean (± SD) volume of infarct and/or hypoperfusion for patients without egocentric neglect (N=20) was 15.15 ± 19.7 cc; median 7 cc, and for patients without allocentric neglect 15.31 ± 20.69 cc; median 17.4 cc; N=17. Therefore, the volume of infarct/hypoperfusion in patients with allocentric neglect was not larger than that of patients without neglect. However, patients with both types of neglect had larger areas of ischemia.
Discussion
Neglect is most commonly reported following RBD, although several studies have reported neglect after LBD (Sunderland, Wade & Langton-Hewer, 1987; Vallar, Rusconi, Geminiani, & Berti, 1991; Denes, Semenza, Stoppa, & Lis, 1982). The results of these studies and one meta-analysis (Bowen, McKenna, & Tallis, 1999) are summarized in Table 3. It is important to note the wide range in time frames of testing, tasks, scoring, and criteria for neglect determination across reported studies. Our results provide additional evidence that there may be more spatial representation within the left hemisphere than previously imagined (see also Ogden, 1985). However, it is quite possible, as indicated by previous studies (e.g. Denes et al. 1982), that neglect recovers more rapidly after LBD than RBD; we have not yet had the opportunity to conduct a follow-up study in these patients.
Table 3.
Summary of the frequency of neglect after LBD and RBD in previous studies.
| Paper | N=RBD | RBD with Neglect | N=LBD | LBD with Neglect | Delay in testing | Comment |
|---|---|---|---|---|---|---|
| Kinsella & Ford 1980 | 14 | 8 (57%) | 17 | 0 | Assessed at 4,8,12 weeks post stroke | |
| Sunderland et al., 1987 | 82 | 11 (13%) | 106 | 4 (4%) | Assessed 3 weeks post stroke using two tasks | Neglct=Position preference on a copying task |
| Vallar et al., 1991 | 66 | 23 (35%) | 44 | 2 (4%) | Assessed a average of 2weeks after stroke, range 1–30 days (mean 14 ± 8) | Neglect= Left bias in picking up balls arranged left to right |
| Beis et al., 2004 | 78 | 8–10 (10–13%) | Assessed in sub acute stroke; days/weeks post-onset not specified | Neglect = left bias on ≥2tasks; (Left bias on ≥1 task =43.5%) | ||
| Denes et al., 1982 | 24 | 8 (33%) | 24 | 5 (21%) | Follow-up assessment of hemiplegic patients 6months after injury | Patients who might have had neglect but no hemiplegia were excluded |
| Albert, M.L. 1973 | 30 | 11 (37%) | 36 | 11 (30%) | >3 weeks post surgery | Neglect=failure to cross out at least one line in line cancellation |
| Ogden, J.A. | 45 | 20 (44%) | 56 | 28 (58%) | Within the hospital, no time post onset specified (included all unilateral lesions) | Left USN more severe than right USN. Included lesions from any source (hemorrhagic/ischemic/tumor ) |
| Mattingley & Bradshaw 1994 | 78 | 64 (82%) | 20 | 13 (65%) | 1–26 weeks post stroke | Neglect= errors on grey scales task |
| Stone et al., 1993 | 69 | 56 (82%) | 102 | 66 (65%) | 2–3 days post stroke | Identified other symptoms associated with neglect |
| Stone et al., 1991 | 16 | 13 (81%) | 21 | 16 (76%) | 3 days post stroke | Neglect= Left bias on 1+egocentric tasks |
After both RBD and LBD, detection of USN is increased by using multiple tests. The previous studies examining LBD and USN have not investigated distinct types of neglect defined by reference frame, although some have reported differences in the tasks that are sensitive to right versus left USN (refer to Table 4; Edmans & Lincoln, 1987). This is an important distinction, because the previously reported data showed the same egocentric versus allocentric dissociations between right and left hemisphere strokes as our current data. To illustrate the importance of this distinction, if only line cancellation had been used by Edmans and Lincoln (1987), neglect would have been found to be much more common in RBD (31%) versus LBD (8%). In contrast, when tasks examining allocentric spatial representation (e.g. copying words) and egocentric spatial representation (e.g. line cancellation) were combined the percentage of neglect was equal for RBD (31%) and LBD (32%).
Table 4.
Differences across tests in detection of neglect for patients with RBD and LBD (adapted from Edmans and Lincoln, 1987)
| Task | Type of neglect best identified with task | RBD with Neglect (n=75) | LBD with Neglect (n=75) |
|---|---|---|---|
| Cancellation | Egocentric/ viewer-centered | 23 (31%) | 6 (8%) |
| Copy shapes | Both egocentric and allocentric? | 14 (19%) | 9 (12%) |
| Copy words | Allocentric/ Stimulus- or object-centered | 7 (9%) | 24 (32%) |
In our study, the frequency distribution of each type of neglect associated with LBD was quite different from that reported after RBD (Hillis et al., 2005). This contrast is shown in Figure 3a and 3b. We found that 67% (6/9) patients with neglect due to LBD had allocentric neglect, while only 33% (3/9) patients had right egocentric neglect (and only one had egocentric neglect only). In contrast, previous studies have reported that after RBD, egocentric USN is the most common type of neglect, and often occurs without other types of neglect (Chatterjee1994; Hillis, et al., 2005). These results are consistent with studies that have shown that patients with RBD show more frequent neglect on line cancellation (which might be considered a better test of egocentric neglect than allocentric USN), while patients with LBD show more frequent neglect on copying words (which might be more sensitive to allocentric neglect) (Edmans & Lincoln, 1987; Table 4). Neglect after LBD may have been underestimated in some previous studies by using tests that are only sensitive to egocentric USN.
These results also provide evidence for a possible theory of hemispheric specialization that can account for differences in reported frequency distribution of types of neglect after RBD versus LBD. While the left hemisphere seems to have a limited role in egocentric representation of space, as indicated by the infrequent cases of right egocentric USN, it may have an important role in allocentric representations of words and objects. This difference between hemispheres could reflect left hemisphere specialization for language, such that the left hemisphere allocates spatial attention across individual objects/stimuli like words (which might be considered local, rather than global processing). In contrast, the right hemisphere might allocate spatial attention across space on both sides of the viewer (which might be considered global processing). Therefore, it is possible that with language lateralization in humans, viewer-centered/global spatial processing has shifted toward the right hemisphere, while allocentric processing has shifted toward the left hemisphere (Figure 5).
Fig. 5.
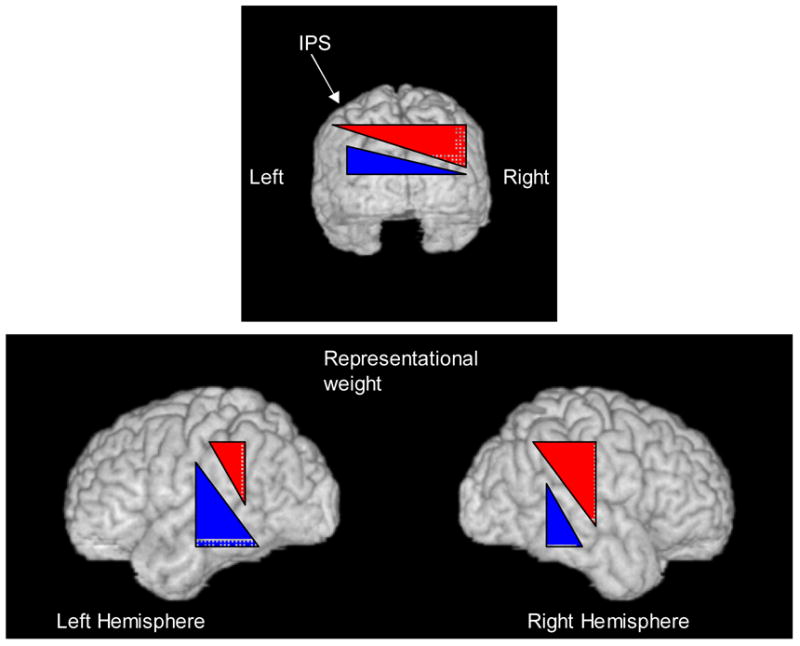
Schematic representations of the bias of the right hemisphere for the egocentric representation (red), and the bias of the left hemisphere for the allocentric representation (blue). This bias is depicted in the left and right hemispheres viewed from the occipital pole (top) and in the lateral views (bottom) by the representational weight (which might reflect the proportion of neurons devoted to spatial processing in each frame of reference in the various regions; see Cate & Behrmann, 2002).indicated by the height of the red or blue triangle.
To account for the spatial bias in allocentric USN, one must make additional assumptions. For example, one could assume that the left hemisphere processes both sides of allocentric spatial representations, while the right hemisphere processes only the left sides, so that a left hemisphere lesion leaves only the right hemisphere to process just the left side of allocentric representations (similar accounts of the disproportionate egocentric neglect after RBD have been proposed by Heilman and Van Den Abell 1980, Mesulam 1981, 1999). Alternatively, each hemisphere might have biased attention toward the opposite side of allocentric spatial representations (and inhibit one another, resulting in balanced attention), but the left hemisphere has a more general (non-spatial role) in processing allocentric spatial representations. On the second hypothesis, damage to the left hemisphere would be more likely to cause frank allocentric neglect because it would cause both a general deficit in processing allocentric spatial representations and a bias in attention to the left (by the intact right hemisphere, no longer inhibited by the left hemisphere). A similar account of the disproportionate frequency and severity of egocentric neglect after RBD has been proposed by Corbetta and colleagues (e.g., Corbetta, Kincade, Ollinger, McAvoy, and Shulman, 2000).
There is evidence from a variety of research methods for the general model of different roles of the two hemispheres in spatial processing schematically depicted in Figure 5. For example, several studies of neurologically impaired patients have shown that the right hemisphere is dominant for global processing, while the left hemisphere is dominant for local processing (e.g. Roberson, Lamb & Knight, 1988). Likewise, studies of event related potentials (ERPs) in normal subjects have revealed right parietal activity with shifts of attention to global forms, and more left posterior temporal activity with shifts of attention to local forms (Yamaguchi, Yamagata & Kobayashi, 2000). Furthermore, in normal subjects inhibitory repetitive transcranial magnetic stimulation (rTMS) over the right parietal lobe disrupts global processing, while inhibitory rTMS over the left posterior temporal cortex disrupts local processing (Ellison, Schindler, Pattison & Milner, 2004). Likewise, inhibitory rTMS over the left parietal cortex in patients with RBD and USN alleviated symptoms of neglect on line bisection tasks (Oliveri et al., 2001). Likewise, viewer-centered (egocentric) neglect has been induced in normal subjects through inhibitory rTMS over right parietal and frontal regions (Brighina et al., 2002, Fierro et al., 2000, Göbel, Calabria, Farné, & Rossetti, 2006). Similarly, inhibitory rTMS over right parietal cortex has induced bisection errors in number line judgments, which are usually described in a spatial manner (Zorzi, Priftis, Meneghello, Marenzi, & Umiltá, 2006).
Although the tasks we administered can distinguish egocentric and allocentric neglect, they may actually be less sensitive to right allocentric USN than left allocentric neglect. A possible explanation for the fact that few patients are described with neglect dyslexia or neglect following LBD is that most words in neglect batteries bias towards substitutions at the beginning of words (Berndt et al. 2005). Our neglect battery, which was initially designed to detect left neglect, was also biased toward making letter substitutions or omission errors (resulting in plausible words) on the left sides versus the right sides of words. For example, the word cage can be read as “age”, “wage”, “page”, “sage”, or “rage” by neglecting (substituting or omitting) the initial letter, but would not form real words by neglecting the final letter. It is therefore possible that the frequency of allocentric neglect reported here was actually lower than would have been reported if the task was not biased toward the left.
Since imaging data that included both PWI and DWI were available for only 22 of the patients, we cannot draw firm conclusions about the brain regions responsible for different types of USN after LBD. It is encouraging that right egocentric neglect was associated with dysfunction in areas similar to previously reported in right hemisphere stroke (Hillis et al., 2005). The two patients with egocentric neglect had imaging abnormalities in BA 40 and 44 (inferior parietal lobule and posterior-inferior frontal gyrus), areas homologous to the areas of dysfunction associated with egocentric (viewer-centered) neglect in patients with RBD (Hillis et al., 2005). In contrast, patients with allocentric neglect showed tissue dysfunction in more ventral (temporo-occipital) cortex, as previously reported for allocentric neglect after RBD (Hillis, et al., 2005). Of course, larger numbers of patients with both types of neglect and with both DWI and PWI are necessary to confirm these observations.
In summary, allocentric USN is more common than egocentric USN after LBD, while the opposite distribution has been reported after RBD in Hillis et al., 2005 (using the same battery of tests). The preliminary observations from lesion analysis in a small number of patients in this study, when combined with data from other studies, are also consistent with the hypothesis that after both LBD and RBD, neural dysfunction of parietal and posterior frontal regions tends to cause egocentric/viewer-centered USN, while neural dysfunction of temporal cortex tends to cause allocentric USN. However, the conclusion regarding this relationship in LBD needs to be confirmed in larger studies. Together, the results from this study and previous studies are consistent with the hypothesis of more global spatial processing in the right hemisphere (and dorsal regions) to guide movements in space relative to the viewer, and more local stimulus- and object-centered spatial processing in the left hemisphere (and ventral regions) for recognition of words and objects. The distinct roles of the two hemispheres in spatial processing should be considered when designing tests to assess USN in LBD patients.
Fig. 2.
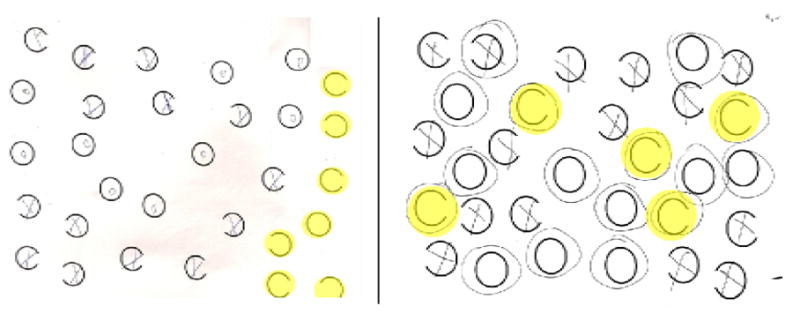
Left panel. Response sheet from the gap detection test of neglect (from Ota, et al. 1995) of a patient with egocentric neglect. Right panel. Response sheet from the gap detection test of a patient with allocentric neglect. The circles highlight errors made on each task.
Fig. 3.
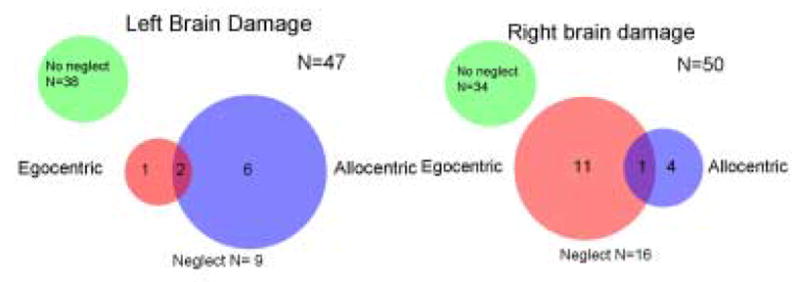
Panel A (left panel). The distribution of neglect types in 47 acute LBD patients, in comparison Panel B (right panel), distribution of neglect types in 50 RBD patients (adapted from Hillis et al. 2005).
Footnotes
Throughout this paper, printed words appear in italics and spoken words appear in quotes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert ML. A simple test of visual neglect. Neurology. 1973;23:658–664. doi: 10.1212/wnl.23.6.658. [DOI] [PubMed] [Google Scholar]
- Arguin M, Bub DN. Evidence for an independent stimulus-centered spatial reference frame from a case of visual hemineglect. Cortex. 1993;29:349–57. doi: 10.1016/s0010-9452(13)80188-8. [DOI] [PubMed] [Google Scholar]
- Barber PA, Darby DG, Desmond PM, Yang Q, Gerraty RP, Jolley D, Donnan G, Tress BM, Davis SM. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology. 1998;51:418–426. doi: 10.1212/wnl.51.2.418. [DOI] [PubMed] [Google Scholar]
- Baxter DM, Warrington EK. Neglect dysgraphia. Journal of Neurology, Neurosurgery and Psychiatry. 1983;45:1073–1078. doi: 10.1136/jnnp.46.12.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ. What is ‘left’ when all is said and done? Spatial coding and hemispatial neglect. In: Karnath HO, Milner D, Vallar G, editors. The cognitive and neural bases of spatial neglect. New York, NY: Oxford Press; 2002. pp. 85–100. [Google Scholar]
- Beis JM, Keller C, Morin N, Bartolomeo P, Bernati T, Chokron S, Leclercq M, Louis-Dreyfus A, Marchal F, Martin Y, Perennou D, Pradat-Diehl P, Prairial C, Rode G, Rousseaux M, Samuel C, Sieroff E, Wiart L, Azouvi P. Right spatial neglect after left hemisphere stroke: qualitative and quantitative study. Neurology. 2004;63:1600–1605. doi: 10.1212/01.wnl.0000142967.60579.32. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Haendiges AN, Mitchum CC. Orthographic effects in the word substitutions of aphasic patients: an epidemic of right neglect dyslexia? Brain and Language. 2005;93:55–63. doi: 10.1016/j.bandl.2004.06.110. [DOI] [PubMed] [Google Scholar]
- Bowen A, McKenna K, Tallis RC. Reasons for variability in the reported rate of occurance of unilateral spatial neglect after stroke. Stroke. 1999;30:1196–1202. doi: 10.1161/01.str.30.6.1196. [DOI] [PubMed] [Google Scholar]
- Brighina F, Bisiach E, Piazza A, Oliveri M, La Bua V, Daniele O, Fierro B. Perceptual and response bias in visuospatial neglect due to frontal and parietal repetitive transcranial magnetic stimulation in normal subjects. Behavioural Neuroscience. 2002;13:2571–2575. doi: 10.1097/00001756-200212200-00038. [DOI] [PubMed] [Google Scholar]
- Butcher KS, Parsons M, MacGregor L, Barber PA, Chalk J, Bladin C, Levi C, Kimber T, Schultz D, Fink J, Tress B, Donnan G, Davis S. Refining the perfusion-diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- Cate A, Behrmann M. Spatial and temporal influences on extinction. Neuropsychologia. 2002;40:2206–2225. doi: 10.1016/s0028-3932(02)00128-8. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. Picturing unilateral spatial neglect: viewer versus object centered reference frames. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:1236–1240. doi: 10.1136/jnnp.57.10.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Cubelli R, Nichelli P, Bonito V, De Tanti A, Inzaghi MG. Different patterns of dissociation in unilateral spatial neglect. Brain and Cognition. 1991;15:139–159. doi: 10.1016/0278-2626(91)90023-2. [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio A. Lesion analysis in neuropsychology. New York: Oxford University Press; 1989. [Google Scholar]
- Denes G, Semenza C, Stoppa E, Lis A. Unilateral spatial neglect and recovery from hemiplegia: a follow-up study. Brain. 1982;105:543–552. doi: 10.1093/brain/105.3.543. [DOI] [PubMed] [Google Scholar]
- Edmans J, Lincoln NB. The frequency of perceptual deficits after stroke. Clinical Rehabilitation. 1987;1:273–281. [Google Scholar]
- Ellis A, Flude B, Young A. "Afferent dysgraphia" in a patient and normal subjects. Cognitive Neuropsychology. 1987;4:465–486. [Google Scholar]
- Ellison A, Schindler I, Pattison LL, Milner AD. An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain. 2004;127:2307–2315. doi: 10.1093/brain/awh244. [DOI] [PubMed] [Google Scholar]
- Fierro B, Brighina F, Oliveri M, Piazza A, La Bua V, Buffa D, Bisiach E. Contralateral neglect induced by right posterior parietal rTMS in healthy subjects. Neuroreport. 2000;11:1519–1521. [PubMed] [Google Scholar]
- Göbel SM, Calabria M, Farné A, Rossetti Y. Parietal rTMS distorts the mental number line: simulating 'spatial' neglect in healthy subjects. Neuropsychologia. 2006;44:860–868. doi: 10.1016/j.neuropsychologia.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Fink GR, Marshall JC, Vallar G. Spatial cognition: evidence from visual neglect. Trends in Cognitive Science. 2003;3:125–133. doi: 10.1016/s1364-6613(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Haywood M, Coltheart M. Neglect dyslexia and the early stages of visual word recognition. Neurocase. 2000;6:33–44. [Google Scholar]
- Heilman KM, Van Den Abell T. Right Hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect) Neurology. 1980;30:327–330. doi: 10.1212/wnl.30.3.327. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Localization of lesions in neglect and related disorders. In: Kertesz A, editor. Localization and neuroimaging in neuropsychology. San Diego: Academic Press; 1994. pp. 495–524. [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect: clinical and anatomic aspects. In: Feinberg TE, Farah MJ, editors. Behavioral neurology and neuropsychology. New York: McGraw Hill; 1997. pp. 309–318. [Google Scholar]
- Hildebrandt H, Schutze C, Ebke M, Brunner-Beeg F, Eling P. Visual search for item- and array-centered locations in patients with left middle cerebral artery stroke. Neurocase. 2005;11:416–426. doi: 10.1080/13554790500263511. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. The graphemic buffer and attentional mechanisms. Brain and Language. 1989;36:208–235. doi: 10.1016/0093-934x(89)90062-x. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. Spatially-specific deficit to stimulus-centered letter shape representations in a case of "unilateral neglect". Neuropsychologia. 1991;29:1223–1240. doi: 10.1016/0028-3932(91)90036-8. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. A framework for interpreting distinct patterns of hemispatial nelgect. Neurocase. 1995a;1:189–207. [Google Scholar]
- Hillis AE, Caramazza A. Spatially-specific deficits in processing graphemic representations in reading and writing. Brain and Language. 1995b;48:263–308. doi: 10.1006/brln.1995.1012. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Rapp BR, Benzing L, Caramazza A. Dissociable coordinate frames of unilateral spatial neglect: viewer-centered neglect. Brain and Cognition. 1998;37:491–526. doi: 10.1006/brcg.1998.1010. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Rapp BR. Unilateral spatial neglect in dissociable frames of reference: a comment on Farah, Brunn, Wong, Wallace, and Carpenter (1990) Neuropsychologia. 1998;36:1257–1262. doi: 10.1016/s0028-3932(98)00009-8. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Barker PB, Beauchamp NJ, Gordon B, Wityk RJ. MR perfusion imaging reveals regions of hypoperfusion associated with aphasia and neglect. Neurology. 2000;55:782–788. doi: 10.1212/wnl.55.6.782. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Barker PB, Beauchamp NJ, Winters BD, Mirski M, Wityk RJ. Restoring blood pressure reperfused Wernicke's area and improved language. Neurology. 2001a;56:670–672. doi: 10.1212/wnl.56.5.670. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, Selnes OA. Hypoperfusion of Wernicke’s area predicts severity of semantic deficit in acute stroke. Annals of Neurology. 2001b;50:561–566. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits E, Degoankar M. Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. Journal of Neuroscience. 2005;25:3161–3167. doi: 10.1523/JNEUROSCI.4468-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE. Neurobiology of unilateral spatial neglect. The Neuroscientist. 2006;2:153–163. doi: 10.1177/1073858405284257. [DOI] [PubMed] [Google Scholar]
- Kinsella G, Ford B. Acute recovery patterns in stroke patients. Medical Journal of Australia. 1980;2:663–666. [PubMed] [Google Scholar]
- Nichelli P, Venneri A, Pentore R, Cubelli R. Horizontal and vertical neglect dyslexia. Brain and Language. 1993;44:264–283. doi: 10.1006/brln.1993.1018. [DOI] [PubMed] [Google Scholar]
- Mattingley JB, Bradshaw JL. How many neglects? Some considerations based on anatomy and information processing. Neuropsychological Rehabilitation. 1994;4:169–172. [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal, and cingulated contributions to the mental representation and attentional targeting of salient extrapersonal events . Philosophical Transaction of the Royal Society of London. Series B: Biological Sciences. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden JA. Anterior-posterior interhemispheric differences in the loci of lesions producing visual hemineglect. Brain and Cognition. 1985;4:59–75. doi: 10.1016/0278-2626(85)90054-5. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Bisiach E, Brighina F, Piazza A, La Bua V, Buffa D, Fierro B. rTMS of the unaffected hemisphere transiently reduces contralesional visuospatial hemineglect. Neurology. 2001;57:1338–1340. doi: 10.1212/wnl.57.7.1338. [DOI] [PubMed] [Google Scholar]
- Ota H, Fujii T, Suzuki K, Fukatsu R, Yamadori A. Dissociation of body-centered and stimulus-centered representations in unilateral neglect. Neurology. 2001;57:2064–2069. doi: 10.1212/wnl.57.11.2064. [DOI] [PubMed] [Google Scholar]
- Riddoch MJ. Neglect and the peripheral dyslexias. London: LEA; 1991. [Google Scholar]
- Robertson LC, Lamb MR, Knight RJ. Effects of lesions of temporal-parietal junction of perceptual and attentional processing in humans. Journal of Neuroscience. 1988;8:3757–3769. doi: 10.1523/JNEUROSCI.08-10-03757.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SP, Wilson B, Wroot A, Halligan PW, Lange LS, Marshall JC, Greenwood RJ. The assessment of visuo-spatial neglect after acute stroke. Journal of Neurology, Neurosurgery, and Psychiatry. 1991;4:345–350. doi: 10.1136/jnnp.54.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SP, Halligan PW, Greenwood RJ. The incidence of neglect phenomena and related disorders in patients with an acute right or left hemisphere stroke. Age and Ageing. 1993;22:46–52. doi: 10.1093/ageing/22.1.46. [DOI] [PubMed] [Google Scholar]
- Subbiah I, Carramazza A. Stimulus-centered neglect in reading and object recognition. Neurocase. 2000;6:13–31. [Google Scholar]
- Sunderland A, Wade DT, Langton-Hewer R. The natural history of visual neglect after stroke: indications from two methods of assessment. Disability Studies. 1987;9:55–59. doi: 10.3109/03790798709166235. [DOI] [PubMed] [Google Scholar]
- Vallar G, Borrini G, Paulesu E. Neglect syndromes: the role of the parietal cortex. Advances in Neurology. 2003;93:293–319. [PubMed] [Google Scholar]
- Vallar G, Rusconi M, Geminiani G, Berti A. Visual and nonvisual neglect after unilateral brain lesions: modulation by visual input. International Journal of Neuroscience. 1991;61:229–239. doi: 10.3109/00207459108990740. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Yamagata S, Kobayashi S. Cerebral asymmetry of the "top-down" allocation of attention to global and local features. Journal of Neuroscience. 2000;20:RC72. doi: 10.1523/JNEUROSCI.20-09-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzi M, Priftis K, Meneghello F, Marenzi R, Umiltá C. The spatial representation of numerical and non-numerical sequences: evidence from neglect. Neuropsychologia. 2006;44:1061–1067. doi: 10.1016/j.neuropsychologia.2005.10.025. [DOI] [PubMed] [Google Scholar]


