Abstract
Isolation and synthesis of isoflavonoids has become a frequent endeavor, due to their interesting biological activities. The introduction of hydroxyl groups into isoflavonoids by the use of enzymes represents an attractive alternative to conventional chemical synthesis. In this study, the capabilities of biphenyl-2,3-dioxygenase (BphA) and biphenyl-2,3-dihydrodiol 2,3-dehydrogenase (BphB) of Burkholderia sp. strain LB400 to biotransform 14 isoflavonoids synthesized in the laboratory were investigated by using recombinant Escherichia coli strains containing plasmid vectors expressing the bphA1A2A3A4 or bphA1A2A3A4B genes of strain LB400. The use of BphA and BphB allowed us to biotransform 7-hydroxy-8-methylisoflavone and 7-hydroxyisoflavone into 7,2′,3′-trihydroxy-8-methylisoflavone and 7,3′,4′-trihydroxyisoflavone, respectively. The compound 2′-fluoro-7-hydroxy-8-methylisoflavone was dihydroxylated by BphA at ortho-fluorinated and meta positions of ring B, with concomitant dehalogenation leading to 7,2′,3′,-trihydroxy-8-methylisoflavone. Daidzein (7,4′-dihydroxyisoflavone) was biotransformed by BphA, generating 7,2′,4′-trihydroxyisoflavone after dehydration. Biotransformation products were analyzed by gas chromatography-mass spectrometry and nuclear magnetic resonance techniques.
Isoflavonoids are one of the six main subclasses of flavonoids and the only one which contains a rearranged C15 skeleton based on 3-phenylchroman. The interesting biological properties described for isoflavonoids include antimicrobial, antioxidant, anti-inflammatory, estrogenic, and cancer chemoprotectant activities (3, 9, 14, 29). Genistein and daidzein have antioxidant properties, leading to the inhibition of lipid peroxidation in a liposomal system, and are quenchers of singlet oxygen (1, 5). The anti-inflammatory activities of isoflavonoids are associated with the inhibition of lipoxygenases and cyclooxygenases (6, 28). The estrogenic activity of genistein, daidzein, and equol are currently being extensively investigated at the molecular, preclinical, and clinical levels to determine their potential for the treatment of chronic diseases such as hormone-dependent cancer, cardiovascular disease, and osteoporosis (31).
To extend the diversity of isoflavonoids and to improve their availability, chemical synthesis is being used. Methods for the chemical synthesis of isoflavonoids have been previously described (17, 25, 30). In addition to chemical synthesis, which often generates toxic waste products, microbial biotransformation has been used (13). Biotransformation is of increasing importance in the chemical industry, and the use of enzymes for the production of organic molecules is an important field of biotechnology (10, 18, 20). Microbial transformation offers the advantages of high stereospecificity, operation at nonextreme pH and near room temperature, and reduced levels of toxic waste products (18). Biotransformation with recombinant microbial enzymes has been widely used, including applications for the production of hormones, antibiotics, and speciality chemicals (10, 20).
Hydroxylation of aromatics by chemical synthesis is difficult and involves diverse reaction steps. Since the conversion of a carbon-hydrogen to a carbon-hydroxyl bond is one of the key features of the oxidative metabolism of many aromatic compounds (12), hydroxylation of these substrates by biotransformation is straightforward. The enzyme biphenyl-2,3-dioxygenase (BphA) of the bacterium Burkholderia sp. strain LB400 is an enzyme with an unusually broad substrate range (11, 21-24). BphA of strain LB400 can oxidize a wide range of aromatic compounds, including a number of substituted biphenyls, unsubstituted dibenzofuran, and dibenzodioxin. In addition, the dehalogenation potential of BphA of strain LB400 toward substituted biphenyls has been observed (11, 22-24). While this work was in preparation, a report was published that described the transformation of flavones by a hybrid biphenyl dioxygenase (7).
Biphenyl-2,3-dihydrodiol 2,3-dehydrogenase (BphB) of the bacterial strain Burkholderia sp. strain LB400 is able to further oxidize diverse dioxygenation products of BphA (21-24). BphA and BphB are the first two enzymes of the upper biphenyl pathway of strain LB400. We describe here the enzymatic transformation of natural and synthetic isoflavonoids catalyzed by BphA alone or by both BphA and BphB of the bacterium Burkholderia sp. strain LB400.
MATERIALS AND METHODS
Isoflavonoid synthesis.
Isoflavonoids (>90% pure) were synthesized in the laboratory by previously described methods (25). Briefly, BF3-diethyl ether (0.88 mol) was added dropwise to a solution of a benzylketone (0.18 mol) in dry N,N-dimethylformamide (200 ml). To this solution, warmed to 50°C, was slowly added a solution of methanesulfonyl chloride (0.56 mol) in N,N-dimethylformamide (100 ml). The resulting mixture was then heated to 100°C for 2 h. After it cooled, it was poured into water (4 liters) and left overnight to yield a precipitate, which was filtered and stirred for 2 h in cold methanol (50 ml), filtered again, and then crystallized in the appropriate solvent.
Bacterial strains, plasmids, and culture conditions.
The Escherichia coli strains used in the present study were BL21(DE3)/pLysS (26) harboring either pT7-6, pAIA111, or pAIA13 plasmids. pT7-6 is the vector devoid of bph genes. pAIA111 and pAIA13 are based on the phage T7 expression vector pT7-6. pAIA111 carries bphA1A2A3A4 (bphA1A2A3A4 are collectively referred to as bphA), and pAIA13 harbors bphAB of Burkholderia sp. strain LB400. The constructions of pAIA111 and pAIA13 have been described previously (16, 23). Bacteria were grown in Luria-Bertani medium (19) at 37°C. Where appropriate, chloramphenicol and ampicillin at concentrations of 20 and 50 μg/ml, respectively, were used for selection.
Preparation of resting cells.
Preparation of resting cells was carried out as previously described (21) with minor modifications. E. coli BL21(DE3)/pLysS cells harboring either pT7-6, pAIA111 (bphA), or pAIA13 (bphAB) were grown in Luria-Bertani medium until the exponential phase was reached (21, 23, 26), followed by further incubation in the presence of 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 30 min at 30°C. Cells were harvested, washed with 50 mM sodium phosphate buffer (pH 7.5), and resuspended in a 1/30 volume of the same buffer.
Biotransformation of isoflavonoids by BphA or BphA-BphB and analysis of products.
Resting cell suspensions (i.e., an optical density at 600 nm of 18) of E. coli BL21(DE3)/pLysS harboring either pAIA111 or pAIA13 were incubated with an isoflavonoid at a nominal concentration of 1 mM on a rotary shaker for 6 h at 30°C. The reaction mixtures were extracted with ethyl acetate at a ratio of 1:0.8. The organic layer was reextracted with 1 volume of 50 mM sodium phosphate buffer (pH 7.5) and dried over magnesium sulfate, and the solvent was removed from the extract.
To obtain trimethylsylil derivatives, the residue was redissolved in 50 μl of N-σ-bis-(trimethylsylil)-trifluoracetamide-trimethylchlorosylane (BSTFA-TMCS) (99:1), and the solution was incubated at 70°C for 30 min. After derivatization, mixtures were evaporated to dryness under a stream of nitrogen and dissolved in 10 μl of n-octane or cyclohexane. Samples (1 μl) were injected in the splitless mode into a gas chromatography-mass spectrometry (GC-MS) system (Perkin-Elmer Autosystem XL gas chromatograph) with a MDN-1 column (Supelco, Bellefonte, Pa.), coupled with a Perkin-Elmer Turbo-Mass mass spectrometer. Helium served as the carrier gas. The mass spectrometer was operated in the electron impact ionization mode at 70 eV.
1H NMR spectra of biotransformation products without further purification were obtained with a Bruker DXP 300 spectrometer. All spectra were recorded in dimethyl sulfoxide-d6.
RESULTS
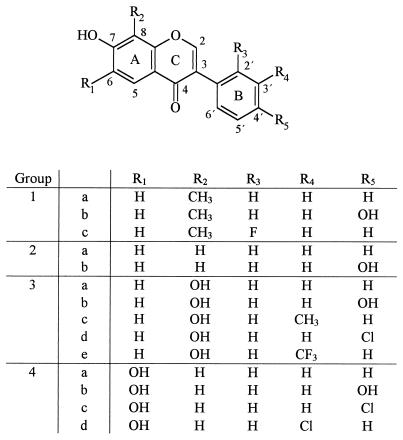
The structural formulas of 14 isoflavonoids synthesized and used for biotransformation experiments are shown in Fig. 1. Incubations of isoflavonoids were carried out with resting recombinant E. coli cells either harboring plasmid pAIA111 and synthesizing BphA or harboring plasmid pAIA13 and synthesizing BphA and BphB of Burkholderia sp. strain LB400. The results of the biotransformation experiments are shown in Tables 1 and 2 . Yields were deduced from total ion chromatography (TIC) peak areas of the products and are based on the assumption that similar amounts of the different products yield similar areas (23). Additionally, for the identification of the products in some cases, NMR analysis was used. Controls showed that E. coli BL21(DE3)/pLysS harboring the pT7-6 vector and devoid of bph genes was unable to biotransform isoflavonoids.
FIG. 1.
Structural formulas and carbon atom numbering of natural and synthetic isoflavonoids synthesized and used for biotransformation experiments. Isoflavonoids were classified into four groups, depending on the substitution pattern of ring A. Group 1 includes 7-hydroxy-8-methylisoflavone (1a), 7,4′-dihydroxy-8-methylisoflavone (1b), and 2′-fluoro-7-hydroxy-8-methylisoflavone (1c). Group 2 includes 7-hydroxyisoflavone (2a), 7,4′-dihydroxyisoflavone, or daidzein (2b). Group 3 includes 7,8-dihydroxyisoflavone (3a), 7,8,4′-trihydroxyisoflavone (3b), 7,8-dihydroxy-3′-methylisoflavone (3c), 4′-chloro-7,8-dihydroxyisoflavone (3d), and 7,8-dihydroxy-3′-trifluoromethylisoflavone (3e). Group 4 includes 6,7-dihydroxyisoflavone (4a), 6,7,4′-trihydroxyisoflavone (4b), 4′-chloro-6,7-dihydroxyisoflavone (4c), and 3′-chloro-6,7-dihydroxyisoflavone (4d).
TABLE 1.
Characterization of products formed from isoflavonoids by dioxygenation catalyzed by the BphA of Burkholderia sp. strain LB400
| Substratea | Products
|
||||
|---|---|---|---|---|---|
| No. | tr (min) | Yieldb (%) | Prominent ionsc (m/z) | Ring B | |
| 7-OH-8-CH3-ISF (1a) | 1 | 27.4 | 30-35 | 412 (M+, 14), 397 (100), 73 (40) | 2′-OHd |
| 2 | 28.5 | 5-8 | 502 (M+, 12), 487 (5), 413 (6), 397 (34), 73 (86) | 2′,3′-Dihydrodiole | |
| 3 | 29.5 | 60-65 | 412 (M+, 60), 397 (45), 73 (100) | 3′-OHd | |
| 2′-F-7-OH-8-CH3-ISF (1c) | 1 | 29.7 | 100 | 500 (M+, 9), 485 (100), 73 (99) | 2′,3′-Di-OHf |
| 7-OH-ISF (2a) | 1 | 26.5 | 35-40 | 398 (M+, 10), 383 (100), 73 (41) | 3′-OHd |
| 2 | 27.8 | 10-15 | 488 (M+, 12), 473 (7), 397 (24), 383 (68), 73 (100) | 3′,4′-Dihydrodiole | |
| 3 | 28.4 | 10-15 | 398 (M+, 93), 383 (90), 73 (84) | 4′-OHd | |
| 4 | 28.6 | 35-40 | 486 (M+, 9), 471 (100), 73 (73) | 3′,4′-Di-OHg | |
| 7,4′-Di-OH-ISF (2b) | 1 | 28.7 | 100 | 486 (M+, 9), 471 (100), 73 (90) | 2′-OHg |
ISF, isoflavone.
Deduced from TIC peak areas.
The mass ion is underlined. The relative abundance(s) (expressed as the percent base peak) of each ion is given in parentheses.
Assignment is based on tr values and on the assumption that this is a dehydration product.
Assignment is based on the identification of the follow up product generated by BphA and BphB.
The defluorinated product generated by BphA must have been attacked at ortho-fluorinated and meta carbons.
Identified by NMR analysis.
TABLE 2.
Characterization of products formed from isoflavonoids catalyzed by the BphA and BphB of Burkholderia sp. strain LB400
| Substratea | Products
|
||||
|---|---|---|---|---|---|
| No. | tr (min) | Yieldb (%) | Prominent ionsc (m/z) | Ring B | |
| 7-OH-8-CH3-ISF (1a) | 1 | 27.3 | 2-4 | 412 (M+, 9), 397 (100), 73 (64) | 2′-OHd |
| 2 | 29.5 | 96-98 | 500 (M+, 9), 485 (79), 73 (100) | 2′,3′-Di-OHe | |
| 7-OH-ISF (2a) | 1 | 26.5 | 10-15 | 398 (M+, 9), 383 (100), 73 (80) | 3′-OHd |
| 2 | 27.8 | 15-20 | 488 (M+, 10), 473 (7), 397 (46), 383 (76), 73 (100) | 3′,4′-Dihydrodiolf | |
| 3 | 28.4 | 10-15 | 398 (M+, 93), 383 (92), 73 (100) | 4′-OHd | |
| 4 | 28.6 | 55-60 | 486 (M+, 9), 471 (100), 73 (60) | 3′,4′-Di-OHg | |
ISF, isoflavone.
Deduced from TIC peak areas.
The mass ion is underlined. The relative abundance(s) (expressed as the percent base peak) of each ion is given in parentheses.
Assignment is based on tr values and on the assumption that this is a dehydration product.
Identified by comparison of the mass spectrum and by chromatographic coelution with the defluorinated product of isoflavone 1c.
Assignment is based on the identification of the follow-up product generated by BphA and BphB.
Identified by NMR analysis.
Isoflavonoids of group 1.
Two isoflavonoids of this group were biotransformed by the enzyme BphA of strain LB400 (Table 1). With isoflavonoids 1a and 1c three and one product were detected, respectively.
Biotransformation of isoflavonoid 1a.
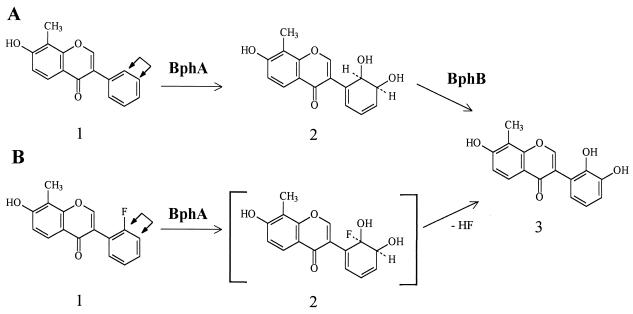
When isoflavonoid 1a was incubated with recombinant E. coli strain synthesizing BphA, three products were observed by GC-MS (Table 1). Product 2 yielded the mass spectrum of an isoflavonoid with a dihydrodiol function on ring B (DHD). Its mass spectrum was characterized primarily by a molecular ion at m/z 502 and the high-mass fragment ions [M-CH3]+ (m/z 487), [M-TMS] (m/z 429), [M-O-TMS]+ (m/z 413), and [M-O-TMS-CH3]+ (m/z 397). Products 1 and 3 yielded mass spectra consistent with isoflavonoids monohydroxylated on ring B (MH). The molecular ion at m/z 412 and the fragment ion [M-CH3]+ (m/z 397) were the principal positively charged species of the mass spectra. The MHs may have been generated by dioxygenation, yielding a DHD, followed by elimination of water (or TMS-OH), respectively. DHDs tend to undergo elimination reactions that regenerate the energetically favorable aromatic system (4). Such an elimination may occur during incubation, during metabolite extraction (particularly under acid conditions), during derivatization, or during GC. After incubation of isoflavonoid 1a with BphA and BphB, two biotransformation products were observed (Table 2). Its mass spectrum was characterized primarily by a molecular ion at m/z 500 and fragment ion [M- CH3] (m/z 485) and corresponds to a catechol. The minor product derivatized with TMS has a mass spectrum with a molecular ion at m/z 412, and the principal fragment ion is [M-CH3] (m/z 397), which are consistent with an isoflavonoid monohydroxylated on ring B.
Biotransfomation of isoflavonoid 1c.
After incubation of isoflavonoid 1c with BphA only one product was observed (Table 1). The product yielded the mass spectrum of an isoflavonoid with a dihydroxylated ring B (DH). The molecular and the fragment ions [M-15]+ (loss of CH3) were the only prominent positively charged species, indicating the loss of the substrate's halogen and the formation of a catechol (15). These results suggest that BphA-catalyzed dioxygenation of isoflavonoid 1c led to the formation of 7,2′,3′-trihydroxy-8-methylisoflavone via elimination of hydrofluoric acid from the ortho-fluorinated ring. In this case, dihydroxylation probably occurs at the ortho-substituted and meta carbons since previous results generally indicate a strong preference for the dioxygenation of ortho and meta carbons on the substituted side of ortho-halogenated rings (24). Both the mass spectrum and GC retention times (tr) of the product of isoflavonoid 1c with BphA were identical to those of the dihydroxylated product obtained from isoflavonoid 1a by cells harboring bphAB. The biotransformation products of isoflavonoid 1c with BphA and isoflavonoid 1a with BphA and BphB coeluted when a mixture was analyzed by GC-MS. Thus, the biotransformation product of isoflavonoid 1a with BphA and BphB was also identified as 7,2′,3′-trihydroxy-8-methylisoflavone.
Isoflavonoids of group 2.
Both isoflavonoids of group 2 were biotransformed by the biocatalyst BphA. Dioxygenation products were analyzed by GC-MS after derivatization with TMS (Table 1) and by NMR.
Biotransformation of isoflavonoid 2a.
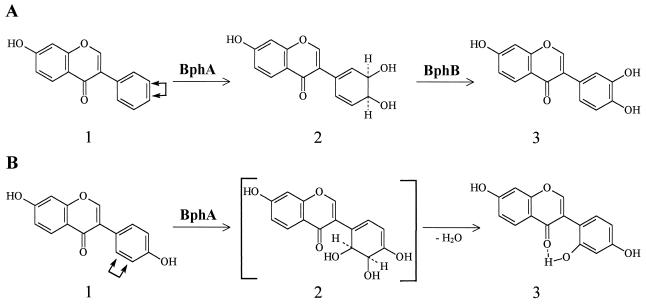
The total ion chromatogram obtained after incubation of isoflavonoid 2a with BphA showed four products (Table 1). Product 2 yielded the mass spectrum of a DHD at ring B. Two products (products 1 and 3) yielded mass spectra consistent with isoflavonoids monohydroxylated on ring B. An isoflavonoid with a dihydroxylated ring B (DH) has also been detected, which should be the result of dehydrogenation probably catalyzed by an E. coli enzymatic activity. In addition to biphenyl-2,3-dihydrodiol, a second metabolite identified as 2,3-dihydroxybiphenyl has also been observed during incubation of E. coli harboring BphA enzyme with biphenyl, probably due to an E. coli enzyme (M. Seeger et al., unpublished results).
The enzymatic hydroxylation by BphA and BphB of 7-hydroxyisoflavone gave a major product with a yield of ca. 55 to 60% of the compound identified as 7,3′,4′-trihydroxyisoflavone, according to GC-MS and 1H NMR analyses. 1H NMR spectral data of 7,3′,4′-trihydroxyisoflavone: δ 8.37 (s, H-2), δ 7.97 (d, J = 8.5 Hz, H-5), δ 7.56 (d,d, J = 8.0 and 2.5 Hz, H-6′), δ 7.53 (d, J = 2.5 Hz, H-2′), δ 7.42 (d, J = 8.0 Hz, H-5′), δ 6.95 (d,d, J = 8.5 and 2.5 Hz, H-6), and δ 6.87 (d, J = 2.5 Hz, H-8). 1H NMR analysis of this product allowed the assignment of the hydroxyl groups to meta and para positions of ring B. Two aromatic ABC systems are present, both with ortho, ortho-meta, and meta couplings (1,3,4-proton pattern). Furthermore, one of these systems shows a proton with a chemical shift of δ 7.97 and J = 8.5 Hz characteristic of H-5 (A-ring). The other system corresponds to a ring B hydroxylated at C-3′ and C-4′. The singlet with δ 8.37 is typical for an H-2 proton. The described result proves that the enzymatic hydroxylation does not take place at ring A. BphA-mediated ring hydroxylation at the meta and para positions has been reported for the bph-encoded enzyme from strain LB400 (11, 23). In addition, three other products were observed by GC-MS (Table 2). In the NMR spectrum, other signals belonging probably to minor products observed by GC-MS are present but could not be clearly assigned.
Biotransformation of isoflavonoid 2b.
The enzymatic hydroxylation by BphA of 7,4′-dihydroxyisoflavone (daidzein) leads to the production of 7,2′,4′-trihydroxyisoflavone as the only compound as determined by GS-MS and 1H NMR data analysis. The total ion chromatogram obtained after incubation of isoflavonoid 2b with BphA detected only one product (Table 1). The mass spectrum of the TMS derivative of this product is consistent with an isoflavonoid monohydroxylated (MH) probably on the ring B. The molecular ions and the fragment ions [M-15]+ (m/z 471) and [M-TMS-CH3] (m/z 398) were the principal positively charged species of the mass spectrum. 1H NMR spectral data of 7,2′,4′-trihydroxyisoflavone: δ 12.55 (s, 2′-OH), δ 7.95 (s, H-2), δ 7.94 (d, J = 9.0 Hz, H-5), δ 6.88 (d, J = 8.0 Hz, H-6′), δ 6.81 (d, J = 1.5 Hz, H-3′), δ 6.78 (d,d, J = 8.0 and 1.5 Hz, H-5′), δ 6.35 (d,d, J = 9.0 and 2.0 Hz, H-6), and δ 6.23 (d, J = 2.0 Hz, H-8). 1H NMR analysis demonstrated that the B ring contained the 2′,4′-resorcine structure. Here we also introduce two ABC systems: H-5 of ring A with a chemical shift at δ 7.94, (doublet, J = 9.0 Hz) and H-2 of ring C resonating at δ 7.95 (sharp singlet). At δ 12.55, we observed the signal of an hydroxyl proton (C-2′-OH) that made a hydrogen bridge with the carbonyl group at C-4.
Isoflavonoids of groups 3 and 4.
Under the same experimental conditions BphA of Burkholderia sp. strain LB400 was unable to biotransform 6,7-dihydroxyisoflavone (3a), 7,8-dihydroxyisoflavone (4a), and related substituted isoflavones 3b-3e and 4b-4d.
DISCUSSION
In the present study, whole recombinant E. coli cells expressing the bphA or bphAB genes of Burkholderia sp. strain LB400 were used. We showed that the recombinant enzymes BphA and BphB of bacterial strain LB400 are useful for the generation of isoflavonoids with hydroxy groups at 2′,3′ and/or 4′ (ring B). For biotransformation, the use of enzymes in whole cells (instead of purified enzyme preparations) is simpler, less expensive, and often more convenient because enzyme isolation and purification is tedious and has a significant cost and because purified enzyme preparations may lead to a rapid loss of enzyme activity (8, 20) In fact, BphA of strain LB400 loses rapidly its activity during biotransformation experiments (J. D. Haddock, unpublished data). In addition, enzyme BphA requires NADH as a cofactor, which should be regenerated. In spite of the fact that cofactor regeneration is possible in vitro, it is easier and less expensive to regenerate cofactors in metabolically active cells (20).
Bacterial aromatic-ring-hydroxylating dioxygenases have been shown to oxidize, in addition to several unsubstituted compounds, a large number of differently substituted derivatives. Previous studies have shown that BphA of Burkholderia sp. strain LB400 is able to oxidize a wide range of polychlorinated biphenyls (11, 21-23), other substituted biphenyls (24), and unsubstituted dibenzofuran and dibenzodioxin (24).
The present study shows that the Burkholderia sp. strain LB400 BphA enzyme is able to biotransform different compounds belonging to the class of the isoflavonoids, compounds that are found in nature as the secondary metabolites of plants. BphA of LB400 is able to dihydroxylate isoflavonoids at the ortho (unsubstituted or halogenated) and meta positions or at meta and para positions of ring B. 7-Hydroxy-8-methylisoflavone was oxidized by BphA at the ortho and meta positions of ring B generating a DHD (Fig. 2A). The compound 2′-fluoro-7-hydroxy-8-methylisoflavone was dihydroxylated by BphA at ortho-halogenated and meta positions with concomitant dehalogenation, leading to 7,2′3′-trihydroxy-8-methylisoflavone (Fig. 2B). The defluorination observed with isoflavonoid 1c is in accordance with previous reports: dehalogenation by the action of strain LB400 BphA of chlorinated (11, 22, 23), as well as of brominated and fluorinated biphenyls (24), has been described. Denitration and dehydroxylation of substituted biphenyls by this enzyme have also been observed (24). 7-Hydroxyisoflavone was the only isoflavonoid dihydroxylated by BphA at the meta and para positions of ring B (Fig. 3A). This type of attack by this enzyme has been observed with other aromatic compounds (11, 23, 24). Daidzein was biotransformed by BphA, generating 7,2′4′-trihydroxyisoflavone after dehydration (Fig. 3B). This compound has been observed by the plant Phaseolus vulgaris after fungal infection (32) and has been used in chemical synthesis for substituted pterocarpans (27).
FIG. 2.
Biotransformation of 7-hydroxy-8-methylisoflavone (1a) and 2′-fluoro-7-hydroxy-8-methylisoflavone (1c). (A) Biotransformation of 7-hydroxy-8-methylisoflavone by BphA and BphB. Compounds: 1, isoflavonoid 1a; 2, DHD; 3, 7,2′3′-trihydroxy-8-methylisoflavone. (B) Biotransformation of 2′-fluoro-7-hydroxy-8-methylisoflavone. Compounds: 1, isoflavonoid 1c; 2, compound in bracket denote proposed unstable intermediate; 3, 7,2′3′-trihydroxy-8-methylisoflavone.
FIG. 3.
Biotransformation of 7-hydroxyisoflavone (2a) and 7,4′-dihydroxyisoflavone (2b). (A) Biotransformation of 7-hydroxyisoflavone by BphA and BphB. Compounds: 1, isoflavonoid 2a; 2, DHD; 3, 7,3′4′-trihidroxysoflavone. (B) Biotransformation of 7,4′-dihydroxyisoflavone by BphA. Compounds: 1, isoflavonoid 2b; 2, compound in brackets denotes a proposed unstable intermediate; 3, 7,2′4′-trihydroxyisoflavone.
The dehydrogenase BphB of strain LB400 is able to further oxidize isoflavonoids dihydroxylated by BphA at the ortho and meta carbons and at the meta and para positions. The enzyme BphB of Burkholderia sp. strain LB400 can biotransform at least two of the isoflavonoids oxidized by BphA. BphB further oxidized the BphA oxidation products of 7-hydroxy-8-methylisoflavone and 7-hydroxyisoflavone to 7,2′3′-trihydroxy-8-methylisoflavone (Fig. 2A) and 7,3′4′-trihydroxyisoflavone (Fig. 3A), respectively. Recently, it has been shown that strain LB400 BphB is able to catalyze the dehydrogenation at, in addition to the ortho and meta positions, the meta and para positions of 3,4-dihydro-3,4-dihydroxy-2,2′,5,5′-tetrachlorobiphenyl (2). Chun et al. reported the biotransformation of flavones by using a hybrid dioxygenase expressed in a gram-positive bacteria (7). In that study, flavones with a dihydrodiol function on a ring, which are the products of dioxygenation, were not observed, and only mono- and dihydroxylated derivatives were detected. BphA of LB400 was able to dihydroxylate isoflavonoids at substituted ring B and to dehalogenate an isoflavanoid, generating a dihydroxylated aromatic ring B. Both properties were not previously observed with the hybrid dioxygenase (7).
6,7-Dihydroxyisoflavone and 7,8-dihydroxyisoflavone and seven related substituted isoflavones belonging to groups 3 and 4 are not biotransformed by BphA of strain LB400. This illustrates the influence of the substitution pattern of ring A for the reactivity of isoflavonoids with BphA. Both groups of isoflavonoids with a catechol group on ring A are not substrates of BphA. This could be due to steric or electronic influences of A ring on the binding to the active site or on the reactivity of the oxidized ring.
The selective modification of isoflavonoids by these enzymes is a powerful tool that deserves further analysis. The hydroxylation by biotransformation of ring B of isoflavonoids are likely to improve their antioxidant properties (1, 5). The activity as inhibitors of lipoxygenases (6, 28) of these modified isoflavonoids should be addressed. Future studies will be needed to analyze the biological properties of the new isoflavonoids generated.
Acknowledgments
We thank Victor Wray (GBF, Germany) for help with generation of the proton NMR data and Bruce Cassels (Universidad de Chile) for editorial assistance.
This work was supported by the following grants: USM of the Universidad Técnica Federico Santa María, FONDECYT 1990808 and 1020221 to M.S., ICM-P99-031 to M.S., and DICYT 02-9891 to S.S.-B.
REFERENCES
- 1.Arora, A., M. G. Nair, and G. M. Strasburg. 1998. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch. Biochem. Biophys. 356:133-141. [DOI] [PubMed] [Google Scholar]
- 2.Barriault, D., M. Vedadi, J. Powlowski, and M. Sylvestre. 1999. cis-2,3-Dihydro-2,3-dihydroxybiphenyl dehydrogenase and cis-1,2-dihydro-1,2-dihydroxynaphthalene dehydrogenase catalyze dehydrogenation of the same range of substrates. Biochem. Biophys. Res. Commun. 260:181-187. [DOI] [PubMed] [Google Scholar]
- 3.Birt, D., S. Hendrich, and W. Wang. 2001. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol. Ther. 90:157-177. [DOI] [PubMed] [Google Scholar]
- 4.Boyd, D. R., J. Blacker, B. Byrne, M. Dalton, V. Hand, S. C. Kelly, R. A. More, O'Ferrall, S. Nagaraja Rao, N. D. Sharma, and G. N. Sheldrake. 1994. Acid-catalyzed aromatization of benzene cis-1,2-dihydrodiols: a carbocation transition state poorly stabilized by resonance. J. Chem. Soc. Chem. Commun. 1994:313-314. [Google Scholar]
- 5.Briviba, K., S. Sepulveda-Boza, F. Zilliken, and H. Sies. 1997. Isoflavonoids as inhibitors of lipid peroxidation and quenchers of singlet oxygen, p. 295-302. In C. A. Rice-Evans and L. Packer (ed.), Flavonoids in health and disease. Marcel Dekker, Inc., New York, N.Y.
- 6.Chia, Y., H. Jonga, K. Sonb, H. Change, S. Kangd, and H. Kima. 2001. Effects of naturally ocurring prenylated flavonoids on enzymes metabolizing arachidonic acid: ciclooxygenases and lipoxygenases. Biochem. Pharmacol. 62:1185-1191. [DOI] [PubMed] [Google Scholar]
- 7.Chun, H.-K., Y. Ohnishi, K. Shindo, N. Misawa, K. Furukawa, and S. Horinouchi. 2003. Biotransformation of flavone and flavanone by Streptomyces lividans cells carrying shuffled biphenyl dioxygenase genes. J. Mol. Catalysis B Enzymatic 21:113-121. [Google Scholar]
- 8.Duetz, W. A., J. B. van Beilen, and B. Witholt. 2001. Using proteins in their natural environment: potential and limitations of microbial whole-cell hydroxylations in applied biocatalysis. Curr. Opin. Biotechnol. 12:419-425. [DOI] [PubMed] [Google Scholar]
- 9.El-Sebakhy, N. A., A. M. Asaad, R. M. Abdallah, S. M. Toaima, M. S. Abdel-Kader, and F. R. Stermitz. 1994. Antimicrobial isoflavans from Astragalus species. Phytochemistry 36:1387-1389. [DOI] [PubMed] [Google Scholar]
- 10.Faber, K. 1999. Biotransformations in organic chemistry: a textbook, 4th ed. Springer, Berlin, Germany.
- 11.Haddock, J. D., J. R. Horton, and D. T. Gibson. 1995. Dihydroxylation and dechlorination of chlorinated biphenyls by purified biphenyl 2,3-dioxygenase from Pseudomonas sp. strain LB400. J. Bacteriol. 177:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holland, H., and H. K. Weber. 2000. Enzymatic hydroxylation reactions. Curr. Opin. Biotechnol. 11:547-553. [DOI] [PubMed] [Google Scholar]
- 13.Hosny, M., and J. P. N. Rosazza. 1999. Microbial hydroxylation and methylation of genistein by streptomycetes. J. Nat. Prod. 62:1609-1612. [Google Scholar]
- 14.Hosny, M., and J. P. N. Rosazza. 2002. New isoflavone and triterpene glycosides from soybeans. J. Nat. Prod. 65:805-813. [DOI] [PubMed] [Google Scholar]
- 15.Massé, R., F. Messier, C. Ayotte, M. F. Lévesque, and M. Sylvestre. 1989. A comprehensive gas chromatographic/mass spectrometric analysis of 4-chlorobiphenyl bacterial degradation products. Biomed. Environ. Mass Spectrom. 18:27-47. [Google Scholar]
- 16.McKay, D. B., M. Seeger, M. Zielinski, B. Hofer, and K. N. Timmis. 1997. Heterologous expression of biphenyl dioxygenase-encoding genes from a gram-positive broad-spectrum polychlorinated biphenyl degrader and characterization of chlorobiphenyl oxidation by the gene products. J. Bacteriol. 179:1924-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mejías, L., M. H. Reihmann, S. Sepulveda-Boza, and H. Ritter. 2002. New polymers from natural phenols using horseradish or soybean peroxidase. Macromol. Biosci. 2:24-32. [Google Scholar]
- 18.Rathbone, D. A., and N. C. Bruce. 2002. Microbial transformation of alkaloids. Curr. Opin. Microbiol. 5:274-281. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 20.Schmid, A., J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts, and B. Witholt. 2001. Industrial biocatalysis today and tomorrow. Nature 409:258-268. [DOI] [PubMed] [Google Scholar]
- 21.Seeger, M., K. N. Timmis, and B. Hofer. 1995. Conversion of chlorobiphenyls into phenylhexadienoates and benzoates by the enzymes of the upper pathway for polychlorobiphenyl degradation encoded by the bph locus of Pseudomonas sp. strain LB400. Appl. Environ. Microbiol. 61:2654-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeger, M., K. N. Timmis, and B. Hofer. 1995. Degradation of chlorobiphenyls catalyzed by the bph-encoded biphenyl-2,3-dioxygenase and biphenyl-2,3-dihydrodiol-2,3-dehydrogenase of Pseudomonas sp. LB400. FEMS Microbiol. Lett. 133:259-264. [DOI] [PubMed] [Google Scholar]
- 23.Seeger, M., M. Zielinski, K. N. Timmis, and B. Hofer. 1999. Regioespecificity of dioxygenation of di- to pentachlorobiphenyls and their degradation to chlorobenzoates by the bph-encoded catabolic pathway of Burkholderia sp. strain LB400. Appl. Environ. Microbiol. 65:3614-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seeger, M., B. Cámara, and B. Hofer. 2001. Dehalogenation, denitration, dehydroxylation, and angular attack on substituted biphenyls and related compounds by a biphenyl dioxygenase. J. Bacteriol. 183:3548-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sepulveda-Boza, S., G. H. Walizei, M. C. Rezende, Y. Vásquez, C. Mascayano, and L. Mejías. 2001. The preparation of new isoflavones. Synth. Commun. 31:1993. [Google Scholar]
- 26.Studier, F. W. 1991. Use of bacteriophage T7 lyzozyme to improve an inducible T7 expression system. J. Mol. Biol. 219:37-44. [DOI] [PubMed] [Google Scholar]
- 27.Visser, F. R., and G. A. Lane. 1987. Selectivity of the hydrogenation of 2′,4′,7-tribenzyloxyisoflavone. Aust. J. Chem. 40:1705-1711. [Google Scholar]
- 28.Voss, C., S. Sepulveda-Boza, and F. W. Zilliken. 1992. New isoflavonoids as inhibitors of porcine 5-lipoxygenase. Biochem. Pharm. 44:157-162. [DOI] [PubMed] [Google Scholar]
- 29.Weidenbörner, M., H. Holger, H. C. Jha, P. Tsotsonos, and H. Egge. 1989. Antifungal activity of isoflavonoids against storage fungi of the genus Arpergillus. Phytochemistry 28:3317-3319. [Google Scholar]
- 30.Whalley, J. L., M. F. Oldfield, and N. P. Botting. 2000. Synthesis of [4-13C]isoflavonoid phytoestrogens. Tetrahedron 56:455-460. [Google Scholar]
- 31.Wiseman, H. 2000. The therapeutic potential of phytoestrogens. Exp. Opin. Investig. Drugs 9:1829-1840. [DOI] [PubMed] [Google Scholar]
- 32.Woodward, M. D. 1980. Phaseolin formation and metabolism in Phaeolus vulgaris. Phytochemistry 19:921-927. [Google Scholar]