Abstract
This study evaluates the occurrence of Cryptosporidium oocysts and Giardia cysts in reclaimed effluents if method 1623 with the Envirochek capsule filters (standard and high-volume [HV] filters) and a modified version of the Information Collection Rule method (ICR) with the polypropylene yarn-wound cartridge filter are used. The recovery efficiency of the analytical methods was evaluated with samples of reagent, tap, and reclaimed water by using flow cytometer-sorted spike suspensions. (Oo)cyst recovery efficiency determined filter performance and method reproducibility in the water matrix tested. Method 1623 with the Envirochek HV capsule filter generated significantly higher recovery rates than did the standard Envirochek filter and the modified ICR method. Notwithstanding, large variations in recovery rates (>80%) occurred with samples of reclaimed water, and none of the water quality parameters analyzed in the reclaimed effluents could explain such variability. The highest concentrations of indigenous oocysts were detected by method 1623 with the HV filter, which provided a sufficient number of oocysts for further confirmation of infectious potential. Confirmation of species and potential infectivity for all positive protozoan samples was made by using a nested PCR restriction fragment polymorphism assay and the focus detection method most-probable-number assay, respectively. The methodology and results described in the present investigation provide useful information for the establishment of pathogen numeric standards for reclaimed effluents used for unrestricted irrigation.
Standardized methods used for surface and drinking waters are available to evaluate the occurrence of protozoan pathogens in water supplies, wastewater effluents, and reclaimed effluents. Reclaimed effluents can be defined as those waters that have received a combination of physical, chemical, and biological processes and operations to remove settleable, suspended, and dissolved solids, organic matter, metals, nutrients, and pathogens from wastewater. The major uses of reclaimed effluents include agricultural and landscape irrigation, groundwater recharge, industrial use, and surface water replenishment (2, 16).
Cryptosporidium parvum and Giardia lamblia, according to some public health officials, are no more prevalent in reclaimed effluents than in other irrigation waters. Occasional findings of (oo)cysts in reclaimed water may, however, present a health risk due to the potential high viability and various routes of exposure. Public concern about the health risk has prompted many states, including Florida, to add periodic sampling for Giardia and Cryptosporidium to present reuse rules (10).
Monitoring for protozoan pathogens is necessary for estimating the risk of infection resulting from exposure to reclaimed water. Presently, there are no approved methods for sampling and detecting protozoan pathogens in reclaimed effluents. With the development of new methods for detecting waterborne Cryptosporidium and Giardia, there is a great interest in applying these methods for the evaluation of pathogen reductions by wastewater reclamation processes and for compliance monitoring of effluents from reclamation facilities that provide water for public access irrigation.
The objective of this study was to evaluate the occurrence of Cryptosporidium oocysts and Giardia cysts in reclaimed effluents by using method 1623 of the U.S. Environmental Protection Agency (22) and a modified version of the Information Collection Rule (ICR) method. The study was based on an experimental design aimed to (i) assess the reproducibility of both methods in reclaimed water matrices by using a combination of experimental conditions; (ii) determine viability/infectivity of Cryptosporidium oocysts isolated from reclaimed effluents; (iii) characterize Cryptosporidium isolates by PCR; (iv) compare the occurrence between Cryptosporidium and Giardia at four water reclamation facilities; and (iv) determine the relationships, if any, among parasites and effluent characteristics associated with operating conditions. The results of this investigation provide new insights on waterborne Cryptosporidium in reclaimed wastewater used for public access irrigation.
MATERIALS AND METHODS
Reclaimed water samples.
Water samples were collected from four water reclamation facilities located in one metropolitan area of Florida. These are conventional activated sludge facilities that provide filtration to secondary treated effluents for reduction of pathogens and particles prior to disinfection with chlorine gas. The filtration system of the reclamation process includes deep-bed dual medium filters (anthracite, sand, and gravel; facilities A and B) and a shallow-bed sand filter (anthracite and/or sand; facilities C and D).
The overall quality of water produced from each water reclamation facility generally met the Florida Department of Environmental Protection minimum standards for public access reuse systems: chloride, less than 600 mg/liter; chlorine residual, 4 mg/liter or greater; total suspended solids (single-sample maximum), 5 mg/liter or less; turbidity, 2.5 nephelometric units [NTUs]; and fecal coliforms, less than detectable levels of fecal coliforms in at least 75% of observations [no single observation may exceed 25 fecal coliforms per 100 ml]) (10). These water quality parameters along with fecal coliform data are sampled on a monthly and/or quarterly basis in the reclaimed water. These data were provided upon request by plant operators at the different facilities. Levels of fecal coliforms were exceeded in reclaimed effluents at one or more of the facilities tested.
Recovery efficiency experiments.
These experiments were carried out as described in the April 1999 version of method 1623 (22) to demonstrate acceptable method performance and included (i) initial precision and recovery (IPR) tests (ii) ongoing precision and recovery tests, (iii) matrix spikes, and (iv) method blanks. From these experiments, mean percent recoveries and relative standard deviations (RSD) were calculated. A combination of filtered tap water and distilled water was used as the reagent water sample for blanks, IPR, and ongoing precision and recovery tests. Unfiltered tap water and reclaimed wastewater were used as matrix spikes.
Three types of filters and two spike suspensions were used in seeding experiments to evaluate mean (oo)cyst recoveries based on a three-factorial treatment design with two levels for filter type and spike and three levels for filter type, spike, and organism. Water samples were filtered through Gelman Envirochek sampling capsules (standard and high-volume [HV] filters) (Pall Gelman Sciences, Inc., Ann Arbor, Mich.) and polypropylene yarn-wound cartridge filters (Filterite, Timonium, Md.). For seeded and unseeded experiments, water volumes of 10 liters were filtered through the Envirochek sampling capsule while 100 liters was filtered through the Envirochek HV capsule and the polypropylene yarn-wound cartridge filter. Seeded matrix samples were filtered on site, and the filters were transported to the laboratory on ice. ColorSeed C&G (BTF Decisive Microbiology, North Ryde, New South Wales, Australia) and flow cytometer-sorted Cryptosporidium oocysts and Giardia cysts obtained from the Wisconsin State Laboratory of Hygiene (WSLH) Flow Cytometry Unit (Madison, Wis.) were used as the spike suspensions in IPR tests and recovery efficiency experiments with tap water samples.
ColorSeed C&G was used in the recovery efficiency experiments carried out with samples of reclaimed water to differentiate indigenous and seeded (oo)cysts. ColorSeed C&G spikes consist of hundreds of red fluorescently labeled and gamma-irradiated Cryptosporidium oocysts and Giardia cysts in approximately 1 ml of saline solution. They can be used as internal quality control parameters to determine the performance or percent recovery achieved with every test (http://www.biotechfrontiers.com). For each spiked sample, between 10 and 100 liters of water (depending on the type of filter) was pumped into a 100-liter plastic trash can. Giardia cysts and Cryptosporidium oocysts spikes were vortexed for 3 min and were added to the 10- or 100-liter sample according to the ColorSeed C&G matrix spike package insert (BTF; Decisive Microbiology). Filters were processed following the steps described below, and the recovery efficiency of (oo)cysts (percentages) achieved by the experiments was calculated as the number of spiked (oo)cysts detected, divided by the ColorSeed C&G number, and multiplied by 100. The adjusted level of (oo)cysts was calculated to reflect the number of (oo)cysts that would be estimated to be in the sample if there had been 100% recovery efficiency. Specifically, the adjusted levels were obtained by dividing the number of indigenous (oo)cysts by the percentage of recovery efficiency and multiplying by 100.
Method 1623.
All water samples were filtered following standard procedures. Envirochek filtration was performed with an automatic-demand diaphragm pump, and flow rates were maintained at 2 to 3.5 liters m−1. After filtration, the capsules were immediately processed or placed on ice and were transported to the laboratory.
Immunomagnetic separation (IMS) of (oo)cysts was performed with a Dynal GC-Combo kit (product no. 730.02; DYNAL A.S., Oslo, Norway) and included two dissociation steps with 100 μl of 0.1 N HCl instead of 50 μl as recommended in the manufacturer's instructions. IMS concentrates (100 to 200 μl) were fixed with absolute methanol and were stained with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (MAbs).
Two commercially available MAbs were used in the immunofluorescence assay (IFA) and were evaluated for detection sensitivity: (i) EasyStain (BTF; Decisive Microbiology) and (ii) Giardia-a-Glo/Crypto-a-Glo (Waterborne Inc., New Orleans, La.). Confirmation of (oo)cysts was accomplished through vital dye staining (4′,6′-diamidino-2-phenylindole [DAPI]/propidium iodide [PI]) along with Nomarski differential interference contrast (DIC) microscopy to look at the internal morphology of (oo)cysts.
Modified ICR method.
Yarn-wound cartridge filtration was carried out by using a gasoline-powered portable water pump with flow rates maintained at 8 to 10 liters m−1. The method for processing samples (filtration, elution, and sample concentration) included most of the steps of the ICR protozoan method for detecting Giardia cysts and Cryptosporidium oocysts in water with a fluorescent antibody procedure, EPA/814-B95-003 (23), plus some modifications. IMS instead of Percoll-sucrose flotation was used as the clarification technique for selective separation of cysts and oocysts from debris. Identification and confirmation of (oo)cysts were accomplished through epifluorescence microscopy, vital dye staining, and DIC microscopy as described above.
Vital dye and cell culture infectivity assays.
Water sample concentrates were analyzed for the presence of viable and infectious Cryptosporidium oocysts by using the vital dye assay following the method described by Campbell et al. (3) with slight modifications and the focus detection method-most probable number (FDM-MPN) method developed by Slifko et al. (20) and modified by Gennaccaro et al. (11). For the vital dye staining assay, the modifications included incubation of (oo)cysts with the dyes for 10 min at room temperature. Previous work found that this time was optimal for maximal dye uptake and that no differences occurred during the length of time described in the protocol of Campbell et al. (3).
For the FDM-MPN assay, 25% of the IMS concentrate (50 μl) was treated (8 min at room temperature) by using 10.5% (vol/vol) sodium hypochlorite (Sigma-Aldrich, St. Louis, Mo.) in phosphate-buffered solution (pH 7.2). The sample was washed once by centrifugation and was suspended in 1 ml of growth medium (RPMI 1640; Fisher Scientific, Pittsburgh, Pa.) supplemented with 10% fetal bovine serum (Invitrogen Life Technologies, Carlsbad, Calif.) and other additives (2% 1 M HEPES and 2 mM l-glutamine plus 10% Optimem). Aliquots of this suspension were inoculated onto human ileocecal adenocarcinoma cell (HCT-8 cells) monolayers cultivated in eight-well chamber slides (LabTech II; Nalgene Nunc, Naperville, Ill.). Uninoculated cell monolayers were included on each well slide as negative controls. After 90 min, more growth medium was added to each well and the slide contents were incubated in a 5% CO2 atmosphere at 37°C for 48 h. Well chamber slides were fixed with 100% methanol for 8 min and were labeled by using the previously described indirect antibody procedure FDM (20). Staining was performed with rat anti-C. parvum sporozoite (Waterborne Inc.) followed by a second labeling with anti-rat immunoglobulin G FITC (Sigma Aldrich, Inc., St. Louis, Mo.). Chamber slides were examined under epifluorescence and DIC microscopy, and each well was scored as positive or negative for infection. The numbers of positive wells for each sample was entered into the MPN program (http://www.epa.gov/nerlcwww/mpn/exe) to determine the number of infectious oocysts per milliliter. The MPN program determined the detection limit for the assay where no infectious oocysts were observed. The concentration of infectious oocysts was expressed as the number of infectious oocysts per 100 liters on the basis of the equivalent volume examined.
DNA extraction and molecular characterization of Cryptosporidium species and genotypes.
DNA was extracted from the remaining 25% of the IMS concentrates (50 μl) by using the Chelex resin freeze-thaw method described by Di Giovanni et al. (7). Briefly, IMS concentrates were centrifuged (10,000 × g for 3 min) and were resuspended in 50 μl of molecular-grade water. Water concentrates were mixed with 10 μl of 1:1 ratio (vol/vol) of Chelex resin-Tris-EDTA buffer and were subjected to eight cycles of freezing and thawing. DNA was recovered from the supernatant after a quick spin step and was stored at −20°C before it was used for PCR analysis. Samples of Cryptosporidium-free molecular-grade water were included randomly as negative controls during the DNA extraction procedures.
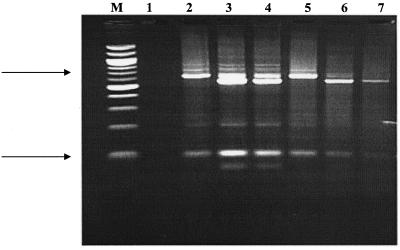
Molecular characterization of Cryptosporidium species and genotypes was carried out by using a nested PCR-restriction fragment length polymorphism assay of the 18S small-subunit rRNA gene fragment and the restriction enzymes SspI and VspI (24, 25). Results are shown in Fig. 1. Some modifications to this protocol were included. The PCR amplification reaction mixtures contained 1× PCR buffer (Qiagen [Valencia, Calif.] 10× PCR buffer with 15 mM MgCl2), 200 μM (each) deoxynucleoside triphosphate (Amersham Biosciences, Piscataway, N.J.), 100 nM (each) primer, 2.5 U of Hot Start Taq polymerase (Qiagen), and 5 and 50 μl of DNA template in total 50- and 100-μl reaction mixtures, respectively. Positive and negative PCR controls were run in parallel with each set of samples. PCR-positive controls for the initial amplification reaction consisted of molecular-grade water and various amounts of C. parvum template DNA. PCR-negative controls contained various amounts of molecular-grade water. Primary PCR was performed with primers 5′-TTCTAGACCTAATACATGCG-3′ and 5′-CCCATTTCCTTCGAAACAGGA-3′. Forty PCR cycles (94°C for 45 s, 55°C for 60 s, and 72°C for 90 s) were carried out in an Eppendorf thermal cycler (Eppendorf AG) with an initial host start at 95°C for 15 min and a final extension at 72°C for 1 min 30 s. For the secondary PCR product, 5 μl of the primary PCR product was amplified with nested primers 5′-GGAAGGGTTGTATTTATTAGATAAAG-3′ and 5′-AAGGAGTAAGGAACAACCTCCA-3′. Cycling conditions were identical to those used for the primary PCR. PCR products were analyzed on 1.5% agarose gels containing 0.5 μg per ml of ethidium bromide in 1× Tris-borate-EDTA buffer (45 mM Tris-borate and 2 mM EDTA). Resulting bands were visualized by UV transillumination. The detection limit of the PCR technique was tested with flow cytometer-counted oocyst suspensions (WSLH) containing 10 oocysts.
FIG. 1.
Genotyping of Cryptosporidium isolates in reclaimed effluents with the small-subunit rRNA-based PCR-restriction fragment length polymorphism technique. Differentiation of Cryptosporidium genotypes was demonstrated by digestion of the secondary PCR product with VspI. Lane 1, no sample; lanes 2 and 5, C. parvum bovine genotype; lanes 3 and 4, C. parvum human and bovine genotypes; lanes 6 and 7, C. parvum human genotype; and lane M, 100-bp DNA ladder. Upper and lower arrows correspond to band sizes of 600 and 100 bp, respectively.
Secondary PCR products were purified by using the QIAquick PCR purification kit (Qiagen) and were eluted in Tris buffer (10 mM Tris-Cl, pH 8.5) prior to restriction fragment analysis and sequencing to remove deoxynucleoside triphosphates, polymerases, salts, and primers. For restriction fragment analysis, 20 μl of the secondary PCR product was digested in a 25-μl (total volume) reaction mixture containing 20 U of SspI (New England BioLabs, Beverly, Mass.) for species diagnosis or 20 U of VspI (MBI Fermentas Inc., Hanover, Md.) for genotyping of C. parvum and the appropriate amount of restriction buffer at 37°C for 1 h. Digested products were fractioned on a 2.0% agarose gel and were visualized by ethidium bromide staining. The patterns of DNA bands were used to differentiate the species and genotypes of Cryptosporidium parasites according to methodology described by Xiao et al. (24, 25). Automated sequencing was performed on the ABI PRISM 3100 Genetic Analyzer (Applied Biosystems) of the Genomic Technology Support Facility at Michigan State University. The resulting sequences were compared with partial sequences available in the GenBank database to identify possible matches with the sequences of the species of Cryptosporidium obtained from samples of reclaimed effluents used for public access irrigation.
Statistical analysis.
A three-way analysis-of-variance task along with multiple-comparison procedures (Bonferroni and Tukey tests) was used to analyze the experimental data generated from the study. A level of significance of 5% (α = 0.05) was used to test for statistical differences. The statistical tools, where applicable, evaluated the effects of each factor (filter type, spike, or organism) individually or the interactions between factors on the recovery efficiency of (oo)cysts. The same approach was used to test for statistical significance for the number of indigenous (oo)cysts detected in reclaimed effluents. For the statistical analysis, recovery efficiency percentages were transformed to arcsine and the number of indigenous organisms was transformed to the natural logarithm (ln y + 1). A correlation analysis was used to evaluate the association between the oocyst recovery and water quality parameters. Analysis was done by using Statistical Analysis System (SAS) software 8.02 (SAS Institute, Inc.).
RESULTS
Recovery efficiency of (oo)cysts in reagent water samples.
Table 1 shows the results of the initial precision and recovery tests carried out to demonstrate acceptable method performance. Recoveries of (oo)cysts were higher when using method 1623 with the Envirochek capsule filters than when using the modified ICR method with the yarn-wound polypropylene cartridge filters. Both the Envirochek HV and the regular Envirochek filters recovered on average between 37 and 62% of spiked (oo)cysts, while the yarn-wound polypropylene cartridge recovered on average between 4 and 13%. Mean Cryptosporidium recoveries with the Envirochek HV filter were 57% (RSD, 40%) with ColorSeed and 45% (RSD, 39%) with WSLH suspensions. Mean percent recoveries for Giardia were 37% (RSD 40%) with ColorSeed and 57% (RSD 33%) with WSLH spikes. Similar data were obtained with the regular Envirochek capsule filter for Cryptosporidium oocysts. With ColorSeed suspensions, mean Cryptosporidium and Giardia recoveries were 62% (RSD, 38%) and 58% (RSD, 40%), respectively. For Giardia cysts, the mean percentage efficiency of recovery with ColorSeed was 45% (RSD, 41%); the mean percent recovery with WSLH suspensions was 49% (RSD, 39%). With the polypropylene yarn-wound cartridge filter, the mean percentage efficiencies of Cryptosporidium and Giardia recoveries were notably lower than those obtained with the previous methods. With ColorSeed spikes, the mean percent recovery was 4% (RSD, 200%) while the mean percentage of oocysts with WSLH spikes was 13% (RSD, 138%). Mean percent recovery of Giardia was 10% (RSD, 150%) with ColorSeed and 5% (RSD, 180%) with WSLH spikes.
TABLE 1.
Summary of the IPR tests for Cryptosporidium and Giardia with different methods and spike suspensions
| Method | Protozoan | Spike suspension dataa
|
|||
|---|---|---|---|---|---|
| ColorSeedb
|
WSLHc
|
||||
| Mean Recovery (% [n = 4]) | Precision (RSD)a | Mean recovery (% [n = 4]) | Precision (RSD) | ||
| ICR (yarn-wound polypropylene filter) | Cryptosporidium | 4 | 200 | 13 | 138 |
| Giardia | 10 | 150 | 5 | 180 | |
| Method 1623 (Envirochek HV) | Cryptosporidium | 57 | 40 | 45 | 39 |
| Giardia | 37 | 40 | 57 | 33 | |
| Method 1623 (Envirochek) | Cryptosporidium | 62 | 38 | 58 | 40 |
| Giardia | 45 | 41 | 49 | 39 | |
Spike suspensions.
Biotechnology Frontiers (North Ryde, Australia) Cryptosporidium count = 98 ± 1.5; Giardia count = 98 ± 1.5.
WSLH Flow Cytometry Unit Cryptosporidium count = 198 ± 2.30; Giardia count = 201 ± 3.83.
Immunofluorescent staining with two FITC-conjugated MAbs.
The application of two different MAbs in the IFA demonstrated that both MAbs were equally effective in detecting Cryptosporidium oocysts and Giardia cysts in positive controls and duplicate samples of reclaimed water; however, staining with the EasyStain MAb resulted in lower levels of background fluorescence and nonspecific binding than when staining with Waterborne MAbs. As a result, the enumeration of (oo)cysts as well as the differentiation of (oo)cysts from nontarget organisms and background debris was markedly improved with EasyStain. These improvements were more evident and useful when evaluating samples of reclaimed water with high levels of algae and mineral particles. Therefore, the EasyStain MAb was used throughout the study.
Some other observations on the use of two different MAbs were noteworthy. The (oo)cysts used in the recovery efficiency experiments were analyzed in duplicate with the two MAbs before and after processing. The microscopic analysis of processed and unprocessed Giardia cysts revealed some degree of uneven immunofluorescence staining with the two antibodies tested, as if portions of the cyst wall had been partially removed. In some instances, the cysts did not stain well with the FITC-conjugated MAbs. This uneven staining pattern occurred only for Giardia cysts obtained from ColorSeed C&G spike suspensions. No differences were detected for Cryptosporidium oocysts, whose fluorescent intensity was similar in both processed and unprocessed controls. These results indicated that none of the methods used for sample processing was detrimental to Cryptosporidium oocyst staining; however, Giardia cysts were somehow affected either during sample processing or by the combined immunofluorescent labeling. Using the appropriate filter, the (oo)cysts from ColorSeed could be easily identified against the background and differentiated from indigenous (oo)cysts.
(Oo)cyst recoveries in seeded tap water and reclaimed water.
The recovery efficiency of Cryptosporidium oocysts and Giardia cysts in tap water varied among filters and spike suspensions used in the experiments; these results are summarized in Table 2. Five replicates for each filter and spike suspension were used in the experiments.
TABLE 2.
Mean (oo)cyst percent recoveries and RSD of tap water samples processed by method 1623 and the modified ICR methodc
| Method (filter type) | Spike suspension data
|
|||
|---|---|---|---|---|
| ColorSeeda
|
WSLHb
|
|||
| Cryptosporidium | Giardia | Cryptosporidium | Giardia | |
| Method 1623 HV (n = 5) | 75 ± 16 | 55 ± 22 | 76 ± 9 | 49 ± 49 |
| Envirochek (n = 5) | 90 ± 6 | 64 ± 16 | 63 ± 5 | 39 ± 26 |
| Modified ICR (yarn wound) (n = 5) | 25 ± 44 | 23 ± 39 | 11 ± 55 | 13 ± 46 |
Cryptosporidium count: 98 ± 1.5; Giardia count: 98 ± 1.5 cysts.
Cryptosporidium count: 198.25 ± 2.3; Giardia count: 174 ± 1.6 cysts.
The recovery rates in the table are expressed as the mean ± RSD.
Mean (oo)cyst recovery efficiency percentages and RSD were 75% ± 16% for Cryptosporidium oocysts and 55% ± 22% for Giardia cysts when the Envirochek HV capsule filter was applied and when ColorSeed C&G was used as the spike suspension. Mean percent recoveries of tap water samples seeded with WSLH spike suspensions were 76% ± 9% for Cryptosporidium and 49% ± 49% for Giardia. The standard Envirochek filter recovered on average 90% ± 6% of oocysts and 64% ± 16% of cysts from ColorSeed C&G. Mean percent recoveries with WSLH suspensions averaged 63% ± 5% and 39% ± 26% for oocysts and cysts, respectively. The polypropylene yarn-wound cartridge filter recovered on average 25% ± 44% of seeded oocysts and 23% ± 39% of seeded cysts from ColorSeed. The recoveries obtained with WSLH suspensions were 11% ± 55% and 13% ± 46% for oocysts and cysts, respectively.
The results of the statistical tests demonstrated that mean percent recoveries with the two different spike suspensions were significantly higher when using the Envirochek capsule filters than when using the polypropylene cartridge filter (P < 0.0001). Statistical significance was found when the interaction between filter and organism was evaluated (P = 0.0008); these results demonstrated that Envirochek filtration provided significantly higher recoveries of Cryptosporidium oocysts than of Giardia cysts in samples of spiked tap water (P < 0.0001). Percent recoveries of Cryptosporidium and Giardia were both low with the modified version of the ICR method applied with the polypropylene yarn-wound cartridge filter.
The interaction between filter and spike suspension was analyzed, and the results demonstrated that such an interaction was statistically significant (P = 0.0013). ColorSeed C&G performed better than WSLH suspensions with the Envirochek capsule filter (P < 0.0001) and the polypropylene cartridge filter (P < 0.0001). However, there were no statistical differences between spike suspensions when the Envirochek HV capsule filter was used.
Table 3 summarizes the results of the recovery efficiency experiments in samples of reclaimed water, including the number of indigenous oocysts and estimations of the adjusted number of indigenous oocysts detected based on the recovery rates obtained for every sample. Prestained (oo)cysts from ColorSeed C&G spike suspensions were included as internal quality controls to differentiate spiked from indigenous (oo)cysts. For these experiments, the percent recovery efficiency of Cryptosporidium oocysts varied from 1 to 84% with the HV filter (32 ± 97 [mean ± RSD]). The number of oocysts detected ranged from 2 to 107 per 100 liters, and the adjusted levels ranged from 7 to 264 oocysts per 100 liters. The percent recovery efficiency of Giardia cysts with the same filter varied from 1 to 68% (27 ± 94 [mean ± RSD]) with levels of cysts detected between 6 and 36 and adjusted levels of cysts ranging from 18 to 309. The percent recovery efficiency of (oo)cysts obtained with the standard Envirochek filter was slightly similar to the results obtained with the HV filter. The recovery rates ranged from 16 to 85% (40 ± 67 [mean ± RSD]) for Cryptosporidium, while recovery rates for Giardia ranged from 2 to 49% (26 ± 84 [mean ± RSD]). The corresponding number of oocysts detected ranged from 14 to 90, and the adjusted numbers ranged from 16 to 219 per 100 liters. The number of cysts detected ranged from 20 to 130, while the adjusted number ranged from 69 to 1800. With the polypropylene cartridge filter, the percent recovery efficiencies of (oo)cysts were relatively lower than with previous methods. Cryptosporidium recovery rates ranged from 2 to 27% (15 ± 60 [mean ± RSD]), and the number of oocysts detected plus the adjusted numbers ranged from 1 to 26 and from 10 to 96, respectively. Giardia recovery rates ranged from 0 to 20% (11 ± 66 [mean ± RSD]), and the number of cysts detected ranged from 2 to 14. Adjusted levels of cysts with the polypropylene cartridge filter were between 42 and 70 per 100 liters. Despite large variations observed in samples of reclaimed water, the results of these experiments indicated that filtration with the Envirochek capsule filters produced significantly higher recovery rates than filtration with the polypropylene cartridge filters (P < 0.0001). Both the HV filter and polypropylene cartridge filter allowed the collection of 100 liters of reclaimed water; however, due to low recoveries obtained with the polypropylene yarn-wound cartridge filter, its use for protozoan analysis in reclaimed effluents is not recommended. The volume of water achievable with the standard Envirochek filter never exceeded 20 liters. In some instances, the increased pressure throughout the filtration components precluded the collection of 10 liters of reclaimed water.
TABLE 3.
Mean oocyst recoveries and levels of indigenous oocysts in samples of reclaimed water processed by method 1623 and the modified ICR method
| Method (filter type) | Protozoan result
|
|||||
|---|---|---|---|---|---|---|
|
Cryptosporidium
|
Giardia
|
|||||
| No. of oocysts/100 liters | %a | Adjusted level | No. of cysts/100 liters | % | Adjusted level | |
| Method 1623 (HV) (n = 5) | 74 | 28 | 264 | 36 | 24 | 150 |
| 20 | 19 | 95 | 34 | 11 | 309 | |
| 2 | 1 | 200 | <1 | 1 | NAd | |
| 2 | 28 | 7 | 6 | 34 | 18 | |
| 107 | 84 | 127 | 34 | 68 | 50 | |
| 32b | 27b | |||||
| 97c | 94c | |||||
| Envirochek (n = 5) | 20 | 25 | 80 | 20 | 29 | 69 |
| 90 | 41 | 219 | 90 | 5 | 1,800 | |
| <10 | 16 | NA | <10 | 2 | NA | |
| <10 | 34 | NA | 70 | 47 | 149 | |
| 14 | 85 | 16 | 130 | 49 | 265 | |
| 40b | 26b | |||||
| 67c | 84c | |||||
| Modified ICR (yarn wound) (n = 5) | 1 | 2 | 50 | 2 | 0 | NA |
| 26 | 27 | 96 | 14 | 20 | 70 | |
| 2 | 19 | 10 | <1 | 13 | NA | |
| <1 | 12 | NA | <1 | 9 | NA | |
| 5 | 16 | 31 | 5 | 12 | 42 | |
| 15b | 11b | |||||
| 60c | 66c | |||||
Percentage of recovery efficiency based on recoveries obtained with ColorSeed spikes.
Mean.
RSD.
NA, not applicable.
The data obtained from these analyses revealed that the concentrations of indigenous (oo)cysts expressed per 100 liters were relatively similar with the Envirochek capsule filters and that there were no statistical differences when comparing concentrations of (oo)cysts with both filters. Both Cryptosporidium and Giardia were detected in the reclaimed effluents at similar concentrations and frequencies.
Indigenous (oo)cysts in reclaimed effluents.
Based on previous results, method 1623 with the Envirochek HV capsule filter was chosen for further analysis of protozoans in reclaimed effluents. Table 4 summarizes the results of the pathogen-monitoring program aimed at the determination of Cryptosporidium oocysts and Giardia cyst occurrence in the final effluent of four water reclamation facilities. ColorSeed C&G spike suspensions were used as internal quality controls in these analyses. IMS concentrates (200 μl) were stained with fluorescent MAbs plus fluorochromes (DAPI/PI) and were analyzed for oocysts with epifluorescence microscopy. Three samples per facility were analyzed, and the level of (oo)cysts was expressed per 100 liters plus the adjusted levels of (oo)cysts based on the recovery efficiency percentages obtained for the same batch of samples. All of the samples collected were positive for Cryptosporidium and Giardia regardless of the type of filtration system used at each facility. The levels of Cryptosporidium oocysts detected without adjustment ranged from 2 to 209 per 100 liters, while the number of Giardia cysts ranged from 13 to 118 per 100 liters.
TABLE 4.
Levels of Cryptosporidium oocysts and Giardia cysts in reclaimed effluents based on results obtained from recovery efficiency experiments with ColorSeed as an internal quality control system
| Filtration system at the facility | Protozoan | Level of indigenous (oo)cysts per 100 liters | Recovery efficiency (%)a | Adjusted level of (oo)cysts per 100 liters |
|---|---|---|---|---|
| Deep bed, multiple media (facilities A and B) | Cryptosporidium | 8 | 31 | 26 |
| 209 | 31 | 674 | ||
| 36 | 14 | 257 | ||
| 2 | 8 | 25 | ||
| 35 | 11 | 318 | ||
| 40 | 5 | 800 | ||
| Shallow bed automatic backwash (facilities C and D) | 28 | 27 | 102 | |
| 11 | 10 | 110 | ||
| 77 | 31 | 248 | ||
| 75 | 18 | 417 | ||
| 20 | 19 | 105 | ||
| 106 | 14 | 757 | ||
| Deep bed, multiple media (facilities A and B) | Giardia | 35 | 24 | 146 |
| 13 | 2 | 650 | ||
| 11 | 10 | 110 | ||
| 66 | 24 | 275 | ||
| 20 | 19 | 105 | ||
| 40 | 42 | 95 | ||
| Shallow bed automatic backwash (facilities C and D) | 10 | 31 | 32 | |
| 85 | 43 | 198 | ||
| 118 | 38 | 310 | ||
| 106 | 14 | 757 | ||
| 35 | 11 | 318 | ||
| 75 | 18 | 417 |
Percentage of recovery efficiency.
Further protozoan analyses were carried out to determine the infectious potential of Cryptosporidium isolates by using nested PCR and tissue cell culture. Duplicate samples were spiked with ColorSeed C&G to determine the recovery efficiency in order to avoid misinterpretation of the infectious potential of the Cryptosporidium isolates. Previous assays indicated that the sporozoites of irradiated oocysts were able to excyst and invade HCT-8 cells. However, the developmental stages (Meront types I and II) required to determine the occurrence of foci and therefore oocyst infectivity in cell culture were not observed in the wells. The FDM-MPN assay was used for detecting and enumerating infectious oocysts in those samples in which ColorSeed spikes were not included.
Application of vital dye assay to determine potential viability of Cryptosporidium oocysts and Giardia cysts in water samples.
The vital dye assay was applied to determine potential viability of isolated oocysts. Three populations or categories of oocysts were present in water sample concentrates obtained from reclaimed effluents: (i) PI-positive (PI+) oocysts, (ii) DAPI-negative PI-negative (DAPI− PI−) oocysts, and (iii) DAPI-positive PI-negative (DAPI+ PI−) oocysts. PI+ oocysts were the population most frequently detected and accounted for more than 80% of the oocysts present in all reclaimed effluent samples analyzed. These are considered dead oocysts by the criteria established for this assay by Campbell et al. (3). DAPI− PI− and DAPI+ PI− oocysts accounted for less than 20% (14 to 20%) and 5% (0.5 to 5%), respectively. PI+ oocysts seen under DIC microscopy were characterized for having a disorganized appearance and lack of any distinct sporozoite structure. All DAPI− PI− oocysts were empty with no internal sporozoite structure; these oocysts have been described as “ghosts” in previous studies (1, 3). DAPI+ PI− oocysts satisfied the criteria of Campbell et al. (3) for potentially infectious oocysts, and DIC microscopy revealed intact sporozoite structures.
Giardia cyst populations or categories were predominantly PI+ (100%), which indicated that most of the cysts were inactivated or nonviable. DIC microscopy revealed that for the majority of the cysts the internal morphology was completely destroyed.
Cell culture assay for testing potential infectivity of Cryptosporidium oocysts in water samples.
The FDM-MPN assay allowed the detection and quantification of infectious oocysts in 50% (n = 12) of the samples analyzed from the reclaimed effluents (Table 5). The concentration of oocysts detected by IFA was calculated from the equivalent volume examined and expressed as levels of oocysts per 100 liters before and after adjustment of these levels to the recovery efficiency rates obtained from spiked samples. Concentrations of oocysts similar to or greater than 100 oocysts/100 liters produced foci in HCT-8 cells. These concentrations were obtained from Cryptosporidium IFA counts between 26 and 152 oocysts prior to considering the equivalent volume examined. The levels of oocysts detected by IFA and the FDM-MPN assay were as follows: 209 oocysts/100 liters and 8 MPN/100 liters (facility A); 162 oocysts/100 liters and 5 MPN/100 liters (facility B); 319 oocysts/100 liters and 2 MPN/100 liters (facility C); and 120 oocysts/100 liters and 4 MPN/100 liters, 53 oocysts/100 liters and 7 MPN/100 liters, and 104 oocysts/100 liters and 18 MPN/100 liters (facility D). Based on the results of the recovery efficiency experiments and adjusted levels of oocysts, the number of infectious oocysts found in reclaimed effluents ranged between 17 and 27 MPN/100 liters.
TABLE 5.
Occurrence of infectious Cryptosporidium oocysts in effluents of water reclamation facilities that provide filtration and combined chlorine disinfection to secondary effluents
| Facility | IFA results
|
Infectious oocysts MPN/ 100 litersd | Species | |||
|---|---|---|---|---|---|---|
| No. of oocysts | % REa | Levelb | Adjusted levelc | |||
| A | 4 | —e | 8 | — | <1.3 | *f |
| 107 | 31 | 209 | 674 | 8 (26) (0.8, 22) | C. parvumg | |
| 5 | 22 | 61 | 277 | <2.5 | * | |
| 5 | — | 11 | — | <1.5 | * | |
| B | 83 | 29 | 162 | 286 | 5 (17) (0.3, 12) | C. parvumh |
| C | 151 | 12 | 319 | 1,258 | 2 (17) (0.4, 8) | C. parvumg |
| 26 | 27 | 55 | 204 | <3.2 | * | |
| D | 3 | 8 | 6 | 75 | <1.8 | * |
| 2 | 5 | 8 | 160 | <2.5 | * | |
| 26 | — | 53 | — | 7 (0.8, 16.9) | C. parvumi | |
| 18 (5.5, 41.4) | C. parvumh | |||||
| 40 | 15 | 120 | 800 | 4 (27) (0.5, 25) | C. parvumi | |
Percentage recovery efficiency.
Levels of oocysts per 100 liters.
Adjusted levels of oocysts based on the recovery efficiency obtained from experiments performed the same day of sample collection.
The numbers in boldface correspond to levels of infectious oocysts based on the recovery efficiency. Numbers in parentheses represent the 95% confidence interval.
—, not done.
*, no amplification.
C. parvum human and bovine genotypes.
C. parvum human genotype.
C. parvum bovine genotype.
Molecular characterization of Cryptosporidium isolates.
Table 5 shows the species and genotypes of Cryptosporidium isolates obtained from samples of reclaimed water. Six of the six samples that tested positive for infectious Cryptosporidium by the FDM-MPN assay produced positive PCR amplification by nested PCR. Restriction digestion of the secondary PCR products with SspI yielded restriction patterns similar to those previously described for the species C. parvum by Xiao et al. (24, 25, 26). Differentiation of C. parvum genotypes was accomplished through digestion with VspI, and the restriction patterns obtained with this enzyme revealed the presence of the C. parvum human and bovine genotypes (Fig. 1, genotypes 1 and 2, respectively). Two of the six samples contained both C. parvum genotypes, as determined by the presence of four bands that were similar in size to those described for the aforementioned C. parvum genotypes by Xiao et al. (24). The sequence identities as determined by BlastN search for the amplicons obtained from these isolates were between 91% and 100% homologous to nucleotide sequences of the species C. parvum (GenBank accession no. AF481962).
DISCUSSION
The present study was designed to evaluate the reproducibility of method 1623 and a modified version of the ICR method by using a combination of experimental conditions aimed at the detection of infectious Cryptosporidium oocysts in samples of reclaimed water. Additional information on the recovery and detection of Giardia cysts was also included.
The performance of both methods was initially evaluated with reagent water (IPR tests), and the results obtained indicated that the acceptance criteria described in the 1999 version of method 1623 for Cryptosporidium (RSD, 40%) and Giardia (RSD, 41%) was met in using both the standard Envirochek and HV filters. Conversely, the modified ICR method with the polypropylene yarn-wound cartridge filter did not generate acceptable recovery rates. Despite the latter results, further analyses were carried out with both methods to evaluate filtration performance and method reproducibility in samples of tap and reclaimed water.
(Oo)cyst recovery efficiency was the key parameter used in this study to assess filtration performance and method reproducibility with the two water matrices. The results indicated that the Envirochek HV capsule filter generated significantly higher recoveries of spiked and indigenous (oo)cysts than did the standard Envirochek and polypropylene yarn-wound cartridge filter. Oocyst recovery rates in reclaimed water varied widely (1 to 84% for Cryptosporidium; 1 to 68% for Giardia) with the HV filter. Relatively less variation in recovery rates was observed with the standard Envirochek and the polypropylene cartridge filter; however, the concentration of (oo)cysts detected with these two filters was consistently lower than with the HV filter. It is noteworthy since the data shown in Table 5 indicate that Cryptosporidium IFA counts between 26 and 152 oocysts produced foci in HCT-8 cells, which corresponded to the numbers of oocysts that produced positive PCR amplification by nested PCR. These oocyst counts occurred in 50% of the IMS concentrate; the remaining 50% was further divided into two aliquots and was used for nested PCR and tissue cell culture. Since 25% of the IMS concentrate obtained from these samples could potentially hold between 5 and 10 oocysts, the results of this study would suggest that a minimum of DNA equivalent to approximately 5 to 10 oocysts is required for molecular characterization of Cryptosporidium isolates and assessment of oocyst infectious potential with tissue cell culture. The concentration of oocysts detected with the standard Envirochek and polypropylene cartridge filter never exceeded 30 oocysts. Therefore, the information on Cryptosporidium in reclaimed effluents provided with the standard Envirochek filter and polypropylene cartridge filter may be limited to the IFA.
Water quality parameters in the final effluent such as turbidity, total suspended solids, pH, and ammonia-nitrogen (NH3-N) determined at the water reclamation facilities were not associated with variations in (oo)cyst recoveries. However, a significant correlation was found between the level of indigenous Cryptosporidium oocysts and carbonaceous biological oxygen demand concentrations (r = 0.84; P < 0.0001). Further research is required to understand more about the significance of this relationship. Carbonaceous biological oxygen demand is the amount of oxygen required to oxidize any organic matter present in the water biochemically; it is an indirect indication of the concentration of organic contamination in the water (17).
The use of the appropriate filtration system is critical in the analysis of waterborne protozoan pathogens, since it is the initial step involved in the concentration of the organisms. The Envirochek HV capsule filter has been demonstrated to perform better than the standard Envirochek filter in natural waters (6). The results of the present study indicated that the filtration capacity of the HV filter was relatively higher than with the standard Envirochek filter; however, oocyst recovery rates were not significantly different for method 1623 with either Envirochek capsule filter. Water matrix components associated with samples of reclaimed water might account for inconsistencies in method reproducibility (low and variable oocyst recoveries) observed in the present study. DiGiorgio et al. (6) reported that the filtration capacity of the HV filter can be affected by high turbidities (88 to 99 NTUs) and water matrix components that can lead to low and variable (oo)cyst recovery rates. Turbidity of the reclaimed water samples analyzed in this study never exceeded 1.75 NTUs. Further research is needed to determine the matrix components of treated wastewater that affect the recovery efficiency of (oo)cysts in order to provide better methods for assessment of water quality used for unrestricted irrigation.
Limitations inherent to the ICR method have been previously reported (9, 13). In the present study, IMS was used as the oocyst purification step in the modified version of the ICR method. IMS allows for more efficient, significant, and reproducible recoveries than Percoll-sucrose flotation (5, 8, 15). The recovery rates in reclaimed water matrices were low and variable with the polypropylene cartridge filter, thereby indicating that previous concentration steps (filtration, elution, and centrifugation) have a much higher impact in oocyst losses than downstream steps (purification and IFA).
Several modifications and alternatives to method 1623 and the ICR method have been proposed in an attempt to optimize the initial concentration process involving filtration. For example, Simmons et al. (19) reported 42 to 46% recovery for Cryptosporidium oocysts when they used a disposable polysulfone hollow-fiber ultrafilter for concentrating oocysts from seeded surface and reagent water. Kuhn and Oshima (12) reported mean oocyst recoveries of 55% with hollow-fiber ultrafiltration for concentrating oocysts from seeded tap and surface water of various turbidities. The results of collaborative trials of (oo)cyst recovery from source water with a modified Filta-Max elution and concentration technique (14) generated recoveries of Cryptosporidium oocysts that ranged from 12.4 to 36.5%, while Giardia cyst recovery percentages ranged from 22.7 to 68.3%. An alternative collection method for the recovery of Cryptosporidium oocysts from large volumes of water involves continuous-flow centrifugation. Continuous-flow centrifugation concentrates particles by size and weight; and recovery of oocyst averages 14% (21). Process time is short, but this intensive equipment-based technique is not field applicable.
Although the results of previous studies seems promising, neither of the filters mentioned above has been tested in reclaimed water matrices. The recovery efficiencies of (oo)cysts reported in the present investigation for method 1623 with the HV filter may be highly variable; however, the method with additional modifications still provides information on the occurrence of infectious oocysts that can be further characterized by molecular techniques to the genotype level.
The inclusion of prestained (oo)cysts (ColorSeed) as an internal quality control enabled the assessment of method performance at the point and time of sample collection, which is important to evaluate not only ongoing method and laboratory performance but also to identify methodology deficiencies that can occur when evaluating protozoan pathogens for monitoring compliance. The latter has also important implications in the establishment of numeric pathogen standards for reclaimed water.
The present study demonstrates the presence of viable and infectious Cryptosporidium oocysts in the reclaimed effluents tested. The FDM-MPN assay was used to provide quantitative information of infectious oocysts, which is important to determine public health risks. The percentage of samples positive for infectious oocysts was 50% (6 of 12), and the numbers detected were below the numeric pathogen standard (maximum limit of 22 viable oocysts/100 liters) proposed for reclaimed effluents by York and Walker-Coleman (27). Notwithstanding, the recovery efficiency data indicated that these concentrations might be underestimations, since the method still is limited by unknown matrix components present in reclaimed water matrices.
Previous investigations have shown that the FDM-MPN method is an excellent and reproducible assay for quantifying oocyst infectivity in vitro (20). The results of this and a previous study (11) demonstrate the sensitivity of the cell culture infectivity assay for determining low numbers of infectious oocysts in reclaimed effluents. The infectivity measured in HCT-8 cell cultures, however, is prone to variability, and the percent oocyst infectivity in our laboratory can range between 0.3 and 40%. Among others, variations in infectivity within the same Cryptosporidium genotype have been reported elsewhere (4, 8, 18, 20). Therefore, one expects to find that the Cryptosporidium isolates obtained from reclaimed effluents may show the same degree of variability in infectivity. Rochelle et al. (18) demonstrated that incubation of oocysts in 0.75% sodium taurocholate prior to inoculation of cell monolayers resulted in increased infectivity in cell cultures. The application of this approach to maximize the sensitivity of the assay in order to determine potential infectivity of Cryptosporidium isolates from reclaimed effluents needs further research. Notwithstanding and despite the variations in recovery efficiency and infectivity in cell culture, the results of this investigation revealed the potential application of advanced Cryptosporidium detection methods required for the establishment of a more useful risk assessment approach for reclaimed water.
Acknowledgments
This work was supported partially by Tampa Bay Water.
We thank the staff of the different water reclamation facilities for their logistic support and Debra E. Huffman of the College of Marine Science at University of South Florida and George D. Di Giovanni of the Agricultural Research and Extension Center at the Texas A&M University System for providing technical information applicable to the cell culture and molecular assays.
REFERENCES
- 1.Anguish, L. J., and W. C. Ghiorse. 1997. Computer-assisted laser scanning and video microscopy for analysis of Cryptosporidium parvum oocysts in soil, sediment, and feces. Appl. Environ. Microbiol. 63:724-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asano, T., and A. D. Levine. 1996. Wastewater reclamation, recycling and reuse: past, present, and future. Water Sci. Technol. 33:1-14. [Google Scholar]
- 3.Campbell, A. T., L. J. Robertson, and H. V. Smith. 1992. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl. Environ. Microbiol. 58:3488-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapell, C. L., M. M. Marshall, G. Widmer, P. C. Okhuysen, and C. R. Sterling. 1999. Cryptosporidium parvum (genotype 2) isolates vary in their capacity to infect cultured enterocytes and animal models, p. 1-5. In Proceedings of the International Symposium on Waterborne Pathogens. American Water Works Association, Milwaukee, Wis.
- 5.Connell, K., C. C. Rodgers, H. L. Shank-Givens, J. Sheller, M. L. Pope, K., and K. Miller. 2000. Building a better protozoa data set. J. Am. Water Works Assoc. 92:30-43. [Google Scholar]
- 6.DiGiorgio, C. L., D. A. Gonzalez, and C. C. Huitt. 2002. Cryptosporidium and Giardia recoveries in natural waters by using Environmental Protection Agency method 1623. Appl. Environ. Microbiol. 68:5952-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Giovanni, G. D., M. R. Karim, M. W. LeChevalier, J. R. Weihe, F. A. Abrams, M. L. Spinner, S. N. Boutros, and J. S. Chandler. 2002. Overcoming molecular sample processing limitations: quantitative PCR. Publication 00-HHE-2b. Water Environment Research Foundation, Alexandria, Va.
- 8.Di Giovanni, G. D., F. H. Hashemi, N. J. Shaw, F. A. Abrahams, M. W. LeChevalier, and M. Abbaszadegan. 1999. Detection of infectious Cryptosporidium parvum oocysts in surface and filter backwash water samples by immunomagnetic separation and integrated cell culture-PCR. Appl. Environ. Microbiol. 65:3427-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dufour, A. P., M. Feige, A. H. D. Linquist, M. Messner, S. Regli, C. Rodgers, F. W. Schaefer, S. Schaub, J. Sinclair, and L. J. Wymer. 1999. Criteria for evaluation of proposed protozoan detection methods. EPA 815-K-99-02. Office of Research and Development, U.S. Environmental Protection Agency, Cincinnati, Ohio.
- 10.Florida Department of Environmental Protection. 1999. Reuse of reclaimed water and land application. Chapter 62-610, Florida Administrative Code. Florida Department of Environmental Protection, Tallahassee, Fla.
- 11.Gennaccaro, A. L., M. R. McLaughlin, W. Quintero-Betancourt, D. E. Huffman, and J. B. Rose. 2003. Infectious Cryptosporidium parvum oocysts in final reclaimed effluents. Appl. Environ. Microbiol. 69:4983-4984. [DOI] [PMC free article] [PubMed]
- 12.Kuhn, R. C., and K. H. Oshima. 2002. Hollow-fiber ultrafiltration of Cryptosporidium parvum oocysts from a wide variety of 10-L water samples. Can. J. Microbiol. 48:542-549. [DOI] [PubMed] [Google Scholar]
- 13.LeChevalier, M. W., W. D. Norton, J. E. Siegel, and M. Abbaszadegan. 1995. Evaluation of the immunofluorescence procedure for detection of Giardia cysts and Cryptosporidium oocysts in water. Appl. Environ. Microbiol. 61:690-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCuin, R. M., and J. L. Clancy. 2003. Modifications to United States Environmental Protection Agency methods 1622 and 1623 for detection of Cryptosporidium oocysts and Giardia cysts in water. Appl. Environ. Microbiol. 69:267-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCuin, R. M., Z. Bukhari, J. Sobrinho, and J. L Clancy. 2001. Recovery of Cryptosporidium oocysts and Giardia cysts from source water concentrates using immunomagnetic separation. J. Microbiol. Methods 45:69-76. [DOI] [PubMed] [Google Scholar]
- 16.Mujeriego, R., and T. Asano. 1999. The role of advanced treatment in wastewater reclamation and reuse. Water Sci. Technol. 4-5:1-9. [Google Scholar]
- 17.Ray, B. T. (ed.). 1995. Environmental engineering, p. 221-261. PWS Publishing Co., Boston, Mass.
- 18.Rochelle, P. A., M. M. Marshall, J. R. Mead, A. M. Johnson, D. G. Korich, J. S. Rosen, and R. De Leon. 2002. Comparison of in vitro cell culture and a mouse model assay for measuring infectivity of Cryptosporidium parvum. Appl. Environ. Microbiol. 68:3809-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simmons, O. D., III, M. D. Sobsey, C. D. Heany, F. W. Schaefer, and D. S. Francy. 2001. Concentration and detection of Cryptosporidium oocysts in surface water samples by method 1622 using ultrafiltration and capsule filtration. Appl. Environ. Microbiol. 67:1123-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slifko, T. R., D. R. Huffman, and J. B. Rose. 1999. A most-probable-number assay for enumeration of infectious Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 65:3936-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swales, C., and S. Wright. 2000. Evaluation of a continuous flow centrifuge for recovery of Cryptosporidium oocysts from large volume water samples. Water Res. 34:1962-1966. [Google Scholar]
- 22.U.S. Environmental Protection Agency. 1999. Method 1623: Cryptosporidium and Giardia in water by filtration/IMS/FA. EPA/821/R-99/006. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 23.U.S. Environmental Protection Agency. 1995. ICR protozoan method for detecting Giardia cysts and Cryptosporidium oocysts in water by a fluorescent antibody procedure. EPA/814-B-95-003. U.S. Environmental Protection Agency, Cincinnati, Ohio.
- 24.Xiao, L., U. M. Morgan, J. Limor, A. Escalante, M. Arrowood, W. Shulaw, R. C. A. Thompson, R. Fayer, and A. A. Lal. 1999. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl. Environ. Microbiol. 65:3386-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao, L., K. Alderisio, J. Limor, M. Royer, and A. Lal. 2000. Identification of species and sources of Cryptosporidium oocysts in storm waters with a small-subunit rRNA-based diagnostic and genotyping tool. Appl. Environ. Microbiol. 66:5492-5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao, L., A. Singh, J. Limor, T. K. Graczyk, S. Gradus, and A. Lal. 2001. Molecular characterization of Cryptosporidium oocysts in samples of raw surface water and wastewater. Appl. Environ. Microbiol. 67:1097-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.York, D., and L. Walker-Coleman. 2000. Pathogen standards for reclaimed water. Water Environ. Technol. 12:58-61. [Google Scholar]