Abstract
Strains of Flavobacterium psychrophilum were studied for their ability to adhere and cause agglutination of erythrocytes and yeast cells. Strains of the serotype Th showed low or no hemagglutinating (HA) properties toward human, avian, bovine, and rainbow trout erythrocytes, whereas strains of serotype Fd and FpT exhibited distinct HA properties. None of the strains was able to cause agglutination of yeast cells. Greater adherence specificity toward rainbow trout blood cells was seen for the HA-positive strains. Growth at 5°C, compared to that at 15°C, induced an increase in the hemagglutination of some strains. HA activities of F. psychrophilum were inhibited only by sialic acid (N-acetyl-neuraminic acid), heat treatment at 65°C, and proteinase K treatment and not by any of seven other carbohydrates, periodate oxidation, or treatment with trypsin. The supernatant from washed bacterial cells also showed some HA properties. All strains were shown to be highly hydrophobic by the hydrophobic interaction chromatography test, although some contradictions to the results of the salt aggregation test (showing some strains as less hydrophobic) were seen. These results indicate that the aggregation of F. psychrophilum and erythrocytes depend on a lectin present on the surface of HA-positive F. psychrophilum strains and absent on HA-negative strains. This lectin reacts specifically with sialic acid. The adhesion differences observed for F. psychrophilum strains do not appear to correlate with the virulence but still provide insights into the interaction of F. psychrophilum and rainbow trout.
Flavobacterium psychrophilum (2) is a gram-negative, rod-shaped bacterium that has been known to cause fish disease since the 1940s and is the cause of bacterial coldwater disease (3), also called rainbow trout fry syndrome (17). F. psychrophilum primarily affects juvenile salmonids and results in high mortality rates among fry and fingerlings in hatcheries. In Europe this bacterium constitutes a large problem for the aquacultural production of rainbow trout (Oncorhynchus mykiss) (5). Outbreaks of the disease are mainly seen at low temperatures, with the most serious problems occurring at temperatures below 15°C (17). Adhesion of a bacterium to the surface of its host is considered to be an important factor for the primary colonization and the subsequent steps in the pathogenicity of the pathogen. Bacterial surface structures, i.e., lipopolysaccharide, capsular polysaccharides, pili, and flagella, have been shown to act as adhesins of many bacteria (13, 24, 25). The adhesion may also be influenced in a nonspecific matter determined through hydrophobic and ionic interactions (25). Specific carbohydrate-binding proteins or lectins have been shown to act as adhesins or hemagglutinins, which adhere to erythrocytes and cause a hemagglutination (HA) (25). These lectins are often associated with pili, and the expression is often influenced by growth conditions or thermoregulated (12, 22). Although previous studies have suggested some kind of weak adhesin or hemagglutinin in F. psychrophilum (17), the distribution and specificity of such adhesins or lectins have not been reported.
F. psychrophilum is generally considered to possess a polysaccharide-rich capsular structure, although the direct evidence of capsular material during different growth conditions is lacking. A loosely attached outer layer has been described for F. psychrophilum (4); however, the adhesive effects of this layer as well as its presence and expression in different strains are unknown. Another fish pathogenic species, Flavobacterium columnare, possesses carbohydrate-binding lectins as well as a capsular layer (6, 7).
The aim of this study was to investigate the presence of surface properties on F. psychrophilum that influence the adhesive abilities of this fish pathogen and may elucidate pathogenic mechanism of importance for the virulence of this bacterium. The effect of different carbohydrates and proteolytic treatments was studied to show whether or not a specific adhesin or lectin was present. The influence of different growth conditions on the surface properties was also investigated, as adhesion and adhesin expression may be regulated by specific growth and temperature conditions.
MATERIALS AND METHODS
Bacterial strains.
F. psychrophilum strains used were selected according to isolation source, virulence, serotype, ribotype, plasmid content, and elastin-degrading ability (5, 19) to ensure that a variety of different characteristics were represented. The strains were the type strain NCMB1947 (serotype FpT), two well-studied Danish strains (950106-1/1 and 900406-1/3, representing serotypes Fd and Th, respectively) (5), 18 strains isolated from rainbow trout from five different freshwater farms in 1994 and 1995 (5, 19), and one strain without plasmid (910611-1) (17). Strains were maintained in tryptone yeast extract salt (TYES) broth (11) with 15 to 20% glycerol at −80°C. The strains were incubated at 15°C for 36 to 48 h in TYES, and subsequently 200 μl was spread on TYES broth with 1.1% agar (TYES-A), unless otherwise stated. Agar plates were incubated at 15 or 5°C for 1 week. Bacteria were harvested from TYES-A with 2 ml of TYES broth and were transferred to sterile glass tubes and placed in a water bath of the same temperature as during growth (15 or 5°C). The cell density was adjusted with TYES broth to a CFU count of 109/ml (optical density at 590 nm [OD590] = 1.6 ± 0.05 [subsequently controlled by CFU plate counts]).
HA.
Rainbow trout, bovine, chicken, and human blood (type O) cells, as well as yeast cells, were tested to show if the bacterial strains had various patterns of binding toward the different blood cell types. Bovine blood was purchased from the Danish Veterinary Institute, and the other types of blood were collected by venipuncture by using a syringe containing Alsever's solution (1). Rainbow trout blood samples were obtained from the caudal vein of anesthetized (MS-222; Sigma-Aldrich Chemie GmbH, Steinheim, Germany) rainbow trout (2 to 3 kg) or rainbow trout fingerlings (6 to 7 g) that had been kept under laboratory conditions and without any previous infection caused by F. psychrophilum. Blood was washed and resuspended three times in phosphate-buffered saline (PBS) and was packed by centrifugation (1,380 × g/10 min/4°C). A 3% (vol/vol) erythrocyte suspension was prepared in PBS, stored at 4°C, and used within a maximum of 2 days. Yeast (Saccharomyces cerevisiae) (Danish Distillers A/S, Copenhagen, Denmark) was washed twice in PBS (1,380 × g/6 min/4°C) before the cells were packed (620 × g/10 min/4°C). A 3% (vol/vol) yeast suspension was made in PBS, stored at 4°C, and used within 2 days.
Prior to the HA test, the OD590-adjusted bacterial suspensions (as described above) were diluted twofold from 1:2 to 1:512. The HA test was performed either on glass tiles or in 96-well polystyrene unabsorbable microtiter plates (Greiner, Frickenhausen, Germany) for five strains. The glass tile procedure was as follows: 50 μl of diluted bacterial suspension was mixed with 50 μl of erythrocyte or yeast suspension before the glass tile plate was incubated for 5 min at 15°C or on ice (0 to 5°C) with rotation at 160 rpm. Following incubation, the HA titer was determined as the reciprocal of the highest dilution to yield a positive agglutination result (+). A positive result was noted when the mixture of erythrocytes (or yeast) and bacteria, after 5 min of incubation, resulted in a slightly shadowed background with a granular appearance. A clear background with aggregated erythrocytes or yeast cells was noted as very positive (++). All agglutinations were compared with negative and positive controls, performed as described above but without added bacteria (negative control) or with a positive bacterial strain (Vibrio anguillarum serogroup O1 [strain 830407-1/7]) (15). The procedure in microtiter plates was as follows: 70 μl of bacterial suspension was added to each well in the first row, and subsequently twofold dilutions were made by transferring 35 μl from the first row into the next row containing 35 μl of TYES broth. Following the dilutions, 35 μl of the erythrocyte suspension was added to each well, which each contained 35 μl of diluted bacterial suspension, and the plate was incubated for 5 min at 15°C/160 rpm. A dissection microscope (×15) was used to determine the HA titers as described above. The use of 96-well microtiter plates for HA testing is less laborious, as bacterial dilutions can be made directly in the microtiter plate. However, in our study, a marked decrease in HA titer was noted for the tested strains when the test was performed in a microtiter plate compared to the result found with glass tiles (data not shown). In addition, a decrease in the HA titers was detected for all HA-positive strains when polystyrene Eppendorf tubes were used instead of glass tubes for the bacterial dilutions (data not shown). Based on these results, all materials used to handle the bacterial suspensions were glass, in an attempt to avoid plastic surface adhesion interfering with the test results.
The HAs of 16 F. psychrophilum strains were tested by using the different erythrocyte and yeast suspensions. The HA of 22 F. psychrophilum strains, incubated at 5 and 15°C, was tested by using rainbow trout erythrocytes. HA titers were assessed as the mean of duplicate studies, where the bacteria were grown separately and tested against two different blood or yeast samples. Triplicate studies were however performed in the experiment concerning the effect of growth temperature. The supernatant, from bacterial samples that were cell density adjusted to a CFU count of ∼109/ml, was likewise tested for HA activity.
One strain (950106-1/1) was grown in TYES broth with agitation, on TYES-A with 1.1 or 0.9% agar and was incubated at 15°C for 1 week to assess HA differences following growth on solid surfaces versus broth. HA titers were determined as described above.
Inhibition of HA.
Inhibition studies were performed as previously described (14) on four HA-positive strains (NCMB1947, 950106-1/1, 43/2A, and 66/3A). Following incubation for 1 week at 15°C on TYES-A, the cells were harvested in 2 ml of TYES broth. Equal amounts of bacterial cell suspension and carbohydrate solution were mixed and incubated at 15°C for 60 min, before the cells were washed once in TYES broth. Cells were then resuspended in TYES broth and adjusted to a CFU count of ∼109/ml as described above, diluted to four times the minimal hemagglutinating dose (4× MHD), and tested against rainbow trout erythrocytes. d-Glucose (Merck, Darmstadt, Germany), d-galactose (Fluka, Buchs, Switzerland), l-fucose, d-mannose, l-rhamnose, N-acetyl-d-glucosamine, N-acetyl-d-galactosamine, and N-acetyl-neuraminic acid (sialic acid) (all from Sigma-Aldrich) were tested for their inhibitory effect at a final concentration of 100 mM. The effects of sodium periodate (Fluka), proteinase K (Sigma-Aldrich), and trypsin (Sigma-Aldrich) (all at a final concentration of 10 mg/ml) were tested as previously described (7). The inhibitory effect of different high- and low-temperature treatments (37, 55, and 65°C for 15 min) was tested by subjecting the bacterial cells to the different temperatures before adjusting bacterial cell density to 4× MHD. The MIC of sialic acid was determined by incubating bacterial cells in twofold serial dilutions of sialic acid (final concentrations of 100, 50, 25, 12.5, and 6.25 mM) for 60 min at 15°C. Subsequently the bacteria were washed, and the cell density was adjusted to 4× MHD before HA was tested with a 3% (vol/vol) rainbow trout erythrocyte suspension.
Oxidative treatment with periodate was also performed on two nonhemagglutinating strains (900406-1/3 and 69/3A) to assess whether or not any hemagglutinating properties were covered by a polysaccharide capsular layer.
HA with sialidase-treated rainbow trout erythrocytes.
Sialic acid, present on the rainbow trout erythrocytes, was destroyed with sialidase as described for human erythrocytes (27), except that the incubation temperature was lowered to 15°C as rainbow trout erythrocytes cannot be handled at 37°C. In brief the procedure was as follows: washed and packed erythrocytes were incubated at 15°C in a 38% (vol/vol) PBS suspension containing 150 mU of Vibrio cholerae sialidase (Sigma-Aldrich) per ml of packed cells. After 1, 2 , 5, 10, 20, and 48 h of incubation with sialidase, the erythrocyte suspension was further diluted with PBS to 3% (vol/vol) and HA was tested toward four HA-positive F. psychrophilum strains diluted to 4× MHD.
, 5, 10, 20, and 48 h of incubation with sialidase, the erythrocyte suspension was further diluted with PBS to 3% (vol/vol) and HA was tested toward four HA-positive F. psychrophilum strains diluted to 4× MHD.
Analysis of cell surface hydrophobicity.
The hydrophobicity of the 22 F. psychrophilum strains was comparatively tested by two methods, i.e., the salt aggregation test (SAT) and hydrophobic interaction chromatography (HIC). SAT measures the hydrofobicity of the cells by precipitating cells in increasing molar salt concentrations (16). The most hydrophobic cells are precipitated at low salt concentrations, and results are expressed as the lowest molarity of ammonium sulfate that results in visible cell aggregates. SAT values that are <0.1 indicate highly hydrophobic strains, and values between 0.1 and 1.0 indicate hydrophobic strains. Strains with SAT values that are >1.0 are considered to be hydrophilic. HIC measures the amount of cells retained by a hydrophobic gel (Octyl-Sepharose CL 4B; Amersham Pharmacia Biotech AB, Uppsala, Sweden) as previously described (23). The cellular concentration of the test suspension was adjusted to an OD590 of 1.1 ± 0.05. HIC values that are >70% indicate highly hydrophobic strains. V. anguillarum (strain 830407-1/7) was included as standard control, having SAT and HIC values of 1.25 and 55%, respectively. SAT values were assessed following incubation at 15°C for all 22 strains. Ten F. psychrophilum strains were incubated at both 5 and 15°C before SAT testing.
Statistical analyses.
The two-way analysis of variance (ANOVA) was used to test for significant difference of HA titers following incubation at 5 and 15°C and between growth in TYES broth and on 1.1% TYES-A and 0.9% TYES-A. The t test was used to test the difference between 1.1% TYES-A and 0.9% TYES-A.
RESULTS
HA.
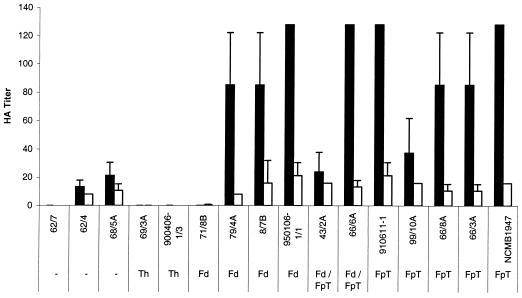
Increased adherence specificity toward rainbow trout erythrocytes was observed for 12 out of 16 F. psychrophilum strains when compared to specificity toward bovine (Fig. 1), avian, or human erythrocytes (data not shown). The remaining strains were unable to cause agglutination of any of the four types of erythrocytes tested. No strains were capable of agglutinating yeast cells.
FIG. 1.
HA titers of 16 F. psychrophilum strains, grown at 15°C, measured by using rainbow trout (▪) and bovine erythrocytes (□). Rainbow trout erythrocytes were significantly more agglutinated than bovine erythrocytes (ANOVA; P < 0.01). Bars represent the means of duplicates plus or minus standard deviations. Dashes indicate no reaction for any tested serotype.
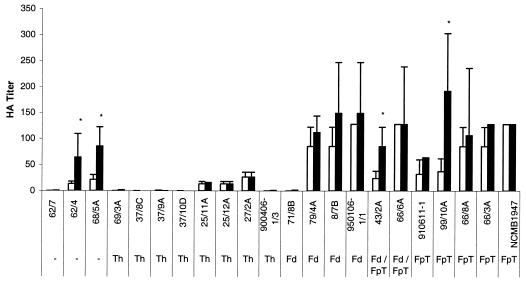
The capacity of the bacteria to cause HA of rainbow trout erythrocytes was tested for 22 strains grown at 5 and 15°C (Fig. 2). The HA titers obtained following incubation at 15°C divided the strains into two different groups with significantly different HA properties (ANOVA; P < 0.01). The first group of strains showed no or low HA properties (HA titers below 50), and the second group of strains showed marked HA properties (HA titers above 50). Following growth at 5°C, the strains generally seemed to possess higher HA properties, being significantly higher for four strains (ANOVA; P < 0.01). Similar HA titers were obtained when rainbow trout erythrocytes isolated from fish weighing 2 to 3 kg and from fingerlings were tested (data not shown). The culture supernatant, excluding bacterial cells, agglutinated the rainbow trout erythrocytes, although the HA titers of the supernatants generally were four to eight times lower (data not shown).
FIG. 2.
HA titers of 22 F. psychrophilum strains following growth at 15 (□) and 5°C (▪). * indicates a statistically significant difference in HA titers between 15 and 5°C (ANOVA; P < 0.01). Bars represent the mean of three replicates plus or minus standard deviation.
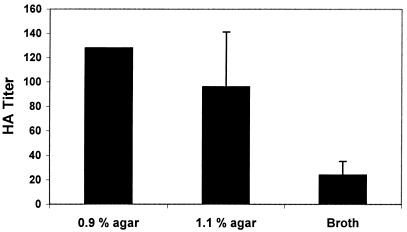
Growth in TYES broth culture resulted in a significant (ANOVA; P < 0.01) decrease in the HA titer of F. psychrophilum strain 950106-1/1 compared to the HA titer following growth on 1.1 and 0.9% TYES-A (Fig. 3). No significant effect was observed between growth on 1.1% agar and 0.9% agar (t test; P > 0.05), although bacteria grown on agar containing 0.9% agar appeared to have slightly increased HA properties.
FIG. 3.
HA titers of F. psychrophilum strain 950106-1/1 following growth on TYES-A (0.9% agar and 1.1% agar) and in an agitated broth culture. Bars represent the mean of duplicates plus or minus standard deviation.
The specificity of the adherence was investigated on a low-agglutinating strain (43/2A) and three high-agglutinating strains (950106-1/1, NCMB1947, and 66/3A). All four strains showed the same pattern of HA following the different treatments. Incubation with sialic acid completely impaired the adhesion, resulting in a total inhibition of the HA of the four tested strains. None of the seven other carbohydrates tested was able to block the adherence and thus cause an inhibition of the HA. Inhibition was also achieved following heat treatment at 65°C as well as proteolytic treatment with proteinase K. Treatments at 55°C or lower temperatures, oxidation of surface polysaccharides with periodate, or proteolytic treatment with trypsin showed no inhibition of the HA. In order to assess if the sensitivity of the sialic acid inhibition varied among the four tested strains, the MIC of sialic acid was tested. All tested strains were impaired in the agglutinating capacity after treatment with 25 mM sialic acid, and furthermore strain 43/2A showed a partial inhibition after treatment with 12.5 mM sialic acid (Table 1). Oxidizing polysaccharides on the surface of two nonagglutinating strains (900406-1/3 and 69/3A) with periodate did not affect the HA.
TABLE 1.
MIC of sialic acid needed to inhibit the agglutination of F. psychrophilum and rainbow trout erythrocytesa
| Bacterial strain | Result at various sialic acid concns (mM)
|
|||||
|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | Control (PBS) | |
| NCMB1947 | − | − | − | ++ | ++ | ++ |
| 950106-1/1 | − | − | − | ++ | ++ | ++ |
| 43/2A | − | − | − | + | ++ | ++ |
| 66/3A | − | − | − | ++ | ++ | ++ |
++, total HA; +, partial HA; −, no HA.
Treatment of blood cells with sialidase destroys the sialic acid present on the erythrocyte surface. The protocol for releasing the sialic acid from rainbow trout erythrocytes was adapted from an assay with human erythrocytes by lowering the temperature to 15°C. A reduced effect of the sialidase was therefore to be expected, and as a consequence a time study was conducted (Table 2). The sialic acid-specific adherence of F. psychrophilum to rainbow trout erythrocytes was not affected after 2 h, whereas treatment of the erythrocytes for 5 h led to partial inhibition of the HA for one strain (43/2A). Sialidase treatment for 10 h further affected the agglutination of one other strain (950106-1/1), and following 20 h of treatment, the agglutination of rainbow trout erythrocytes and these two strains was further impaired. None of the other strains tested showed a decrease in their HA following sialidase treatment of rainbow trout erythrocytes compared to the result with untreated rainbow trout erythrocytes.
h, whereas treatment of the erythrocytes for 5 h led to partial inhibition of the HA for one strain (43/2A). Sialidase treatment for 10 h further affected the agglutination of one other strain (950106-1/1), and following 20 h of treatment, the agglutination of rainbow trout erythrocytes and these two strains was further impaired. None of the other strains tested showed a decrease in their HA following sialidase treatment of rainbow trout erythrocytes compared to the result with untreated rainbow trout erythrocytes.
TABLE 2.
HA of F. psychrophilum and sialidase-treated rainbow trout erythrocytesa
| Bacterial strain | Incubation time and erythrocyte type
|
|||||||
|---|---|---|---|---|---|---|---|---|
1 and 2 h h
|
5 h
|
10 h
|
20 and 48 h
|
|||||
| Control | Sialidase treated | Control | Sialidase treated | Control | Sialidase treated | Control | Sialidase treated | |
| 900406-1/1 | − | − | − | − | − | − | − | − |
| 69/3A | − | − | − | − | − | − | − | − |
| 66/3A | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 43/2A | ++ | ++ | ++ | + | ++ | + | ++ | − |
| 950106-1/1 | ++ | ++ | ++ | ++ | ++ | +* | ++ | + |
| NCMB1947 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
Time intervals depict the incubation times of erythrocytes with sialidase before testing HA. Control erythrocytes were incubated with PBS. ++, total HA; +, partial HA; −, no HA. *, 950106-1/1 caused a weak HA in one of two experiments. All other samples showed the same result in duplicate experiments.
Cell surface hydrophobicity.
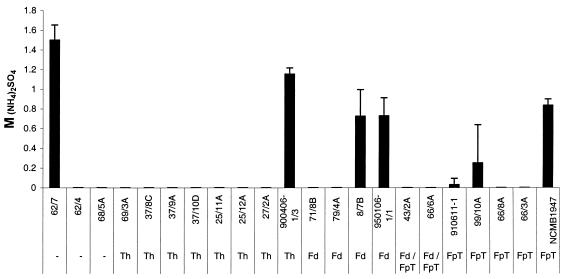
The 22 different strains of F. psychrophilum, which were tested in this study, exhibited a high or relatively high surface hydrophobicity. The HIC test showed all 22 strains to be highly hydrophobic, as all strains were retained 100% in the octyl-Sepharose column. The SAT, on the other hand, showed a slight variation among the strains (Fig. 4). Still, 16 of the 22 strains were classified as highly hydrophobic, as they aggregated in less than 0.1 M ammonium sulfate. Out of the remaining six strains, four were relatively hydrophobic and aggregated in 0.1 to 0.9 M ammonium sulfate and two were relatively hydrophilic as they aggregated in 1.0 to 1.5 M ammonium sulfate. Similar results were achieved for testing of bacteria incubated at 5 or 15°C.
FIG. 4.
SAT values of 22 F. psychrophilum strains representing the cell surface hydrophobicity and expressed as molar concentration of (NH4)2SO4 necessary to cause agglutination of the bacterial cells. Bars represent the mean of three replicate experiments plus or minus standard deviation.
DISCUSSION
The adhesive properties due to the presence of adhesins or lectins on the surface of bacteria have been studied intensively for numerous bacteria, as well as the effect of these structures on bacterial virulence (12, 13, 24, 25). In our study concerning the adhesive properties of the fish pathogenic bacterium F. psychrophilum, hemagglutinating studies divided the strains into two groups. One group of strains (HA positive) adhered to erythrocytes, whereas the other group of strains (HA negative) was either negative or greatly reduced in its adhesive capacity. Strains of the virulent serotype Th were shown to either lack hemagglutinating ability or to only express this ability to a very low degree. The picture of HA capacities was not as clear for serotypes Fd and FpT. Strains of these serotypes could generally be characterized as HA positive, although some strains expressed low HA abilities at 15°C. Previously eight of the strains used in this study have been examined for virulence following intraperitoneal injections showing three strains to be virulent (900406-1/3, 69/3A, and 950106-1/1) and five strains to be nonvirulent (62/4, 68/5A, 99/10A, 66/8A, and NCMB1947) (18, 19). Out of these three virulent strains, two were HA negative and one HA positive and the nonvirulent strains were shown to be both HA negative as well as HA positive. Accordingly no direct correlation could be made between the previously tested virulence and the ability to hemagglutinate. The present study showed an increased adherence ability of bacteria grown at 5°C and on solid surfaces, and since the bacterial cultures used to test virulence were grown at 15°C and in broth, further studies would be needed to determine the importance of HA as a virulence factor. Previously ribotypes, elastin-degrading properties, and plasmid content have been examined for the different strains (5, 18, 19), but no apparent correlation could be made with any of these characteristics and the adhesive properties of the HA-positive and HA-negative strains. Neither could any correlation be made to the size of the fish nor to any particular organ from which the strain was isolated.
The higher adhesive abilities of F. psychrophilum grown on solid surfaces indicated an increased expression of an adhesive factor, e.g., a lectin. Most bacterial lectins are organized as the thin threadlike organelles referred to as pili or fimbriae (12). No reports have been made about pilus expression on the surface of F. psychrophilum, and Flavobacteria (formerly known as Cytophaga) are generally considered to lack pilus expression (9, 20, 26). One member of this group of bacteria, Flavobacterium branchiophilum, the fish pathogenic agent of bacterial gill disease, has nevertheless been reported to possess pili (10). Studies of the human pathogen Haemophilus influenzae type b have shown that strains without pili are more resistant to complement-mediated bacteriolysis in vitro and thus cause higher mortality in vivo (21). The low expression of the HA-responsible adhesin among the F. psychrophilum strains of the virulent serotype Th as well as the high expression in the nonvirulent serotype FpT might influence the pathogenicity mechanisms of this bacterium in a similar manner.
The proteinaceous nature of the structure responsible for HA suggested that the HA-positive F. psychrophilum possessed a lectin on its surface, which was lacking on the surface of HA-negative strains. This lectin interacts specifically to sialic acid and may be involved in the virulence and pathogenicity of F. psychrophilum as well as play a role in the immune response of the host. The association of F. psychrophilum and rainbow trout phagocytes has been suggested to be influenced by sialic acid (29), and sialic acid-specific lectins have been reported for other bacteria, e.g., K99 on Escherichia coli (13, 22). In the present study we treated rainbow trout erythrocytes with sialidase in order to destroy the sialic acids present on the erythrocytes. An inhibition of the HA was observed for two of the four tested strains with use of lower temperatures and longer incubation times than previously reported. The conformation of sialic acids has been reported as essential for the adhesion of sialic acid-binding lectins (22), and further studies should be conducted to determine the exact effect of sialidase on the sialic acids of rainbow trout erythrocytes.
A polysaccharide-thick slime or capsular layer that was released to the culture medium has been reported for F. psychrophilum (4). The amount and expression of this capsular layer in several F. psychrophilum strains as well as under different growth conditions have, to our knowledge, not been studied, and this polysaccharide-rich layer might affect the HA as well as the hydrophobic properties. The HA-responsible lectin could be incorporated into the capsule, or the adhesion might be assisted by the polysaccharide chains that make up the capsule. The adhesion of some bacteria has been shown to be destroyed by oxidation with sodium periodate (6, 7, 8), including the adhesion of F. psychrophilum to rainbow trout phagocytes (29), indicating the importance of capsular polysaccharides for these bacteria. Still, we found no differences in HA properties following oxidation of polysaccharides on the surface of F. psychrophilum. An agglutinating ability was, however, found for the supernatant, which indicated at least some release of adhesin from the bacterial surface.
F. psychrophilum has previously been reported to possess limited HA properties without major adherence differences between strains (17); however, these studies were conducted in microtiter plates and the observed difference in HA titers from our studies in microtiter plates and on glass tiles could explain the discrepancy between these prior results and the results of this study. The bacterial adhesion to plastic surfaces could likewise be caused by the high hydrophobicity observed in almost all the tested strains. A high degree of hydrophobicity with no strain variation has previously been reported for F. psychrophilum (28).
The evidence presented here suggests that a sialic acid-binding lectin is involved in F. psychrophilum adhesion. This lectin may only be present on some F. psychrophilum strains, as some strains are impaired in their adhesive abilities. Although these adhesion differences do not appear to correlate with the virulence of this fish pathogenic bacterium, these results provide insights into interaction between F. psychrophilum and rainbow trout as well as demonstrate the broad adhesion varieties among strains of one bacterial species.
Acknowledgments
This work was supported by The Danish Institute for Fisheries Research and The Danish Research Academy.
We thank Kirsten Kaas for her skillful technical assistance. The provision of the plasmid-free strain 910611-1 by Ellen Lorenzen is greatly appreciated.
REFERENCES
- 1.Alsever, J. B., and R. B. Ainslie. 1941. A new method for the preparation of dilute blood plasma and the operation of a complete transfusion service. N. Y. State J. Med. 41:126-131. [Google Scholar]
- 2.Bernardet, J.-F., P. Segers, M. Vancanneyt, F. Berthe, K. Kersters, and P. Vandamme. 1996. Cutting a Gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (basonym, Cytophaga aquatilis Strohl and Tait 1978). Int. J. Syst. Microbiol. 46:128-148. [Google Scholar]
- 3.Borg, A. F. 1960. Studies on myxobacteria associated with diseases in salmonid fishes. J. Wildl. Dis. 8:1-85. [Google Scholar]
- 4.Crump, E. M., M. B. Perry, S. C. Clouthier, and W. W. Kay. 2001. Antigenic characterization of the fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 67:750-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalsgaard, I., and L. Madsen. 2000. Bacterial pathogens in rainbow trout, Oncorhynchus mykiss (Walbaum), reared at Danish freshwater farms. J. Fish Dis. 23:199-209. [Google Scholar]
- 6.Decostere, A., F. Haesebrouck, G. Charlier, and R. Ducatelle. 1999. The association of Flavobacterium columnare strains of high and low virulence with gill tissue of black mollies (Poecilia sphenops). Vet. Microbiol. 67:287-298. [DOI] [PubMed] [Google Scholar]
- 7.Decostere, A., F. Haesebrouck, E. Van Driessche, G. Charlier, and R. Ducatelle. 1999. Characterization of the adhesion of Flavobacterium columnare (Flexibacter columnaris) to gill tissue. J. Fish Dis. 22:465-474. [Google Scholar]
- 8.Haque, M. A., F. Qadri, K. Ohki, and O. Kohashi. 1995. Surface components of Shigellae involved in adhesion and hemagglutination. J. Appl. Bacteriol. 79:186-194. [DOI] [PubMed] [Google Scholar]
- 9.Henrichsen, J., and J. Blom. 1975. Examination of fimbriation of some gram-negative rods with and without twitching and gliding motility. Acta Pathol. Microbiol. Scand. 83:161-170. [DOI] [PubMed] [Google Scholar]
- 10.Heo, G.-J., H. Wakabayashi, and S. Watabe. 1990. Purification and characterization of pili from Flavobacterium branchiophila. Fish Pathol. 25:21-27. [Google Scholar]
- 11.Holt, R. A., J. S. Rohovec, and J. L. Fryer. 1993. Bacterial coldwater disease, p. 3-23. In V. Inglis, R. J. Roberts, and N. R. Bromage (ed.), Bacterial diseases of fish. Blackwell Scientific Publications, Oxford, United Kingdom.
- 12.Jacques, M., and S. E. Paradis. 1998. Adhesin-receptor interactions in Pasteurellaceae. FEMS Microbiol. Rev. 22:45-59. [DOI] [PubMed] [Google Scholar]
- 13.Klemm, P., and M. A. Schembri. 2000. Bacterial adhesins: function and structure. Int. J. Med. Microbiol. 290:27-35. [DOI] [PubMed] [Google Scholar]
- 14.Korhonen, T. K., and J. Finne. 1985. Agglutination assays for detecting bacterial binding specificities, p. 301-313. In T. K. Korhonen, E. A. Dawes, and P. H. Mäkelä (ed.), Enterobacterial surface antigens: methods for molecular characterisation. Elsevier Science Publishers B. V., Amsterdam, Holland.
- 15.Larsen, J. L., and J. E. Olsen. 1991. Occurrence of plasmids in Danish isolates of Vibrio anguillarum serovars O1 and O2 and association of plasmids with phenotypic characteristics. Appl. Environ. Microbiol. 57:2158-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindahl, M., A. Faris, T. Wadstrom, and S. Hjerten. 1981. A new test based on ′salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim. Biophys. Acta 677:471-476. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzen, E., I. Dalsgaard, and J. Bernardet. 1997. Characterization of isolates of Flavobacterium psychrophilum associated with coldwater disease or rainbow trout fry syndrome I: phenotypic and genomic studies. Dis. Aquat. Org. 31:197-208. [Google Scholar]
- 18.Madsen, L., and I. Dalsgaard. 1998. Characterization of Flavobacterium psychrophilum: comparison of proteolytic activity and virulence of strains isolated from rainbow trout (Oncorhynchus mykiss), p. 45-52. In A. C. Barnes, G. A. Davidson, M. P. Hiney, and D. McIntosh (ed.), Methodology in fish diseases research. Fisheries Research Services, Aberdeen, United Kingdom.
- 19.Madsen, L., and I. Dalsgaard. 2000. Comparative studies of Danish Flavobacterium psychrophilum isolates: ribotypes, plasmid profiles, serotypes and virulence. J. Fish Dis. 23:211-218. [Google Scholar]
- 20.McBride, M. J. 2001. Bacterial gliding motility: multiple mechanisms for cell movement over surfaces. Annu. Rev. Microbiol. 55:49-75. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki, S., K. Tateda, T. Matsumoto, N. Furuya, and K. Yamaguchi. 1999. The pathogenic role of fimbriae of Haemophilus influenzae type b in murine bacteraemia and meningitis. J. Appl. Bacteriol. 48:383-388. [DOI] [PubMed] [Google Scholar]
- 22.Mouricout, M. 1997. Interactions between the enteric pathogen and the host. An assortment of bacterial lectins and a set of glycoconjugate receptors. Adv. Exp. Med. Biol. 412:109-123. [PubMed] [Google Scholar]
- 23.Mozes, N., and P. G. Rouxhet. 1987. Methods for measuring hydrophobicity of microorganisms. J. Microbiol. Methods 6:99-112. [Google Scholar]
- 24.Ofek, I., and E. H. Beachey. 1980. Bacterial adherence. Adv. Intern. Med. 25:505-532. [PubMed] [Google Scholar]
- 25.Ofek, I., and R. J. Doyle. 1994. Bacterial adhesion to cells and tissues. Chapman & Hall, Inc., New York, N.Y.
- 26.Pate, J. L. 1985. Gliding motility in Cytophaga. Microbiol. Sci. 2:289-295. [PubMed] [Google Scholar]
- 27.Paulson, J. C., and G. N. Rogers. 1987. Resialylated erythrocytes for assessment of the specificity of sialyloligosaccharide binding proteins. Methods Enzymol. 138:162-168. [DOI] [PubMed] [Google Scholar]
- 28.Vatsos, I. N., K. D. Thompson, and A. Adams. 2001. Adhesion of the fish pathogen Flavobacterium psychrophilum to unfertilized eggs of rainbow trout (Oncorhynchus mykiss) and n-hexadecane. Lett. Appl. Microbiol. 33:178-182. [DOI] [PubMed] [Google Scholar]
- 29.Wiklund, T., and I. Dalsgaard. Association of Flavobacterium psychrophilum with rainbow trout (Oncorhynchus mykiss) kidney phagocytes in vitro. Fish Shellfish Immunol., in press. [DOI] [PubMed]