Abstract
Vibrio cholerae is a versatile bacterium that flourishes in diverse environments, including the human intestine, rivers, lakes, estuaries, and the ocean. Surface attachment is believed to be essential for colonization of all of these natural environments. Previous studies have demonstrated that the vps genes, which encode proteins required for exopolysaccharide synthesis and transport, are required for V. cholerae biofilm development in Luria-Bertani broth. In this work, we showed that V. cholerae forms vps-dependent biofilms and vps-independent biofilms. The vps-dependent and -independent biofilms differ in their environmental activators and in architecture. Our results suggest that environmental activators of vps-dependent biofilm development are present in freshwater, while environmental activators of vps-independent biofilm development are present in seawater. The distinct environmental requirements for the two modes of biofilm development suggest that vps-dependent biofilm development and vps-independent biofilm development may play distinct roles in the natural environment.
Vibrio cholerae is the agent of the dreaded waterborne disease cholera. Cholera is characterized by massive diarrhea, resulting in severe dehydration and even death. When V. cholerae is not wreaking havoc in the human intestine, it may be found in diverse aquatic environments, such as the ocean, estuaries, rivers, and lakes (5, 10, 26, 30, 36). Adhesion to surfaces both in the human intestine and in the aquatic environment plays an important role in V. cholerae's success as a pathogen and an environmental organism.
Bacteria attached to a surface, which are collectively termed a bacterial biofilm, have been a subject of intense interest in recent years due to the predominance of biofilm-associated bacteria in natural environments, the complex developmental pathway that bacteria follow in forming a biofilm, and the role of biofilm formation in antibiotic-resistant bacterial infections (7, 22). Many gram-negative bacteria use flagella and/or pili to associate with surfaces (2, 9, 15, 16, 25, 28, 31, 32, 40, 41). The subsequent accumulation of bacteria on surfaces with the characteristic biofilm architecture of pillars of bacteria surrounded by water channels is thought to require exopolysaccharide synthesis (6, 40-43). Surface adhesion and exopolysaccharide production by bacteria have been shown to depend on the inorganic and organic compositions of the bathing medium. In particular, high concentrations of Na+ and Ca2+ in growth media have been shown to enhance biofilm development by diverse bacteria (17, 18, 34, 35). Furthermore, the carbohydrate composition of the growth medium influences exopolysaccharide production and biofilm development by many bacterial species (1, 3, 4, 21, 24, 35).
Exopolysaccharide-dependent and -independent biofilms have been described for both Pseudomonas aeruginosa and Escherichia coli. In a rich growth medium, wild-type P. aeruginosa and E. coli form thick biofilms with pillars and channels. When quorum sensing is eliminated in P. aeruginosa by mutation of the lasI gene, however, a flat, densely packed biofilm is formed. This biofilm exhibits increased susceptibility to detergents (8). In E. coli, a similar flat biofilm is observed when genes for synthesis of the exopolysaccharide colanic acid are disrupted (6).
V. cholerae biofilm development has previously been studied in Luria-Bertani (LB) broth, a complex, undefined medium containing 100 mM NaCl, 1.0% yeast extract, and 1.5% tryptone (12, 27, 37, 39, 40, 43). In this medium, V. cholerae forms a thick biofilm containing pillars of bacteria surrounded by water channels (41). The VPS exopolysaccharide is required for formation of this biofilm structure. This exopolysaccharide is synthesized and exported by proteins encoded by genes both in the vps locus and in other loci (27, 43). In the absence of the vps genes, cells form only a monolayer on the surface (40, 43). Furthermore, vps transcription is greater in biofilm-associated cells than in planktonic cells, suggesting that vps gene transcription is activated by surface association (12).
In this study, we explored the environmental activators of V. cholerae biofilm development in the context of a defined growth medium. We obtained qualitative and quantitative evidence that V. cholerae forms vps-dependent biofilms and vps-independent biofilms in response to distinct environmental signals. V. cholerae is found in both freshwater and seawater environments. Our findings suggest that only vps-dependent biofilm development is possible in freshwater environments, while both vps-dependent biofilm development and vps-independent biofilm development are possible in seawater environments. Our results suggest that vps-dependent surface adhesion and vps-independent surface adhesion play different roles in aquatic environments.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains used in our experiments are described in Table 1. Biofilms were formed in a medium supplemented with either 1% yeast extract (Difco) and 1.5% tryptone (Difco) (YT) or 1% vitamin assay Casamino Acids (CAA) (Difco). Various salt mixtures were added to this base, as follows: 10 mM NaCl, 100 mM NaCl, commercial artificial seawater (Instant Ocean; Aquarium Systems), a defined mixture of the salts reported to be in Instant Ocean (defined seawater [DSW], containing 468 mM NaCl, 55 mM MgSO4 · 7H2O, 3.0 mM NaHCO3, 9.9 mM CaCl2 · 2H2O, 10.3 mM KCl, 0.14 mM Na2B4O7 · 10H2O, 0.1 mM SrCl2 · 6H2O, 0.03 mM NaBr, 0.002 mM NaI, and 0.026 mM LiCl), autoclaved river water (Charles River, Newton, Mass.), and autoclaved real seawater (Crane Beach, Mass.). Where indicated below, 0.16% monosaccharides (0.015% glucose, 0.048% galactose, 0.098% mannose) was added to the growth medium. This monosaccharide mixture was determined to be equivalent to that found in a mixture of 1% yeast extract and 1.5% tryptone based on a monosaccharide analysis performed on the reagents used in our laboratory.
TABLE 1.
Bacterial strains and plasmid used
| Strain or plasmid | Characteristics | Reference |
|---|---|---|
| Strains | ||
| V. cholerae MO10 | 1992 clinical isolate of V. cholerae O139 from India; Smr | 38 |
| PW328 | MO10 ΔvpsL, Smr | 12 |
| PW357 | MO10 lacZ::vpsLp→lacZ, Smr | 12 |
| PW396 | MO10 ΔvpsA, Smr | This study |
| PW397 | MO10 ΔvpsLΔvpsA, Smr | This study |
| Plasmid pAJH9 | pWM91 carrying a fragment of the vpsA operon harboring an internal, unmarked deletion | This study |
Construction of a vpsA deletion mutant.
A large operon required for VPS synthesis extends from VC0917 to VC0927 (vpsA operon). A 407-bp fragment located between positions 979148 and 979555 on chromosome 1 and a 462-bp fragment located between positions 990697 and 991099 on chromosome 1 were amplified by PCR. The internal primers included a complementary 15-bp sequence at the 3′ and 5′ ends, respectively, as previously described (12). These two fragments were joined by using the technique of splicing by overlap extension, which resulted in construction of a fragment with an 11,141-bp deletion in the vpsA operon (14). The fragment containing the deletion was gel purified and ligated into pCR2.1TOPO (Invitrogen). This fragment was then removed from pCR2.1TOPO by digestion with SpeI and XhoI and ligated into pWM91 to create a suicide plasmid, pAJH9. This plasmid was used to create vpsA operon deletions in the relevant strains by double homologous recombination and sucrose selection as previously described (12).
Biofilm assays.
Borosilicate glass tubes were filled with 300 μl of the growth medium as indicated below and inoculated with the strain under study. For reproducibility, experiments were initiated at an optical density at 595 nm (OD595) of 0.02. Unless specifically indicated otherwise, cells were allowed to adhere to the surface during 18 h of incubation at 27°C. Biofilms were visualized by incubation with a 1-mg/ml aqueous solution of crystal violet as previously described (12). Surface-adherent cells were quantified as follows. Following formation of the biofilm, planktonic cells were removed, and the tubes were rinsed and filled with 300 μl of fresh medium. An equivalent volume of 1-mm-diameter borosilicate glass beads (Biospec) was added to each tube. Biofilm-associated cells were mechanically removed and dispersed by vortexing in the presence of these beads for approximately 10 s. Finally, the OD595 of the planktonic and biofilm cell suspensions were measured. This rapid method of quantifying biofilm-associated cells was quite reproducible, and the results compared favorably with measurements based on total protein and viable cell counts. Furthermore, in these assays, this method was more accurate than quantification of biofilm-associated crystal violet by solubilization with dimethyl sulfoxide.
Fluorescence microscopy and quantitative analysis of biofilm structure.
Fluorescence microscopy was used to evaluate the three-dimensional biofilm structure. The biofilms used for fluorescence microscopy were formed on borosilicate glass coverslips that were placed in 50-ml Falcon tubes containing 7.0 ml of medium. Bacteria were inoculated into the medium to obtain an initial OD595 of 0.05, and biofilms were formed over a 24-h period. 4′,6-Diamidino-2-phenylindole (DAPI), which penetrates bacterial cell membranes to form a fluorescent complex with DNA, was used to visualize biofilms. Seven microliters of a 1-mg/ml aqueous solution of DAPI was added directly to each culture following formation of the biofilm. Then the coverslip, with its associated biofilm, was incubated with DAPI for 10 min.
The coverslip was removed, rinsed with fresh medium, and placed on a concave microscope slide filled with sterile medium. An Eclipse TE-200 inverted fluorescence microscope (Nikon) equipped with an Orca digital charge-coupled device camera (Hamamatsu), VVM-D1 shutter drivers (Uniblitz), and a focus motor was used to collect a stack of evenly spaced transverse sections through the biofilm. The image stacks were subjected to nearest-neighbor deconvolution and three-dimensional image reconstruction by using the Metamorph Imaging software (Universal Imaging). Various attributes of the biofilm structure were then quantified by using the COMSTAT software developed by Heydorn and colleagues (13). In this study, total biofilm biomass, substratum coverage, average biofilm thickness, and maximum biofilm thickness were used to quantitatively describe the biofilm architecture. The total biofilm biomass was determined by dividing the volume of cells in the three-dimensional biofilm by the substratum surface area analyzed. The substratum coverage was the proportion of the total substratum surface area that was covered by cells. The values reported below are averages for six image stacks (three image stacks collected in two independent experiments).
β-Galactosidase measurements.
β-Galactosidase assays were performed as previously described (12), with the following modifications. For measurements of vpsL promoter activity in both planktonic and biofilm-associated cells, wild-type V. cholerae was incubated at 27°C for 24 h in 50-ml Falcon tubes containing 3 ml of growth medium. After transfer of planktonic cells to new tubes, the biofilms were rinsed with sterile growth medium and removed from the surface by agitation with 1-mm-diameter borosilicate beads (BioSpec) as described above. The OD595 of each resulting suspension was measured, and biofilm-associated cells were then gently pelleted and resuspended in 1 ml of Z buffer. The cells were then lysed by three freeze-thaw cycles and centrifuged to remove the cell debris. A 100-μl aliquot of the lysate was set aside for protein determination with a Bradford assay kit (Pierce), and o-nitrophenyl-β-d-galactopyranoside (Sigma) was added to the remaining lysate. Samples were incubated for 18 h at 37°C to allow a yellow color to develop, and the A405 of each sample was measured. To obtain a relative β-galactosidase activity measurement for each sample, the A405 was multiplied by 1,000 and then divided by the calculated protein concentration or the OD595. All experiments were performed in triplicate.
Biofilm matrix isolation and analysis.
V. cholerae biofilms were formed in LB broth or commercial artificial seawater supplemented with CAA over a period of 72 h. V. cholerae ΔvpsL, ΔvpsA, or ΔvpsLA mutant biofilms were formed under similar conditions in commercial artificial seawater supplemented with CAA. In all experiments, planktonic cells were removed, and biofilm cells were dispersed and separated from the biofilm matrix by bead beating at 5,000 rpm for 10 s with 1/3 volume of 1-mm-diameter borosilicate beads (BioSpec). Intact V. cholerae cells were removed by centrifugation at 4,000 × g. Three volumes of 95% ethanol was added to the supernatant, and exopolysaccharide and other components of the biofilm matrix were then allowed to precipitate overnight at 4°C. The precipitate was collected by centrifugation and sent to the Glycobiology Core at the University of San Diego for monosaccharide analysis.
Analysis of river and seawater compositions.
Elemental and osmolarity analyses were performed on aliquots of Charles River water and Massachusetts seawater. An elemental analysis was performed by a commercial laboratory (Alpha Analytical Laboratories, Westborough, Mass.). Osmolarity was measured with a freezing point osmometer (model 3300; Advanced Instruments).
RESULTS AND DISCUSSION
V. cholerae is a human pathogen and a natural inhabitant of riverine, estuarine, and marine environments. In these varied environments, bacteria are principally found in association with a surface. We hypothesized that the ionic and nutrient compositions of the bathing medium might determine the nature of the biofilm formed. In particular, we were interested in identifying the environmental determinants of V. cholerae biofilm development, with the ultimate goal of relating these findings to freshwater and marine environments.
V. cholerae biofilm development is vps dependent in 10 mM NaCl-YT and vps independent in artificial seawater.
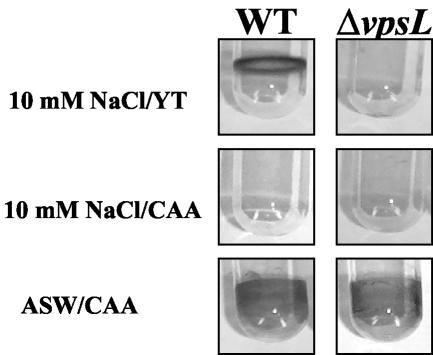
It has been demonstrated previously that the wild-type V. cholerae O139 biofilm formed in LB broth depends on the vpsL gene product (12). LB broth, however, is a complex, poorly defined medium consisting of 100 mM NaCl, the products of a tryptic digest of casein, and the products of an ethanol extract of Saccharomyces cerevisiae. In order to understand the environmental enhancers of biofilm development, our goal was to develop a better defined growth medium that activated V. cholerae biofilm development. Because V. cholerae is found in both freshwater and seawater environments, we first compared V. cholerae biofilm development in growth media consisting of an inorganic base, either 10 mM NaCl or commercial artificial seawater, supplemented with either YT or CAA. Crystal violet-stained biofilms resulting from culture of wild-type V. cholerae and a ΔvpsL mutant in these media are shown in Fig. 1. Wild-type V. cholerae, but not the ΔvpsL mutant, was able to form a robust biofilm in 10 mM NaCl supplemented with YT. These results are similar to the results of previous studies of V. cholerae biofilm development in LB broth (40, 41). Amino acids in the form of peptides and proteins are a major component of yeast extract and tryptone. Thus, we tested whether addition of CAA alone to 10 mM NaCl supported wild-type V. cholerae biofilm development. In this medium, however, no biofilm was observed. Thus, supplementation with amino acids alone was not sufficient to induce wild-type V. cholerae biofilm development in 10 mM NaCl. However, biofilm development by wild-type V. cholerae and the ΔvpsL mutant in artificial seawater medium supplemented with CAA was surprisingly different. Commercial artificial seawater supplemented with CAA induced robust biofilm development by both wild-type V. cholerae and the ΔvpsL mutant. The same result was observed for biofilms formed in commercial artificial seawater supplemented with YT (results not shown). The bacterial growth in all media containing YT as a nutrient source was approximately four times that in media containing CAA. This probably reflects the B vitamins, iron, and other nutrients that are abundant in YT but not in vitamin assay CAA.
FIG. 1.
Representative wild-type V. cholerae strain MO10 (WT) and ΔvpsL mutant PW328 biofilms formed over 18 h in 10 mM NaCl or commercial artificial seawater (ASW) supplemented with either YT or CAA. Biofilms were visualized by crystal violet staining.
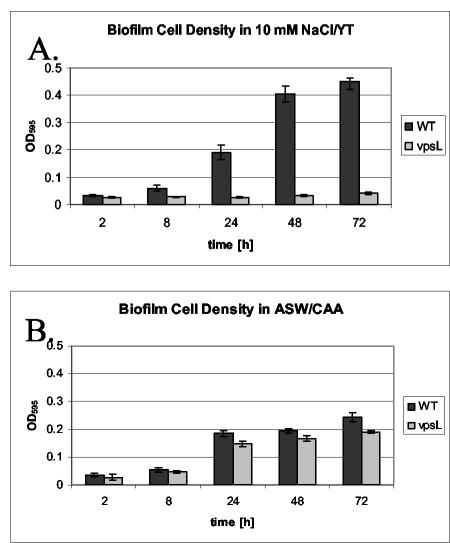
In order to quantify surface attachment, we harvested biofilms from borosilicate tubes at various times after inoculation. The increases in surface-attached cells over time in the various media are shown in Fig. 2. Wild-type V. cholerae was able to accumulate on borosilicate surfaces bathed in 10 mM NaCl supplemented with YT, but the ΔvpsL mutant was not (Fig. 2A). In 10 mM NaCl supplemented with CAA, neither wild-type V. cholerae nor ΔvpsL mutant cells accumulated on the borosilicate surface in significant numbers. After 72 h, the OD595 of surface-associated cells did not exceed 0.05 (data not shown). In contrast, when commercial artificial seawater was supplemented with CAA, the numbers of surface-associated wild-type V. cholerae and ΔvpsL mutant cells were similar (Fig. 2B). It should be noted that the amount of surface-attached cells in the biofilm formed in commercial artificial seawater supplemented with CAA was only one-half the amount of surface-attached cells in the biofilm formed in 10 mM NaCl supplemented with YT. This suggests that there was either an increased rate of cell attachment to or a decreased rate of cell detachment from the biofilm formed in 10 mM NaCl supplemented with YT compared with the rate for the biofilm formed in commercial artificial seawater.
FIG. 2.
Accumulation over time of wild-type V. cholerae strain MO10 (WT) and a ΔvpsL mutant (PW328) on a borosilicate surface in two types of media, 10 mM NaCl-YT (A) and commercial artificial seawater (ASW) supplemented with CAA (B).
From these experiments, we formulated the following three hypotheses: (i) biofilm development in 10 mM NaCl supplemented with YT requires the vps genes and, therefore, is vps dependent, (ii) biofilm development in artificial seawater does not require the vps genes and, therefore, is vps independent, and (iii) vps-dependent biofilm development requires a component of YT that is not present in CAA.
The vps-dependent and -independent biofilms differ in architecture.
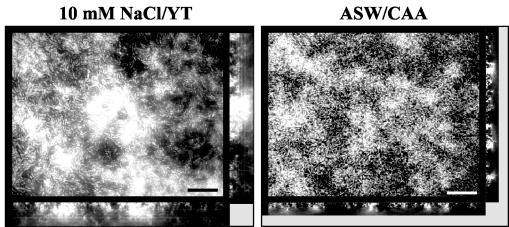
Exopolysaccharide is thought to be required for the prototypical biofilm structure consisting of pillars of bacteria surrounded by water channels. Thus, we hypothesized that the structure of the vps-independent biofilm might differ from that of the vps-dependent biofilm (6). In order to test this hypothesis, deconvolution fluorescence microscopy was used to visualize biofilms formed in 10 mM NaCl-YT and artificial seawater supplemented with CAA. As shown in Fig. 3, substantial V. cholerae surface accumulation was observed in both growth media. While the biofilm formed in 10 mM NaCl supplemented with YT consisted of tall pillars interspersed with water channels, the biofilm formed in commercial artificial seawater supplemented with CAA had smaller, more closely spaced pillars.
FIG. 3.
Transverse and vertical cross sections through DAPI-stained wild-type V. cholerae strain MO10 biofilms formed in 10 mM NaCl-YT and commercial artificial seawater (ASW) supplemented with CAA. Transverse cross sections were obtained at the level of the substratum. Bars = 10 μm.
In order to more objectively assess our qualitative impressions of the vps-dependent and -independent biofilm architectures, we used the Comstat program designed by Heydorn et al. (13). Biomass, substratum coverage, average biofilm thickness, and maximum biofilm thickness were used to characterize each biofilm (Tables 2 and 3). When normalized to surface area, the total biomass of the wild-type V. cholerae biofilm formed in 10 mM NaCl supplemented with YT was 2.5 times that of the biofilm formed in commercial artificial seawater supplemented with CAA. Although the surface coverage values were similar for the two biofilms, the average biofilm thickness and the maximum biofilm thickness of the biofilm formed in 10 mM NaCl supplemented with YT were approximately three and two times, respectively, greater than those of the biofilm formed in commercial artificial seawater supplemented with CAA. These results substantiated our qualitative impressions based on microscopy.
TABLE 2.
Quantitative comparison of the architectures of wild-type V. cholerae biofilms formed in various model freshwater mediaa
| Medium | Biomass (μm3/μm2) | Fraction of substratum covered by cells | Avg thickness (μm) | Maximum thickness (μm) |
|---|---|---|---|---|
| 10 mM NaCl/YT | 1.93 ± 0.66 | 0.58 ± 0.06 | 2.83 ± 1.02 | 10.19 ± 1.15 |
| 10 mM NaCl/CAA | 0.11 ± 0.02 | 0.08 ± 0.01 | 0.16 ± 0.02 | 1.49 ± 0.31 |
| 10 mM NaCl/CAA/CRB | 1.85 ± 0.27 | 0.42 ± 0.05 | 2.17 ± 0.31 | 11.38 ± 0.69 |
| CRW/CAA | 0.4 ± 0.06 | 0.37 ± 0.04 | 0.45 ± 0.06 | 2.64 ± 0.84 |
Biofilms were formed in either 10 mM NaCl supplemented with YT (10 mM NaCl/YT), 10 mM NaCl supplemented with CAA (10 mM NaCl/CAA), 10 mM NaCl supplemented with CAA and monosaccharides (10 mM NaCl/CAA/CRB), or Charles River water supplemented with CAA (CRW/CAA). Values were calculated by using the Comstat program (13).
TABLE 3.
Quantitative comparison of the architectures of wild-type V. cholerae and ΔvpsL mutant biofilms formed in three model seawater mediaa
| Medium | Strain | Biomass (μm3/μm2) | Fraction of substratum covered by cells | Avg thickness (μm) | Maximum thickness (μm) |
|---|---|---|---|---|---|
| ASW/CAA | Wild type | 0.76 ± 0.01 | 0.59 ± 0.06 | 0.96 ± 0.07 | 5.39 ± 0.24 |
| ΔvpsL | 0.45 ± 0.01 | 0.62 ± 0.02 | 0.48 ± 0.04 | 2.62 ± 0.03 | |
| DSW/CAA | Wild type | 0.29 ± 0.02 | 0.44 ± 0.03 | 0.41 ± 0.05 | 2.0 ± 0.48 |
| ΔvpsL | 0.23 ± 0.05 | 0.41 ± 0.05 | 0.30 ± 0.05 | 1.76 ± 0.25 | |
| RSW/CAA | Wild type | 0.41 ± 0.08 | 0.42 ± 0.05 | 0.55 ± 0.08 | 6.66 ± 0.4 |
| ΔvpsL | 0.40 ± 0.02 | 0.45 ± 0.08 | 0.51 ± 0.05 | 5.64 ± 1.77 |
Biofilms were formed in either commercial artificial seawater supplemented with CAA (ASW/CAA), seawater prepared from defined components supplemented with CAA (DSW/CAA), or seawater from the Massachusetts coast supplemented with CAA (RSW/CAA). Values were calculated by using the Comstat program (13).
Monosaccharides are critical activators of vps-dependent biofilm formation.
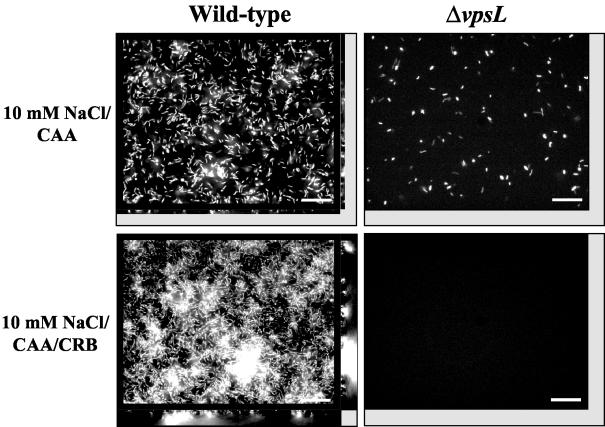
We observed that supplementation of 10 mM NaCl with CAA did not activate vps-dependent biofilm development by wild-type V. cholerae, while addition of YT did. Thus, we hypothesized that a nonproteinaceous component of YT must be an activator of and/or a substrate for VPS synthesis. In an attempt to activate vps-dependent biofilm development, B vitamins, choline, iron, and nucleic acids were added to 10 mM NaCl supplemented with CAA at concentrations similar to those reported for YT. However, no V. cholerae biofilm was observed in the presence of these supplements (data not shown). Monosaccharide analysis of YT revealed the presence of mannose, glucose, and galactose. Because the presence of monosaccharides in growth medium has been shown to influence exopolysaccharide production and biofilm formation by a wide variety of bacteria, we tested whether supplementation of 10 mM NaCl-CAA with monosaccharides activated vps-dependent biofilm formation by V. cholerae. In fact, when wild-type V. cholerae was cultured in 10 mM NaCl supplemented with CAA, 0.098% mannose, 0.015% glucose, and 0.048% galactose, a biofilm formed (Fig. 4). The vps mutant, however, formed no biofilm in 10 mM NaCl supplemented with CAA and monosaccharides. Both glucose alone and mannose alone were able to activate vps-dependent biofilm development. Galactose, however, augmented vps-dependent biofilm development only in the presence of glucose or mannose (data not shown).
FIG. 4.
Architecture of V. cholerae biofilms formed in 10 mM NaCl supplemented with monosaccharides and/or CAA: transverse and vertical cross sections through DAPI-stained wild-type V. cholerae strain MO10 and ΔvpsL mutant PW328 biofilms in 10 mM NaCl supplemented with CAA and 10 mM NaCl supplemented with CAA and monosaccharides (CRB). Transverse sections were obtained at the level of the substratum. Bars = 10 μm.
We also used quantitative biofilm parameters to compare the structures of wild-type V. cholerae biofilms formed in 10 mM NaCl supplemented with YT, with CAA, or with CAA and monosaccharides (Table 2). The biomass of the biofilm formed in 10 mM NaCl supplemented with CAA was 17-fold less than that of the biofilm formed in 10 mM NaCl supplemented with YT. Substratum coverage, average thickness, and maximum thickness were also severalfold less. However, the quantitative parameters describing the biofilm formed in 10 mM NaCl supplemented with CAA and monosaccharides were remarkably similar to those describing the biofilm formed in 10 mM NaCl supplemented with YT. These results support the hypothesis that the polysaccharide component of YT induces vps-dependent biofilm development by V. cholerae.
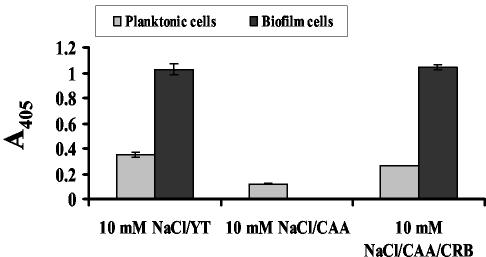
Because monosaccharides are the substrates for exopolysaccharide synthesis, we questioned whether monosaccharide-rich growth medium activated transcription of vps genes or merely accelerated exopolysaccharide synthesis by providing an abundance of monosaccharide building blocks. To determine this, we measured vpsL transcription in biofilm-associated and planktonic cells grown in 10 mM NaCl supplemented with CAA in the presence and absence of monosaccharides and compared the results to the results obtained for vpsL transcription of planktonic and biofilm-associated cells grown in 10 mM NaCl supplemented with YT. As shown in Fig. 5, vpsL transcription in biofilms formed in 10 mM NaCl supplemented with YT was 3-fold higher than vpsL transcription in the neighboring planktonic cells and 10-fold higher than vpsL transcription in planktonic cells grown in 10 mM NaCl supplemented with CAA. However, when the monosaccharide mixture was added to 10 mM NaCl supplemented with CAA, the pattern of vpsL transcription observed for planktonic and biofilm-associated cells was similar to the pattern observed for planktonic and biofilm-associated cells grown in 10 mM NaCl supplemented with YT. These data suggest that in addition to serving as the building blocks of VPS, monosaccharides activate vps transcription in both planktonic and biofilm-associated cells.
FIG. 5.
Normalized β-galactosidase activities of biofilm-associated and planktonic wild-type V. cholerae strain PW357 cells grown in 10 mM NaCl-YT, 10 mM NaCl-CAA, and 10 mM NaCl supplemented with CAA and carbohydrates (CRB). This strain carries a chromosomal fusion of the vpsL promoter to lacZ.
Calcium is required for formation of the vps-independent biofilm.
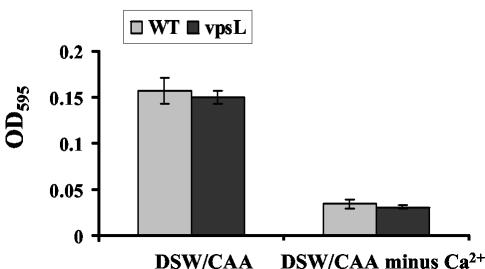
We hypothesized that one or several components of artificial seawater were responsible for induction of the vps-independent biofilm. We initially used commercial artificial seawater in our experiments. Commercial artificial seawater, however, is intended for salt water aquariums rather than for laboratory experiments. In order to create a better defined medium whose composition could be altered, we purchased high-purity inorganic components and combined them in the concentrations reported to be in commercial artificial seawater to obtain DSW. The biofilms formed by wild-type V. cholerae and a ΔvpsL mutant in DSW supplemented with CAA are shown in Fig. 6. The biofilms formed in DSW exhibited the vps independence observed for biofilms formed in commercial artificial seawater. We also used quantitative parameters to compare wild-type V. cholerae and ΔvpsL mutant biofilms formed in commercial artificial seawater or DSW supplemented with CAA (Table 3). Interestingly, this analysis underscored differences between the biofilms formed in commercial artificial seawater and DSW. The biofilm formed in commercial artificial seawater was 2.7-fold thicker and contained 2.6-fold more biomass than the biofilm formed in DSW. Furthermore, the wild-type V. cholerae biofilm formed in commercial artificial seawater contained 1.7-fold more biomass than the biofilm formed by the ΔvpsL mutant, suggesting that there was moderate vps dependence. No vps dependence was observed in the structure of the biofilm formed in DSW. Quantification of the cells associated with wild-type V. cholerae and ΔvpsL mutant biofilms formed in DSW supplemented with CAA confirmed the vps independence (Fig. 7). We attributed the vps dependence of the biofilm formed in commercial artificial seawater to unreported organic components of this preparation. Furthermore, we noted that the ΔvpsL mutant biofilm formed in commercial artificial seawater contained almost twice as much biomass as the ΔvpsL mutant biofilm formed in DSW. We hypothesized that unreported components of artificial seawater enhance vps-independent biofilm development, as well as vps-dependent biofilm development.
FIG. 6.
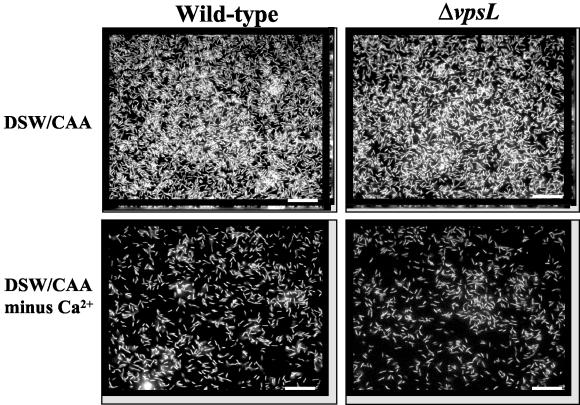
Architecture of biofilms formed in DSW supplemented with CAA with and without Ca2+: transverse and vertical cross sections through DAPI-stained wild-type V. cholerae strain MO10 and ΔvpsL mutant PW328 biofilms. Transverse sections were obtained at the level of the substratum. Bars = 10 μm.
FIG. 7.
Quantification of wild-type V. cholerae strain MO10 and ΔvpsL mutant PW328 surface-associated cells in DSW supplemented with CAA with and without Ca2+.
In order to identify the component(s) of defined seawater that were required for vps-independent biofilm development, we first combined every additional component of DSW singly with a solution containing 468 mM NaCl and 10.3 mM KCl supplemented with 1% CAA. We found, however, that no single component of DSW, when combined with 468 mM NaCl, 10.3 mM KCl, and 1% CAA, restored surface attachment by wild-type V. cholerae (data not shown). We then hypothesized that several components of DSW and rich medium might be required for biofilm development but might not be sufficient to activate a vps-independent biofilm. To test this hypothesis, we added each component of DSW to LB broth. Biofilm development by wild-type V. cholerae and a ΔvpsL mutant was tested in all these media. We observed that Ca2+ was required for vps-independent biofilm formation in LB broth. We then grew wild-type V. cholerae in a medium containing all elements present in defined seawater except Ca2+. In this medium, very few attached cells were observed, and these cells were arranged in a flat monolayer (Fig. 6). Furthermore, quantification of surface association demonstrated that there were very few attached cells (Fig. 7). Thus, we concluded that Ca2+ is the component of DSW that enables vps-independent biofilm formation.
Transcription of vps genes is low in artificial seawater supplemented with CAA.
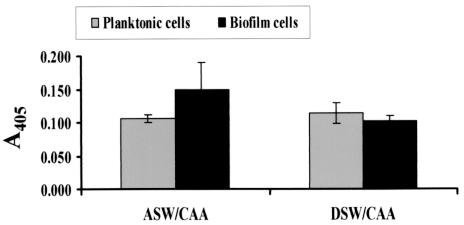
Because biofilms formed in artificial seawater and defined seawater supplemented with CAA displayed vps independence, we predicted that the vpsL genes would not be highly transcribed by planktonic cells or biofilm cells grown in these media. To test this prediction, we measured vpsL transcription by planktonic and surface-associated wild-type V. cholerae and ΔvpsL mutant cells. As shown in Fig. 8, vpsL transcription was quite low in cells associated with the vpsL-independent biofilms formed in both artificial seawater and DSW supplemented with CAA.
FIG. 8.
Normalized β-galactosidase activities of biofilm-associated and planktonic wild-type V. cholerae strain PW357 cells grown in commercial artificial seawater (ASW) supplemented with CAA and DSW supplemented with CAA. This strain carries a chromosomal fusion of the vpsL promoter to lacZ.
A distinct vps-independent exopolysaccharide is not observed in the extracellular matrix of V. cholerae biofilms formed in artificial seawater.
We observed that the V. cholerae ΔvpsL mutant formed a biofilm in commercial artificial seawater supplemented with CAA. Because biofilm formation often requires an extracellular matrix containing exopolysaccharide, we hypothesized that the extracellular matrix of the Δvps mutant biofilm might contain a novel exopolysaccharide that was independent of the vps genes. To test this hypothesis, we isolated the extracellular matrices of a wild-type V. cholerae biofilm formed in LB broth and wild-type V. cholerae and Δvps mutant biofilms formed in commercial artificial seawater supplemented with CAA and determined their monosaccharide compositions. The V. cholerae biofilm matrix formed in LB broth contained monosaccharides in the following molar ratio: N-acetylglucosamine, 0.49; galactose, 0.13; glucose, 0.23; and mannose, 0.15. This composition is similar to that reported for the biofilm matrix of a rugose variant of V. cholerae El Tor, although our biofilm matrix contained considerably more glucose (43). The wild-type V. cholerae biofilm formed in commercial artificial seawater supplemented with CAA had a monosaccharide composition similar to that of the biofilm formed in LB broth, except that significant amounts of N-acetylgalactosamine were present. The exact molar ratio of monosaccharides for this biofilm matrix was as follows: N-acetylglucosamine, 0.25; galactose, 0.08; glucose, 0.19; mannose, 0.11; and N-acetylgalactosamine, 0.26. Because N-acetylgalactosamine was not present in the matrix of the wild-type V. cholerae biofilm formed in artificial seawater supplemented with YT, we hypothesized that this component is the result of the nutrient source provided rather than the result of the components of artificial seawater (data not shown). The presence of polysaccharide in the matrix of the wild-type V. cholerae biofilm formed in artificial seawater is consistent with the vps dependence noted by Comstat analysis of this biofilm. Analysis of the Δvps mutant biofilm matrix formed in commercial artificial seawater did not suggest that there was an alternative vps-independent exopolysaccharide matrix. Rather, the number of moles of monosaccharide per microgram of the extracellular matrix isolated from the Δvps mutant biofilms was approximately 20-fold less than that of the extracellular matrix isolated from the wild-type V. cholerae biofilm formed in seawater. N-Acetylgalactosamine was always absent from the matrix of the Δvps mutant biofilm, suggesting that this component was dependent on the vps genes as well. Thus, we found no evidence that there was a novel vps-independent exopolysaccharide in the extracellular matrix of the Δvps mutant biofilm formed in commercial artificial seawater.
vps-dependent biofilm development occurs in a freshwater-based medium, while vps-independent biofilm development occurs in a seawater-based medium.
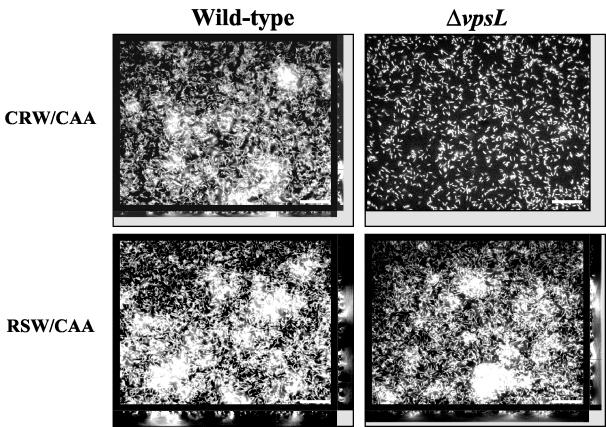
Because calcium is scarce in freshwater and abundant in seawater, we hypothesized that only vps-dependent surface adhesion might be available to V. cholerae in freshwater environments, while both vps-dependent and vps-independent biofilm development would be possible in marine environments. We collected local freshwater and seawater from the Charles River and from the Massachusetts coast, respectively. In order to test our hypothesis, we formed wild-type V. cholerae and ΔvpsL mutant biofilms in true freshwater and seawater supplemented with CAA and studied their vps dependence. As shown in Fig. 9, the wild-type V. cholerae biofilm formed in freshwater had pillars, while the ΔvpsL mutant biofilm consisted only of a monolayer of cells. Thus, formation of the three-dimensional structure of the V. cholerae biofilm formed in this freshwater medium required the vps genes. In contrast, both wild-type V. cholerae and a ΔvpsL mutant formed robust biofilms in true seawater (Fig. 9). Thus, a vps-independent biofilm was formed in true seawater. We performed Comstat analysis on the wild-type V. cholerae and ΔvpsL mutant biofilms formed in true freshwater and seawater (Tables 2 and 3). Although the freshwater biofilm exhibited vps dependence, the biomass and average thickness of the wild-type V. cholerae biofilm formed in freshwater supplemented with CAA were 4.8- and 6.2-fold less, respectively, than those of the wild-type V. cholerae biofilm formed in 10 mM NaCl supplemented with CAA and monosaccharides. This most likely reflected the relative paucity of monosaccharides present in our freshwater sample. In contrast, the quantitative measurements of the wild-type V. cholerae and ΔvpsL mutant biofilms formed in true seawater supplemented with CAA suggested that there was no vps dependence (Table 3). The structure of the V. cholerae biofilm formed in true seawater differed significantly from the structure of the biofilm formed in DSW. Although the overall biomass of the biofilm formed in true seawater was only 1.4-fold greater than the overall biomass of the biofilm formed in DSW, the maximum thickness of the former biofilm was 3-fold greater than the maximum thickness of the latter biofilm. Thus, the biofilm formed in true seawater contained a few tall pillars. We hypothesized that true seawater contains organic or inorganic components that promote the formation of pillars without a requirement for the VPS polysaccharide.
FIG. 9.
Architecture of wild-type V. cholerae and ΔvpsL mutant biofilms formed in water from the Charles River in Massachusetts (CRW) and in water from the Massachusetts coast (RSW) supplemented with CAA: transverse and vertical cross sections through DAPI-stained biofilms. Transverse sections were obtained at the level of the substratum. Bars = 10 μm.
In order to determine if the activators of vps-dependent and -independent biofilms were present in true freshwater and seawater, respectively, we analyzed the inorganic and organic constituents of our true freshwater and seawater. The results of the inorganic analysis are shown in Table 4. We found that the Ca2+ concentration of true freshwater was quite low, as required for formation of a vps-dependent biofilm. The Na+ concentration and osmolarity were quite low, as expected for freshwater. The inorganic composition and osmolarity of our DSW were very similar to those of our true seawater. In particular, the Ca2+ concentration was high, as required for vps-independent biofilm development.
TABLE 4.
Inorganic components of true river water, DSW, and true seawater
| Water | Concn (mM) of:
|
Osmolarity (mosM) | ||
|---|---|---|---|---|
| Na+ | K+ | Ca2+ | ||
| Charles River | 8.7 | 0.23 | 0.47 | 45 |
| DSW | 468 | 10.31 | 9.95 | 990 |
| Massachusetts coast | 435 | 15.64 | 9.5 | 968 |
Potential environmental roles for vps-dependent and vps-independent biofilm development.
In this work, we identified and characterized a vps-dependent V. cholerae biofilm and a vps-independent V. cholerae biofilm. The vps-dependent biofilm predominated in growth media containing monosaccharides. We hypothesized, therefore, that vps-dependent biofilm development occurs only in nutrient-rich environments. This type of biofilm development may facilitate V. cholerae's colonization of favorable aquatic environments. Conversion of environmental monosaccharides into an exopolysaccharide matrix may even serve as a form of nutrient storage. In contrast, millimolar Ca2+ concentrations, which are present in seawater but not in freshwater, are required for vps-independent biofilm development. Thus, we hypothesized that vps-independent biofilm development occurs primarily in marine environments. Furthermore, in contrast to the variability of organic nutrients in aquatic environments, the concentration of Ca2+ is uniformly high in the marine environment. Thus, we speculated that V. cholerae surface adhesion in marine environments may be less discriminating.
Cholera epidemics have been associated with heavy rainfall and increases in sea surface height (19, 20). Both of these conditions are predicted to alter the organic and inorganic compositions of an estuary, which is the primary interface between humans and the marine environment (11, 23, 29, 33). We hypothesized that changes in the organic and inorganic compositions of the aquatic environment may alter the nature of V. cholerae's association with surfaces. It is interesting to speculate that these types of transitions may play some role in the initiation of cholera epidemics.
Acknowledgments
We thank Arne Heydorn, Soren Molin, and their colleagues for generous sharing of expertise and the Comstat software. We also thank Anne Kane of the GRASP Center and her staff for their patience with the development of the various growth media and their expert preparation of many reagents, which greatly accelerated the course of our experiments.
This work was supported by grant NIH R01 AI50032 to P.I.W. and also by pilot project grant P30 DK34928 from the New England Medical Center GRASP Center (NIH/NIDDK).
REFERENCES
- 1.Allison, D. G., and M. J. Goldsbrough. 1994. Polysaccharide production in Pseudomonas cepacia. J. Basic Microbiol. 34:3-10. [DOI] [PubMed] [Google Scholar]
- 2.Bechet, M., and R. Blondeau. 2003. Factors associated with the adherence and biofilm formation by Aeromonas caviae on glass surfaces. J. Appl. Microbiol. 94:1072-1078. [DOI] [PubMed] [Google Scholar]
- 3.Bryan, B. A., R. J. Linhardt, and L. Daniels. 1986. Variation in composition and yield of exopolysaccharides produced by Klebsiella sp. strain K32 and Acinetobacter calcoaceticus BD4. Appl. Environ. Microbiol. 51:1304-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burdman, S., E. Jurkevitch, B. Schwartsburd, M. Hampel, and Y. Okon. 1998. Aggregation in Azospirillum brasilense: effects of chemical and physical factors and involvement of extracellular components. Microbiology 144:1989-1999. [DOI] [PubMed] [Google Scholar]
- 5.Colwell, R. R., and W. M. Spira. 1992. The ecology of Vibrio cholerae, p. 107-127. In D. Barua and W. B. I. Greenough (ed.), Cholera. Plenum, New York, N.Y.
- 6.Danese, P. N., L. A. Pratt, and R. Kolter. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 9.Di Martino, P., N. Cafferini, B. Joly, and A. Darfeuille-Michaud. 2003. Klebsiella pneumoniae type 3 pili facilitate adherence and biofilm formation on abiotic surfaces. Res. Microbiol. 154:9-16. [DOI] [PubMed] [Google Scholar]
- 10.Garay, E. A., A. Arnau, and C. Amaro. 1985. Incidence of Vibrio cholerae and related vibrios in a coastal lagoon and seawater influenced by lake discharges along an annual cycle. Appl. Environ. Microbiol. 50:426-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han, W. Q., and P. J. Webster. 2002. Forcing mechanisms of sea level interannual variability in the Bay of Bengal. J. Phys. Oceanogr. 32:216-239. [Google Scholar]
- 12.Haugo, A. J., and P. I. Watnick. 2002. Vibrio cholerae CytR is a repressor of biofilm development. Mol. Microbiol. 45:471-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 14.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 15.Kachlany, S. C., P. J. Planet, R. DeSalle, D. H. Fine, and D. H. Figurski. 2001. Genes for tight adherence of Actinobacillus actinomycetemcomitans: from plaque to plague to pond scum. Trends Microbiol. 9:429-437. [DOI] [PubMed] [Google Scholar]
- 16.Kang, Y., H. Liu, S. Genin, M. A. Schell, and T. P. Denny. 2002. Ralstonia solanacearum requires type 4 pili to adhere to multiple surfaces and for natural transformation and virulence. Mol. Microbiol. 46:427-437. [DOI] [PubMed] [Google Scholar]
- 17.Kim, S. H., S. Ramaswamy, and J. Downard. 1999. Regulated exopolysaccharide production in Myxococcus xanthus. J. Bacteriol. 181:1496-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knobloch, J. K., K. Bartscht, A. Sabottke, H. Rohde, H. H. Feucht, and D. Mack. 2001. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J. Bacteriol. 183:2624-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipp, E. K., A. Huq, and R. R. Colwell. 2002. Effects of global climate on infectious disease: the cholera model. Clin. Microbiol. Rev. 15:757-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lobitz, B., L. Beck, A. Huq, B. Wood, G. Fuchs, A. S. G. Faruque, and R. Colwell. 2000. Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc. Natl. Acad. Sci. USA 97:1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Looijesteijn, P. J., I. C. Boels, M. Kleerebezem, and J. Hugenholtz. 1999. Regulation of exopolysaccharide production by Lactococcus lactis subsp. cremoris by the sugar source. Appl. Environ. Microbiol. 65:5003-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 23.Mahadevan, A., and B. Subramanian. 1999. Seasonal and diurnal variation of hydrobiological characters of coastal water of Chennai (Madras), Bay of Bengal. Indian J. Mar. Sci. 28:429-433. [Google Scholar]
- 24.Mozzi, F., G. Rollan, G. S. de Giori, and G. Font de Valdez. 2001. Effect of galactose and glucose on the exopolysaccharide production and the activities of biosynthetic enzymes in Lactobacillus casei CRL 87. J. Appl. Microbiol. 91:160-167. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, T. F., and C. Kirkham. 2002. Biofilm formation by nontypeable Haemophilus influenzae: strain variability, outer membrane antigen expression and role of pili. BMC Microbiol. 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myatt, D. C., and G. H. Davis. 1989. Isolation of medically significant Vibrio species from riverine sources in south east Queensland. Microbios 60:111-123. [PubMed] [Google Scholar]
- 27.Nesper, J., C. M. Lauriano, K. E. Klose, D. Kapfhammer, A. Kraiss, and J. Reidl. 2001. Characterization of Vibrio cholerae O1 El tor galU and galE mutants: influence on lipopolysaccharide structure, colonization, and biofilm formation. Infect. Immun. 69:435-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 29.Padmavathi, D., and D. Satyanarayana. 1999. Distribution of nutrients and major elements in riverine, estuarine and adjoining coastal waters of Godavari, Bay of Bengal. Indian J. Mar. Sci. 28:345-354. [Google Scholar]
- 30.Perez-Rosas, N., and T. C. Hazen. 1989. In situ survival of Vibrio cholerae and Escherichia coli in a tropical rain forest watershed. Appl. Environ. Microbiol. 55:495-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Planet, P. J., S. C. Kachlany, D. H. Fine, R. DeSalle, and D. H. Figurski. 2003. The widespread colonization island of Actinobacillus actinomycetemcomitans. Nat. Genet., 34:193-198. [DOI] [PubMed]
- 32.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 33.Reemtsma, T., V. Ittekkot, M. Bartsch, and R. R. Nair. 1993. River inputs and organic-matter fluxes in the northern Bay of Bengal. Chem. Geol. 103:55-71. [Google Scholar]
- 34.Rose, R. K., and S. J. Turner. 1998. Extracellular volume in streptococcal model biofilms: effects of pH, calcium and fluoride. Biochim. Biophys. Acta 1379:185-190. [DOI] [PubMed] [Google Scholar]
- 35.Sheikh, J., S. Hicks, M. Dall'Agnol, A. D. Phillips, and J. P. Nataro. 2001. Roles for Fis and YafK in biofilm formation by enteroaggregative Escherichia coli. Mol. Microbiol. 41:983-997. [DOI] [PubMed] [Google Scholar]
- 36.Venkateswaran, K., C. Kiiyukia, M. Takaki, H. Nakano, H. Matsuda, H. Kawakami, and H. Hashimoto. 1989. Characterization of toxigenic vibrios isolated from the freshwater environment of Hiroshima, Japan. Appl. Environ. Microbiol. 55:2613-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wai, S. N., Y. Mizunoe, A. Takade, S. I. Kawabata, and S. I. Yoshida. 1998. Vibrio cholerae O1 strain TSI-4 produces the exopolysaccharide materials that determine colony morphology, stress resistance, and biofilm formation. Appl. Environ. Microbiol. 64:3648-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waldor, M. K., and J. J. Mekalanos. 1994. Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J. Infect. Dis. 170:278-283. [DOI] [PubMed] [Google Scholar]
- 39.Watnick, P. I., K. J. Fullner, and R. Kolter. 1999. A role for the mannose-sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor. J. Bacteriol. 181:3606-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watnick, P. I., C. M. Lauriano, K. E. Klose, L. Croal, and R. Kolter. 2001. Absence of a flagellum leads to altered colony morphology, biofilm development, and virulence in V. cholerae O139. Mol. Microbiol. 39:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yildiz, F. H., N. A. Dolganov, and G. K. Schoolnik. 2001. VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPSETr-associated phenotypes in Vibrio cholerae O1 El Tor. J. Bacteriol. 183:1716-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yildiz, F. H., and G. K. Schoolnik. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. USA 96:4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]