Abstract
Hydroxycinnamates are plant products catabolized through the diphenol protocatechuate in the naturally transformable bacterium Acinetobacter sp. strain ADP1. Genes for protocatechuate catabolism are central to the dca-pca-qui-pob-hca chromosomal island, for which gene designations corresponding to catabolic function are dca (dicarboxylic acid), pca (protocatechuate), qui (quinate), pob (p-hydroxybenzoate), and hca (hydroxycinnamate). Acinetobacter hcaC had been cloned and shown to encode a hydroxycinnamate:coenzyme A (CoA) SH ligase that acts upon caffeate, p-coumarate, and ferulate, but genes for conversion of hydroxycinnamoyl-CoA to protocatechuate had not been characterized. In this investigation, DNA from pobS to an XbaI site 5.3 kb beyond hcaC was captured in the plasmid pZR8200 by a strategy that involved in vivo integration of a cloning vector near the hca region of the chromosome. pZR8200 enabled Escherichia coli to convert p-coumarate to protocatechuate in vivo. Sequence analysis of the newly cloned DNA identified five open reading frames designated hcaA, hcaB, hcaK, hcaR, and ORF1. An Acinetobacter strain with a knockout of HcaA, a homolog of hydroxycinnamoyl-CoA hydratase/lyases, was unable to grow at the expense of hydroxycinnamates, whereas a strain mutated in HcaB, homologous to aldehyde dehydrogenases, grew poorly with ferulate and caffeate but well with p-coumarate. A chromosomal fusion of lacZ to the hcaE gene was used to monitor expression of the hcaABCDE promoter. LacZ was induced over 100-fold by growth in the presence of caffeate, p-coumarate, or ferulate. The protein deduced to be encoded by hcaR shares 28% identity with the aligned E. coli repressor, MarR. A knockout of hcaR produced a constitutive phenotype, as assessed in the hcaE::lacZ-Kmr genetic background, revealing HcaR to be a repressor as well. Expression of hcaE::lacZ in strains with knockouts in hcaA, hcaB, or hcaC revealed unambiguously that hydroxycinnamoyl-CoA thioesters relieve repression of the hcaABCDE genes by HcaR.
Synthesized by plants and comprising an aromatic ring with a propenoate unit at the 1 position, hydroxycinnamates play diverse, critical roles in plant architecture and defense. They are present as structural components of cell walls, of the protective matrix suberin, and of lignin, they contribute to allelopathic interactions among plants, and they are precursors in the synthesis of flavonoids. Nutritional opportunities presented by hydroxycinnamates contribute to bacterial diversity. Particular interest has been directed to pathways of hydroxycinnamate dissimilation that generate aldehyde intermediates, such as vanillin, with biotechnological potential.
Acinetobacter sp. strain ADP1 is capable of utilizing the hydroxycinnamates ferulate, p-coumarate, and caffeate as sole sources of carbon and energy. The strain, derived from strain BD413, which was isolated by Elliot Juni (29), is remarkable in its capacity for high-efficiency natural transformation by DNA (36). The chromosome of strain ADP1 contains an “island of catabolic diversity” which encodes many catabolic enzymes and related proteins. Central to this genetic island are the pca genes, essential for catabolism of the diphenolic compound protocatechuate, along with the linked qui (quinate/shikimate) genes and adjacent pob (p-hydroxybenzoate) genes. Dissimilation of both quinate and p-hydroxybenzoate occurs via protocatechuate. At the other end of the cluster, dca genes for catabolism of adipate and other straight-chain, saturated dicarboxylic acids have been identified (45) (Fig. 1A).
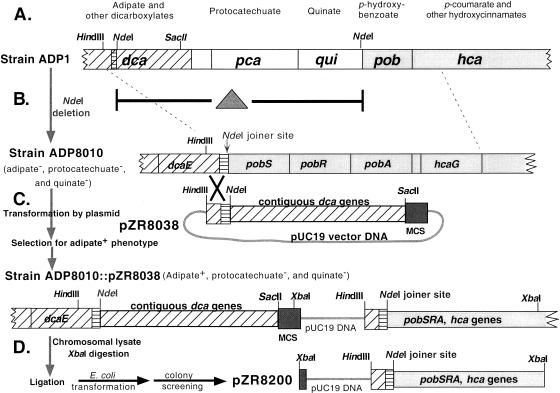
FIG. 1.
Physical arrangement of the dca-pca-qui-pob-hca genetic cluster of Acinetobacter sp. strain ADP1 and the strategy used to capture the hca genes. (A) Organization of the genetic regions surrounding the pca gene cluster in the wild-type strain. (B) Introduction of an NdeI deletion that removed DNA from an intergenic dca region up to the pobS gene to form strain ADP8010, defective in adipate, protocatechuate, and quinate catabolism. The small segment of DNA on the left of some NdeI sites (▤) is part of a dca intergenic region. (C) Transformation of strain ADP8010 by pZR8038, followed by selection on adipate, yielding heterogenotes in which a single crossover restored the ability of cells to utilize adipate. MCS, multiple cloning site. (D) Digestion of a lysate of the heterogenote ADP8010::pZR8038 with XbaI followed by ligation and transformation of E. coli, yielding plasmid pZR8200, which was found to have a 16.5-kb insert containing all of the pob and hca genes.
Interest in discovering the extent of the pca-centered catabolic cluster led to the sequencing of plasmid pZR9500, which contains pobA and flanking DNA from strain ADP1. Genetic evidence revealed that downstream of pobA and transcribed in the opposite orientation lie genes for enzymes of hydroxycinnamate metabolism that route substrates through degradative pathways to protocatechuate (55). In addition to hcaG, encoding an esterase that breaks down chlorogenate to produce quinate and caffeate, the stretch beyond the pobA gene contains the hcaD gene encoding an acyl-coenzyme A (acyl-CoA) dehydrogenase homolog that enables ADP1 cells to generate hydroxycinnamoyl-CoA thioesters from corresponding compounds with a saturated propanoate side chain. Upstream of hcaD is hcaC, which encodes a hydroxycinnamate:CoASH ligase for the initial step in dissimilation of caffeate, p-coumarate, and ferulate. At the distal end of the insert in pZR9500 is a partial open reading frame (ORF) with similarity to aldehyde dehydrogenase genes (55) (Fig. 2).
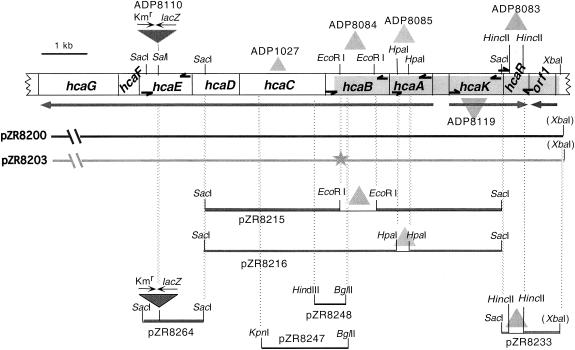
FIG. 2.
Physical map of the hca genes showing mutations of strains and some of the subclones used in strain construction or characterization. Shading indicates the newly captured hca DNA present in pZR8200. Acinetobacter hca DNA on plasmid pZR9500, characterized in a previous study (55), ended near the 3′ terminus of hcaB, where the shading begins. The star on pZR8203 marks the location of a frameshift mutation. Arrows denote direction of transcription but are not necessarily transcriptional units, triangles mark the sites of deletion mutations, and half-arrows mark the sites of primers used to verify mutations.
Evidence from overflow metabolites in another Acinetobacter strain led to proposed pathways for the catabolism of p-coumarate via p-hydroxybenzoate and of ferulate via vanillate (13). Supporting evidence for similar metabolic routes has come from work with bacteria of other genera. Some or all of the genes involved in the breakdown of ferulate to vanillate have been characterized from Pseudomonas sp. strain HR199 (41, 48), Pseudomonas putida WCS358 (59), Pseudomonas fluorescens AN103 (20), Amycolatopsis sp. strain HR167 (1), and Sphingomonas paucimobilis SYK6 (34).
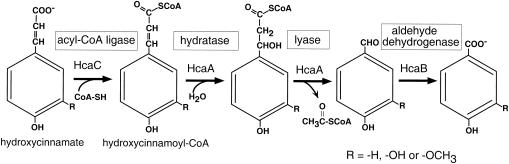
Gasson et al. discovered that conversion of feruloyl-CoA to vanillin in P. fluorescens was catalyzed by an unusual, bifunctional enzyme, an enoyl-CoA hydratase/lyase (20). In spite of the potential diversity of pathways, the same type of hydratase/lyase reaction was found to mediate ferulate to vanillin conversion in Pseudomonas sp. strain HR199 (41), the gram-positive bacterium Amycolatopsis sp. strain HR167 (1), and S. paucimobilis (34). The pathway for hydroxycinnamate dissimilation common to these diverse microbes is shown in Fig. 3.
FIG. 3.
Pathway predicted to transform the hydroxycinnamates p-coumarate, caffeate, and ferulate to the respective intermediates p-hydroxybenzoate, protocatechuate, and vanillate in Acinetobacter sp. strain ADP1. Homologs of HcaA, shown as two reaction products, have been shown to be bifunctional enzymes that participate in the metabolism of ferulate by other organisms, as noted in the text.
The Acinetobacter DNA insert in pZR9500 did not contain all of the genes required for conversion of hydroxycinnamates to protocatechuate. This communication describes the capture of the rest of the hca genes via endogenous vector integration, the newly cloned hca genes, the mechanism of regulatory control over them, and the structure of the inducer responsible for triggering expression of the hca genes.
MATERIALS AND METHODS
Strains, media, and growth of cells.
Table 1 presents strains and plasmids used in this study. Cells were cultured in Luria-Bertani medium (54) or minimal medium (46). Sigma Chemical Co. and Aldrich Chemical Co. were the sources of hydroxy-trans-cinnamates and other growth substrates. Solidified minimal medium contained succinate at 10 mM or p-coumarate or ferulate at 2 mM. Comparative growth yields of Acinetobacter strains in liquid medium were carried out with stock solutions of substrates prepared at a concentration of 1.0 M in dimethyl sulfoxide, and hydroxycinnamates were provided in doses of 2 mM.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Genotype | Reference or source |
|---|---|---|---|
| E. coli DH5α | F−φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mK−) supE44 λ−thi-1 gyrA96 relA1 | Invitrogen | |
| Acinetobacter sp. strains | |||
| ADP1 | Wild-type strain (also known as BD413) | 29 | |
| ADP992 | 90-bp deletion in pobA; block in p-hydroxybenzoate hydroxylase | ΔpobA992 | D. A. D'Argenio, unpublished data |
| ADP1027 | 100-bp deletion in hcaC; block in hydroxycinnamate: CoASH ligase | ΔhcaC1 | 55 |
| ADP8010 | 25.8-kb NdeI deletion from pZR8033 with endpoints in a dca intergenic region (between dcaECHF and dcaAKIJPQ) and the pobS gene; genes deleted include those required for dicarboxylate, protocatechuate, and quinate catabolism | Δ(dca-quiA)1 | This study |
| ADP8078 | Kmr; Δ (hcaR orf1)::sacB-Kmr from pZR8231 introduced into the ADP1 chromosome | Δ(hcaR-orf1)::sacB-Kmr | This study |
| ADP8079 | Kmr; Δ(hcaKAB)::sacB-Kmr from pZR8227 introduced into the ADP1 chromosome | Δ(hcaKAB)1::sacB-Kmr | This study |
| ADP8081 | Kmr; ΔhcaB1::lacZ-Kmr from pZR8238 in the chromosome of ADP1 | ΔhcaB1::lacZ-Kmr | This study |
| ADP8083 | 217-bp HincII deletion in hcaR from pZR8233 in the ADP8078 chromosome | ΔhcaR1 | This study |
| ADP8084 | 600-bp EcoRI deletion in hcaB from pZR8215 in the chromosome of ADP8079 | ΔhcaB3 | This study |
| ADP8085 | 163-bp HpaI deletion in hcaA from pZR8216 in the chromosome of ADP8079 | ΔhcaA1 | This study |
| ADP8088 | Kmr; Δ(hcaR-orf1)::sacB-Kmr from pZR8231 in the chromosome of ADP8083 | ΔhcaR1 ΔhcaB1::lacZ-Kmr | This study |
| ADP8101 | Kmr; Δ(hcaRorf1)::sacB-Kmr from pZR8246 in the chromosome of ADP1027 | ΔhcaC1 Δ(hcaR-orf1)::sacB-Kmr | This study |
| ADP8102 | Kmr; hcaK::sacB-Kmr from pZR8246 in ADPI background | hcaK1::sac-Kmr | This study |
| ADP8110 | Kmr; hcaE::lacZ-Kmr from pZR8264 in the chromosome of ADP1 | hcaE1::lacZ-Kmr | This study |
| ADP8113 | Kmr; hcaE::lacZ-Kmr from pZR8264 in the chromosome of ADP1027 | ΔhcaC1 hcaE1::lacZ-Kmr | This study |
| ADP8114 | Kmr; hcaE::lacZ-Kmr from pZR8264 in the chromosome of ADP8085 | ΔhcaA1 hcaE1::lacZ-Kmr | This study |
| ADP8116 | Kmr; hcaE::lacZ-Kmr from pZR8264 in the chromosome of ADP8084 | ΔhcaB3 hcaE1::lacZ-Kmr | This study |
| ADP8117 | Kmr; hcaE::lacZ-Kmr from pZR8264 in the chromosome of ADP8083 | ΔhcaR1 hcaE1::lacZ-Kmr | This study |
| ADP8119 | Kmr; ΔhcaK from pZR8271 in the chromosome of ADP8102 | ΔhcaK3 | This study |
| ADP8121 | ΔhcaR1 from pZR8233 in the chromosome of ADP8101 | ΔhcaC1 ΔhcaR1 | This study |
| ADP8135 | Δ(hcaRKABCDEFG) from pZR8207 in the chromosome of ADP8102; hydroxycinnamate negative | Δ(hcaRKABCDEFG)1 | This study |
| ADP8136 | Kmr; hcaE::lacZ-Kmr from pZR8264 in the chromosome of ADP8121 | ΔhcaC1 ΔhcaR1 hcaE1::lacZ-Kmr | This study |
| Plasmids | |||
| pBKS | Apr; narrow host range cloning vector | Stratagene | |
| pBKSΔ1 | Ap; pBKS which is ΔSmaI-HincII | This study | |
| pKOK6 | Apr Kmr Tcr; contains 4.6-kb promoterless lacZ-Kmr cassette for constructing operon fusions | 31 | |
| pRMJ1 | Apr Kmr; contains sacB-Kmr cassette for genetic replacement by positive selection | 27 | |
| pRK415 | Tcr; broad-host-range vector | 30 | |
| pUC4K | Apr Kmr; contains antibiotic resistance cassette | Amersham Pharmacia Biotech | |
| pUC18 and pUC19 | Apr; narrow-host-range cloning vectors | 61 | |
| pZR8000 | Chlr; 34.8-kb Acinetobacter sp. strain ADP1 SacI insert in pBBR1MCS containing dca, pca, qui, and pob genes | 45 | |
| pZR8033 | Chlr; 25.8-kb NdeI deletion in pZR8000 | This study | |
| pZR8038 | Apr; 8.9-kb HindIII-SacII insert of dca genes from pZR8000 in pUC19 | 45 | |
| pZR8200 | Apr; 16.5-kb HindIII-XbaI insert of DNA from strain ADP8010::pZR8038, which encompasses 1-kb of dca DNA, the pob and hca genes; in pUC19 | This study | |
| pZR8203 | Apr; pZR8203 with a mutation introduced at the unique XcmI site in hcaB | This study | |
| pZR8207 | ΔhcaRKABCDEFG of pZR8200 created by exonuclease activity of Klenow fragment | This study | |
| pZR8210 | Apr; 6.5-kb SacI insert with hca genes from pZR8200 in pBKSΔ1 | This study | |
| pZR8215 | Apr; 600-bp EcoRI deletion of hcaB in pZR8210 | This study | |
| pZR8216 | Apr; 163-bp HpaI deletion of hcaA in pZR8210 | This study | |
| pZR8219 | Apr; 1.66-kb SacI subclone of pZR8200 in pBKSΔ1 | This study/PICK> | |
| pZR8220 | Apr; 2.4-kb PstI deletion from hcaB to hcaK in pZR8210 | This study | |
| pZR8224 | Apr; 1.6-kb SacI subclone of pZR8200 containing hcaR and ORF1 in pBKSΔ1 | This study | |
| pZR8227 | Apr; Kmr; sacB-Kmr cassette of pRMJ1 inserted at the site of the PstI deletion in pZR8220 | This study | |
| pZR8231 | Apr; sacB-Kmr cassette of pRMJ1 inserted at the site of a 0.63-kb BglII deletion in pZR8224 | This study | |
| pZR8233 | Apr; 217-bp HincII deletion within hcaR in pZR8224 | This study | |
| pZR8238 | TcrKmr; lacZ-Kmr cassette from pKOK6 in the ΔBglII site of hcaB in pZR8223; lacZ expression is in the same direction as that of hcaB | This study | |
| pZR8239 | Tcr; 1.2-kb HindIII-SacI insert of hcaK′ in pRK415 | This study | |
| pZR8240 | Tcr; 3.3-kb SacI-BglII insert of hcaC plus the 3′ part of hcaB | This study | |
| pZR8246 | Tcr Kmr; pRMJ1 sacB-Kmr cassette in PstI site of hcaK in pZR8239 | This study | |
| pZR8247 | Apr; 2.1-kb KpnI-BglII subclone of pZR8240 in pUC18 | This study | |
| pZR8248 | Apr; 0.95-kb HindIII-BglII subclone of pZR8240 which includes 268 bp of hcaC and part of hcaB in pUC18 | This study | |
| pZR8264 | Apr Kmr; lacZ-Kmr cassette from pKOK6 in the SalI site of hcaE in pZR8219; lacZ expression is in the same direction as that of hcaE | This study | |
| pZR8265 | Apr; 1.2-kb HindIII-SacI insert of pZR8239 in pUC18 | This study | |
| pZR8271 | Apr; 775-bp BsrGI deletion within hcaK in pZR8265 | This study |
Luria-Bertani medium contained kanamycin and ampicillin concentrations of 15 μg ml−1 and 100 μg ml−1, respectively, for the screening of Acinetobacter cells for antibiotic resistance markers. Because of natural low-level resistance of ADP1 cells to ampicillin, it was necessary to streak to isolated single colonies to screen for ampicillin resistance. Selection against cells that carried a sacB-Kmr cassette (27) was applied on Luria-Bertani medium containing 5% sucrose. When Escherichia coli cells were under selection, Luria-Bertani medium was supplemented with kanamycin or ampicillin at 25 or 100 μg ml−1, respectively.
Growth was monitored by measuring turbidity as cultures were incubated at 37°C and 250 rpm. To determine growth rate constants, succinate-grown inocula in late exponential phase were diluted 20-fold into 10 ml of medium in 50 ml Erlenmeyer flasks. For comparison of lag times, succinate-grown inocula were from overnight stationary-phase cultures.
General methods of strain and plasmid construction.
Molecular biology manipulations followed standard techniques (5, 51). Natural transformation of Acinetobacter strains was accomplished by published methods (28). Crude lysates were prepared by resuspension of pelleted cells in 500 μl of lysis buffer which contains 0.15 M NaCl, 0.015 M Na+ citrate, and 0.05% sodium dodecyl sulfate (28), followed by incubation at 60°C for 1 h. Lysates of cells for PCRs were derived from 1 ml of a succinate overnight culture. The ability of mutant strains of ADP1 to take up DNA carried on plasmids in E. coli by replica plating has been described (6, 45).
Enzyme assays.
LacZ assays were conducted according to the method of Miller (37). Acinetobacter cells containing an hcaE::lacZ-Kmr mutation were grown overnight in minimal medium containing 10 mM succinate, 100 μl was inoculated into 5 ml of fresh succinate medium with or without an aromatic compound, and cells were grown at 250 rpm and 37°C for 4.5 h. Cultures were harvested as previously described (44). In the special case of ADP8110 cells grown at the expense of hydroxycinnamates in the absence of succinate, cells were grown under similar conditions, but the inocula, cells in late exponential phase of growth on succinate, were diluted only 10-fold. The latter cells were grown at the expense of 4 mM caffeate, p-coumarate, or ferulate, each provided in separate doses of 2 mM.
Cloning the DNA upstream of hcaC.
Figure 1 outlines the strategy used to clone DNA upstream of the hcaC gene. Plasmid pZR8000 (45) contains a large insert spanning dca, pca, qui, and pob genes. A 25.8-kb NdeI deletion in pZR8000 removed part of an intergenic region between two divergently transcribed dca operons, as well as all intervening DNA up to the pobS gene (Fig. 1B). Introduction of the NdeI deletion into strain ADP1 created strain ADP8010, which was unable to utilize protocatechuate, quinate, or the dicarboxylate adipate.
Plasmid pZR8038 contains an insert of Acinetobacter dca DNA, which includes 1 kb of DNA downstream from the dca intergenic NdeI site; the 1-kb region is the only Acinetobacter DNA segment in the insert homologous to the chromosome of ΔNdeI strain ADP8010. Cells of ADP8010 were transformed with pZR8038, and 200 μl of the transformant culture was inoculated into 5 ml of liquid minimal medium supplemented with 5 mM adipate. The rationale for the adipate selection is that, theoretically, growth of ADP8010 on adipate can be accomplished only by integration of pZR8038 into the chromosome by means of a single crossover at the 1-kb homologous region of DNA, as shown in Fig. 1C. After overnight incubation, the cells were centrifuged and resuspended in lysis buffer, and a lysate was prepared. Digestion of the chromosomal lysate with XbaI was followed by precipitation, threefold dilution, heat inactivation, ligation, concentration, and transformation of E. coli DH5α cells chemically prepared to be highly competent (24).
Colonies that appeared on the vector-specific antibiotic plates were replica plated onto additional plates for further testing. They were screened for the presence of the pobA gene by transformation of the Acinetobacter pobA mutant strain ADP992. Their ability to convert p-coumarate to protocatechuate was screened by replica plating colonies onto Luria-Bertani antibiotic medium containing 1 mM p-coumarate as well as ferric chloride and p-toluidine, which is chromogenic for diphenolics (43).
Construction of hca mutant strains of ADP1.
Mutations in hca genes were designed as deletions in order to minimize the possibility of downstream transcription termination effects which were noted with the pUC4K Kmr marker in dca genes (45), to allow selection for a lacZ-Kmr cassette, and to facilitate the eventual construction of multiply mutated strains. Strains carrying a sacB cassette fail to grow in the presence of 5% sucrose, and deletion strains were constructed by replacement of the sacB-Kmr cassette of pRMJ1 (27) in the targeted site of recipient strains as summarized in Table 1. It was necessary to do the sacB selection at room temperature (27), and colonies that grew in the presence of 5% sucrose were screened for the absence of the Kmr marker present in the cassette as well as absence of the vector antibiotic resistance marker.
Table 1 details the construction of strains used in this investigation. Strain ADP8083 (ΔhcaR1) was created by transformation of the competent sacB-Kmr strain ADP8078 with plasmid pZR8233 (Table 1; Fig. 2). Strains ADP8084 (ΔhcaB3) and ADP8085 (ΔhcaA1) were created by transformation of competent ADP8079 cells with plasmids pZR8215 and pZR8216, respectively (Table 1; Fig. 2). Strains ADP8110, ADP8113, ADP8114, ADP8116, and ADP8117 contain hcaE::lacZ-Kmr in different genetic backgrounds, as described in Table 1. Insertion and deletion mutations in the Acinetobacter chromosome were confirmed by PCR (Fig. 2).
PCRs.
PCR products were produced by using dilute chromosomal lysates as templates. The usual PCR conditions were 94°C for 3 min, followed by 30 cycles of denaturing at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for an appropriate length of time. Primer sets (as possible, noted in Fig. 2) were as follows: MGZR80T38 and MGZR80F7 for ΔNdeI in ADP8010; DPZR82R7 and DPZR82F2 for analysis of ΔhcaR1 in ADP8083 and ADP8136; PPABR1 and DPZR82F8 for ΔhcaB3 in ADP8084; DPZR82R3 and DPZR82F6 for ΔhcaA1 in ADP8085; DPZR82R5 and DPZR82F3 for ΔhcaK3 in ADP8119; and VD902R3 and MS902F1 for hcaE::lacZ-Kmr in ADP8110, ADP8113, ADP8114, ADP8116, ADP8117, and ADP8136. Oligonucleotide sequences of these primers are as follows: MGZR80T38, 5′-ATAGTAGATTGCTATAGCGAAATATAGAGA-3′; MGZR80F7, 5′-CCTGCTTGTAATCCAGTGAGATGA-3′; DPZR82R7, 5′TCAGAAAGCCCAAACACCCTGTA-3′; DPZR82F2, 5′-TTCTTCACCATTAATTCAAGGTTATTAC-3′; PPABR1, 5′-TGCTTCATCGTGTACGGTTGCACC-3′; DPZR82F8, 5′-CAAAATACCGAAAAGTTTATCGAAATC-3′; DPZR82R3, 5′-TCTTCATTCTGATCCCATGTCAGCT-3′; DPZR82F6, 5′-GGGTCACACTTAATCGTCCTCACAA-3′; DPZR82R5, 5′-GAATGCGTGCCGAATTTATGTTAGA-3′; DPZR82F3, 5′-ACAGGGTGTTTGGGCTTTCTGAC-3′; VD902R3, 5′-CGATGAGCAGTCCTGTTTCACC-3′; and MS902F1, 5′-GAAGGTGTTGAAAAAGGACGTTCG-3′.
DNA sequencing.
The region of pZR8200 that contained newly cloned Acinetobacter DNA (Fig. 2) was sequenced by primer walking at the Yale Keck Biotechnology Resource Lab. Standard ABI PRISM terminator cycle sequencing with AmpliTaq DNA polymerase was used.
Nucleotide sequence accession number.
Accession no. L05770 in the GenBank database contains the DNA sequence for the hca genes from Acinetobacter sp. strain ADP1 up to an XbaI site.
RESULTS
Isolation of clone pZR8200.
Two factors influenced the vector integration cloning strategy for Acinetobacter. The fact that commonly used plasmids with the ColE1 origin of replication are able to replicate in ADP1 (22) is a disadvantage when the goal is to create heterogenotes. A second consideration is that most of the Acinetobacter strains used in our investigations are not cleanly sensitive to ampicillin and certain other drugs used for plasmid selections. By using a recipient Acinetobacter strain with a designed deletion and correcting that deletion through heterogenote formation alone (Fig. 1), it was possible to apply a nutritional selection for formation and maintenance of the heterogenote. A lysate of the heterogenote-containing strain provided a source of material for cloning the full suite of hca genes in E. coli.
As summarized in Fig. 1 and described in detail in Materials and Methods, strain ADP8010, which is missing DNA from an intergenic dca region up to the pobS gene by virtue of a large NdeI deletion, was transformed with plasmid pZR8038. This plasmid contains the dca genes deleted from strain ADP8010 as well as the adjacent 1-kb region of dca DNA, which provided a region of homology between pZR8038 and ADP8010 for crossover to occur. Only cells that had undergone heterogenote formation could grow at the expense of adipate (Fig. 1C). Following transformation of ADP8010 with pZR8038, selection for heterogenotes on adipate, and preparation of a lysate of them, the lysate was digested with XbaI. The XbaI fragments were circularized with ligase and used to transform E. coli. Selection for Apr yielded colonies that were screened for the presence of the pobA gene, which was not present in plasmid pZR8038 (Fig. 1C). Of the screened E. coli colonies, 6% were pobA+; the others were presumed to have been transformed with pZR8038, which was carried by the ADP8010 transformants. All of the pobA+ colonies also converted p-coumarate to protocatechuate as judged by accumulation of a characteristic deep purple color around colonies in a p-toluidine test for diphenolics (43). Plasmid pZR8200 (Fig. 1 and 2), which included 4.8 kb of uncharacterized DNA, was isolated from one of the positive colonies.
Sequence analysis of the new Acinetobacter DNA captured in pZR8200.
The newly captured DNA of pZR8200 (Fig. 2) was deduced to contain five new ORFs that were analyzed using the BLAST program (4) against the National Center for Biotechnology Information database. Upstream of hcaC by 66 bp is a 1.45-kb ORF, designated hcaB, transcribed in the same direction, which encodes a protein with 59% identity to the aligned vanillin dehydrogenase from Pseudomonas sp. strain HR199 (accession no. CAA72286) (48). The sequencing of plasmid pZR9500 had uncovered 446 bp at the 3′ end of this ORF (55). The protein encoded by hcaB is presumed to be a dehydrogenase that acts upon aldehyde intermediates in hydroxycinnamate catabolism.
Upstream of hcaB by 36 bp is a 0.83-kb ORF termed hcaA which is positioned at the beginning of the hca structural gene transcript (Fig. 2). A homolog of hydroxycinnamoyl-CoA hydratase/lyases, HcaA is identical at 79% of the residues when aligned with the homolog from P. fluorescens (accession no. CAA73502) (20).
An intergenic distance of 256 bp separates hcaA from its upstream, divergently transcribed neighbor, an ORF deduced to be 1.23 kb and termed hcaK. HcaK is homologous to the aromatic compound transporters PcaK (accession no. Q51955 and AAC37151) (11, 23, 32), BenK (accession no. 030513) (9), and VanK (accession no. AAC27108) (52). These transporters are members of the aromatic acid transporter subgroup within the major facilitator superfamily, which displays considerable divergence among its members (42). PcaK and VanK from ADP1, both of which mediate transport of protocatechuate (11), share 31% identity along their polypeptides, while HcaK has 29 and 24% identity, respectively, with them. Typical for members of the family, HcaK is predicted to possess 12 transmembrane helices.
Translation of an ORF termed hcaR, which lies 63 bp downstream of hcaK, generates a protein with similarity to the E. coli repressor MarR (accession no. P27245) (8). HcaR is 159 amino acid residues long, compared to 144 residues for MarR, and these proteins share 28% identical residues along the aligned portions. Although a common Shine-Dalgarno sequence is absent upstream of the predicted ATG start codon for hcaR, the sequence AAG 7 bp upstream of the ATG is presumed to comprise a ribosomal binding domain (60).
Transcribed towards hcaR is ORF1, whose deduced translation product is a 205-residue protein. The putative translational start codon for ORF1 is TTG (12), 7 bp downstream from a GGA sequence predicted to be part of the Shine-Dalgarno sequence. ORF1 possesses 45% sequence similarity and 26% identity with the aligned primary sequence of the E. coli repressor TetR (accession no. S07359) (58). The XbaI site at the end of the insert in pZR8200 is situated 272 bp from the 5′ end of ORF1.
Phenotypes of hcaA, hcaB, hcaK, and hcaR deletion strains.
Since pZR8200 conferred upon E. coli the ability to convert p-coumarate to protocatechuate, it can be deduced that the hydratase/lyase (HcaA) and aldehyde dehydrogenase (HcaB) homologs act upon p-coumaroyl-CoA and p-hydroxybenzaldehyde, respectively.
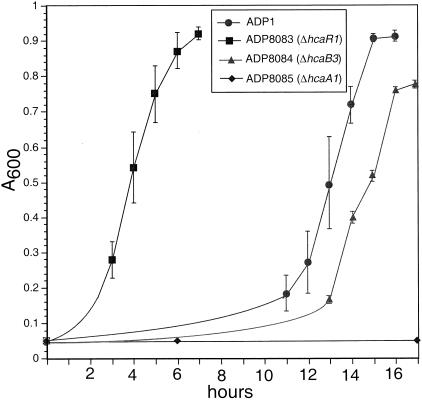
Further analysis of the roles of HcaA and HcaB in hydroxycinnamate dissimilation was carried out following creation of Acinetobacter strains with deletions of each gene. Deletions (Fig. 2; Table 1), designed to totally eliminate activity of the gene products, were predicted to produce truncated versions of the wild-type proteins: 27% of HcaA, 59% of HcaB, 39% of HcaK, and 17% of HcaR. Strain ADP8085 (ΔhcaA1) is unable to grow at the expense of p-coumarate, ferulate, or caffeate (Table 2; Fig. 4).
TABLE 2.
Growth of wild-type and mutant strains with hydroxycinnamates
| Strain | Mutation | Growth rate constant (h−1) for growth witha:
|
|||
|---|---|---|---|---|---|
| Succinate | Caffeate | p-Coumarate | Ferulate | ||
| ADP1 | None | 1.55 | 0.44 | 0.58 | 0.32 |
| ADP8083 | ΔhcaR1 | 1.55 | 0.54 | 0.64 | 0.19 |
| ADP8084 | ΔhcaB3 | ND | 0.30 | 0.58 | <0.09 |
| ADP8085 | ΔhcaA1 | ND | <0.09 | <0.03 | <0.09 |
ND, not determined.
FIG. 4.
Growth curves of strains utilizing p-coumarate as the sole carbon source. The growth curves of cultures used as inocula, grown in parallel on succinate to stationary phase, were superimposable. Error bars show the standard deviations for three independent data sets.
Strain ADP8084 (ΔhcaB3) grows poorly at the expense of ferulate and has a leaky phenotype on caffeate and an almost wild-type phenotype on p-coumarate (Table 2; Fig. 4). Impairment of strain ADP8084 on p-coumarate, relative to that of ADP1, is manifested in a slight growth lag out of stationary phase and reduced final cell density. A likely source of the leaky phenotypes is the activity of aldehyde dehydrogenase(s) associated with other pathways, such as the one that acts on benzaldehyde (26).
Additional genetic tests were carried out to confirm that HcaB indeed acts on a metabolite of p-coumarate. To rule out the possibility that the E. coli DH5α(pZR8200) bioconversion of p-coumarate to protocatechuate depends upon an E. coli aldehyde dehydrogenase that can fill in for HcaB, an hcaB mutation in pZR8200, making plasmid pZR8203, was used. As assessed by the p-toluidine test (43), E. coli carrying pZR8203 produces protocatechuate from p-hydroxybenzoate but fails to produce it from p-coumarate. Further proof that the only relevant pZR8203 mutation blocking the p-coumarate to protocatechuate pathway is the mutation in hcaB came from the ability of pZR8203 to transform strain ADP8135 (ΔhcaRKABCDEFG) to a p-coumarate-positive growth phenotype.
Although sequence similarities to transporters of aromatic compounds suggest that HcaK is involved in transport of hydroxycinnamates across the inner cell membrane, ΔhcaK3 mutant strain ADP8119 exhibited an unaltered phenotype when grown at the expense of 2 mM p-coumarate, ferulate or caffeate. The role of hcaK in transport of hydroxycinnamates at lower concentrations is under further investigation. A strain carrying a deletion in ORF1 exhibited a growth phenotype similar to that of ADP1.
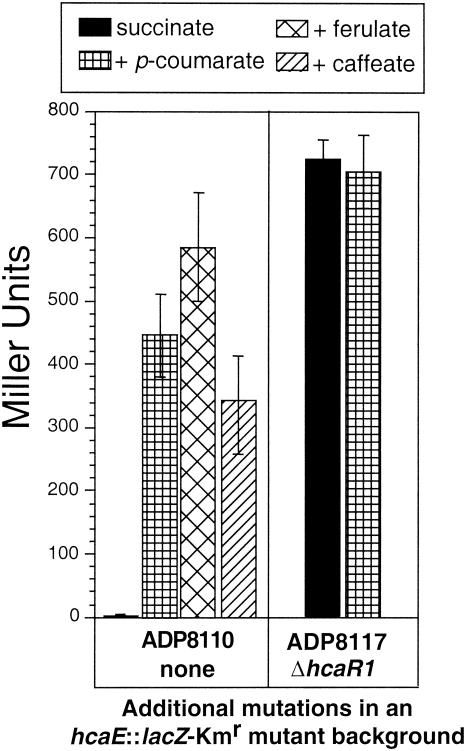
Strain ADP8083 (ΔhcaR1) grew well at the expense of p-coumarate, caffeate, and ferulate (Table 2). In a further examination of growth kinetics, succinate-grown, stationary-phase cultures of strains ADP1 and ADP8083 were inoculated into medium containing p-coumarate as the sole carbon source. Another aliquot of the cells was inoculated into succinate to serve as a control for the possibility that the initial viable cell numbers were different for the two strains. Whereas the strains thus inoculated had superimposable kinetics on succinate (data not shown), the hcaR knockout mutant strain ADP8083 had a greatly reduced lag time compared to ADP1 when inoculated into p-coumarate (Fig. 4), consistent with the phenotype of a mutant strain that is constitutive for enzymes of p-coumarate catabolism.
Analysis of the role of hcaR.
Further analysis of the hcaR mutant phenotype was conducted in cells that contained a promoterless lacZ-Kmr cassette in the chromosomal hcaE gene (Fig. 2). The product of this gene is an apparent porin precursor that is not required for growth with hydroxycinnamates at 2 mM, as used in this investigation. Figure 5 presents data on the expression of lacZ in cells grown for 4 h at the expense of succinate alone or with succinate plus a hydroxycinnamate. Strain ADP8110 (hcaE::lacZ-Kmr in a wild-type background) expressed at least a 100-fold increase in LacZ in response to the addition of p-coumarate, ferulate, or caffeate. In the presence or absence of hydroxycinnamate, regulatory mutant strain ADP8117 (ΔhcaR hcaE::lacZ-Kmr) expressed LacZ at a level that was 300-fold higher than that in succinate-grown ADP8110 cultures (Fig. 5). It should be noted that ADP8110 cells grown at the expense of hydroxycinnamates in the absence of succinate had somewhat higher LacZ levels: 930 Miller units when grown at the expense of p-coumarate alone, 900 Miller units when grown at the expense of caffeate alone, and 830 Miller units when grown at the expense of ferulate alone.
FIG. 5.
Role of HcaR in expression off the hca promoter. Promoter activity was measured by β-galactosidase activity (Miller units) by using strains with the chromosomal insertion hcaE::lacZ. Cultures were grown on succinate with or without the addition of 1 mM caffeate, p-coumarate, or ferulate. Not shown are the LacZ levels for ADP8110 cells grown at the expense of hydroxycinnamates in the absence of succinate: 928 Miller units with p-coumarate alone, 899 Miller units with caffeate alone, and 825 Miller units with ferulate alone. Data are averages based on three independent trials; error bars indicate standard deviations.
Introduction of ΔhcaR into the chromosome entailed selection for loss of a sacB-Kmr cassette, and this was accomplished by transformation with pZR8233 (Fig. 2), which contained the hcaR1 deletion. Thus, there was no selection or screening related to the resultant constitutive phenotype. Reintroduction of the wild-type hcaR gene by transformation of ADP8117 cells with pZR8224, the parental plasmid of ΔhcaR plasmid pZR8233, eliminated the constitutive phenotype and restored inducibility; transformation with pZR8233 yielded no such transformants.
As noted in the sequence analysis section above, ORF1 is homologous to the E. coli repressor TetR. If this gene encodes a repressor of hca genes, its knockout would be expected to produce a constitutive phenotype; if it encodes an activator of hca genes, its elimination should cause a hydroxycinnamate-negative phenotype. A strain bearing hcaE::lacZ-Kmr and a deletion in ORF1 displayed the wild-type growth phenotype on hydroxycinnamates, and it had a wild-type phenotype on succinate plates containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) plus caffeate, p-coumarate, or ferulate (D. Parke, unpublished data), leading to the conclusion that ORF1 is not an hca gene.
Identification of the inducer responsible for derepression of the hca genes.
In order to determine the nature of the inducer that governs the hca genes, the hcaE::lacZ-Kmr construction was introduced into strains in which different steps of hydroxycinnamate dissimilation were blocked.
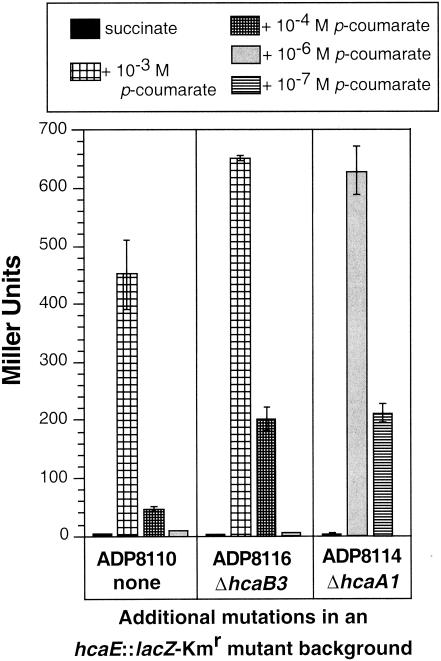
Strain ADP8114 (ΔhcaA1 hcaE::lacZ-Kmr), in which enoyl-CoA hydratase/lyase was blocked, expressed high levels of LacZ activity in the presence of as little as 10−6 M p-coumarate, whereas LacZ activity in strain ADP8110, which possessed a wild-type background, fell dramatically when the concentration of p-coumarate was reduced from 10−3 to 10−4 or 10−6 M (Fig. 6). The results for strain ADP8116 (ΔhcaB3 hcaE::lacZ-Kmr) were similar to those for ADP8110 (Fig. 6). Because ADP8116 exhibits an impaired growth phenotype only when grown at the expense of ferulate or, to a lesser extent, caffeate, the LacZ data for ADP8116 cells induced in the presence of these two hydroxycinnamates are presented in Fig. 7. Unlike the ADP8114 results, no induction occurred at the low concentration. Moreover, the aldehyde catabolite vanillin, tested as an inducer of hcaE::lacZ, did not elicit LacZ expression in strain ADP8116 (ΔhcaB hcaE::lacZ-Kmr) (data not shown).
FIG. 6.
Identification of p-coumaroyl-CoA as a true inducer of the hca genes. Cultures were grown on succinate with the addition of compounds as noted. Not shown because they would compress the uninduced bars are results for strain ADP8114 grown in the presence of 10−4 M p-coumarate (1,160 Miller units of LacZ activity). Promoter activity was measured, and the averages of three independent trials are presented with their standard deviations.
FIG. 7.
Absence of induction at low concentrations of caffeate or ferulate in strain ADP8116, with a block in HcaB, the aldehyde dehydrogenase. Cultures were grown on succinate with the addition of compounds as noted. ADP8116 cells grown in the presence of 1 mM vanillin, a catabolite of ferulate, had LacZ levels close to those grown on succinate alone (data not shown). Promoter activity was measured, and the averages of three independent trials are shown with their standard deviations.
Evidence pointing to hydroxycinnamoyl-CoA thioesters as inducers of the hca genes was supported by results of experiments with cells blocked in the activation of hydroxycinnamates. Strain ADP8113 (ΔhcaC1 hcaE::lacZ-Kmr) possessed no LacZ activity in response to the presence of p-coumarate (Fig. 8). To confirm that the lack of expression was due to the ligase mutation and not to instability related to the hcaE::lacZ-Kmr construction or its expression, strain ADP8113 was subjected to separate transformations by pZR8247, which carries most of the ligase gene, and pZR8248, which carries a truncated version (Fig. 2). Struck to single colonies on a plate containing X-Gal, the ADP8113 population transformed with pZR8247 gave rise to blue, growing colonies on medium with 2 mM p-coumarate, and LacZ+ blue colonies in a field of white ones were observed on medium containing succinate plus p-coumarate. The ADP8113 population transformed with pZR8248, the negative control, failed to grow at the expense of p-coumarate alone or to show blue color on either medium.
FIG. 8.
Failure of unactivated p-coumarate to elicit expression of hcaE::lacZ or hcaB::lacZ in strains impaired in hydroxycinnamate:CoASH ligase. Succinate-grown cells were amended with p-coumarate as noted. LacZ levels for the two constitutive strains with the ΔhcaR1 mutation provide the respective values to be expected for induced cells analogous to what was seen with ADP8110 and ADP8117 cells in Fig. 5. Promoter activity was measured, and the averages of three independent trials are shown with their standard deviations.
The low LacZ activity with strain ADP8113 could also be interpreted as being caused by a polar effect of the frameshift created by ΔhcaC1 on expression of downstream genes in an operon (2). Strain ADP8136 (ΔhcaC1 ΔhcaR1 hcaE::lacZ-Kmr) differs from ADP8113 in having an hcaR knockout, thus making expression of LacZ constitutive. As shown in Fig. 8, constitutive LacZ expression of ADP8136 cells is greatly reduced from that found in ADP8117, indicating that polarity caused by the ΔhcaC1 mutation indeed compromises expression of hcaE::lacZ. If p-coumarate itself were an actual inducer, cells of ADP8113 should exhibit an induced level similar to the constitutive level in ADP8136. The total absence of LacZ activity in ADP8113 indicates that the unactivated compound does not have an inductive role.
Further support for this conclusion was obtained with strains ADP8081 (ΔhcaB1::lacZ-Kmr) and ADP8088 (ΔhcaR1 ΔhcaB1:: lacZ-Kmr). Since the lacZ-Kmr cassette contains the phage fd terminator (31), there should be minimal expression of genes downstream of the cassette, provided that the downstream genes are part of the operon into which the cassette is inserted. Due to this polar effect, strains ADP8081 and ADP8088 are effectively negative for the downstream hcaC gene. The constitutive level of LacZ expression found with strain ADP8088 is unmatched by comparable induction of LacZ in ADP8081 cells (Fig. 8), echoing the conclusion that activation of the hydroxycinnamate is required for induction.
Results on solidified minimal medium containing succinate, a hydroxycinnamate, and X-Gal served to confirm and extend the quantitative LacZ results. On plates containing succinate plus p-coumarate, ferulate, or caffeate at a concentration of 1 mM, strains ADP8110 (hcaE::lacZ-Kmr), ADP8114 (ΔhcaA1 hcaE::lacZ-Kmr), and ADP8116 (ΔhcaB3 hcaE::lacZ-Kmr) formed blue colonies, indicating cleavage of X-Gal by LacZ and thus expression of hcaE::lacZ off the hca promoter, whereas strain ADP8113 (ΔhcaC1 hcaE::lacZ-Kmr) colonies appeared pale. Strains ADP8110, ADP8114, and ADP8116 were also tested on succinate medium containing caffeate, p-coumarate, or ferulate at 10−5 and 10−6 M. Only ADP8114 (ΔhcaA1 hcaE::lacZ-Kmr) formed deep blue colonies on media containing the low concentrations of hydroxycinnamates. Thus, the data show conclusively that thioester intermediates are the true inducers of the operon that includes hcaABCDE.
Genes associated with the major Acinetobacter hca transcript.
The major hca transcript is assumed to include hcaA and hcaB, given that they are separated by only 36 bp. As noted above, the lacZ-Kmr cassette used in this work contains a transcription terminator that should have a polar effect. Unlike hcaB deletion strain ADP8084, strain ADP8081 (ΔhcaB::lacZ-Kmr) and constitutive strain ADP8088 (ΔhcaR1 ΔhcaB::lacZ-Kmr) fail to grow on p-coumarate. This phenotype can only be due to the absence of expression of HcaC in the latter strain, because, of all the genes between hcaB and pobA (Fig. 1B and 2), only hcaC is required for growth at the expense of hydroxycinnamates (55; Parke, unpublished). The result indicates that hcaC is part of the hcaAB transcript. Finally, polarity of the ΔhcaC1 frameshift mutation demonstrated in strain ADP8136 (ΔhcaC1 ΔhcaR1 hcaE::lacZ-Kmr) (Fig. 8) compared to expression of hcaE::lacZ in ADP8117 (ΔhcaR1 hcaE::lacZ-Kmr) (Fig. 6) indicates that hcaC and hcaE share a common transcript. Thus, at a minimum, the major hca transcript includes hcaABCDE.
DISCUSSION
Chromosome striding by means of an endogenous vector.
Introduction of a vector into a chromosomal site that flanks DNA targeted for cloning is an effective and simple method for capturing DNA that neighbors a region already cloned (35). One advantage of this method over PCR for chromosome walking, aside from simplicity, yield, and fidelity to the wild-type sequence, is in the length of DNA that can be cloned. The insert of pZR8200 captured by this method was 16.5 kb. The stored lysate from the heterogenote Acinetobacter strain that contained the endogenous pUC vector for the cloning of pZR8200 was used to walk further down the chromosome. By this means, an insert was isolated that totaled 23 kb yet still maintained the functional hca genes that allowed E. coli to convert p-coumarate to protocatechuate (Parke, unpublished).
One potential outcome of vector insertional cloning, which is likely to occur with a strain in which the chosen vector can replicate, is the reisolation of the starting plasmid. The application of a selection for heterogenote formation (Fig. 1C) combined with a screen of E. coli colonies for the desired clones enabled this cloning method to work effectively.
Comparative organization of the genes for hydroxycinnamate dissimilation.
In addition to having clustered genetic units that mediate related physiological functions, Acinetobacter sp. strain ADP1 has a remarkable tendency to exert unified control over related catabolic genes, exemplified by the pcaIJFBDKCHG operon (32). This trait continues with the hcaABCDE operon. The order of the hcaAB genes is similar to that of the homologous genes of Pseudomonas sp. strain HR199 (41, 48), P. putida WCS358 (59), and P. fluorescens AN103 (20), but a gene corresponding to hcaC does not appear to be linked to the other two in these strains. In Amycolatopsis sp. strain HR167 (1) and S. paucimobilis SYK-6 (34), a feruloyl-CoA synthetase gene lies immediately downstream of a gene homologous to hcaA. However, the ancestries of the latter ligase genes are remote from those of strains ADP1 or HR199. Organization of apparent hca gene homologs in the recently sequenced genome of P. putida KT2440 (25, 39) reveals an order similar to that of the Acinetobacter hcaABC genes. A divergently transcribed gene encodes a homolog of hcaR; nonetheless, the predicted product of this gene is remote from HcaR, with only 29% of the aligned residues being identical.
The results presented here establish that the major Acinetobacter hca operon consists of at least hcaABCDE. Only 36 bp separate hcaA and hcaB, a transcription terminator in hcaB has a downstream effect on hcaC, and the ΔhcaC1 frameshift mutation has a polar effect that extends to hcaE and possibly beyond it (Fig. 7). Sequence analysis of the previously isolated Acinetobacter hcaCDEFG genes revealed that hcaF represented a 390-bp ORF of unknown function (55). Because it is not known if hcaF is a functional ORF or part of an intergenic region between hcaE and hcaG, it is not yet clear whether the hcaABCDE operon extends beyond hcaE.
Identification of the thioester inducer of hca genes.
Growth of Acinetobacter with caffeate, p-coumarate, and ferulate induces HcaG, chlorogenate esterase, whose level was monitored qualitatively by means of a T7-TAG marker (55). In a ligase-deficient strain, cells failed to grow at the expense of chlorogenate even though they were able to utilize quinate. An explanation for this observation was that a catabolite of caffeate may induce HcaG (55). This was the first suggestion that a hydroxycinnamate catabolite may trigger induction of an hca gene.
The more detailed analysis carried out with hcaE::lacZ-Kmr strain constructions for the present communication has pinpointed the inducing metabolite for the hcaABCDE promoter to be the thioester product of the hydroxycinnamate:CoASH ligase reaction. The results with HcaG (55) suggest that its gene may be governed in concert with the other hca genes, but it remains to be determined whether hcaG is part of the larger hca structural gene transcript. Although vanillin was observed to elicit expression of vdh, the vanillin dehydrogenase gene, in Pseudomonas sp. strain HR199 (48), the aldehyde had no inducing role in strain ADP1 (Fig. 6).
The simplest interpretation of the results with strain ADP8114 (ΔhcaA hcaE::lacZ-Kmr), in which induction was triggered by 10−7 M p-coumarate (Fig. 6), is that hydroxycinnamoyl-CoA interacts directly with HcaR. A few other regulatory molecules that appear to interact with aromatic thioesters have been identified. Catabolism of phenylacetic acid in E. coli and P. putida is carried out by the paa gene products through thioester formation, hydroxylation, and β-oxidation (17, 40). Repression of the paaABCDEFGHIJK operon mediated by PaaX is countered by the inducer phenylacetyl-CoA (18, 19). BadR, a MarR homolog atypical in being an activator, controls expression of genes involved in anaerobic benzoate dissimilation, and benzoyl-CoA may be its coinducer molecule (15).
HcaR, a novel member of the MarR family of regulators.
MarR negatively regulates multiple antibiotic resistance and oxidative stress operons in Salmonella and E. coli (3). Diverse compounds relieve repression by the protein (53), and among these are aromatic compounds (57). Involved in regulating genes related to antibiotic resistance, the MarR homologs EmrR from E. coli (7, 33) and MexR from Pseudomonas aeruginosa (16, 47, 56) also appear to respond to specific aromatic compounds.
Within the highly divergent MarR evolutionary family are other regulators, in addition to BadR and HcaR, which govern aromatic catabolism in bacteria. CinR from Butyrivibrio fibrisolvens E14, which represses a cinnamoyl ester hydrolase gene, responds in vitro to sugars that contain ferulate (10). The repressor HpcR from E. coli strain C controls genes for catabolism of homoprotocatechuate (50). Although apparently not the primary regulator of the cbaABC genes for chlorobenzoate catabolism in Comamonas testosteroni BR60, CbaR, encoded by a divergently transcribed gene, responds to chlorobenzoate and may have a role in modulating expression (49). With respect to HcaR, hydroxycinnamates tend to inhibit cell growth at higher concentrations. Thus, the evolutionary relationship between MarR and a regulator like HcaR, which itself evolved to allow its host to cope with two demands, detoxification and nutrition, is intriguing.
In the context of the 56-kb Acinetobacter island of catabolic diversity, HcaR is an evolutionarily atypical regulator. The four regulators that govern the pca (21), qui, pob (14), and dca (Parke, unpublished) structural genes are all members of the small IclR family, and the two that govern the aromatic catabolic genes are known to be activators. Located elsewhere on the chromosome and required for catabolism of ferulate, the vanillate genes (52) are regulated by VanR, a repressor unrelated to HcaR (38).
Regulatory controls for hydroxycinnamate catabolic genes cloned from other bacteria have not been established. It will be interesting to learn whether the conservation observed in structural genes is echoed in common elements of regulatory control. To our knowledge, HcaR is a novel member of the MarR family in possessing the dual characteristics of being a repressor and recognizing an aromatic thioester. Given the apparently broad specificity of the hydroxycinnamate catabolic enzymes, product induction may well serve to enhance the specificity of induction, thus circumventing dissimilation of hydroxy- and methoxycinnamates that produce dead-end catabolites.
Acknowledgments
We thank R. M. Jones and P. A. Williams for generously providing plasmid pRMJ1 and helpful information about its application prior to publication and D. E. Cameron for conducting PCR analysis of the ADP8084 hcaB mutation. Sequence data were generated by the Yale Keck Biotechnology Resource Lab.
Grant DAAG55-98-1-0232 from the Army Research Office funded the research.
REFERENCES
- 1.Achterholt, S., H. Priefert, and A. Steinbuchel. 2000. Identification of Amycolatopsis sp. strain HR167 genes, involved in bioconversion of ferulic acid to vanillin. Appl. Microbiol. Biotechnol. 54:799-807. [DOI] [PubMed] [Google Scholar]
- 2.Adhya, D., and M. Gottesman. 1978. Control of transcription termination. Annu. Rev. Biochem. 47:967-996. [DOI] [PubMed] [Google Scholar]
- 3.Alekshun, M. N., S. B. Levy, T. R. Mealy, B. A. Seaton, and J. F. Head. 2001. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 Å resolution. Nat. Struct. Biol. 8:710-714. [DOI] [PubMed] [Google Scholar]
- 4.Altschul, S. F., T. L. Madden, A. A. Schafer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1991. Current protocols in molecular biology. Wiley, New York, N.Y.
- 6.Averhoff, B., L. Gregg-Jolly, D. Elsemore, and L. N. Ornston. 1992. Genetic analysis of supraoperonic clustering by use of natural transformation in Acinetobacter calcoaceticus. J. Bacteriol. 174:200-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooun, A., J. J. Tomashek, and K. Lewis. 1999. Purification and ligand binding of EmrR, a regulator of a multidrug transporter. J. Bacteriol. 181:5131-5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen, S. P., H. Hachler, and S. B. Levy. 1993. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J. Bacteriol. 175:1484-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collier, L. S., N. N. Nichols, and E. L. Neidle. 1997. benK encodes a hydrophobic permease-like protein involved in benzoate degradation by Acinetobacter sp. strain ADP1. J. Bacteriol. 179:5943-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalrymple, B. P., and Y. Swadling. 1997. Expression of a Butyrivibrio fibrisolvens E14 gene (cinB) encoding an enzyme with cinnamoyl ester hydrolase activity is negatively regulated by the product of an adjacent gene (cinR). Microbiology 143:1203-1210. [DOI] [PubMed] [Google Scholar]
- 11.D'Argenio, D. A., A. Segura, W. M. Coco, P. V. Bünz, and L. N. Ornston. 1999. The physiological contribution of Acinetobacter PcaK, a transport system that acts upon protocatechuate, can be masked by the overlapping specificity of VanK. J. Bacteriol. 181:3505-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Date, T., K. Tanihara, and N. Numura. 1990. Construction of Escherichia coli vectors for expression and mutagenesis: synthesis of human c-Myc protein that is initiated at a non-AUG codon in exon 1. Gene 90:141-144. [DOI] [PubMed] [Google Scholar]
- 13.Delneri, D., G. Degrassi, R. Rizzo, and C. V. Bruschi. 1995. Degradation of trans-ferulic and p-coumeric acid by Acinetobacter calcoaceticus DSM 586. Biochim. Biophys. Acta 1244:363-367. [DOI] [PubMed] [Google Scholar]
- 14.DiMarco, A. A., B. Averhoff, and L. N. Ornston. 1993. Identification of the transcriptional activator pobR and characterization of its role in the expression of pobA, the structural gene for p-hydroxybenzoate hydroxylase in Acinetobacter calcoaceticus. J. Bacteriol. 175:4499-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egland, P. G., and C. S. Harwood. 1999. BadR, a new MarR family member, regulates anaerobic benzoate degradation by Rhodopseudomonas palustris in concert with AadR, an Fnr family member. J. Bacteriol. 181:2102-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans, K., L. Adewoye, and K. Poole. 2001. MexR repressor of the mexAB-oprM multidrug efflux operon of Pseudomonas aeruginosa: identification of MexR binding sites in the mexA-mexR intergenic region. J. Bacteriol. 183:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrández, A., J. L. García, and E. Díaz. 1998. Catabolism of phenylacetic acid in Escherichia coli. Characterization of a new aerobic hybrid pathway. J. Biol. Chem. 273:25974-25986. [DOI] [PubMed] [Google Scholar]
- 18.Ferrández, A., J. L. García, and E. Díaz. 2000. Transcriptional regulation of the divergent paa catabolic operons for phenylacetic acid degradation in Escherichia coli. J. Biol. Chem. 275:12214-12222. [DOI] [PubMed] [Google Scholar]
- 19.García, B., E. R. Olivera, B. Miñambres, D. Carnicero, C. Muñiz, G. Naharro, and J. M. Luengo. 2000. Phenylacetyl-coenzyme A is the true inducer of the phenylacetic acid catabolism pathway in Pseudomonas putida U. Appl. Environ. Microbiol. 66:4575-4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasson, M. J., Y. Kitamura, W. R. McLauchlan, A. Narbad, A. J. Parr, E. L. H. Parsons, J. Payne, J. C. Rhodes, and N. J. Walton. 1998. Metabolism of ferulic acid to vanillin. A bacterial gene of the enoyl-SCoA hydratase/isomerase superfamily encodes an enzyme for the hydration and cleavage of a hydroxycinnamic acid SCoA thioester. J. Biol. Chem. 273:4163-4170. [DOI] [PubMed] [Google Scholar]
- 21.Gerischer, U., A. Segura, and L. N. Ornston. 1998. PcaU, a transcriptional activator of genes for protocatechuate utilization in Acinetobacter. J. Bacteriol. 180:1512-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gralton, E. M., A. L. Campbell, and E. L. Neidle. 1997. Directed introduction of DNA cleavage sites to produce a high-resolution genetic and physical map of the Acinetobacter sp. strain ADP1 (BD413UE) chromosome. Microbiology 143:1345-1357. [DOI] [PubMed] [Google Scholar]
- 23.Harwood, C. S., N. N. Nichols, M. K. Kim, J. L. Ditty, and R. E. Parales. 1994. Identification of the pcaRKF gene cluster from Pseudomonas putida: involvement in chemotaxis, biodegradation, and transport of 4-hydroxybenzoate. J. Bacteriol. 176:6479-6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoue, H., H. Nojima, and H. Okayama. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 25.Jiménez, J. I., B. Miñambres, J. L. García, and E. Díaz. 2002. Genomic analysis of the aromatic catabolic pathways from Pseudomonas putida KT2440. Environ. Microbiol. 4:824-841. [DOI] [PubMed] [Google Scholar]
- 26.Jones, R. M., L. S. Collier, E. L. Neidle, and P. A. Williams. 1999. areABC genes determine the catabolism of aryl esters in Acinetobacter sp. strain ADP1. J. Bacteriol. 181:4568-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones, R. M., and P. A. Williams. 2003. Mutational analysis of the critical bases involved in activation of the AreR-regulated σ54-dependent promoter in Acinetobacter sp. strain ADP1. Appl. Environ. Microbiol. 69:5627-5635. [DOI] [PMC free article] [PubMed]
- 28.Juni, E. 1972. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J. Bacteriol. 112:917-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juni, E., and A. Janick. 1969. Transformation of Acinetobacter calcoaceticus (Bacterium anitratum). J. Bacteriol. 98:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 31.Kokotek, W., and W. Lotz. 1989. Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene 84:467-471. [DOI] [PubMed] [Google Scholar]
- 32.Kowalchuk, G. A., G. B. Hartnett, A. Benson, J. E. Houghton, K.-L. Ngai, and L. N. Ornston. 1994. Contrasting patterns of evolutionary divergence within the Acinetobacter calcoaceticus pca operon. Gene 146:23-30. [DOI] [PubMed] [Google Scholar]
- 33.Lomovskaya, O., K. Lewis, and A. Matin. 1995. EmrR is a negative regulator of the Escherichia coli multidrug resistance pump EmrAB. J. Bacteriol. 177:2328-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masai, E., Y. Harada, and X. Peng. 2002. Cloning and characterization of the ferulic acid catabolic genes of Sphingomonas paucimobilis SYK-6. Appl. Environ. Microbiol. 68:4416-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mejean, V., J. P. Claverys, H. Vasseghi, and A. M. Sicard. 1981. Rapid cloning of specific DNA fragments of Streptococcus pneumoniae by vector integration into the chromosome followed by endonucleolytic excision. Gene 15:289-293. [DOI] [PubMed] [Google Scholar]
- 36.Melnikov, A., and P. J. Youngman. 1999. Random mutagenesis by recombinational capture of PCR products in Bacillus subtilis and Acinetobacter calcoaceticus. Nucleic Acids Res. 27:1056-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 38.Morawski, B., A. Segura, and L. N. Ornston. 2000. Repression of Acinetobacter vanillate demethylase synthesis by VanR, a member of the GntR family of transcriptional regulators. FEMS Microbiol. Lett. 187:65-68. [DOI] [PubMed] [Google Scholar]
- 39.Nelson, K. E., C. Weinel, I. T. Paulsen, R. J. Dodson, H. Hilbert, V. A. P. Martins dos Santos, D. E. Fouts, S. R. Gill, M. Pop, M. Holmes, L. Brinkac, M. Beanan, R. T. DeBoy, S. Daugherty, J. Kolonay, R. Madupu, W. Nelson, O. White, J. Peterson, H. Khouri, I. Hance, P. Chris Lee, E. Holtzapple, D. Scanlan, K. Tran, A. Moazzez, T. Utterback, M. Rizzo, K. Lee, D. Kosack, D. Moestl, H. Wedler, J. Lauber, D. Stjepandic, J. Hoheisel, M. Straetz, S. Heim, C. Kiewitz, J. Eisen, K. N. Timmis, A. Düsterhöft, B. Tümmler, and C. M. Fraser. 2002. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 4:799-808. [DOI] [PubMed] [Google Scholar]
- 40.Olivera, E. R., B. Miñambres, B. García, C. Muñiz, M. A. Moreno, A. Ferrández, E. Diaz, J. L. Garcia, and L. M. Luengo. 1998. Molecular characterization of the phenylacetic acid catabolic pathway in Pseudomonas putida U: the phenylacetyl-CoA catabolon. Proc. Natl. Acad. Sci. USA 95:6419-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Overhage, J., H. Priefert, and A. Steinbuchel. 1999. Biochemical and genetic analysis of ferulic acid catabolism in Pseudomonas sp. strain HR199. Appl. Environ. Microbiol. 65:4837-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pao, S. S., I. T. Paulsen, and M. H. Saier, Jr. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parke, D. 1992. Application of p-toluidine in chromogenic detection of catechol and protocatechuate, diphenolic intermediates in catabolism of aromatic compounds. Appl. Environ. Microbiol. 58:2694-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parke, D. 1996. Characterization of PcaQ, a LysR-type transcriptional activator required for catabolism of phenolic compounds, from Agrobacterium tumefaciens. J. Bacteriol. 178:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parke, D., M. A. Garcia, and L. N. Ornston. 2001. Cloning and genetic characterization of dca genes required for beta-oxidation of straight-chain dicarboxylic acids in Acinetobacter sp. strain ADP1. Appl. Environ. Microbiol. 67:4817-4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parke, D., and L. N. Ornston. 1984. Nutritional diversity of Rhizobiaceae revealed by auxanography. J. Gen. Microbiol. 130:1743-1750. [Google Scholar]
- 47.Poole, K., K. Tetro, Q. Zhao, S. Neshat, D. E. Heinrichs, and N. Bianco. 1996. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob. Agents Chemother. 40:2021-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Priefert, H., J. Rabenhorst, and A. Steinbuchel. 1997. Molecular characterization of genes of Pseudomonas sp. strain HR199 involved in bioconversion of vanillin to protocatechuate. J. Bacteriol. 179:2595-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Providenti, M. A., and R. C. Wyndham. 2001. Identification and functional characterization of CbaR, a MarR-like modulator of the cbaABC-encoded chlorobenzoate catabolism pathway. Appl. Environ. Microbiol. 67:3530-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roper, D. I., T. Fawcett, and R. A. Cooper. 1993. The Escherichia coli C homoprotocatechuate degradative operon: hpc gene order, direction of transcription and control of expression. Mol. Gen. Genet. 237:241-250. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 52.Segura, A., P. V. Bünz, D. A. D'Argenio, and L. N. Ornston. 1999. Genetic analysis of a chromosomal region containing vanA and vanB, genes required for conversion of either ferulate or vanillate to protocatechuate in Acinetobacter. J. Bacteriol. 181:3494-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seoane, A. S., and S. B. Levy. 1995. Characterization of MarR, the repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli. J. Bacteriol. 177:3414-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 55.Smith, M. A., V. B. Weaver, D. M. Young, and L. N. Ornston. 2003. Genes for chlorogenate and hydroxycinnamate catabolism (hca) are linked to functionally related genes in the dca-pca-qui-pob-hca chromosomal cluster of Acinetobacter sp. strain ADP1. Appl. Environ. Microbiol. 69:524-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srikumar, R., C. J. Paul, and K. Poole. 2000. Influence of mutations in the mexR repressor gene on expression of the MexA-MexB-OprM multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 182:1410-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sulavik, M. C., L. F. Gambino, and P. F. Miller. 1995. The MarR repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli: prototypic member of a family of bacterial regulatory proteins involved in sensing phenolic compounds. Mol. Med. 1:436-446. [PMC free article] [PubMed] [Google Scholar]
- 58.Unger, B., G. Klock, and W. Hillen. 1984. Nucleotide sequence of the repressor gene of the RA1 tetracycline resistance determinant: structural and functional comparison with three related Tet repressor genes. Nucleic Acids Res. 12:7693-7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venturi, V., F. Zennaro, G. Degrassi, B. C. Okeke, and C. V. Bruschi. 1998. Genetics of ferulic acid bioconversion to protocatechuic acid in plant-growth-promoting Pseudomonas putida WCS358. Microbiology 144:965-973. [DOI] [PubMed] [Google Scholar]
- 60.Whitehorn, E. A., K. J. Livak, and S. R. Petteway. 1985. The effect of hybrid ribosome-binding site variants on the expression of human interferon-β in Escherichia coli. Gene 36:375-379. [DOI] [PubMed] [Google Scholar]
- 61.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]