Abstract
Currently, there is no consensus concerning the geographic distribution and extent of endemism in Antarctic cyanobacteria. In this paper we describe the phenotypic and genotypic diversity of cyanobacteria in a field microbial mat sample from Lake Fryxell and in an artificial cold-adapted sample cultured in a benthic gradient chamber (BGC) by using an inoculum from the same mat. Light microscopy and molecular tools, including 16S rRNA gene clone libraries, denaturing gradient gel electrophoresis, and sequencing, were used. For the first time in the study of cyanobacterial diversity of environmental samples, internal transcribed spacer (ITS) sequences were retrieved and analyzed to complement the information obtained from the 16S rRNA gene. Microscopy allowed eight morphotypes to be identified, only one of which is likely to be an Antarctic endemic morphotype. Molecular analysis, however, revealed an entirely different pattern. A much higher number of phylotypes (15 phylotypes) was found, but no sequences from Nodularia and Hydrocoryne, as observed by microscopy, were retrieved. The 16S rRNA gene sequences determined in this study were distributed in 11 phylogenetic lineages, 3 of which were exclusively Antarctic and 2 of which were novel. Collectively, these Antarctic sequences together with all the other polar sequences were distributed in 22 lineages, 9 of which were exclusively Antarctic, including the 2 novel lineages observed in this study. The cultured BGC mat had lower diversity than the field mat. However, the two samples shared three morphotypes and three phylotypes. Moreover, the BGC mat allowed enrichment of one additional phylotype. ITS sequence analysis revealed a complex signal that was difficult to interpret. Finally, this study provided evidence of molecular diversity of cyanobacteria in Antarctica that is much greater than the diversity currently known based on traditional microscopic analysis. Furthermore, Antarctic endemic species were more abundant than was estimated on the basis of morphological features. Decisive arguments concerning the global geographic distribution of cyanobacteria should therefore incorporate data obtained with the molecular tools described here.
Cyanobacteria have often been recorded as the dominant phototrophs in Antarctic terrestrial and freshwater ecosystems. The greatest accumulation of biomass of cyanobacterial communities occurs in the benthic habitats of lakes and ponds, where the organisms form microbial mats, which are highly pigmented and structured biofilms covering the substrate (63). The compositions of cyanobacterial communities in mats in a number of Antarctic lakes, including Lake Fryxell, have been described on the basis of microscopic observations (66). In addition, the 16S rRNA gene sequences of 11 cultured oscillatorian strains from mat samples from the McMurdo Ice Shelf were determined by Nadeau et al. (37). However, there have been no molecular ecology studies based on culture-independent approaches that focused on the cyanobacterial diversity of these biotopes.
Molecular ecology studies have greatly enhanced our ability to detect and identify microorganisms in nature (1). Moreover, the need for molecular tools to study the genotypic relationships of cyanobacteria, to reconstruct their evolution, and to improve their taxonomy has long been recognized (69). Garcia-Pichel et al. (17) pointed out that the cyanobacterial genotypic diversity and the diversity inferred from identification of morphotypes may or may not coincide depending on the specific case analyzed and suggested that the correlation between morphotypes and phylotypes became gradually better as more complex forms were considered.
One of the questions that now can be addressed with molecular tools is the geographic distribution of the cyanobacteria and the existence of endemic taxa in Antarctica. Currently, there is no consensus concerning the extent of endemism of cyanobacteria in polar environments (62, 63). Arguments against the existence of endemic cyanobacterial species in Antarctica are related to the efficient dispersal abilities of the organisms and the relatively young age of the ice-free area. Moreover, most of the Antarctic cyanobacteria identified to date based on morphological features have apparently cosmopolitan distributions (62). However, Komárek (27) attributed the assignment of Antarctic cyanobacterial taxa to cosmopolitan species to the use of taxonomic keys developed for temperate or tropical microflora. In support of the hypothesis that there are endemic cyanobacteria in polar environments (63) are the fact that Antarctica has been more isolated than other parts of the world for several million years, the fact that dispersal processes which favor local species are more efficient than long-range dispersal processes, and the observation that there has probably been environmental selection for adaptative strategies. Accordingly, on the basis of morphology, Komárek (27) determined that about 60% of the species in various microbiotopes of ice-free areas of King George Island are probably endemic. However, it is necessary to obtain sequence data because there is evidence that morphological features do not reflect the real genetic and physiological divergence (63). Recent sequence information (37, 44, 52) has shown that there are clusters containing only Antarctic sequences. This could support the hypothesis that there is cyanobacterial endemism. Hence, molecular techniques can provide new insights into the genotypic diversity of the Antarctic cyanobacterial communities and their affinities with Arctic and nonpolar communities. These techniques provide a different way of sampling microbial diversity that is complementary to cultivation and microscopy (65).
This study was part of a comprehensive biodiversity study of a microbial mat from Lake Fryxell performed in the framework of the joint research project MICROMAT (Biodiversity of Microbial Mats in Antarctica [http://www.nerc-bas.ac.uk/public/mlsd/micromat/]). The same mat sample was shared by the different research groups and was used for determinations of bacterial and archaeal diversity (8, 59, 61), as well as an inoculum for culturing mat communities with a benthic gradient chamber (BGC) (11). The artificial communities cultured in the BGC at 5°C were clearly cold adapted, as oxygenic photosynthesis was optimal at 10°C, and they shared several features with the field mat, such as pinnacle formation and pigment composition (11, 43).
To describe the morphological and genotypic diversity of cyanobacteria from a Lake Fryxell mat sample, we used a polyphasic approach. The morphological diversity was assessed by light microscopy, and the genotypic diversity was studied by examining clone libraries and by performing denaturing gradient gel electrophoresis (DGGE) based on rRNA gene amplicons. The internal transcribed spacer (ITS) has proven to be useful for taxonomic studies of cyanobacterial cultures (3, 7, 13), as well as for bacterial community studies (5). Therefore, we retrieved ITS sequences from the cyanobacterial communities to complement the information obtained from the 16S rRNA gene. Using the same approach, we analyzed the cyanobacteria from the BGC mat, which originated from Lake Fryxell and showed functional similarities with the natural community. Finally, by performing a detailed phylogenetic analysis we compared the cyanobacteria observed in this study with previously described polar cyanobacteria to study possible endemism in Antarctica and to make comparisons between polar regions.
MATERIALS AND METHODS
Sampling and samples studied.
Lake Fryxell (77°37′S, 163°07′E) is located at the eastern end of the Taylor Valley in southern Victoria Land, Antarctica. It is a 7-km2, 18.5-m-deep brackish and meromictic lake with a perennial 3- to 4.5-m-thick ice cover. However, each summer a peripheral moat area opens up around the edge of the lake ice cover (55). Microbial mat samples were collected in January 1999 from this shallow moat area of Lake Fryxell close to the inflow of Huey Creek and Canada Stream. Mat samples were shipped refrigerated to the University of Nottingham, and from there they were sent frozen to the University of Liege and the University of Bordeaux.
Cultivation of artificial mats.
An artificial microbial mat was cultured in a BGC with opposing oxygen and sulfide gradients at 5°C for 8.5 months at the University of Bordeaux. Further details of the cultivation procedure and a structural description of the mat have been provided by Buffan-Dubau et al. (11). The artificial mat grew mainly above the sand surface and reached a final thickness of 2.5 to 5 mm. The surface of the mat was dark green with a few light green and brown patches. Pinnacle formation similar to that described for Antarctic mats was observed on the surface. At the end of the culture period the BGC was opened, and the artificial microbial mat was collected. A subsample was stored in 50% ethanol and was sent to the University of Liege for subsequent analyses.
Microscopic observations.
Immediately upon receipt, subsamples were fixed with 4% formaldehyde. The major cyanobacterial taxa were observed with a Wild MS-20 microscope equipped with a screw micrometer (68). The diacritical morphological traits used for botanical species descriptions were considered (cell shape for both intercalary and end cells; width and length of intercalary cells; presence or absence of constriction at the cross wall, of necridic cells, and of a sheath; color of the sheath; number of trichomes per filament; presence or absence of heterocysts; width and length of heterocysts). For each biometrical character, 30 measurements were obtained from cells, heterocysts, and filaments sampled at random. The taxonomic works of Geitler (18), Anagnostidis and Komárek (2), and Komárek and Anagnostidis (26), as well as descriptions in the Antarctic literature (9, 10, 33), were used.
Isolation of DNA.
The DNA extraction protocol used in this study was derived from that of Smalla et al. (51). A 0.5-g mat sample was suspended in 0.5 ml of SNT solution (500 mM Tris-HCl, 100 mM NaCl, 25% saccharose) supplemented with 26 μl of freshly added lysozyme (25%, wt/vol). The resulting suspension was shaken and incubated for 30 min at 37°C. After incubation, 0.5 ml of solution II (500 mM Tris base, 500 mM EDTA, 1% sodium dodecyl sulfate, 6% phenol) and 0.25 g of glass beads (diameter, 0.17 to 0.18 mm; Braun Biotech) were added to the sample and shaken for 60 s in a bead beater (Braun Biotech). The resulting suspension was placed on ice for 1 h and vortexed every 10 min. After incubation, the suspension was centrifuged for 10 min at 720 × g (MinifugeT; Heraeus), and 1 ml of the aqueous phase was mixed with an equal volume of phenol, after which it was centrifuged for 5 min at 13,600 × g. The supernatant was then transferred into new tubes, extracted with equal volumes of phenol-chloroform-isoamyl alcohol (25:24:1), and reextracted with equal volumes of chloroform-isoamyl alcohol (24:1). Then a standard Na acetate-ethanol precipitation was performed, and the dried pellet was resuspended in 100 μl of TE buffer (10 mM Tris-Cl, 1 mM EDTA; pH 8).
Cloning of the ribosomal DNA and screening by amplified ribosomal DNA restriction analysis (ARDRA).
The crude DNA preparations were subjected to several cesium chloride (CsCl)- and potassium acetate-based purifications steps by using the protocol of Smalla et al. (51), except for a few modifications. Two hundred milligrams of CsCl was added and mixed, and the tubes were centrifuged for 10 min at 13,600 × g after 2 h of incubation at 20°C (with mixing every 30 min). Three volumes of water and 0.6 volume of 80% isopropanol were added to the supernatant, and the tubes were centrifuged for15 min at 13,600 × g after 30 min of incubation at 20°C. After this the supernatant was discarded, and the pellet was dried. The DNA was dissolved by adding 100 μl of TE buffer, followed by addition of 20 μl of 8 M potassium acetate and 1.5 h of incubation at 20°C. After 15 min of centrifugation at 13,600 × g, 0.6 volume of 80% isopropanol was added to the supernatant, mixed, and incubated for 30 min at 20°C. After centrifugation for 15 min at 13,600 × g and 4°C, the supernatant was discarded, and the pellet was dried. The DNA was dissolved in 200 μl of TE buffer.
PCR amplification of a cyanobacterial 16S rRNA gene plus the ITS was performed in a 50-μl (total volume) reaction mixture containing 0.5 μl of mat DNA, 1× Super Taq Plus PCR buffer, each deoxynucleoside triphosphate at a concentration of 0.2 mM, 0.5 μM primer 16S27F (Table 1), 0.5 μM primer 23S30R (Table 1), 1 mg of bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.) ml−1, and 1 U of Super Taq Plus polymerase with proofreading activity (HT Biotechnology, Cambridge, United Kingdom). Amplification was carried out with a Gene Cycler (Bio-Rad, Hercules, Calif.) as follows: one cycle of 5 min at 94°C; 10 cycles of 45 s at 94°C, 45 s at 57°C, and 2 min at 68°C; 25 cycles of 45 s at 92°C, 45 s at 54°C, and 2 min at 68°C; and a final elongation step of 7 min at 68°C. PCR products were purified with a Quantum Prep PCR Kleen Spin column (Bio-Rad). Poly(A) extension for 20 min at 72°C with dATP and the Goldstar polymerase (Eurogentec, Seraing, Belgium) was performed.
TABLE 1.
Primer sequences and target sites
| Primera | Sequence (5′ → 3′) | Target sitec | Reference |
|---|---|---|---|
| 16S27F | AGA GTT TGA TCC TGG CTC AG | 7-27 | 71 |
| 16S378F | GGG GAA TTT TCC GCA ATG GG | 359-378 | 40 |
| 16S1114F | GTC CCG CAA CGA GCG CAA CCC | 1094-1114 | 70 |
| 16S1407F | TGT ACA CAC CGC CCG TC | 1391-1407 | 24 |
| 16S781R(a)b | GAC TAC TGG GGT ATC TAA TCC CAT T | 781-805 | 40 |
| 16S781R(b)b | GAC TAC AGG GGT ATC TAA TCC CTT T | 781-805 | 40 |
| 16S784R | GGA CTA CWG GGG TAT CTA ATC CCd | 784-806 | 40e |
| 16S1494R | GTA CGG CTA CCT TGT TAC GAC | 1494-1514 | 70 |
| 23S30R | CTT CGC CTC TGT GTG CCT AGG T | 30-52 | 28 |
R (reverse) and F (forward) designations refer to the primer orientation in relation to the rRNA.
A 38-nucleotide GC-rich sequence (5′-CGC CCG CCG CGC CCC GCG CCC GTC CCG CCG CCC CCG CC-3′) is attached to the 5′ end of the reverse primers.
E. coli numbering of 16S rRNA or 23S rRNA nucleotides.
W indicates a A/T nucleotide degeneracy.
Derived from a study by Nübel et al. (40).
Cloning of the PCR products was done with a TOPO TA cloning kit (Invitrogen BV, Breda, The Netherlands) by following the manufacturer's instructions and using a vector/insert ratio of 1:4. White and light blue transformants were purified twice by streaking and then were screened by performing colony PCR with the M13 forward and reverse primers. The amplification conditions described above were used, except that 0.8 U of Taq polymerase (Promega, Madison, Wis.) was used and amplification was carried out as follows: incubation for 10 min at 94°C; 20 cycles of 1 min at 94°C, 1 min at 55°C, and 2 min at 68°C; 15 cycles of 1 min at 90°C, 1 min at 55°C, and 2 min at 68°C; and a final elongation step of 10 min at 68°C.
Plasmid DNAs were extracted with a Quantum Prep Plasmid Miniprep kit (Bio-Rad) by following the manufacturer's instructions. The inserted 16S rRNA gene plus ITS was reamplified with primers 16S27F and 23S30R as described above and subjected to ARDRA to screen the clone libraries. For the ARDRA, all steps were performed as previously described (49), except for the following changes: MboI and HpaII (Gibco Life Sciences) were used as restriction enzymes, and electrophoresis was performed at a constant voltage of 3 V cm−1 for 235 min.
For each ARDRA type, partial 16S rRNA gene sequences that were at least 400 bp long were determined for one to three clones by using sequencing primer 16S378F and/or primer 16S784R (Table 1). Sequencing was carried out by GenomeExpress (Paris, France) with an ABI PRISM system 377 (PE Applied Biosystems, Foster City, Calif.). Complete 16S rRNA gene sequences (Escherichia coli positions 27 to 1492) were determined for one DNA strand for one clone selected at random from among the clones identified as members of the same phylotype. We define phylotype as a group of sequences that exhibit more than 97.5% similarity with each other.
We set the threshold at 97.5% for two reasons. First, for complete 16S rRNA gene sequences, a binary similarity value less than 97.5% likely corresponds to a DNA-DNA hybridization value less than 70%, and therefore the sequences probably correspond to two different species (56). Second, there is a good correlation between complete and partial 16S rRNA gene sequence similarity values. We calculated binary similarity values for all complete 16S rRNA gene sequences of cyanobacterial strains available from GenBank (indels were not taken into account). The 53 strains with binary similarity values between 96 and 98% were selected, and their partial 16S rRNA gene sequence similarities were calculated (for E. coli positions 378 to 784). The average similarities were 97.05 and 97.01% for complete and partial sequences, respectively. Hence, on average, partial sequence similarity reflected the complete sequence similarity well, although the standard deviation was 1.24.
The sequencing was carried out with primers 16S1494R and 16S784R (Table 1). The consensus sequences were obtained with the software AlignIR (LI-COR). In addition, the complete ITS sequences were determined by using primer 23S30R and/or primer 16S1407F (Table 1) for the 16 clones with complete 16S rRNA gene sequences, as well as for nine closely related clones. Six clones with very similar ITS and different 16S rRNA genes were subjected to an additional control by sequencing ca. 750 bp with primer 16S1114F (Table 1).
DGGE analysis.
The crude DNA preparations were subjected to a purification step by using the Wizard DNA Clean-up system (Promega) and following the manufacturer's instructions.
16S rRNA gene fragments that were 422 bp long were generated by seminested PCR. The primers used for the first PCR were 16S378F and 23S30R (Table 1). The PCR conditions were similar to those used when the forward primer was 16S27F, but the reaction was carried out as follows: incubation for 5 min at 94°C, followed by 30 cycles of 45 s at 94°C, 45 s at 54°C, and 2 min at 68°C and then a final elongation step of 7 min at 68°C. The resulting PCR products (0.5 μl) served as templates for the second PCR, which was performed with forward primer 16S378F and reverse primers 16S781R(a) and 16S781R(b) (Table 1). A 38-nucleotide GC-rich sequence was attached to the 5′ end of each of the reverse primers. The reaction conditions were the same as those described above except that amplification was carried out as follows: incubation for 5 min at 94°C, followed by 35 cycles of 1 min at 94°C, 1 min at 60°C, and 1 min at 68°C and then a final elongation step of 7 min at 68°C. Two distinct reactions were performed for each reverse primer. The negative control for the first PCR was used in the second PCR to check for contamination.
DGGE was carried out with a Dcode gene system (Bio-Rad) and was performed as described by Nübel et al. (40), with the following modifications. The PCR products obtained with primers 16S781R(a) and 16S781R(b) were applied separately to the polyacrylamide gel. The gel contained a linear 40 to 65% denaturant gradient, the pH of the TAE buffer was adjusted to 7.4, and electrophoresis was performed for 16 h at 45 V and 60°C.
The DGGE bands were excised with a surgical scalpel. Each small gel block was placed in 100 μl of sterile water for 2 h at room temperature. Each solution was used as a template for PCR amplification as described above. The PCR products were then electrophoresed under the DGGE conditions described above to confirm their positions relative to the bands from which they were excised and to detect potential heteroduplexes. A partial ca. 350-bp 16S rRNA gene sequence from primer 16S378F or 16S784R was determined.
Analysis of sequence data.
The sequences were initially analyzed by a similarity search by using the BLAST software, widely available on the Internet, and chimera detection was performed by using Check Chimera in the Ribosomal Database Project (30). The sequences determined in this study were included in the database of the ARB software package (29) available at http://www.arb-home.de and were aligned with the cyanobacterial sequences available from GenBank.
A distance tree was constructed with the software package TREECON for Windows 1.3b (60). The dissimilarity values were corrected for multiple substitutions by the method of Jukes and Cantor (25) and were used to calculate a distance matrix, and a tree was constructed by the neighbor-joining method (48). Aligned partial 16S rRNA gene sequences corresponding to E. coli sequence positions 405 to 780 were used, but the indels were not taken into account. A bootstrap analysis was performed that involved construction of 500 resampled trees. The tree comprised all sequences determined in this study (clones and DGGE bands) and all the sequences of polar cyanobacteria available in databases, as well as the related sequences (as indicated by BLAST) that exhibited more than 97.5% similarity with the sequences mentioned above. However, large groups of sequences that exhibited more than 97.5% similarity were represented by a limited number of selected sequences (e.g., Nostoc sp.). In addition, we included in the tree the two nearest neighbors indicated by BLAST when no sequences were related at a similarity level of more than 97.5%. If these two sequences were from uncultured clones, we added the sequences of the two closest cultured strains indicated by BLAST. In addition, we included at least one sequence for each of the clusters defined by Wilmotte and Herdmann (67).
To check the robustness of this analysis, other phylogenetic trees were constructed by neighbor-joining, maximum-likelihood, and maximum-parsimony methods by using the ARB software as described by Garcia-Pichel et al. (16).
Nucleotide sequence accession numbers.
New sequence data were deposited in the GenBank database. Sixteen almost complete 16S rRNA gene sequences were deposited under accession numbers AY151721 to AY151736, 33 partial 16S rRNA gene sequences were deposited under accession numbers AY151737, AY151738, and AY151740 to AY151770, and 24 ITS sequences were deposited under accession numbers AF547626 to AF547637, AF547639 to AF547649, and AY188584.
RESULTS
Microscopic diversity.
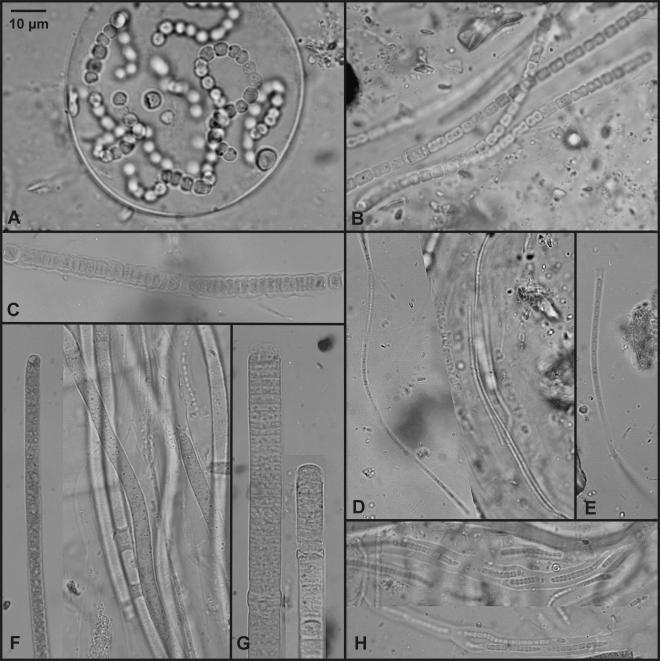
Eight morphotypes were described, and their corresponding taxonomic assignments are shown in Table 2. Photomicrographs are shown in Fig. 1.
TABLE 2.
Cyanobacterial morphotypes distinguished in the field sample and in the BGC sample
| Morphotypea | Descriptionb | Classical designation | Order |
|---|---|---|---|
| A | Heterocystous and filamentous; cells subspherical, 3.93 ± 0.51 μm wide and 3.84 ± 0.81 μm long; heterocysts 5.56 ± 0.41 μm wide and 5.75 ± 0.57 μm long; confluent gel holds trichome masses in spherical hyaline or brown colonies | Nostoc sp. | Nostocales |
| B | Heterocystous and filamentous; several trichomes in one common hyaline sheath; cells barrel shaped 3.34 ± 0.19 μm wide and 3.73 ± 0.41 μm long; heterocysts cylindrical, 4.83 ± 0.47 μm wide and 7.54 ± 0.92 μm long | Hydrocoryne cf. spongiosa Schwabe | Nostocales |
| C | Heterocystous and filamentous; filaments 5.86 ± 0.57 μm wide; cells disk shaped, 4.33 ± 0.19 μm wide and 1.53 ± 0.38 μm long; heterocysts 5.60 ± 0.53 μm wide and 4.97 ± 0.41 μm long | Nodularia cf. harveyana Thuret | Nostocales |
| D | Filamentous; trichomes slightly constricted at the cross walls; cells 0.77 ± 0.06 μm wide and 4.26 ± 0.77 μm long; end cells rounded | Leptolyngbya sp. 1 | Oscillatoriales |
| E | Filamentous, ensheathed, slightly constricted at the cross walls; cells 1.45 ± 0.17 μm wide and 2.32 ± 0.56 μm long; attenuated and curved end cells | Leptolyngbya sp. 2 | Oscillatoriales |
| F | Filamentous, ensheathed or not ensheathed; filaments 5.04 ± 0.47 μm wide; trichomes sometimes slightly curved at the end without constrictions at the cross walls; cells 4.16 ± 0.57 μm wide and 2.54 ± 0.75 μm long; calyptra present or not present (but end cells rounded) | Phormidium cf. autumnale (Agardh) Gomont | Oscillatoriales |
| G | Filamentous, ensheathed or not ensheathed; filaments 10.60 ± 0.56 μm wide; trichomes not constricted to slightly constricted at the cross walls, briefly attenuated at the end; cells disk shaped, 8.30 ± 0.69 μm wide and 1.65 ± 0.34 μm long; necridic cells present; end cells rounded | Oscillatoria cf. subproboscidea West & West | Oscillatoriales |
| H | Filamentous, ensheathed; several trichomes in one common hyaline sheath; constrictions at the cross walls; cells 1.78 ± 0.16 μm wide and 1.43 ± 0.22 μm long; end cells rounded to conical | Schizothrix sp. | Oscillatoriales |
Morphotypes E, F, and H were present in the BGC sample.
Cell measurements are averages ± standard deviations.
FIG. 1.
Diversity of cyanobacterial morphotypes identified in the artificial and natural microbial mats. (A) Nostoc sp.; (B) Hydrocoryne cf. spongiosa; (C) Nodularia cf. harveyana; (D) Leptolyngbya sp. 1; (E) Leptolyngbya sp. 2; (F) Phormidium cf. autumnale; (G) Oscillatoria cf. subproboscidea; (H) Schizothrix sp.
Hydrocoryne cf. spongiosa has rarely been observed in Antarctica (it has been found in soil samples in South Victoria Land [42]), while Nostoc sp., Nodularia cf. harveyana, Oscillatoria cf. subproboscidea, Phormidium cf. autumnale, Schizothrix sp., and several thin oscillatorians resembling Leptolyngbya sp. 1 and Leptolyngbya sp. 2 have been found frequently in different Antarctic biotopes, including Canada Stream (9, 33), which flows into Lake Fryxell near our sampling site. However, with the exception of Oscillatoria cf. subproboscidea, which is apparently endemic to Antarctica (27), these morphotypes have been found in various biotopes outside Antarctica.
The eight morphotypes were observed in the field sample, but only three of them (morphotypes E, F, and H) were found in the BGC sample (Table 2). The major differences between samples were the absence of the three heterocystous taxa (Nostoc sp., Nodularia cf. harveyana, and Hydrocoryne cf. spongiosa) and the great abundance of Phormidium cf. autumnale in the BGC sample. Phormidium cf. autumnale, which was a minor component in the original sample, was the dominant morphotype in the BGC. This species is one of the most frequently reported taxa observed in Antarctic biotopes, and it comprises numerous morphotypes and ecotypes (27).
Clone libraries.
Altogether, 87 clones with an insert of the correct size were obtained; 45 of these clones produced 28 different ARDRA patterns for the field sample, and 42 clones produced 14 different ARDRA patterns for the BGC sample.
To assign clones to broad taxonomic clusters, 42 partial 16S rRNA gene sequences were determined for one representative of each ARDRA pattern. In addition, two cyanobacterial sequences obtained by Brambilla et al. (8) from a clone library of bacterial sequences for the field sample were added. Five sequences of chimeric origin, one plastid sequence, and three sequences resulting from cloning rearrangements were detected and excluded from the analysis. Finally, 35 partial cyanobacterial sequences were obtained.
To evaluate the levels of discrimination of the ARDRA with the restriction enzymes MboI and HpaII, partial 16S rRNA gene sequences were determined for nine additional clones selected at random from among seven ARDRA patterns represented by several clones. On average, 16S rRNA gene sequences (E. coli positions 405 to 780) of two clones with identical ARDRA patterns showed only a single substitution (i.e., 99.75% similarity). Hence, in the rest of our analysis we considered that clones with identical ARDRA patterns had virtually identical DNA partial sequences.
The 23 sequences (38 clones) obtained for the field sample and the 12 sequences (40 clones) obtained for the BGC sample were grouped in 15 phylotypes (Table 3) by using a threshold of 97.5% similarity (E. coli positions 405 to 780). Eleven phylotypes were detected only in the original sample and two phylotypes were detected only in the BGC sample, whereas two cyanobacterial phylotypes were present in both samples.
TABLE 3.
Summary of the data obtained from the clone libraries and DGGE
| Phylotype | Sequenced clones and DGGE bandsa | Total no. of clones | Closest GenBank relative (% similarity)b | Closest GenBank cultured relative (% similarity)b | Clones with the following ITS types:
|
Clusterc | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Unique | ||||||
| 1 | Fr397, Fr114, Fr311, Fr401, Fr025, Fr252 | 10 | Uncultured Antarctic bacterium LB3-46 (99) | Oscillatoria sp. strain OH25 (90) | Fr397, Fr311 | XII | ||||||
| 2 | Fr032, Fr288, FrF4 | 2 | Phormidium mucicola M221 (95) | Fr032 | XIV | |||||||
| 3 | Fr239 | 1 | Phormidium mucicola (95) | Fr239 | XIV | |||||||
| 4 | Fr094, Fr297, Fr246, Fr350, Fr396 | 13 | Uncultured Antarctic bacterium CSC1 (96-99) | Leptolyngbya sp. strain PCC9207 (90) | Fr297, Fr350 | Fr094 | Fr246 | XI | ||||
| 5 | Fr132, Fr044, BGC-, Fr019, FrE203, FrF5 | 4 | Uncultured bacterium WH12 (92) | Leptolyngbya sp. strain PCC9207 (91) | Fr132 | X | ||||||
| 6 | Fr285 | 1 | Uncultured bacterium WH12 (93) | Leptolyngbya sp. strain PCC9207 (91) | X | |||||||
| 7 | Fr023 | 2 | Uncultured Antarctic bacterium LB3-76 (99) | Oscillatoria amphigranulata strain 11-3 (91) | Fr121 | IX | ||||||
| Fr121 | Uncultured Antarctic bacterium CSC14 (98) | Oscillatoria amphigranulata strain 11-3 (91) | ||||||||||
| 8 | Fr304 | 1 | Nostoc commune NIVA-CYA308 (99) | Fr304 | VII | |||||||
| FrF1, FrF1bis | Nostoc sp. (Nephroma helveticum cyanobion) strain 33 (99) | |||||||||||
| 9 | BGC-Fr056, BGC-Fr023, BGC-Fr005, BGC-Fr020, BGC-Fr067, FrF3 | 6 | Geitlerinema sp. strain PCC9222 (97-99) | BGC-Fr056, BGC-Fr005, BGC-Fr067 | BGC-Fr023 | V | ||||||
| 10 | BGC-Fr032, BGC-Fr025, BGC-Fr068, BGC-Fr072, BGC-Fr078, BGC-Fr080 | 10 | Phormidium sp. strain Ant-Orange (99) | BGC-Fr068 | BGC-Fr032, BGC-Fr080 | Fr048 | I | |||||
| Fr048 | Lyngbya sp. strain UTCC296 (99) | |||||||||||
| 11 | BGC-Fr054, BGC-Fr006, BGC-Fr030, BGC-Fr044, BGC-Fr060, BGC-Fr073 | 24 | Uncultured Antarctic bacterium LB3-1 (97-98) | LPP group Antarctic cyanobacterium QSSC8cya (97-98) | BGC-Fr054 | BGC-Fr060 | XXI | |||||
| 12 | Fr147 | 1 | Uncultured soil crust cyanobacterium lichen 4 (96) | Phormidium ambiguum M71 (92) | Fr147 | III | ||||||
| 13 | Fr005 | 1 | Uncultured soil crust cyanobacterium lichen 4 (96) | Phormidium ambiguum M71 (92) | Fr005 | III | ||||||
| 14 | FrE313 | 1 | Pseudanabaena sp. strain PCC6903 (98%) | XIII | ||||||||
| 15 | Fr127 | 1 | Uncultured Antarctic bacterium CSC17 (94) | Phormidium mucicola M221 (90) | Fr127 | IX | ||||||
The 16S rRNA genes of the following clones have been completely sequenced: Fr397, Fr032, Fr239, Fr094, Fr297, Fr132, Fr121, Fr304, BGC-Fr056, BGC-Fr032, Fr048, BGC-Fr054, Fr147, Fr005, FrE313, and Fr127. The following clones are duplicate clones according to their ARDRA patterns: Fr025, Fr252, Fr396, BGC-Fr005, BGC-Fr020, BGC-Fr067, BGC-Fr078, BGC-Fr080, and BGC-Fr073. Clones Fr019 and Fr285 were detected as chimeras in the complete 16S rRNA gene sequence. FrF4, FrF5, FrF1, FrF1bis, and FrF3, are DGGE bands.
Levels of similarity were determined by BLAST.
See Fig. 2.
The accumulation curves (data not shown) based on the number of clones per phylotype showed a hyperbolic tendency towards a saturation curve, even though the accumulation curve calculated for the field sample suggested that new phylotypes could be obtained by studying more clones. In addition, the coverage index indicated that more than three-quarters of the total diversity in the field clone library and almost all the diversity in the BGC clone library were detected.
The total number of phylotypes, the Shannon-Wiener diversity index, and the Berger-Parker dominance index (Table 4) showed that the genotypic diversity was twice as high in the field sample as in the BGC sample and that the BGC sample was dominated by a smaller subset of taxa.
TABLE 4.
Richness. coverage, Shannon-Wiener, and Berger-Parker indices
The coverage index (C) (19) was calculated as follows: C = (1 − n/N) × 100, where n is the number of phylotypes composed of a single clone and N is the total number of clones.
The Shannon-Wiener index (H) (31) was calculated as follows: H = −∑ pi log2 pi, where pi is the number of clones belonging to the ith phylotype.
The Berger-Parker index (d) (32) was calculated as follows: d = N/Nmax, where N is the total number of clones and Nmax is the number of clones belonging to the dominant phylotype.
The new sequences exhibited levels of 16S rRNA gene similarity (E. coli positions 405 to 780) with their closest relatives deposited in GenBank ranging from 90 to 99% (Table 3). Seven phylotypes contained only sequences that exhibited more than 2.5% dissimilarity with GenBank sequences. Four of the eight remaining phylotypes were related exclusively to Antarctic environmental clones or strains.
For each of the 15 phylotypes, one or two complete 16S rRNA gene sequences were determined. Three complete sequences determined to be chimeras were discarded. This was also the case for clone Fr285, and phylotype 6 was therefore represented by only the partial sequence of Fr285 (E. coli positions 405 to 780). Hence, 16 complete 16S rRNA gene sequences that belonged to 14 phylotypes were obtained.
For the first time in a study of cyanobacterial diversity with environmental samples, ITS sequences were exploited to complement the information obtained from the 16S rRNA gene. ITS sequences of 26 clones were determined, including 2 sequences that were determined to be chimeras and discarded.
An alignment of the ITS sequences (http://www.ulg.ac.be/cingprot/ITS%20alignment.pdf) was constructed on the basis of conserved domains (24) and tRNAs. Groups of ITS sequences in which the alignment seemed meaningful (72) were distinguished, and we defined nine different ITS types (Table 3). ITS sequences belonging to the same ITS type exhibited between 94.3 and 100% similarity (average, 97.9%). Both tRNA genes were found in ITS types 1 to 5, as well as in the ITS sequences of clones Fr032 and Fr121, whereas the ITS sequence of clone Fr147 had only the tRNAIle gene and ITS type 6 did not have any tRNA genes. Table 3 shows the ITS types that were observed for the different phylotypes. For five phylotypes, the ITS sequences of several clones were obtained. Four of these phylotypes comprised a number of clones with different ITS types (Phy4/ITS4-5-6, Phy9/ITS1-2, Phy10/ITS1-3, Phy11/ITS2-3), while only a single ITS type (4) was found for phylotype 1. Unexpectedly, the same ITS types were found for clones that belonged to different phylotypes (ITS1/Phy9-10-11, ITS2/Phy9-11, ITS3/Phy10-11, ITS4/Phy1-4-13-15, ITS5/Phy1-3-4-8-10, ITS6/Phy4-5).
DGGE.
A total of 43 bands were observed in the DGGE patterns (data not shown). Theoretically (14, 45), if bands at the same position had identical sequences, these bands corresponded to 35 genotypes (21 for the field sample and 22 for the BGC sample, with 8 genotypes identical for the two samples).
Altogether, five DGGE band sequences (FrF1, FrF1bis, FrF3, FrF4, FrF5) belonging to four phylotypes were obtained for the field sample (Table 3). Two phylotypes (2 and 8) were found only in the clone library of the field sample, one phylotype (5) was found in both libraries, and interestingly, the last phylotype (9) was found in only the BGC clone library. Based on the number of clones in each phylotype (Table 3), we noticed that our DGGE band sequences did not belong to the phylotypes (1, 4, 10, and 11) which comprised the highest numbers of clones. No DGGE band sequences were obtained from the BGC patterns despite several attempts.
Phylogenetic analysis.
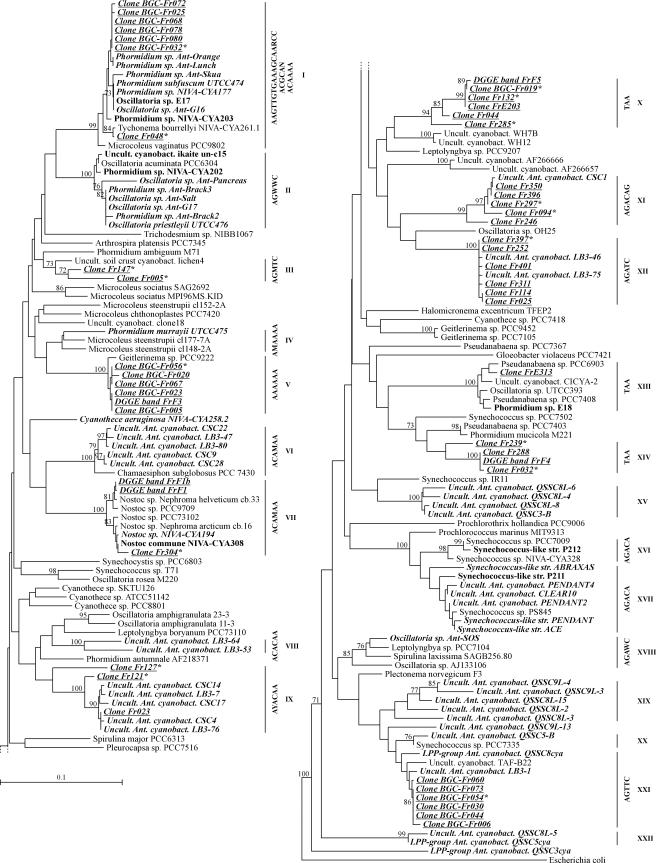
As shown in the distance tree in Fig. 2, all polar sequences (including the sequences obtained in this study) were distributed in 22 lineages; two of the lineages (clusters X and XIV) were novel, and seven (clusters VIII, IX, XI, XII, XV, XIX, and XXII) contained Antarctic representatives exclusively. Table 5 shows the origins of the polar sequences. The sequences obtained in this study were distributed in 11 lineages, which included the 2 novel lineages and 3 lineages that were exclusively composed of Antarctic representatives. The same novel and Antarctic clades were obtained with different methods of tree construction.
FIG.2.
Neighbor-joining tree based on partial 16S rRNA gene sequences corresponding to E. coli positions 405 to 780, with the exception of the sequences of the uncultured Antarctic cyanobacteria CLEAR-10, PENDANT-2, −4, and QSSC (E. coli positions 519 to 780). The tree includes the 49 sequences of clones and DGGE bands determined in the present study (boldface, italic, underlined type), 125 previously published sequences (Antarctic sequences are in boldface italic type, and Arctic sequences are in boldface roman type), and the E. coli sequence used as an outgroup. Bootstrap values equal to or greater than 70% are indicated at the nodes. The evolutionary distance between two sequences is obtained by adding the lengths of the horizontal branches connecting them and using the scale bar (0.1 mutation per position). Signatures are shown next to the cluster numbers. Abbreviations: Uncult., uncultured; cyanobact., cyanobacterium; Ant., Antarctic; str., strain.
TABLE 5.
Origins of the polar sequences
| Sequence(s) (cluster)a | Location | Reference |
|---|---|---|
| Antarctic sequences | ||
| C. aeruginosa NIVA-CYA 258.2 | Dronning Mauds Land | 47 |
| LPP group cyanobacterium QSSC5cya (XXII), QSSC8cya (XXI), QSSC3cya | Quartz stones, Vestfold Hills | 52 |
| Nostoc sp. strain NIVA-CYA 194 (VII) | Dronning Mauds Land | 47 |
| Oscillatoria priestleyii UTCC476 (II) | Pond, McMurdo Ice Shelf | Casamatta and Vis, unpublished data |
| Oscillatoria sp. strains Ant-G16 (I), Ant Salt, Ant-G17, Ant-Pancreas (II), Ant-SOS (XVIII) | Pond, Bratina Island, McMurdo Ice Shelf | 37 |
| Phormidium murrayi UTCC475 (VI) | Pond, McMurdo Ice Shelf | Casamatta and Vis, unpublished data |
| Phormidium sp. strains Ant-Lunch, Ant-Orange, Ant-Skua (I), Ant-Brack2, Ant-Brack3 (II) | Pond, Bratina Island, McMurdo Ice Shelf | 37 |
| Phormidium sp. strain NIVA-CYA 177 (I) | Dronning Mauds Land | 47 |
| Phormidium subfuscum UTCC474 (I) | Lake, McMurdo Ice Shelf | Casamatta and Vis, unpublished data |
| Synechococcus-like strains ACE, PENDANT, ABRAXAS (XVII) | Lakes, Vestfold Hills | 64 |
| Uncultured clones CLEAR, PENDANT (XVII) | Lakes, Vestfold Hills | 6 |
| Uncultured clones CSC9, CSC22, CSC28 (VI), CSC4, CSC14, CSC17 (IX), CSC1 (XI) | Cryoconite holes, Canada Glacier, McMurdo Dry Valleys | 12 |
| Uncultured clones LB3-47, LB3-80 (VI), LB3-7, LB3-76 (IX), LB3-64, LB3-53 (VIII), LB3-46, LB3-75 (XII), LB3-I (XXI) | Ice cover of Lake Bonney, McMurdo Dry Valleys | 44 |
| Uncultured clones QSSC3-B, QSSC8L-4, QSSC8L-6 and QSSC8L-8 (XV), QSSC9L-4, QSSC9L-3, QSSC8L-15, QSSC8L-2, QSSC8L-3 (XIX), QSSC8L-5 (XXII), QSSC5-B (XX), QSSC9L-13 (XXI) | Quartz stones, Vestfold Hills | 52 |
| Arctic sequences | ||
| Nostoc sp. strain NIVA-CYA 308 (VII) | Norwegian arctic | 47 |
| Oscillatoria sp. strain E17 (I) | Pond, Canadian arctic | 37 |
| Phormidium sp. strain E18 (XIII) | Pond, Canadian arctic | 37 |
| Phormidium sp. strain NIVA-CYA 203 (I), NIVA-CYA 202 (II) | Norwegian arctic | 47 |
| Synechococcus-like strains P212 (XVI), P211 (XVII) | Ponds, Bylot Islands, high Canadian arctic | 64 |
| Uncultured clone ikaite un-c15 (II) | Ikaite tuffa column, Ikka Fjord, Greenland | 57 |
See Fig. 2 for clusters.
Novel clusters.
Cluster X comprised four clone sequences and one DGGE band sequence from the field sample, as well as one clone sequence from the BGC sample. Within this cluster, the sequences exhibited at least 94.5% similarity and belonged to phylotypes 5 and 6. All of them exhibited at least 7% dissimilarity with the sequences available in the databases. Cluster XIV contained two clone sequences and one DGGE band sequence isolated from the field sample. The minimum level of similarity between sequences in this cluster was 95%, and the sequences belonged to phylotypes 2 and 3. All these sequences exhibited at least 5% dissimilarity with the database sequences. These findings suggest that clusters X and XIV represented novel evolutionary branches which have not been characterized previously.
Antarctic clusters.
Clusters IX, XI, and XII comprised sequences of clones isolated from the field sample from Lake Fryxell and other sequences of uncultured Antarctic cyanobacteria. Sequences belonging to these three clusters exhibited at least 8% dissimilarity with their closest relatives from nonpolar environments available in the databases. Therefore, we propose that these clusters are Antarctic lineages. The minimum level of sequence similarity within cluster IX was 91.5%, and sequences in this cluster belonged to phylotypes 7 and 15. Clusters VIII, XV, XIX, and XXII comprised only previously published Antarctic sequences. The closest nonpolar relatives exhibited at least 6% dissimilarity with the sequences belonging to these clusters. In addition, two previously described Antarctic strains, Cyanothece aeruginosa NIVA-CYA 258.2 and the LPP group cyanobacterium QSSC3cya, exhibited at least 9 and 12% dissimilarity, respectively, with all other database sequences.
Other clusters.
The rest of the sequences obtained in this study fell into clusters I, III, V, VII, XIII, and XXI. Six clone sequences from the BGC sample and one clone sequence from the field sample fell in cluster I and were grouped with polar and nonpolar sequences. Three of the Antarctic sequences were from the psychrophilic organisms Phormidium sp. strains Ant-Lunch and Ant-Orange and Oscillatoria sp. strain ANT-G16. Cluster III comprised two clone sequences from the field sample, as well as the sequence of the uncultured cyanobacterium cl lichen 4 (46). The levels of sequences similarity within this cluster ranged from 94.9 to 96.9%, and the clone sequences belonged to phylotypes 12 and 13. Furthermore, the most closely related strain exhibited at least 8% sequence dissimilarity. Interestingly, cluster V comprised five BGC clone sequences and only one sequence from the field sample obtained from a DGGE band. A single sequence from a nonpolar organism fell in this cluster; this was the sequence of Geitlerinema sp. strain PCC 9222 (35), which exhibited levels of similarity ranging from 98 to 99% with our sequences. Cluster VII corresponded to the order Nostocales. One clone sequence and two DGGE band sequences from the field sample fell in this cluster and were grouped together with other polar and nonpolar sequences of Nostoc strains. Cluster XIII contained one clone sequence from the field sample obtained with bacterial primers (8), the Arctic sequences of Phormidium sp. strain E18, and nonpolar sequences. Cluster XXI comprised the sequences of six BGC clones, the uncultured Antarctic clone LB3-1, the LPP group cyanobacterium QSSC8cya, and the nonpolar uncultured cyanobacterium TAF-B22 (41), which exhibited levels of similarity ranging from 96.5 to 97.8% with the Antarctic sequences of this cluster. In clusters II, IV, VI, XVI, XVII, XVIII, and XX, previously described Antarctic and/or Arctic sequences were grouped with nonpolar sequences.
Nadeau et al. (37) pointed out that there is a rare 11-nucleotide insertion (5′-AGTTGTGAAAG-3′) in the 16S rRNA genes of the Antarctic isolates Ant-Lunch and Ant-Orange and the Arctic isolate NIVA-CYA 203. We found this insertion in the 16S rRNA genes of clones BGC-Fr025, BGC-Fr032, BGC-Fr068, BGC-Fr072, BGC-Fr078, and BGC-Fr080 in cluster I. In addition, the Microcoleus vaginatus PCC9802 sequence and many sequences recently deposited in the databases also have this insertion (7, 16, 46; D. A. Casamatta and M. L. Vis, unpublished data). We found that there was a good correlation between the highly variable region corresponding to E. coli positions 463 to 468, where this insertion was located, and the clusters of the tree (Fig. 2). Indeed, for 17 clusters, this region could serve as a signature, because all the sequences in these clusters were identical or nearly identical to each other at E. coli positions 463 to 468. However, the signatures of clones Fr121 and Fr127, which were distantly related to the other clones in cluster IX, were AGAAC and TAA, respectively. In addition, three different signatures were found among the sequences in cluster I (AAGTTGTGAAAGCAARCC, ACGCAN, and ACAAAA).
DISCUSSION
Diversity and endemism.
This was the first study in which morphological characterization and molecular characterization of the cyanobacterial diversity in the microbial mats of an Antarctic lake were combined. Moreover, we compared the diversity and composition of a field sample with the diversity and composition of a derived laboratory community obtained as an artificial cold-adapted mat grown in a BGC.
Based on morphological data, five of the eight species identified in the field sample by using morphological criteria (morphotypes) have been found in Canada Stream, which flows into Lake Fryxell close to our sampling site (9, 33). Even if a comparison based on species inventory had limits, these species were not observed in other mat samples or in the plankton of Lake Fryxell (34, 50, 53, 66).
When geographical distribution was considered, with the exception of Oscillatoria cf. subproboscidea, a species apparently endemic to Antarctica (27), the species found in this study have been found in various biotopes outside Antarctica and appear to have cosmopolitan distributions. Thus, on their own, the morphological results support the idea that endemism is rare among Antarctic cyanobacteria.
Molecular tools, however, revealed an entirely different pattern. Indeed, a higher number of phylotypes (15) than morphotypes (8) was detected. In addition, microdiversity, commonly found in molecular ecology studies (15), was observed. On this basis and considering the small size (0.5 g) of the mat sample studied, we assume that the cyanobacterial diversity in microbial mats of Lake Fryxell is quite high. Brambilla et al. (8) have described the enormous complexity of the eubacterial community of these mats on the basis of 16S rRNA gene sequences of clones and isolates.
The phylogenetic analysis showed that sequences determined in this study along with the polar sequences available from the databases were distributed in 22 lineages, 2 of which were novel and 7 of which were exclusively Antarctic. The sequences obtained in this study were distributed in 11 lineages, which included the 2 novel lineages and 3 lineages that are exclusively Antarctic. Furthermore, five Antarctic sequences determined in the present study and 13 sequences available in the databases were distantly related (less than 97.5% similarity) to the other sequences in their clusters. Therefore, if we considered numbers of phylotypes rather than numbers of lineages, our sequences belonged to 15 phylotypes, 4 of which were closely related to Antarctic sequences only and 7 of which were novel phylotypes. These results suggest that a previously undiscovered diversity was found and that Antarctic endemic species are more abundant than estimated previously on the basis of morphological features. In contrast, the three Nostoc sequences determined in this study belonged to cluster VII, which comprised other Nostoc sequences from polar and nonpolar environments. It seems, therefore, that the genus Nostoc has a more cosmopolitan distribution. However, these conclusions must be considered with care. Indeed, 16S rRNA gene sequence databases have grown very fast during the last decade, but they are certainly not exhaustive.
Nadeau et al. (37) suggested a bipolar distribution for several oscillatorians based on a rare 11-nucleotide insertion (5′-AGTTGTGAAAG-3′) in the 16S rRNA genes of several Antarctic and Arctic strains. However, sequences from nonpolar environments also have this insertion, and a strict bipolar distribution was not observed in our analysis. In contrast to Antarctic sequences, Arctic sequences always clustered with nonpolar sequences. However, as only eight Arctic sequences were available in the databases, the apparent lack of Arctic endemism must be considered with care.
Interestingly, Gordon et al. (20) demonstrated, by using oligonucleotide probes specific for 16S rRNA gene sequences of uncultured Antarctic cyanobacteria isolated from the ice cover of Lake Bonney, that the lake ice microbial community was dominated by organisms that originated elsewhere in the surrounding region (e.g., terrestrial microbial mats). In agreement with this conclusion, some of our sequences clustered with sequences with which the probes were designed (clusters IX and XII).
Finally, as explained above, our findings support the conclusion that there is not a univocal relationship between morphological diversity and molecular diversity. The molecular results displayed greater diversity, and decisive arguments concerning the worldwide geographical distribution of cyanobacteria should therefore include genotypic data. However, as remarked by other authors, it cannot be presumed that the molecular approach provides a complete view of diversity. In the present study, for example, sequences related to the genera Nodularia and Hydrocoryne were not detected, although these genera were observed by light microscopy. To obtain as complete a picture of the diversity as possible, molecular methods and the more traditional methods based on microscopy should be combined.
Molecular tools: DGGE versus clone libraries, ITS sequences, and PCR primers.
The problem of DGGE band reamplification precluded a complete comparison of DGGE and clone libraries in terms of the sequences obtained. However, we observed that the clone libraries provided a more complete picture of the cyanobacterial diversity in Antarctic microbial mats than DGGE provided as they yielded a higher number of different sequences or phylotypes. In addition, Ferris et al. (14) indicated that the DGGE technique could be used to detect the most abundant organisms, but this was not the case in our study.
Since the ITS is less subject to selection pressure, it may accumulate more mutations than functional genes (39) and is therefore a variable marker. Consequently, it has been used successfully in several studies based on restriction fragment length polymorphism (49) or direct sequencing (3, 7, 13) to discriminate cyanobacterial strains at the intra- or interspecific level. In addition, it has proven to be useful in bacterial community studies (5). Here we evaluated the potential of its high variability, first in combination with the 16S rRNA gene to screen our clone libraries by the ARDRA technique and second to reveal finer-scale variations in the genotypic community composition by sequencing. However, the existence of multiple rRNA operons with ITS that may differ in sequence, length, secondary structure, and the presence or absence of tRNA genes in a single organism (7) must be taken into consideration when data are interpreted.
Our analysis showed that different ITS types, which could not be aligned meaningfully, were retrieved from clones belonging to the same phylotype (16S rRNA gene partial sequences that were up to >99.5% similar). In addition, we did not expect to find clones belonging to different phylotypes with identical ITS types (levels of sequence similarity ranging from 94.3 to 100%). As we resequenced the junctions between the 16S rRNA gene and the ITS, this observation could be explained only by the existence of lateral gene transfer or by the presence of undetected chimeras among our clones. The latter hypothesis is more credible as the formation of chimeras is a well-known artifact of PCR (45, 54), and we excluded several such artifacts during this study.
For the first time in community analysis, a PCR primer pair that allowed amplification of the 16S rRNA gene and the ITS that is specific for the cyanobacterial sequences was used. Primer 16S27F is a universal primer for bacteria. Primer 23S30R and its previous version (primer 23S26R) have been used with success with a wide variety of strains since 1993 (28, 38, 70, 71, 72). The high number of cyanobacterial sequences and the absence of other bacterial sequences in our clone libraries confirm the applicability of this primer combination, which performs better than the universal bacterial primers. Indeed, with the latter primers, only five cyanobacterial sequences (including two chimeras) and one plastid sequence were obtained among a total of 325 sequences for a subsample of the same microbial mat from Lake Fryxell (8).
Comparison of the field mat and the cultured artificial mat, linking the community structure of the artificial mat with its functional properties.
Our analysis of the cyanobacterial diversity in a field mat sample from Lake Fryxell was enlarged to include a derived cultured mat from a BGC, since the BGC mat has been extensively studied by using microsensor techniques and pigment analysis (11, 43), which permit linkage of its community structure directly to its functional properties.
In terms of pigment content and primary productivity, Buffan-Dubau et al. (11) have suggested that artificial mats have some characteristics of natural polar mats, although some differences were found.
Not unexpectedly, the present study showed that the BGC culture conditions selected a cyanobacterial mat community which represented some of the diversity observed in the original field mat used as the inoculum. With the exception of one phylotype, the morphotypes and phylotypes retrieved from the BGC sample were present in the field sample. The most notable differences between the two mats were the high number of sequences closely related to psychrophilic oscillatorians, the dominance of Phormidium cf. autumnale, and the apparent absence of Nostocales in the BGC sample. Based on the 16S rRNA gene, psychrophilic oscillatorian strains appear to be restricted to a single monophyletic clade (37), while most polar cyanobacteria are psychrotolerant (58) and the order Oscillatoriales is polyphyletic (23, 38, 67, 69). This suggests that the BGC clones belonging to cluster I represented possibly psychrophilic cyanobacteria. The numerical importance of these clones may explain the observation that oxygenic photosynthesis in the artificial cold-adapted mats was optimal at 10°C, while no oxygenic photosynthesis was detected at temperatures above 15°C. The high concentration of hydrogen sulfide, which was more than 100 μM at the end of the 6-h dark period below a depth of 1 mm (43), along with the presence of oscillatorians able to migrate in the mats depending on the light conditions (4, 36), suggests that Phormidium cf. autumnale (morphotype F) migrated to the surface when the H2S concentration was too high and was therefore selected in the BGC. The apparent absence of heterocystous taxa was also reflected by the scytonemin contents, which were 2 orders of magnitudes lower in the BGC mat than in the field mat. Scytonemin plays an important role in photoprotection against the deleterious effects of UV radiation in Antarctic mats (21) but does not provide a real advantage under culture conditions where artificial light is less harmful (11). This may suggest that members of the Nostocales were overgrown by fast-growing oscillatorians. Most likely, the absence of heterocystous cyanobacteria in the BGC was related to the availability of high levels of bound nitrogen (NH4Cl), as well as the greater sensitivity of the organisms to high sulfide concentrations (22).
This study provided genotypic confirmation of the existence of endemic cyanobacteria in Antarctica, as well as the existence of cosmopolitan taxa that have Antarctic representatives. Additional studies of cyanobacteria living in microbial mats in different regions of Antarctica would be interesting to verify the geographical conclusions of this study and to obtain more information about the ecological range of the cyanobacterial genotypes. Other techniques, like fluorescent in situ hybridization, could also help workers assign morphotypes to clusters containing only sequences of uncultured organisms.
Acknowledgments
This study was funded by the European Union Biotechnology Program through the MICROMAT project (grant BIO4-CT98-0040). Annick Wilmotte is a research associate of the National Fund for Scientific Research (Belgium). Arnaud Taton had a fellowship from the Funds for Research Formation in Industry and Agriculture (Belgium).
We thank Cathy Welsch, who collected the mat material, and the Long Term Ecosystem Research Program, under whose auspices the material was collected. Johanna Laybourn-Parry (University of Nottingham, Nottingham, United Kingdom) organized the transport of samples. Dominic Hodgson (British Antarctic Survey) and Warwick Vincent (Laval University, Quebec, Canada) are acknowledged for stimulating discussions, for reading versions of the manuscript, and for contributing many helpful comments and suggestions. We thank the anonymous reviewers for their corrections.
REFERENCES
- 1.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anagnostidis, K., and J. Komárek. 1988. Modern approach to the classification system of cyanophytes. 3. Oscillatoriales. Arch. Hydrobiol. 50/53(Suppl. 80):327-472. [Google Scholar]
- 3.Baurain, D., L. Renquin, S. Grubisic, and P. Scheldeman. 2002. Remarkable conservation of internally transcribed spacer sequences of Arthrospira (“Spirulina”) (Cyanophyceae, Cyanobacteria) strains from four continents and of recent and 30-year-old dried samples from Africa. J. Phycol. 38:384-393. [Google Scholar]
- 4.Bebout, B. M., and F. Garcia-Pichel. 1995. UV B-induced vertical migrations of cyanobacteria in a microbial mat. Appl. Environ. Microbiol. 61:4215-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benlloch, S., S. G. Acinas, J. Anton, L. Lopez, S. P. Luz, and F. Rodiguez-Valera. 2001. Archeal biodiversity in crystallizer ponds from a solar saltern: culture versus PCR. Microb. Ecol. 41:12-19. [DOI] [PubMed] [Google Scholar]
- 6.Bowman, J. P., S. M. Rea, S. A. McCammon, and T. A. McMeekin. 2000. Diversity and community structure within anoxic sediment from marine salinity meromictic lakes and a coastal meromictic marine basin, Vestfold Hills, Eastern Antarctica. Environ. Microbiol. 2:227-237. [DOI] [PubMed] [Google Scholar]
- 7.Boyer, S. L., J. R. Johansen, V. R. Flechtner, and G. L. Howard. 2002. Phylogeny and genetic variance in terrestrial Microcoleus (Cyanophyceae) species based on sequence analysis of the 16S rRNA gene and associated 16S-23S ITS region. J. Phycol. 38:1222-1235. [Google Scholar]
- 8.Brambilla, E., H. Hippe, A. Hagelstein, B. J. Tindall, and E. Stackebrandt. 2001. 16S rDNA diversity of cultured and uncultured prokaryotes of a mat sample from Lake Fryxell, McMurdo Dry Valleys, Antarctica. Extremophiles 5:23-33. [DOI] [PubMed] [Google Scholar]
- 9.Broady, P. A. 1982. Taxonomy and ecology of algae in a freshwater stream in Taylor Valley, Victoria Land, Antarctica. Arch. Hydrobiol. 32:331-349. [Google Scholar]
- 10.Broady, P. A., and A. L. Kibblewhite. 1991. Morphological characterization of Oscillatoriales (cyanobacteria) from Ross Island and southern Victoria Land, Antarctica. Antarct. Sci. 3:35-45. [Google Scholar]
- 11.Buffan-Dubau, E., O. Pringault, and R. de Wit. 2001. Artificial cold-adaptated microbial mats cultured from Antarctic lake samples. 1. Formation and structure. Aquat. Microb. Ecol. 26:115-125. [Google Scholar]
- 12.Christner, B. C., B. H. Kvitko II, and J. N. Reeve. 2003. Molecular identification of bacteria and eukarya inhabiting an Antarctic cryoconite hole. Extremophiles 7:177-183. [DOI] [PubMed]
- 13.Ernst, A., S. Becker, U. I. A. Wollenzien, and C. Postius. 2003. Ecosystem-dependent adaptive radiations of picocyanobacteria inferred from 16S rRNA and ITS-1 sequence analysis. Microbiology 149:217-228. [DOI] [PubMed] [Google Scholar]
- 14.Ferris, M. J., G. Muyzer, and D. M. Ward. 1996. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl. Environ. Microbiol. 62:340-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furhman, J. A., and L. Campbell. 1998. Microbial microdiversity. Nature 393:410-411. [Google Scholar]
- 16.Garcia-Pichel, F., A. López-Cortés, and U. Nübel. 2001. Phylogenetic and morphological diversity of cyanobacteria in soil desert crusts from the Colorado Plateau. Appl. Environ. Microbiol. 67:1902-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Pichel, F., L. Prufert-Bebout, and G. Muyzer. 1996. Phenotypic and phylogenetic analyses show Microcoleus chthonoplastes to be a cosmopolitan cyanobacterium. Appl. Environ. Microbiol. 62:3284-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geitler, L. 1932. Cyanophyceae. Rabenhorst's Kryptogamen-Flora von Deutschland, Österreich und der Schweiz. Akademische Verlagsgesellschaft, Leipzig, Germany.
- 19.Good, I. J. 1953. The population frequencies of species and the estimation to the population parameters. Biometrika 40:237-264. [Google Scholar]
- 20.Gordon, D. A., J. Priscu, and S. Giovannoni. 2000. Origin and phylogeny of microbes living in permanent Antarctic lake ice. Microb. Ecol. 39:197-202. [DOI] [PubMed] [Google Scholar]
- 21.Hodgson, D. A., W. Vyverman, and K. Sabbe. 2001. Limnology and biology of saline lakes in the Rauer Islands, eastern Antarctica. Antarct. Sci. 13:255-270. [Google Scholar]
- 22.Howsley, R., and H. W. Pearson. 1979. pH dependent sulfide toxicity to oxygenic photosynthesis in cyanobacteria. FEMS Microbiol. Lett. 6:287-292. [Google Scholar]
- 23.Ishida, T., M. M. Watanabe, J. Sugiyama, and A. Yokota. 2001. Evidence for polyphyletic origin of the members of Oscillatoriales and Pleurocapsales as determined by 16S rDNA analysis. FEMS Microbiol. Lett. 201:79-82. [DOI] [PubMed] [Google Scholar]
- 24.Iteman, I., R. Rippka, N. Tandeau de Marsac, and M. Herdman. 2000. Comparison of conserved structural and regulatory domains within divergent 16S rRNA-23S rRNA spacer sequences of cyanobacteria. Microbiology 146:1275-1286. [DOI] [PubMed] [Google Scholar]
- 25.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, New York, N.Y.
- 26.Komárek, J., and K. Anagnostidis. 1989. Modern approach to the classification system of cyanophytes. 4. Nostocales. Arch. Hydrobiol. 56(Suppl. 82/3):247-345. [Google Scholar]
- 27.Komárek, J. 1999. Diversity of cyanoprokaryotes (cyanobacteria) of King George Island, maritime Antarctica—a survey. Arch. Hydrobiol. 94:181-193. [Google Scholar]
- 28.Lepère, C., A. Wilmotte, and B. Meyer. 2000. Molecular diversity of Microcystis strains (Cyanophyceae, Chroococcales) based on 16S rDNA sequences. Syst. Geogr. Plants 70:275-283. [Google Scholar]
- 29.Ludwig, W., O. Strunk, S. Klubauer, M. Weizeneger, J. Neumaier, M. Bachleitner, and K. H. Schleifer. 1998. Bacterial phylogeny based on comparative sequence analysis. Electrophoresis 19:554-568. [DOI] [PubMed] [Google Scholar]
- 30.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margalef, R. 1958. Information theory in ecology. Gen. Syst. 3:36-71. [Google Scholar]
- 32.May, R. M. 1975. Patterns of species abundance and diversity, p. 81-120. In M. L. Cody and J. M. Diamond (ed.), Ecology and evolution of communities. Harvard University Press, Cambridge, Mass.
- 33.McKnight, D. M., A. Alger, C. M. Tate, G. Shupe, and S. A. Spaulding. 1998. Longitudinal patterns in algal abundance and species distribution in meltwater streams in Taylor Valley, southern Victoria Land, Antarctica, p. 109-127. In J. C. Priscu (ed.), Ecosystem dynamics in a polar desert: the McMurdo Dry Valleys, Antarctica. American Geophysical Union, Washington, D.C.
- 34.McKnight, D. M., B. L. Howes, C. D. Taylor, and D. D. Goehringer. 2000. Phytoplankton dynamics in a stability stratified Antarctic lake during winter darkness. J. Phycol. 36:852-861. [Google Scholar]
- 35.Miller, S. R., and R. W. Castenholz. 2001. Ecological physiology of Synechococcus sp. strain SH-94-5, a naturally occurring cyanobacterium deficient in nitrate assimilation. Appl. Environ. Microbiol. 67:3002-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nadeau, T. L., C. Howard Williams, and R. W. Castenholz. 1999. Effects of solar UV and visible irradiance on photosynthesis and vertical migration of Oscillatoria sp. (cyanobacteria) in an Antarctic microbial mat. Aquat. Microb. Ecol. 20:231-243. [Google Scholar]
- 37.Nadeau, T. L., E. C. Milbrandt, and R. W. Castenholz. 2001. Evolutionary relationships of cultivated Antarctic oscillatoriaceans (cyanobacteria). J. Phycol. 37:650-654. [Google Scholar]
- 38.Nelissen, B., R. De Baere, A. Wilmotte, and R. De Wachter. 1996. Phylogenetic relationships of nonaxenic filamentous cyanobacterial strains based on 16S rRNA sequence analysis. J. Mol. Evol. 42:194-200. [DOI] [PubMed] [Google Scholar]
- 39.Normand, P., C. Ponsonnet, X. Nesme, M. Neyra, and P. Simonet. 1996. ITS analysis of prokaryotes, 3.4.5, p. 1-12. In A. D. L. Akkermans, J. D. van Elsas, and F. J. de Bruijn (ed.), Molecular microbial ecology manual. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 40.Nübel, U., F. Garcia-Pichel, and G. Muyzer. 1997. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 63:3327-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Sullivan, L. A., A. J. Weigthman, and J. C. Fry. 2002. New degenerate Cytophaga-Flexibacter-Bacteroides-specific 16S ribosomal DNA-targeted oligonucleotide probes reveal high bacterial diversity in River Taff epilithon. Appl. Environ. Microbiol. 68:201-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prescott, G. W. 1979. A contribution to a bibliography of Antarctic and subantarctic algae, p. 1-312. In J. Cramer (ed.), Bibliotheca phycologica. A. R. Gantner Verlag, Vaduz, Liechtenstein.
- 43.Pringault, O., E. Buffan-Dubau, and R. de Wit. 2001. Artificial cold-adapted microbial mats cultured from Antarctic lake sample. 2. Short-term temperature effects on oxygen turn-over. Aquat. Microb. Ecol. 26:127-138. [Google Scholar]
- 44.Priscu, J. C., C. H. Fristen, E. E. Adams, S. J. Giovannoni, H. W. Paerl, C. P. McKay, P. T. Doran, D. A. Gordon, B. D. Lanoil, and J. L. Pinckney. 1998. Perennial Antarctic lake ice: an oasis for life in a polar desert. Science 280:2095-2098. [DOI] [PubMed] [Google Scholar]
- 45.Qiu, X., L. Wu, H. Huang, P. E. McDonel, A. V. Palumbo, J. M. Tiedje, and J. Zou. 2001. Evaluation of PCR-generated chimeras, mutations, and heteroduplexes with 16S rRNA gene-based cloning. Appl. Environ. Microbiol. 67:880-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redfield, E., S. M. Barns, J. Belnap, L. L. Daane, and C. R. Kuske. 2002. Comparative diversity and composition of cyanobacteria in three predominant soil crusts of Colorado Plateau. FEMS Microbiol. Ecol. 4:55-63. [DOI] [PubMed] [Google Scholar]
- 47.Rudi, K., O. M. Skulberg, F. Larsen, and K. S. Jakobsen. 1997. Strain characterization and classification of oxyphotobacteria in clone cultures on the basis of 16S rRNA sequences from the variable regions V6, V7, and V8. Appl. Environ. Microbiol. 63:2593-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 49.Scheldeman, P., D. Baurain, R. Bouhy, M. Scott, M. Mühling, B. A. Whitton, A. Belay, and A. Wilmotte. 1999. Arthrospira (′Spirulina') strains from four continents are resolved into only two clusters, based on amplified ribosomal DNA restriction analysis of the internally transcribed spacer. FEMS Microbiol. Lett. 172:213-222. [DOI] [PubMed] [Google Scholar]
- 50.Simmons, G. M., Jr., J. R. Vestal, and R. A. Wharton, Jr. 1993. Environmental regulators of microbial activity in continental Antarctic lakes, p. 491-541. In W. J. Green and E. I. Freidmann (ed.), Physical and biogeochemical processes in Antarctic lakes. American Geophysical Union, Washington, D.C.
- 51.Smalla, K., N. Cresswell, L. C. Mendoca-Hagler, A. Wolters, and J. D. van Elsas. 1993. Rapid DNA extraction protocol from soil for polymerase chain reaction-mediated amplification. J. Appl. Bacteriol. 74:78-85. [Google Scholar]
- 52.Smith, M. C., J. P. Bowman, F. J. Scott, and M. A. Line. 2000. Sublithic bacteria associated with Antarctic quartz stones. Antarct. Sci. 12:177-184. [Google Scholar]
- 53.Spaulding, S. A., D. M. McKnight, R. L. Smith, and R. Dufford. 1994. Phytoplankton population dynamics in perennially ice-covered Lake Fryxell, Antarctica. J. Plankton Res. 16:527-541. [Google Scholar]
- 54.Speksnijder, A. G. C. L., G. A. Kowalchuk, S. De Jong, E. Kline, J. R. Stephen, and H. J. Laanbroek. 2001. Microvariation artifacts introduced by PCR and cloning of closely related 16S rRNA gene sequences. Appl. Environ. Microbiol. 67:469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spigel, R. H., and J. C. Priscu. 1998. Physical limnology of the McMurdo Dry Valleys lakes, p. 153-188. In J. C. Priscu (ed.), Ecosystem dynamics in a polar desert: the McMurdo Dry Valleys, Antarctica. American Geophysical Union, Washington, D.C.
- 56.Stackebrandt, E., and B. M. Göbel. 1994. A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 57.Stougaard, P., F. Jørgensen, M. G. Johnsen, and O. C. Hansen. 2002. Microbial diversity in ikaite tufa columns: an alkaline, cold ecological niche in Greenland. Environ. Microbiol. 4:487-493. [DOI] [PubMed] [Google Scholar]
- 58.Tang, E. P. Y., R. Tremblay, and W. F. Vincent. 1997. Cyanobacterial dominance of polar freshwater ecosystems: are high-latitude mat-formers adapted to low temperature? J. Phycol. 33:171-181. [Google Scholar]
- 59.Tindall, B. J., E. Brambilla, M. Steffen, R. Neumann, R. Pukall, R. M. Kroppensted, and E. Stackebrandt. 2000. Cultivable microbial biodiversity: gnawing at the Gordian knot. Environ. Microbiol. 2:310-318. [DOI] [PubMed] [Google Scholar]
- 60.Van de Peer, Y., and R. De Wachter. 1997. Construction of evolutionary distance trees with TREECON for Windows: accounting for variation in nucleotide substitution rate among sites. Comput. Applic. Biosci. 13:227-230. [DOI] [PubMed] [Google Scholar]
- 61.Van Trappen S., J. Mergaert, S. Van Eygen, P. Dawyndt, M. C. Cnockaert, and J. Swings. Diversity of 746 heterotrophic bacteria isolated from microbial mats in Antarctic lakes. Syst. Appl. Microbiol. 25:603-610. [DOI] [PubMed]
- 62.Vincent, W. F. 2000. Cyanobacterial dominance in the polar regions, p. 321-340. In B. A. Whitton and M. Potts (ed.), The ecology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 63.Vincent, W. F. 2000. Evolutionary origins of Antarctic microbiota: invasion, selection and endemism. Antarct. Sci. 12:374-385. [Google Scholar]
- 64.Vincent, W. F., J. P. Bowman, L. M. Rankin, and T. A. McMeekin. 2000. Phylogenetic diversity of picocyanobacteria in Arctic and Antarctic ecosystems, p. 317-322. In R. Bell, C. M. Brylinsky, and M. Johnson-Green (ed.), Microbial biosystems: new frontiers. Proceedings of the 8th International Symposium on Microbial Ecology, Halifax Canada. Atlantic Canada Society for Microbial Ecology, Halifax, Canada.
- 65.Ward, D. M., M. J. Ferris, S. C. Nold, and M. M. Bateson. 1998. A natural view of microbial diversity within hot spring cyanobacterial mat communities. Microbiol. Mol. Biol. Rev. 62:1353-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wharton, R. A., Jr., C. B. Parker, and G. M. Simmons, Jr. 1983. Distribution, species composition and morphology of algal mats in Antarctic Dry Valley lakes. Phycologia 22:355-365. [Google Scholar]
- 67.Wilmotte, A., and M. Herdman. 2001. Phylogenetic relationships among cyanobacteria based on 16S rRNA sequences, p. 487-493. In D. R. Boone and R. W. Castenholz (ed.), Bergey's manual of systematic bacteriology, vol. 1. Springer, New York, N.Y.
- 68.Wilmotte, A. 1988. Growth and morphological variability of six strains of Phormidium cf. ectocarpi (Cyanophyeae) cultivated under different temperatures and light intensities Arch. Hydrobiol. 50/53(Suppl. 80):35-46. [Google Scholar]
- 69.Wilmotte, A. 1994. Molecular evolution and taxonomy of the cyanobacteria, p. 1-25. In A. Bryant (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 70.Wilmotte, A., C. Demonceau, A. Goffart, J.-H. Hecq, V. Demoulin, and A. C. Crossley. 2002. Molecular and pigment studies of the picophytoplankton in a region of Southern Ocean (42-54°S, 141-144°E) in March 1998. Deep-Sea Res. II 49:3351-3363. [Google Scholar]
- 71.Wilmotte, A., G. Van der Auwera, and R. De Wachter. 1993. Structure of the 16S ribosomal RNA of the thermophilic cyanobacterium Chlorogloeopsis HTF (′Mastigocladus laminosus HTF') strain PCC7518, and phylogenic analysis. FEMS Lett. 317:96-100. [DOI] [PubMed] [Google Scholar]
- 72.Wilmotte, A., J.-M. Neefs, and R. De Wachter. 1994. Evolutionary affiliation of the marine nitrogen-fixing cyanobacterium Trichodesmium sp. strain NIBB 1067, derived by 16S ribosomal RNA sequence analysis. Microbiology 140:2159-2164. [DOI] [PubMed] [Google Scholar]