Abstract
The insulin-like growth factors (IGFs) play a central role in controlling somatic growth in mammals and exert anabolic effects on most tissues, including bone. IGF action is mediated by the IGF-I receptor and additionally is regulated by six high-affinity IGF binding proteins (IGFBP-1 through IGFBP-6), of which IGFBP-4 and IGFBP-5 are most abundant in bone. The focus of this brief review is on the role of IGFBP-5 in bone biology. IGFBP-5 has been implicated as a pro-osteogenic factor in several studies but conversely has been shown to act as an inhibitor of bone formation, primarily by interfering with IGF actions on osteoblasts. These potentially contradictory effects of IGFBP-5 in bone are further complicated by observations indicating that IGFBP-5 additionally may function in an IGF-independent way, and may have been accentuated by differences in both experimental design and methodology among published studies. Suggestions are made for a more systematic approach to help discern the true roles of IGFBP-5 in bone physiology.
Introduction
Skeletal development and remodeling are controlled by systemically-derived and local signals mediated by protein hormones, peptide growth factors, and other biologically-active molecules. Among growth factors with actions on bone are the insulin-like growth factors, IGF-I and -II. These molecules are closely-related single-chain secreted proteins that bind with high affinity to the IGF-I receptor (IGF-IR) on the surface of responsive cells, and to a family of soluble IGF binding proteins, IGFBP-1 through -6. It is generally agreed that the IGF-IR, a membrane-spanning ligand-activated tyrosine protein kinase, mediates the biological actions of the two IGFs, while the IGFBPs play more modulating roles, potentially by regulating both IGF half-life and access to the IGF-IR [1–3]. Recent studies additionally support the idea that IGFBPs also may have biological actions that are independent of their ability to bind IGF-I or IGF-II [4], although aspects of this research remain controversial. Among the six IGFBPs, IGFBP-4 and -5 are the most abundant in bone [4,5].
In this review we will focus on the actions of IGFBP-5 in bone. Results from a variety of studies have been interpreted to support the hypothesis that IGFBP-5 enhances bone formation and osteoblast differentiation, while alternatively other experiments have suggested that IGFBP-5 inhibits osteoblast functions, primarily by blocking the effects of IGF-I and IGF-II on these cells. We will discuss each side of this controversy, and will highlight differences in both experimental design and methodology that might explain the conflicting results. Finally, we will offer suggestions for a more systematic approach to help elucidate the true actions of IGFBP-5 in bone physiology.
IGFBP-5: Structural Considerations
IGFBP-5 is a 252 amino acid secreted protein that consists of highly conserved, cysteine-rich, NH2- and COOH-terminal domains, and a less well-conserved central segment [1,6,7]. It has been found that the NH2-terminal domain of IGFBP-5 contains the primary IGF binding site, and mutagenesis experiments directed toward this region of the molecule have demonstrated that 27 amino acids, from valine 49 to leucine 75 of the mature protein, form a hydrophobic patch on the surface of IGFBP-5 that is necessary for IGF binding [1,8]. As shown by NMR spectroscopy, the isolated NH2-terminal segment of IGFBP-5 forms a tight globular structure with three strands of anti-parallel β sheets in the center [9]. The structure of the full-length protein has not been characterized yet.
The COOH-terminal domain of IGFBP-5 contains 6 conserved cysteine residues and binds via a series of basic amino acids to extracellular matrix components such as heparin and hydroxyapatite [10–12]. This segment of the molecule also contains a binding site for acid-labile subunit (ALS), the protein that forms a ternary complex with either IGFBP-3 or IGFBP-5 and IGF-I in the blood [13,14]. A region of basic amino acids within the COOH-terminal region also has been shown to act as a putative nuclear localization sequence in over-expression experiments, but it remains unknown if nuclear accumulation of IGFBP-5 occurs under normal physiological conditions [1,6]. The isolated COOH-terminus of IGFBP-5 cannot bind IGF-I or IGF-II by itself but mutagenesis studies indicate that it contributes to the overall affinity and stability of ligand binding in the full-length protein [15,16]. As the three-dimensional structure of full-length IGFBP-5 has not been resolved yet, the physical nature of interactions between the NH2- and COOH-terminal segments of the protein to define the high-affinity IGF binding site remains conjectural.
The linker (L) domain of IGFBP-5 separates the NH2- and COOH-terminal segments. The L domain contains several potential proteolytic cleavage sites, and is a substrate for at least two distinct proteases that reside in the extra-cellular environment of osteoblasts, matrix metalloprotease-2 and ADAM-9 [17,18]. IGFBP-5 also undergoes several post-translational modifications. Within the L domain are three predicted O-linked glycosylation sites [19], and 12 putative phosphorylation sites [20], although the full extent of phosphorylation has not been established in any tissue in which IGFBP-5 is produced and secreted.
IGFBP-5 and Bone
IGF action enhances bone mass
A number of studies in experimental animals have concluded that IGF action is essential for normal bone formation, growth, and maintenance. For example, mice lacking the IGF-IR in all cell types have retarded skeletal development accompanied by delayed ossification, as well as many other severe systemic defects that contribute to their neonatal death [21]. Targeted loss of the IGF-IR exclusively in osteoblasts also has a bone phenotype. In these mice, bones were of normal length but total trabecular volume was reduced by nearly 50%. The main defect appeared to be a decline in mineral apposition rate [22]. In agreement with the conclusions of these studies, targeted over-expression of IGF-I in mature osteoblasts in mice via the osteocalcin promoter caused an increased rate of bone formation and more extensive mineralization than normal, which resulted in a ~30% increase in trabecular bone volume compared with controls [23]. Targeting IGF-I to osteoblast precursor cells in mice with the rat collagen type I gene promoter also gave rise to a robust bone phenotype, and led to increases in femur length, cortical width, and cross-sectional area [24]. Thus, regardless of the timing of IGF-I over-expression in bone in transgenic mice, net bone formation is enhanced. Therefore, based on several types of evidence, IGF action via the IGF-IR is critical for normal bone development and mineralization.
Many of the effects of growth hormone (GH) on bone are thought to occur as the result of its ability to stimulate the synthesis of IGF-I throughout the body, including in osteoblasts [25]. In agreement with this concept, mice lacking the GH receptor in all tissues have diminished cortical bone growth and trabecular turnover at 6 weeks of age, which could be reversed within two weeks by systemic IGF-I treatment [26].
Expression of IGFBP-5 in bone cells
Soon after its initial discovery, IGFBP-5 was isolated from human bone [27]. During fetal development, it is expressed during endochondral bone formation in differentiating chondrocytes and osteoblasts [28,29], while in primary osteoblast cultures, IGFBP-5 is secreted by pre-osteoblasts but decreases in abundance during their differentiation and maturation [30]. The accumulation of IGFBP-5 also varies in cultured osteoblasts derived from different bone sources, being more abundant in conditioned media from calvarial osteoblasts than in media from cells isolated from the mandible, vertebral bodies, or ribs [31]. It has been proposed that site-dependent differences in IGFBP-5 production by different bones are secondary to alterations in either the growth factor micro-enviornment or in the rate or extent of mechanical loading, but it is additionally possible that osteoblasts from different locations respond differently to the same cues in culture. Many bone cell lines also express IGFBP-5 at varying levels [32].
The production and accumulation of IGFBP-5 in bone cells appears to be regulated by multiple factors [33]. Several hormones, including prostaglandin E2 and parathyroid hormone, enhanced IGFBP-5 gene expression in cultured osteoblasts [34-36], while transforming growth factor-β, platelet-derived growth factor, and basic fibroblast growth factor each reduced IGFBP-5 mRNA abundance in the same cells [37]. IGF-I also has been shown to induce IGFBP-5 mRNA and protein synthesis in cultured osteoblasts [19,33,34,37] and in bone in transgenic mice over-expressing IGF-I in osteoblasts [38]. The amount of IGFBP-5 in bone matrix also may be regulated by its proteolysis [17], which appears to be blocked upon binding of IGF-I.
Effects of IGFBP-5 on bone formation
Positive outcomes
Systemic administration by daily subcutaneous injection of equimolar amounts of IGFBP-5 and IGF-I in combination for 8 weeks led to a 20% increase in cortical bone formation, bone area, and mineral density in mice [39]. Similarly, a 40% rise in local bone area and density was seen after a 19 day course of daily injections of both IGFBP-5 and IGF-I into calvariae (67 or 200 μg/day of IGFBP-5). By contrast, the same doses of IGFBP-5 alone had no effect [40]. However, in other studies, daily subcutaneous injections of E. coli derived IGFBP-5 (50 μg/day for 20 days) in mice led to increases in serum osteocalcin levels (58%) and in femoral bone alkaline phosphatase activity (85%), although direct effects on bone formation were not assessed [41]. It is important to note that systemic treatment with IGF-I or IGFBP-5 may lead to effects on bone that are caused by other factors that may be regulated by either protein. In addition, as IGFBP-5 may undergo proteolytic cleavage after systemic administration, it may not have the same effects on bone as local delivery of the protein. In this regard, observations with IGFBP-4 are instructive, as the protein was rapidly degraded after systemic injection, leading to increased bone formation, while when delivered locally to bone or added to cultured osteoblasts, it was inhibitory [42].
In one group of in vitro experiments, E. coli-derived IGFBP-5 (100 ng/ml) was shown to increase DNA synthesis in the MC3T3 pre-osteoblastic cell line and in primary mouse osteoblasts up to 4-fold compared with vehicle-treated controls, and was equally effective with or without added IGF-I [43]. Similar results were seen using human cell culture-derived IGFBP-5, although a much higher dose was needed (2300 ng/ml), suggesting variability in potency or stability between bacterial and mammalian cell derived IGFBP-5 [16]. Although cell counts were not performed in these experiments, these observations have been interpreted to indicate that IGFBP-5 was able promote osteoblast proliferation.
Very few experiments have shown positive effects of IGFBP-5 on osteoblast differentiation in culture. Treatment of the MG63 osteoblastic cell line or primary human osteoblasts with E. coli-derived IGFBP-5 resulted in modest increases in alkaline phosphatase activity and osteocalcin protein levels [41]. Similar results were observed in mouse osteosarcoma cells transfected with an expression plasmid for IGFBP-5 [44], but no longer-term outcomes were assessed, such as effects on mineralization.
Negative outcomes
Osteoblast-specific over-expression of IGFBP-5 in transgenic mice via the osteocalcin promoter resulted in small decreases in bone mineral apposition rate and in trabecular bone volume by 4–5 weeks of age that were normalized by 24 weeks, coincident with the drop in osteocalcin promoter activity with aging [45]. Even though mineralization was reduced, osteoid width was unchanged, which resembled the phenotype seen in mice lacking the IGF-IR in osteoblasts [22]. As the number of osteoblasts was normal in these mice and osteoclast number and activity were identical to controls [45], the results indicate that IGFBP-5 impaired osteoblast function. Cultured bone marrow stromal cells from these mice also showed diminished mineralization.
In another transgenic mouse model, IGFBP-5 was expressed in multiple tissues via the β-actin gene promoter, leading to up to an 8-fold increase in serum levels compared with controls [46]. Long bone mineral density, as assessed by dual energy X-ray absorptiometry and quantitative computed tomography, was reduced by 30% by 3 weeks of age, although these effects were observed primarily in male mice, where accumulation of IGFBP-5 in the blood was highest. Based on these results, it appears that over-expressed IGFBP-5 reduces net bone mineralization. However, as neither osteoblasts nor osteoclasts were examined directly in these experiments, it is unclear whether the effects on bone were caused by changes in their numbers, function, or both.
Other results using cell culture models support the idea that IGFBP-5 inhibits bone formation. Over-expression of IGFBP-5 in osteosarcoma cells reduced their proliferation by 30% [44]. In another study, addition of mammalian cell culture-derived IGFBP-5 to U2 osteosarcoma cells blocked proliferation induced by IGF-I but not by a derivative, des-1,3-IGF-I, which interacts poorly with IGFBPs [19]. In addition, over-expression of IGFBP-5 in MC3T3 cells using a retroviral vector caused a decline in differentiation as measured by reductions in osteoblast-specific mRNAs and proteins, and decreased mineralization [47].
Bone density is dynamically controlled through contrasting actions of osteoclasts and osteoblasts in the bone microenvironment. Yet only a few studies have examined the effects of IGFBP-5 on osteoclasts. In one group of experiments, IGFBP-5 caused an increase in osteoclast formation and stimulated a 7-fold rise in bone resorbing activity [48], lending further support to an overall inhibitory role for IGFBP-5 in bone.
Evidence for IGF-independent effects of IGFBP-5 in bone
As outlined in preceding sections, IGFBP-5 can play a modulating role on the primarily anabolic effects of IGF-I or IGF-II in bone. There is also some evidence for IGF-independent actions of IGFBP-5 on bone cell function. Major support for this hypothesis derives from studies in osteoblasts isolated from IGF-I-deficient mice. In one series of experiments, addition of E. coli-derived IGFBP-5 resulted in small increases in cell proliferation (< 40%) and in early indicators of differentiation, such as alkaline phosphatase activity (< 40% rise) [40]. Since IGF-II was undetectable in the conditioned media from these cells, the results were interpreted as IGF-independent effects of IGFBP-5. Small increases in alkaline phosphatase activity also were measured in parietal bones of IGF-I knockout mice after IGFBP-5 was injected into the periosteum [41].
Several potential mechanisms have been postulated to be responsible for these putative IGF-independent effects of IGFBP-5 in bone. IGFBP-5 has been shown to bind to an uncharacterized cell surface protein of ~ 450 kDa in osteoblasts [49], but it remains to be determined whether this is a receptor, as to date no IGFBP-5-stimulated intracellular signaling pathways have been identified. It also has been suggested that IGFBP-5 encodes a potential nuclear localization sequence within its COOH-terminus [6], and in one series of studies, after exogenous administration, IGFBP-5 could be detected in the nucleus of human osteoblasts by immunocytochemistry [17]. Whether nuclear accumulation of IGFBP-5 occurs in vivo under physiological conditions remains unknown, and the biological consequences remain to be characterized. Genetic screens using the yeast 2-hybrid assay with IGFBP-5 as ‘bait’, and a cDNA expression library from U2 osteosarcoma cells as ‘prey’, have led to the identification of several putative IGFBP-5 interacting proteins that are primarily expressed intracellularly [4]. At present, despite some intriguing initial observations, the physiological relevance of these interactions and their roles in mediating the effects of IGFBP-5 bone remain uncharacterized.
Summary and perspectives for the future
In this review we describe the potentially conflicting roles for IGFBP-5 as both a facilitator and an inhibitor of bone formation and osteoblast function. In one group of observations, it was shown that IGFBP-5 could collaborate with IGF-I to increase bone formation and enhance bone mineral density in mice [39]. The evidence that IGFBP-5 was effective on its own in vivo was less compelling, as parameters of bone formation were not measured directly in the studies reporting positive outcomes [40]. Conversely, in other experiments, over-expression of IGFBP-5 targeted to osteoblast precursor cells, or delivered systemically, led to a decline in bone formation in mice, and in one group of studies, caused a reduction in long bone mineral density [45,46]. Results of experiments in cultured cells were equivalently contradictory, in some cases showing that IGFBP-5 could increase osteoblast DNA synthesis [16,40,43], and in others demonstrating a decline in cell proliferation [19,44]. Other results showed either increases or decreases in parameters of osteoblast differentiation [40,41,44,47].
How can we explain these very divergent and confounding observations? One possibility is differences in experimental design. As seen in Table 1, this includes use of different osteoblastic cell lines, which may vary in terms of IGF-I receptor expression, in the production and secretion of IGF-I, IGF-II, other growth factors with effects on bone, or other IGFBPs, to name just a few differences. Also, with regard to in vivo experiments, systemic versus local delivery of IGFBP-5 in mice almost certainly will cause variability in outcomes, as will continuous IGFBP-5 over-expression as seen in transgenic models when compared with the intermittent delivery that occurs after repeated injections. Additionally, the source and the extent of purification of exogenous IGFBP-5 could cause variability. IGFBP-5 derived from an E. coli production system may behave differently than IGFBP-5 purified from mammalian sources, in part because of differences in post-translational modifications. In this regard, a comparative study of dose-dependent biological effects of all sources of recombinant IGFBP-5 would be very instructive.
Table 1.
Effects of IGFBP-5 on bone parameters
| System | Source of IGFBP-5 | Effects observed | References | |
|---|---|---|---|---|
| Stimulatory effects | Mouse osteoblasts in primary culture | Mammalian cell culture | Increase in thymidine incorporation into DNA | [16] |
| MC3T3, human osteoblasts in primary culture | E. coli-derived | Increase in thymidine incorporation into DNA | [43] | |
| MG63, human osteoblasts in primary culture | E. coli-derived | Increases in osteocalcin and alkaline phosphatase | [41] | |
| Wild type and IGF-I-deficient mice
In vitro-calvarial osteoblasts In vivo-injection in parietal bones |
E. coli-derived |
In vitro - increase in thymidine incorporation into DNA and alkaline phophatase activity
In vivo - increase in alkaline phophatase and osteocalcin mRNA |
[40] | |
| Local or systemic injections of IGFBP-5 and IGF-I in rats | E. coli-derived | Increase in cortical bone formation, bone area, bone mineral density | [39] | |
| Mouse osteosarcoma cells (OS/50K8) | Expression plasmid | Increase in osteocalcin mRNA | [44] | |
| Inhibitory effects | U2 osteosarcoma cells | Mammalian-cell culture | IGF-I-mediated cell proliferation inhibited | [19] |
| Mouse osteosarcoma cells | Expression plasmid | Increase in cell doubling time (decrease in proliferation by 30%) | [44] | |
| MC3T3 | Retrovirus | Decreased mRNAs of osteoblast specific genes; decreased bone mineralization | [47] | |
| IGFBP-5 transgenic mice | Systemic expression via β-actin promoter | Decrease in bone mineral density and mineralization | [46] | |
| IGFBP-5 transgenic mice | Osteoblast-specific expression | Transient decrease mineral apposition;in decreased trabecular bone volume and osteopenia | [45] |
Other reasons for contradictory outcomes may relate to the parameters being measured. While it should be obvious that increases in rates of DNA synthesis do not necessarily equal alterations in cell number, often the distinctions are blurred. Similarly, changes in serum levels of proteins made in bone are not equivalent to alterations in the extent of new bone formation or changes in bone mineral content measured directly. Suggestions for a more systematic and uniform approach to evaluating the actions of IGFBP-5 in bone are listed in Table 2.
Table 2.
Guidelines for evaluating IGFBP-5 actions in bone
| Parameter | Guidelines |
|---|---|
| IGFBP-5 | Source (bacterial vs. mammalian derived)
Mode of delivery
|
| Cell culture system | Stage of differentiation (pre-osteoblasts vs. osteoblasts)
Abundance of IGF system components (IGF-IR, IGF-I and -II, IGFBP-5 proteases, other IGFBPs) Expression of other factors capable of regulating IGFBP-5 |
| Assessment of osteogenic outcomes |
In vitro
Proliferation - DNA synthesis rates, cell counts, cell doubling time Differentiation - time course studies with standardization of osteogenic markers (gene expression, protein levels, enzymatic assays, etc.) Mineralization rates and extent In vivo Analysis of local bone parameters (bone mineral density, cortical and trabecular bone, mineral apposition rates, etc.) Effects on different bone cell types (osteoblast, osteoclast, osteocyte) |
Finally, while it is clear that IGFBP-5 and other IGFBPs influence the biological effects of IGF-I and IGF-II, it is still unproven how IGFBP-5 might act through IGF-independent mechanisms in bone or in other tissues. Experiments to prove IGF-independent effects of IGFBP-5 are very difficult to perform convincingly, and results to date remain subject to alternative interpretations. If IGFBP-5 acts through a cell-surface receptor, then the responsible signaling pathways need to be characterized. Similarly, if IGFBP-5 acts in the nucleus, then it needs to be shown in as compelling a way as possible that it enters the nucleus under physiologically-relevant conditions, and does not accumulate there secondary to over-expression of either native or modified versions of the protein in cultured cells.
IGFBP-5 is the most conserved of the six IGFBPs, and appears to be present in all vertebrate species which have been examined. It is time to define its actions in bone and in other tissues as completely as possible.
Figure 1.
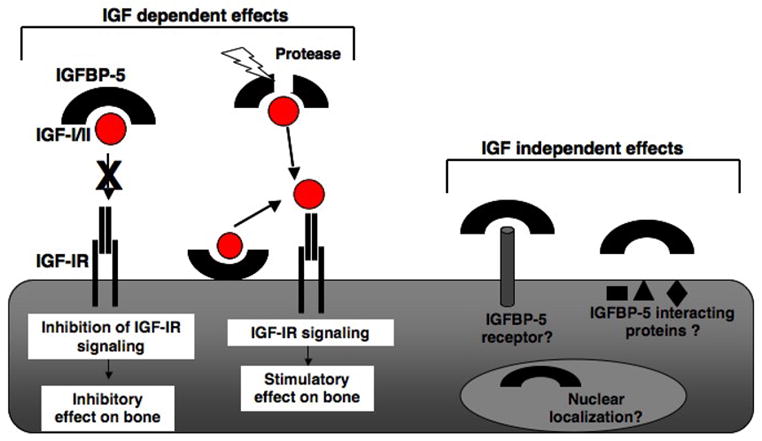
Effects of IGFBP-5 in bone. Illustrated are potential mechanisms by which IGFBP-5 modifies the actions of IGF-I and IGF-II in bone cells. Also shown are putative IGF-independent effects of IGFBP-5. See text for details.
Acknowledgments
This review was supported in part by US National Institutes of Health research grants, RO1 DK63073 and RO1 DK42748 (to P. R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beattie J, Allan GJ, Lochrie JD, Flint DJ. Insulin-like growth factor-binding protein-5 (IGFBP-5): a critical member of the IGF axis. Biochem J. 2006;395:1–19. doi: 10.1042/BJ20060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemmons DR. Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol. 1998;140:19–24. doi: 10.1016/s0303-7207(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 3.LeRoith D, Werner H, Neuenschwander S, Kalebic T, Helman LJ. The role of the insulin-like growth factor-I receptor in cancer. Ann NY Acad Sci. 1995;766:402–408. doi: 10.1111/j.1749-6632.1995.tb26689.x. [DOI] [PubMed] [Google Scholar]
- 4.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 5.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 6.Schedlich LJ, Young TF, Firth SM, Baxter RC. Insulin-like growth factor-binding protein (IGFBP)-3 and IGFBP-5 share a common nuclear transport pathway in T47D human breast carcinoma cells. J Biol Chem. 1998;273:18347–18352. doi: 10.1074/jbc.273.29.18347. [DOI] [PubMed] [Google Scholar]
- 7.Schneider MR, Wolf E, Hoeflich A, Lahm H. IGF-binding protein-5: flexible player in the IGF system and effector on its own. J Endocrinol. 2002;172:423–440. doi: 10.1677/joe.0.1720423. [DOI] [PubMed] [Google Scholar]
- 8.Imai Y, Moralez A, Andag U, Clarke JB, Busby WH, Jr, Clemmons DR. Substitutions for hydrophobic amino acids in the N-terminal domains of IGFBP-3 and -5 markedly reduce IGF-I binding and alter their biologic actions. J Biol Chem. 2000;275:18188–18194. doi: 10.1074/jbc.M000070200. [DOI] [PubMed] [Google Scholar]
- 9.Kalus W, Zweckstetter M, Renner C, Sanchez Y, Georgescu J, Grol M, Demuth D, Schumacher R, Dony C, Lang K, Holak TA. Structure of the IGF-binding domain of the insulin-like growth factor-binding protein-5 (IGFBP-5): implications for IGF and IGF-I receptor interactions. EMBO J. 1998;17:6558–6572. doi: 10.1093/emboj/17.22.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arai T, Parker A, Busby W, Jr, Clemmons DR. Heparin, heparan sulfate, and dermatan sulfate regulate formation of the insulin-like growth factor-I and insulin-like growth factor-binding protein complexes. J Biol Chem. 1994;269:20388–20393. [PubMed] [Google Scholar]
- 11.Parker A, Clarke JB, Busby WH, Jr, Clemmons DR. Identification of the extracellular matrix binding sites for insulin-like growth factor-binding protein 5. J Biol Chem. 1996;271:13523–13529. doi: 10.1074/jbc.271.23.13523. [DOI] [PubMed] [Google Scholar]
- 12.Song H, Beattie J, Campbell IW, Allan GJ. Overlap of IGF- and heparin-binding sites in rat IGF-binding protein-5. J Mol Endocrinol. 2000;24:43–51. doi: 10.1677/jme.0.0240043. [DOI] [PubMed] [Google Scholar]
- 13.Twigg SM, Kiefer MC, Zapf J, Baxter RC. Insulin-like growth factor-binding protein 5 complexes with the acid-labile subunit. Role of the carboxyl-terminal domain. J Biol Chem. 1998;273:28791–28798. doi: 10.1074/jbc.273.44.28791. [DOI] [PubMed] [Google Scholar]
- 14.Twigg SM, Baxter RC. Insulin-like growth factor (IGF)-binding protein 5 forms an alternative ternary complex with IGFs and the acid-labile subunit. J Biol Chem. 1998;273:6074–6079. doi: 10.1074/jbc.273.11.6074. [DOI] [PubMed] [Google Scholar]
- 15.Andress DL, Loop SM, Zapf J, Kiefer MC. Carboxy-truncated insulin-like growth factor binding protein-5 stimulates mitogenesis in osteoblast-like cells. Biochem Biophys Res Commun. 1993;195:25–30. doi: 10.1006/bbrc.1993.2004. [DOI] [PubMed] [Google Scholar]
- 16.Andress DL, Birnbaum RS. Human osteoblast-derived insulin-like growth factor (IGF) binding protein-5 stimulates osteoblast mitogenesis and potentiates IGF action. J Biol Chem. 1992;267:22467–22472. [PubMed] [Google Scholar]
- 17.Mohan S, Thompson GR, Amaar YG, Hathaway G, Tschesche H, Baylink DJ. ADAM-9 is an insulin-like growth factor binding protein-5 protease produced and secreted by human osteoblasts. Biochemistry. 2002;41:15394–15403. doi: 10.1021/bi026458q. [DOI] [PubMed] [Google Scholar]
- 18.Thrailkill KM, Quarles LD, Nagase H, Suzuki K, Serra DM, Fowlkes JL. Characterization of insulin-like growth factor-binding protein 5-degrading proteases produced throughout murine osteoblast differentiation. Endocrinology. 1995;136:3527–3533. doi: 10.1210/endo.136.8.7543045. [DOI] [PubMed] [Google Scholar]
- 19.Conover CA, Kiefer MC. Regulation and biological effect of endogenous insulin-like growth factor binding protein-5 in human osteoblastic cells. J Clin Endocrinol Metab. 1993;76:1153–1159. doi: 10.1210/jcem.76.5.7684391. [DOI] [PubMed] [Google Scholar]
- 20.Coverley JA, Baxter RC. Phosphorylation of insulin-like growth factor binding proteins. Mol Cell Endocrinol. 1997;128:1–5. doi: 10.1016/s0303-7207(97)04032-x. [DOI] [PubMed] [Google Scholar]
- 21.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 22.Zhang M, Xuan S, Bouxsein ML, von SD, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 23.Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141:2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- 24.Jiang J, Lichtler AC, Gronowicz GA, Adams DJ, Clark SH, Rosen CJ, Kream BE. Transgenic mice with osteoblast-targeted insulin-like growth factor-I show increased bone remodeling. Bone. 2006;39:494–504. doi: 10.1016/j.bone.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 25.Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 26.Sims NA, Clement-Lacroix P, Da PF, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, Baron R, Kelly PA. Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest. 2000;106:1095–1103. doi: 10.1172/JCI10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bautista CM, Baylink DJ, Mohan S. Isolation of a novel insulin-like growth factor (IGF) binding protein from human bone: a potential candidate for fixing IGF-II in human bone. Biochem Biophys Res Commun. 1991;176:756–763. doi: 10.1016/s0006-291x(05)80249-9. [DOI] [PubMed] [Google Scholar]
- 28.Olney RC, Mougey EB. Expression of the components of the insulin-like growth factor axis across the growth-plate. Mol Cell Endocrinol. 1999;156:63–71. doi: 10.1016/s0303-7207(99)00144-6. [DOI] [PubMed] [Google Scholar]
- 29.Kiepe D, Andress DL, Mohan S, Standker L, Ulinski T, Himmele R, Mehls O, Tonshoff B. Intact IGF-binding protein-4 and -5 and their respective fragments isolated from chronic renal failure serum differentially modulate IGF-I actions in cultured growth plate chondrocytes. J Am Soc Nephrol. 2001;12:2400–2410. doi: 10.1681/ASN.V12112400. [DOI] [PubMed] [Google Scholar]
- 30.Birnbaum RS, Wiren KM. Changes in insulin-like growth factor-binding protein expression and secretion during the proliferation, differentiation, and mineralization of primary cultures of rat osteoblasts. Endocrinology. 1994;135:223–230. doi: 10.1210/endo.135.1.8013356. [DOI] [PubMed] [Google Scholar]
- 31.Malpe R, Baylink DJ, Linkhart TA, Wergedal JE, Mohan S. Insulin-like growth factor (IGF)-I, -II, IGF binding proteins (IGFBP)-3, -4, and -5 levels in the conditioned media of normal human bone cells are skeletal site-dependent. J Bone Miner Res. 1997;12:423–430. doi: 10.1359/jbmr.1997.12.3.423. [DOI] [PubMed] [Google Scholar]
- 32.Schmid C, Schlapfer I, Gosteli-Peter MA, Froesch ER, Zapf J. Expression, effects, and fate of IGFBP-5 are different in normal and malignant osteoblastic cells. Prog Growth Factor Res. 1995;6:167–173. doi: 10.1016/0955-2235(95)00037-2. [DOI] [PubMed] [Google Scholar]
- 33.Gabbitas B, Canalis E. Insulin-like growth factors sustain insulin-like growth factor-binding protein-5 expression in osteoblasts. Am J Physiol. 1998;275:E222–E228. doi: 10.1152/ajpendo.1998.275.2.E222. [DOI] [PubMed] [Google Scholar]
- 34.McCarthy TL, Casinghino S, Centrella M, Canalis E. Complex pattern of insulin-like growth factor binding protein expression in primary rat osteoblast enriched cultures: regulation by prostaglandin E2, growth hormone, and the insulin-like growth factors. J Cell Physiol. 1994;160:163–175. doi: 10.1002/jcp.1041600119. [DOI] [PubMed] [Google Scholar]
- 35.Pash JM, Canalis E. Transcriptional regulation of insulin-like growth factor-binding protein-5 by prostaglandin E2 in osteoblast cells. Endocrinology. 1996;137:2375–2382. doi: 10.1210/endo.137.6.8641189. [DOI] [PubMed] [Google Scholar]
- 36.Schmid C, Schlapfer I, Gosteli-Peter MA, Hauri C, Froesch ER, Zapf J. 1 alpha,25-dihydroxyvitamin D3 increases IGF binding protein-5 expression in cultured osteoblasts. FEBS Lett. 1996;392:21–24. doi: 10.1016/0014-5793(96)00777-6. [DOI] [PubMed] [Google Scholar]
- 37.Canalis E, Gabbitas B. Skeletal growth factors regulate the synthesis of insulin-like growth factor binding protein-5 in bone cell cultures. J Biol Chem. 1995;270:10771–10776. doi: 10.1074/jbc.270.18.10771. [DOI] [PubMed] [Google Scholar]
- 38.Rutter MM, Markoff E, Clayton L, Akeno N, Zhao G, Clemens TL, Chernausek SD. Osteoblast-specific expression of insulin-like growth factor-1 in bone of transgenic mice induces insulin-like growth factor binding protein-5. Bone. 2005;36:224–231. doi: 10.1016/j.bone.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Bauss F, Lang K, Dony C, Kling L. The complex of recombinant human insulin-like growth factor-I (rhIGF-I) and its binding protein-5 (IGFBP-5) induces local bone formation in murine calvariae and in rat cortical bone after local or systemic administration. Growth Horm IGF Res. 2001;11:1–9. doi: 10.1054/ghir.2000.0181. [DOI] [PubMed] [Google Scholar]
- 40.Miyakoshi N, Richman C, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that IGF-binding protein-5 functions as a growth factor. J Clin Invest. 2001;107:73–81. doi: 10.1172/JCI10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richman C, Baylink DJ, Lang K, Dony C, Mohan S. Recombinant human insulin-like growth factor-binding protein-5 stimulates bone formation parameters in vitro and in vivo. Endocrinology. 1999;140:4699–4705. doi: 10.1210/endo.140.10.7081. [DOI] [PubMed] [Google Scholar]
- 42.Miyakoshi N, Richman C, Qin X, Baylink DJ, Mohan S. Effects of recombinant insulin-like growth factor-binding protein-4 on bone formation parameters in mice. Endocrinology. 1999;140:5719–5728. doi: 10.1210/endo.140.12.7175. [DOI] [PubMed] [Google Scholar]
- 43.Mohan S, Nakao Y, Honda Y, Landale E, Leser U, Dony C, Lang K, Baylink DJ. Studies on the mechanisms by which insulin-like growth factor (IGF) binding protein-4 (IGFBP-4) and IGFBP-5 modulate IGF actions in bone cells. J Biol Chem. 1995;270:20424–20431. doi: 10.1074/jbc.270.35.20424. [DOI] [PubMed] [Google Scholar]
- 44.Schneider MR, Zhou R, Hoeflich A, Krebs O, Schmidt J, Mohan S, Wolf E, Lahm H. Insulin-like growth factor-binding protein-5 inhibits growth and induces differentiation of mouse osteosarcoma cells. Biochem Biophys Res Commun. 2001;288:435–442. doi: 10.1006/bbrc.2001.5785. [DOI] [PubMed] [Google Scholar]
- 45.Devlin RD, Du Z, Buccilli V, Jorgetti V, Canalis E. Transgenic mice overexpressing insulin-like growth factor binding protein-5 display transiently decreased osteoblastic function and osteopenia. Endocrinology. 2002;143:3955–3962. doi: 10.1210/en.2002-220129. [DOI] [PubMed] [Google Scholar]
- 46.Salih DA, Mohan S, Kasukawa Y, Tripathi G, Lovett FA, Anderson NF, Carter EJ, Wergedal JE, Baylink DJ, Pell JM. Insulin-like growth factor-binding protein-5 induces a gender-related decrease in bone mineral density in transgenic mice. Endocrinology. 2005;146:931–940. doi: 10.1210/en.2004-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durant D, Pereira RM, Canalis E. Overexpression of insulin-like growth factor binding protein-5 decreases osteoblastic function in vitro. Bone. 2004;35:1256–1262. doi: 10.1016/j.bone.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Kanatani M, Sugimoto T, Nishiyama K, Chihara K. Stimulatory effect of insulin-like growth factor binding protein-5 on mouse osteoclast formation and osteoclastic bone-resorbing activity. J Bone Miner Res. 2000;15:902–910. doi: 10.1359/jbmr.2000.15.5.902. [DOI] [PubMed] [Google Scholar]
- 49.Andress DL. Insulin-like growth factor-binding protein-5 (IGFBP-5) stimulates phosphorylation of the IGFBP-5 receptor. Am J Physiol. 1998;274:E744–E750. doi: 10.1152/ajpendo.1998.274.4.E744. [DOI] [PubMed] [Google Scholar]


