Abstract
Musty “off-flavor” in pond-cultured channel catfish (Ictalurus punctatus) costs the catfish production industry in the United States at least $30 million annually. The cyanobacterium Oscillatoria perornata (Skuja) is credited with being the major cause of musty off-flavor in farm-raised catfish in Mississippi. The herbicides diuron and copper sulfate, currently used by catfish producers as algicides to help mitigate musty off-flavor problems, have several drawbacks, including broad-spectrum toxicity towards the entire phytoplankton community that can lead to water quality deterioration and subsequent fish death. By use of microtiter plate bioassays, a novel group of compounds derived from the natural compound 9,10-anthraquinone have been found to be much more selectively toxic towards O. perornata than diuron and copper sulfate. In efficacy studies using limnocorrals placed in catfish production ponds, application rates of 0.3 μM (125 μg/liter) of the most promising anthraquinone derivative, 2-[methylamino-N-(1′-methylethyl)]-9,10-anthraquinone monophosphate (anthraquinone-59), dramatically reduced the abundance of O. perornata and levels of 2-methylisoborneol, the musty compound produced by O. perornata. The abundance of green algae and diatoms increased dramatically 2 days after application of a 0.3 μM concentration of anthraquinone-59 to pond water within the limnocorrals. The half-life of anthraquinone-59 in pond water was determined to be 19 h, making it much less persistent than diuron. Anthraquinone-59 appears to be promising for use as a selective algicide in catfish aquaculture.
Farm-raised channel catfish (Ictalurus punctatus) is the largest segment of the aquaculture industry in the United States, and Mississippi supplies well over half of the farm-raised catfish processed every year. The largest economic losses in catfish aquaculture, other than disease-related causes, are due to “off-flavor” problems. Annually, losses caused by all types of off-flavor problems can cost producers up to U.S. $60 million (32). The most common off-flavors in catfish are “musty” and “earthy.” Musty off-flavor in catfish flesh is typically associated with the chemical compound 2-methylisoborneol (MIB), while earthy off-flavor is attributed to the presence of geosmin. Geosmin and 2-methylisoborneol are produced by species of actinomycetes (6, 23, 25), cyanobacteria (8, 9, 18, 24, 25), and fungi (10). However, cyanobacteria are attributed as being the major cause of musty off-flavor in farm-raised channel catfish in the southeastern United States (3), and Oscillatoria perornata (Skuja) [previously referred to as Oscillatoria cf. chalybea (18)] is the most prevalent MIB-producing cyanobacterium found in catfish production ponds in Mississippi (34, 35). Geosmin has been found to be less prevalent than MIB in catfish production ponds in west Mississippi (35). The human sensory threshold for MIB in channel catfish is considered to be about 700 ng/kg (11).
Geosmin and MIB are released into the pond water from producing species of cyanobacteria, and these compounds are quickly absorbed and concentrated into the flesh of catfish (12, 17). Catfish determined to be off-flavor by processors must be held by producers until they are deemed to be “on-flavor.” These delays in harvest can last for several days or weeks, depending upon the lipid content of the catfish, water temperature, and severity and longevity of the musty off-flavor episode in the production pond (13). Such delays result in economic losses to the producer for the following reasons: (i) additional feed costs; (ii) interruption of cash flow; (iii) forfeiture of income from foregone sales; and (iv) potential loss of held fish to disease, deterioration of water quality, and bird depredation (32).
One of the management practices used by producers to prevent musty off-flavor episodes involves the application of algicides to fish ponds in order to kill or help prevent the growth of undesirable cyanobacteria. Copper sulfate, chelated-copper compounds, and diuron (3-[3,4-dichlorophenyl]-1,1-dimethylurea) are the only compounds currently approved by the U.S. Environmental Protection Agency for use in catfish production ponds as algicides. Unfortunately, these compounds have the following undesirable characteristics: (i) broad-spectrum toxicity towards phytoplankton that can result in the death of the entire phytoplankton community and subsequent water quality deterioration that may stress or kill catfish; (ii) lengthy persistence in the environment that creates concerns about environmental safety; and (iii) the public's negative perception of the use of synthetic herbicides in food fish production ponds (32). The discovery of environmentally safe, selective algicides that help prevent the growth of the cyanobacteria responsible for causing musty off-flavor in pond-cultured catfish would greatly benefit the catfish aquaculture industry.
Previous research (26, 28) has identified several natural compounds that are selectively toxic towards O. perornata. One of these compounds is 9,10-anthraquinone, which is found in plant tannin extracts (22), has a high degree of selective toxicity towards O. perornata (28), and inhibits photosynthesis in O. perornata (27).
Anthraquinone is insoluble in water and must be dissolved in ethanol or other solvents. Efficacy testing of 5 μM of 9,10-anthraquinone dissolved in ethanol (final concentration, 0.00001%) and applied to pond water within limnocorrals placed in catfish production ponds (31) did not effectively reduce the abundance of O. perornata or reduce MIB levels, possibly due to precipitation of 9,10-anthraquinone out of solution (K. K. Schrader, unpublished data). Additional efficacy testing of several different formulations of 9,10-anthraquinone (e.g., incorporation with hydroxypropylmethylcellulose; Tween 80 and canola oil emulsion) to maintain toxic levels of anthraquinone in the pond water also did not reduce the abundance of O. perornata or MIB levels (K. K. Schrader, A. M. Rimando, and C. S. Tucker, unpublished data). Subsequently, a different approach was used in which the chemical structure of 9,10-anthraquinone was modified to make it water soluble, thereby maintaining it in a form in the water that is toxic towards O. perornata. In this study, we discuss the results of laboratory and efficacy testing of new synthetic derivatives of 9,10-anthraquinone that dramatically reduce the abundance of O. perornata and MIB levels in catfish pond water.
MATERIALS AND METHODS
Water-soluble analogs of 9,10-anthraquinone.
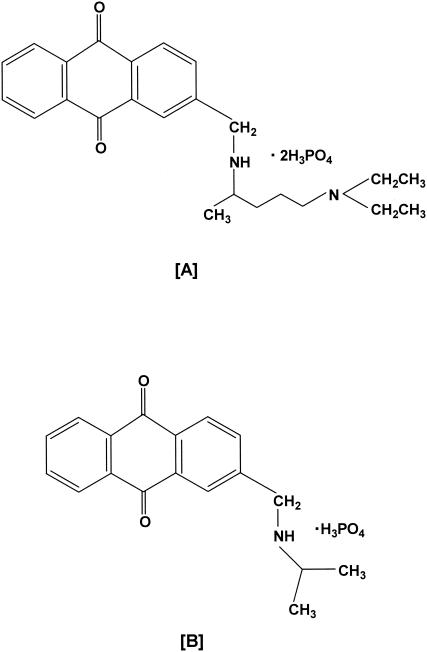
Analogs of modified 9,10-anthraquinone were synthesized in the laboratory. The first analog tested was 2-[methylamino-N-(1′-methyl-4′-N,N-diethylaminobutyl)] anthraquinone diphosphate (Fig. 1A), which has a molecular weight of 574 and is referred to as anthraquinone-19. The second primary analog pursued in this project was 2-[methylamino-N-(1′-methylethyl)]-9,10-anthraquinone monophosphate (Fig. 1B), which has a molecular weight of 377 and is referred to as anthraquinone-59. Both analogs of 9,10-anthraquinone are soluble in water, and their purity (>99%) was verified by high-performance liquid chromatography (HPLC). An additional 34 water-soluble analogs of 9,10-anthraquinone were synthesized in the laboratory. All of the analogs were screened for algicidal activity (30), and anthraquinone-19 and anthraquinone-59 were determined to be the best leads for use as selective algicides based upon laboratory screening results (unpublished observations).
FIG. 1.
Structures of anthraquinone-19 (A) and anthraquinone-59 (B).
Synthesis of anthraquinone-19.
A mixture of 2-(chloromethyl)anthraquinone (20 g) and 2-amino-5-diethylaminopentane (50 ml) was stirred under a nitrogen atmosphere at 80°C for 40 min. The reaction mixture was poured into ice-cold HCl (5%, 500 ml) and extracted with ether (three times, 150 ml). The ether layer was discarded, and the aqueous layer was basified with cold aqueous sodium hydroxide (10%) to pH 12 and extracted with ether (three times, 300 ml). The ether extract was washed with water (three times, 300 ml), dried over anhydrous Na2SO4, and evaporated to dryness under vacuum. The purity and identity of the material was assessed by high-resolution mass spectrometry (HRMS) and nuclear magnetic resonance (NMR).
HRMS data are as follows: m/e 379.2399 (M+H+, C24H31N2O2, calculated, 379.2385). NMR data are as follows: δ (CDCl3) 0.87 (6H, t, J = 7.2 Hz, CH2CH3), 0.99 (3H, d, J = 6.2 Hz, 1′′-CH3), 1.21 (2H, m, 3′-CH2), 1.38 (2H, m, 2H, 2′-CH2), 2.27 (2H, dd, J = 6.0, 8.9, 4′-CH2), 2.38 (4H, q, J = 7.2 Hz, -CH2CH3), 2.58 (1H, m, 1′-CH), 3.75 and 3.82 (1H each, d, J = 14.2 Hz, 2-CH2), 7.60 (3H, m, 3,6,7-H), 8.22-8.29 (4H, m, 1,4,5,8-H).
The crystalline residue obtained was dissolved in methanol (700 ml) and mixed efficiently with phosphoric acid (85%, 25 ml in 75 ml of methanol). The mixture was allowed to stand for 1 h, filtered, washed with methanol (4 × 50 ml), and dried to give 2-[methylamino-N-(1′-methyl-4′-N,N-diethylaminobutyl)]anthraquinone diphosphate as a pale yellow amorphous powder (32.8 g). Analysis, C 50.22, H 6.20, N 4.79, P 10.71%. Calculated for C24H30N2O2 · 2H3PO4, C 50.18, H 6.32, N 4.88, P 10.78%.
Synthesis of anthraquinone-59.
A mixture of 2-chloromethylanthraquinone (10 g), isopropylamine (15 ml), and dimethyl sulfoxide (15 ml) was heated for 30 min at 80°C. The reaction mixture was poured into ice-cold 5% HCl solution (500 ml) and extracted three times with methylene chloride (200 ml). The aqueous layer was basified (to pH 12) with sodium hydroxide solution (10%) and extracted three times with diethyl ether. The combined ether layer was washed with water, dried over sodium sulfate, and evaporated to yield 2-[methylamino-N-(1′-methylethyl)]-9,10-anthraquinone. The purity and identity of the material was assessed by HRMS and NMR.
HRMS data are as follows: m/e 280.1316 (M + H+, C18H18NO2, calculated, 280.1337). NMR data are as follows: δ (CDCl3) 1.05 [6H, d, J = 6.2 Hz, CH(CH3)2], 2.80 [1H, septet, J = 6.2 Hz, CH(CH3)2], 3.84 (2H, s, 2-CH2), 7.65-7.68 (3H, m, 3,6,7-H), 8.07-8.15 (4H, m, 1,4,5,8-H).
This product was dissolved in methanol (500 ml) and treated with methanolic phosphoric acid (10 ml of 85% H3PO4 in 90 ml of methanol) under stirring, left overnight at room temperature, and filtered to give 2-[methylamino-N-(1′-methylethyl)]-9,10-anthraquinone monophosphate (10.5 g). Analysis, C 57.59, H 5.48, N 3.94, P 8.07%. Calculated for C18H17NO2 · H3PO4, C 55.02, H 4.62, N 4.01, P 8.87%.
Laboratory screening of anthraquinone analogs.
The anthraquinone analogs were screened for selective toxicity towards O. perornata using the method of Schrader et al. (30). Water was used to dissolve the two anthraquinone derivatives. The representative green algal species used was Selenastrum capricornutum, a common green alga found in catfish aquaculture ponds. Absorbance readings were graphed, and graphs were used to determine the lowest-observed-effect concentration (LOEC) and the lowest-complete-inhibition concentration (LCIC) for each anthraquinone analog. In addition, a 96-h 50% inhibition concentration (IC50) was determined by using the method described by Schrader et al. (29). Stock solutions of anthraquinone-19 and anthraquinone-59 were prepared so that final concentrations screened for 96-h IC50 determinations were as follows: (i) 0, 0.01, 0.033, 0.1, 0.333, 1.0, 3.3, and 10.0 μM anthraquinone-19 for both O. perornata and S. capricornutum; (ii) 0, 0.003, 0.01, 0.033, 0.1, 0.333, 1.0, and 3.333 μM anthraquinone-59 for O. perornata; and (iii) 0, 0.1, 0.333, 1.0, 3.333, 10.0, 33.333, and 100.0 μM anthraquinone-59 for S. capricornutum. Estimation of the IC50 was determined by plotting 96-h absorbance readings against logarithmic dilution values of the anthraquinone analogs.
Efficacy testing in catfish production ponds.
The “limnocorral” method described by Schrader et al. (31) was used to determine the potential for using the anthraquinone derivatives as selective algicides in catfish aquaculture ponds. All of the catfish ponds used in efficacy tests were maintained using commercial pond management practices and were located at the National Warmwater Aquaculture Center Pond Facility, Mississippi State University, Stoneville, Miss. The ponds used ranged in size from 0.1 to 4 ha (0.25 to 10 acres). Anthraquinone analogs were dissolved in deionized water before application to water within the limnocorrals. Treatment limnocorrals were randomly selected, and control (no test compound applied) limnocorrals were included in each efficacy study. For each sampling, two water samples (250 ml) were obtained from within each limnocorral (approximately 6 to 8 cm below the water surface and from opposite sides of each limnocorral) and mixed together in a 500-ml sample bottle to provide a representative sample of the water contained within the limnocorral.
Efficacy testing of anthraquinone-19.
Three limnocorral efficacy studies were conducted with anthraquinone-19. In the first study, six limnocorrals (open-ended fiberglass cylinders, 2.44 m in diameter and 1.53 m high, enclosed water volume of 5.5 kl; Solar Components Corporation, Manchester, N.H.) were placed in a 4-ha earthen catfish pond. The pond was chosen due to the presence of a bloom of O. perornata. The water within each limnocorral received mixing in the same manner as used by Schrader et al. (31). Three randomly selected limnocorrals were used as treatments (received anthraquinone-19), and the other three limnocorrals were controls. Water samples were taken before application of anthraquinone-19 (2.0 μM [1,148 μg/liter] per enclosure), 30 min after application (for measurement of the test compound levels within the limnocorrals), and at days 1, 3, 8, and 10. Water samples were analyzed for chlorophyll a (chloroform-methanol extraction method followed by spectroscopy) (15), for phytoplankton community structure and enumeration (1), and for geosmin and MIB levels using solid-phase microextraction with gas chromatography-mass spectrometry. Analyses of geosmin and MIB levels were similar to the method of Lloyd et al. (16), but several modifications were made (see below). To perform phytoplankton identification and enumeration, water samples were processed by preserving 50-ml subsamples with Lugol's solution and storing them at 4°C until they could be identified and counted as “natural units” (i.e., colonies, filaments, or unialgal cells) using a Sedgewick-Rafter counting chamber at magnification ×300. Eukaryotic algae were identified to the genus level, and filamentous cyanobacteria were identified to the species level. Water samples were also analyzed for levels of anthraquinone-19 by using HPLC.
In the second study with anthraquinone-19, 12 limnocorrals (the same size as those used in the first study) were placed in another 4-ha earthen catfish pond. The pond also had a bloom of O. perornata. Randomly selected limnocorrals were used as follows: (i) three controls; (ii) three received anthraquinone-19 at an application rate of 1.0 μM (574 μg/liter) per enclosure; (iii) three received anthraquinone-19 at an application rate of 0.3 μM (191 μg/liter) per enclosure; and (iv) three received anthraquinone-19 at an application rate of 0.1 μM (57.4 μg/liter) per enclosure. The same sampling regime and procedures used in the first study were followed in a similar manner, except that water samples were obtained before anthraquinone-19 application, 30 min after application, and at days 1, 2, 4, and 7.
The third study with anthraquinone-19 duplicated the second study in time. Limnocorrals were placed in the same pond, and the same procedures and conditions were used.
Efficacy testing of anthraquinone-59.
Three limnocorral efficacy tests were performed using anthraquinone-59. The first study was a dose-response study and used application rates of 0.1 μM (37.7 μg/liter), 0.3 μM (125 μg/liter), and 1.0 μM (377 μg/liter) of anthraquinone-59 applied to water within limnocorrals (2.44 m in diameter and 1.53 m high, enclosed water volume of 5.5 kl; three limnocorrals per concentration). Three limnocorrals were used as controls. These limnocorrals were set up in a 3.3-ha earthen pond that had a bloom of O. perornata and Anabaena circinalis (geosmin producer). Water within each limnocorral was not mixed by aeration (using air forced through airstones) (31) until the day after the limnocorrals were placed in the pond. Mixing was delayed to allow most of the suspended sediment and organic matter, disturbed during limnocorral placement in the ponds, to settle to the pond bottom. Approximately 30 min after mixing the water within each limnocorral, randomly selected treatment limnocorrals received the appropriate amounts of anthraquinone-59. Water samples were collected before application of the test compound, 20 min after application (for anthraquinone level determination), and 16 h after application. This study only proceeded for 1 day, since a severe thunderstorm disrupted the integrity of the limnocorrals 24 h after application of anthraquinone-59. However, due to the rapid toxicity of anthraquinone-59 towards O. perornata (also observed in previous laboratory tests), treatment-related effects could still be determined 16 h after the initial treatment. The same tests and analytical procedures performed on water samples taken during the efficacy testing of anthraquinone-19 were used in the three efficacy tests undertaken with anthraquinone-59.
In the second efficacy test with anthraquinone-59, six limnocorrals (1.53 m in diameter and 1.53 m high, enclosed water volume of 1.7 kl) were placed in a 0.1-ha earthen pond containing a heavy bloom of O. perornata. As in the first efficacy test, mixing the water within the limnocorrals was delayed until treatment. Water within three randomly selected limnocorrals received 0.3 μM (125 μg/liter) anthraquinone-59, and the other three limnocorrals were controls. Water samples were collected before application of anthraquinone-59, 20 min after application, and at days 1, 2, 3, and 7. The analytical tests and methods performed on water samples were the same as those used in the first efficacy testing of anthraquinone-59.
For the third efficacy test with anthraquinone-59, 12 limnocorrals (2.44 m in diameter and 1.53 m high, enclosed water volume of 5.5 kl) were placed in a 4-ha earthen pond containing a bloom of O. perornata. The same procedures and methods used in the second efficacy test of anthraquinone-59 were used, except that six limnocorrals were randomly selected to receive an application rate of 0.3 μM (125 μg/l) anthraquinone-59, while the other six limnocorrals were controls. Water samples were collected before application of anthraquionone-59, 20 min after application, and at days 1, 2, 3, 4, and 7. The analytical tests and methods performed on water samples were the same as those used in the first efficacy testing of anthraquinone-59.
Analysis of geosmin and MIB levels in water samples.
The solid-phase microextraction procedure used to quantify levels of geosmin and MIB in water was similar to the method of Lloyd et al. (16). Aliquots of water samples were pipetted into 2-ml glass screw-top vials (600 μl/vial), and sodium chloride was added (0.3 g/vial). Vials were then placed in a heated carousel (40°C) for at least 40 min before volatiles were absorbed onto a 100 μm polydimethyl siloxane solid-phase microextraction fiber (Supelco, Bellefonte, Pa.). The fiber assembly (Varian, Sugar Land, Tex.) was shaken for 10 min during the absorption period and then desorbed for 2 min at 250°C in the injection port of an HP 5890 Series II Plus (Hewlett Packard, Palo Alto, Calif.) gas chromatograph operated in selected ion monitoring mode. Gas chromatography conditions were as follows: (i) initial oven temperature of 60°C for 0.5 min; (ii) then, ramp rate of 30°C/min to 100°C; (iii) then, ramp rate of 20°C/min to 300°C with an isotherm time of 2 min; (iv) maintenance of flow pressure at 18 lb/in2; and (v) use of helium as the carrier gas. The molecular ion, base peak, and additional fragment ion at m/z 168, 95, and 135 were monitored for MIB and at m/z 182, 112, and 126 for geosmin. The capillary column used was a DB-5 (5%-phenyl-methylsiloxane, 30 m, 0.25 mm inside diameter, 0.25-μm film thickness; J&W Scientific, Folsom, Calif.). Injection ports were held in splitless mode, and a reduced volume injection sleeve (0.75 mm inside diameter; Supelco, Bellefonte, Pa.) was used. The retention times of MIB and geosmin were 5.2 and 6.8 min, respectively. Standards of MIB and geosmin (both obtained from Wako Chemicals USA, Inc., Richmond, Va.) were prepared (0.1, 0.5, 1.0, and 2.5 μg/liter) in deionized water and run at the beginning, middle, and end of each set of samples (36 samples/set). Each water sample was run in triplicate.
HPLC determination of anthraquinone analog level in water samples.
Approximately 15 ml of water samples designated for HPLC analysis were placed in scintillation vials and stored in a freezer (−4°C). Prior to analysis, water samples were thawed at room temperature, and 5 ml of each sample was then filtered through a nylon membrane filter (13-mm diameter and 0.45 μm; Whatman International, Maidstone, England) using a 5-ml syringe (Hamilton Company, Reno, Nev.) and a 13-mm syringe filter holder (Fisher Scientific Company, Pittsburgh, Pa.). Filtrate (1 ml) was placed in 2-ml vials and capped using Teflon-rubber septum caps (National Scientific Company, Jeddah, Saudi Arabia). This filtrate was the soluble portion (unbound to organic matter, soil particles, etc.) of the anthraquinone analog in the water column. The membrane filter was removed and placed in a scintillation vial to which 2 ml of HPLC-grade methanol (Fisher Scientific, Fair Lawn, N.J.) was added and then sonicated for 5 min. The methanol solution was then filtered using a nylon membrane acrodisc (25 mm and 0.45 μm; Pall Life Sciences, Ann Arbor, Mich.), and the methanol filtrate was placed in separate 2-ml vials. This methanol filtrate was equivalent to the bound or particulate portion of the anthraquinone analog in the water column.
Filtrate samples were analyzed (n = 2) using a 2690 Alliance HPLC containing a 996 photodiode array detector and an XTerra RP 18 column (150 mm by 4.6 mm, 5-μm particle size; Waters Corporation, Milford, Mass.). The mobile phase consisted of 25-mM sodium dihydrogenphosphate in 0.1% phosphoric acid (A) and acetonitrile (B). Gradient elution was performed from 80A/20B in 15 min to 40A/60B. After each run, a 5-min wash with 100% methanol was performed, followed by equilibration of the column for 10 min with 80A/20B. The temperature was set to 40°C, the flow rate was 1 ml/min, the detection wavelength was 256 nm, and the sample volume injected was 10 μl. The calibration levels were prepared by diluting the stock solutions of anthraquinone-19 and anthraquinone-59 with 100% methanol (2.0 to 200.0 μg/ml and 1.07 to 86.60 μg/ml, respectively). The five-point calibration data (n = 3) were obtained as follows. (i) For anthraquinone-19, y = 5.32 × 104 x linear through zero; r2 = 0.9999; and limit of detection = 1.0 ng/ml. (ii) For anthraquinone-59, y = 9.19 × 104 x linear through zero; r2 = 0.9995; and limit of detection = 1.0 ng/ml. All of the solvents used were HPLC grade (Fisher Scientific). For each water sample, results from the analysis of the water-soluble portion and the particulate portion were combined to yield the total content of the anthraquinone analog in the pond water column.
Data analysis.
The experimental design of the limnocorral studies is a split plot. The main unit has a completely random design. Measurements were made over time. Means and standard deviations of data from chlorophyll a measurements and levels of anthaquinone-19 and anthraquinone-59 were determined and graphed. For phytoplankton enumeration data and levels of geosmin and MIB data, means and standard errors were determined and graphed. Analysis of variance was performed on chlorophyll a levels, phytoplankton abundance data, and MIB levels in each study and also on geosmin levels in the first efficacy study of anthraquinone-59 (the only study in which geosmin was detected in water samples). A repeated measure error was used for the subunit time based on a first-order autoregressive error. Mean comparisons were based upon an least-significant-difference (LSD) value (P ≤ 0.05). Data were analyzed using the mixed procedure in SAS, version 6.12 (14).
RESULTS
Laboratory screening.
Table 1 lists the LOEC, LCIC, and IC50 results obtained from the rapid bioassay for anthraquinone-19 and anthraquinone-59. Each analog has a similar degree of toxicity towards the two test organisms in the laboratory. Both compounds are very selectively toxic towards O. perornata, with anthraquinone-19 being 80 times more toxic and anthraquinone-59 approximately 890 times more toxic towards O. perornata than the common green alga S. capricornutum based upon IC50 values.
TABLE 1.
Rapid bioassay screening results of 9,10-anthraquinone analogs
| Compound | Concn (nM) for test organism
|
|||||
|---|---|---|---|---|---|---|
|
O. perornata
|
S. capricornutum
|
|||||
| LOEC | LCIC | IC50 | LOEC | LCIC | IC50 | |
| Anthraquinone-19 | 10 | 100 | 63 | 10,000 | 10,000 | 5,012 |
| Anthraquinone-59 | 10 | 100 | 6.3 | 10,000 | 100,000 | 5,623 |
First efficacy study of anthraquinone-19.
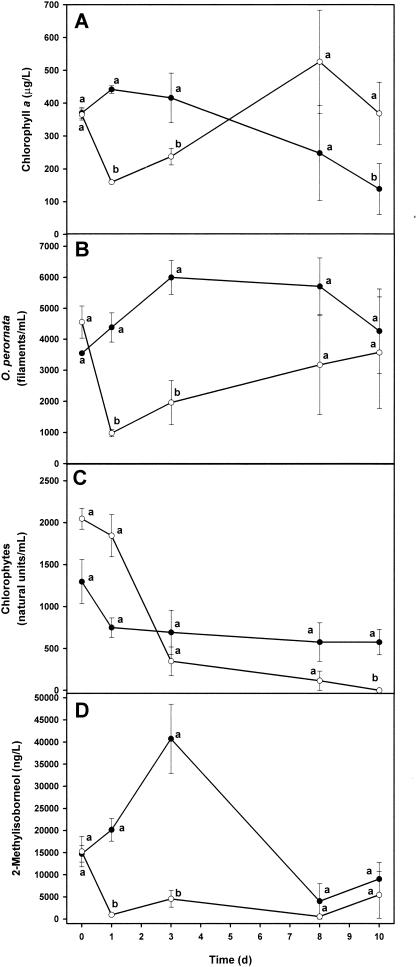
Mean chlorophyll a levels in water from within the limnocorrals are shown in Fig. 2A. Twenty-four hours after application of 2.0 μM anthraquinone-19, chlorophyll a levels decreased more than 50% in water within the treatment limnocorrals before gradually increasing to levels higher than in control limnocorrals on day 8. Figure 2B and C show numbers of O. perornata and green algae, respectively. The abundance of O. perornata was significantly reduced by application of 2.0 μM anthraquinone-19 but began to increase at 3 days after application. Numbers of green algae (division Chlorophyta) in controls and in limnocorrals treated with 2.0 μM anthraquinone-19 were not significantly different based upon LSD values until 10 days after the application of anthraquinone-19 (Fig. 2C). Levels of MIB were significantly reduced, from 15,250 ng/liter to near 970 ng/liter, and remained much lower than MIB levels in the controls for at least 3 days (Fig. 2D). Geosmin was not detected during this study. The most common genera of green algae observed in pond water samples during the three efficacy studies with anthraquinone-19 were Actinastrum, Ankistrodesmus, Closterium, Coelastrum, Crucigenia, Oocystis, Pediastrum, Scenedesmus, Schroederia, and Staurastrum.
FIG. 2.
First efficacy study of the effect of anthraquinone-19 on chlorophyll a levels (A), the abundance of O. perornata (B), the abundance of green algae (C), and 2-methylisoborneol levels (D) in pond water. Each symbol is the mean ± standard deviation of the mean of measurements in three replicate limnocorrals. Means on the same day with the same letter are not significantly different (P ≤ 0.05) based upon LSD values. Symbols: •, controls; ○, 2 μM anthraquinone-19.
Second and third efficacy studies of anthraquinone-19.
Results from the second efficacy study of anthraquinone-19 revealed a lack of reduction of O. perornata filaments and 2-methylisoborneol levels compared to controls at application rates of 1.0, 0.3, and 0.1 μM (data not shown). Similar results were obtained for the third efficacy study (data not shown). Geosmin was not detected in these two studies. It was determined that anthraquinone-19 is effective in selectively reducing numbers of O. perornata and MIB levels in pond water when applied at 2.0 μM but not at the lower application rates of 1.0, 0.3, and 0.1 μM.
First efficacy study of anthraquinone-59.
Compared to values in control limnocorrals, chlorophyll a levels were significantly reduced 16 h after application of anthraquinone-59 at 1.0 and 0.3 μM but not at 0.1 μM (Table 2). Application rates of 0.1, 0.3, and 1.0 μM anthraquinone-59 significantly reduced numbers of O. perornata filaments compared to controls (Table 2). Levels of MIB decreased in all of the treatment and control limnocorrals, but to a significantly greater degree in limnocorrals that received applications of 0.3 and 1.0 μM anthraquinone-59 than in the controls and limnocorrals that received 0.1 μM anthraquinone-59 (Table 2). Geosmin levels also decreased in all of the treatments and controls, but to a significantly greater degree in limnocorrals that received 1.0 μM anthraquinone-59 than in the controls and limnocorrals that received applications of 0.1 and 0.3 μM anthraquinone-59 (Table 2). The abundance of A. circinalis was significantly reduced in limnocorrals that received 1.0 μM anthraquinone-59 from that in the controls and in limnocorrals that received applications of 0.1 and 0.3 μM anthraquinone-59 (Table 2). In fact, in limnocorrals that received 1.0 μM anthraquinone-59, no filaments of A. circinalis were observed in the water samples obtained 16 h after application. This first efficacy study was the only one of the three conducted with anthraquinone-59 in which geosmin was detected in the pond water. Numbers of green algae were not significantly affected by applications of anthraquinone-59 at 0.1, 0.3, and 1.0 μM compared to the controls (Table 2). Numbers of diatoms (division, Chromophyta [= Chrysophyta]; class, Bacillariophyceae) were very low in the water samples, and therefore, the effect of anthraquinone-59 on this group of phytoplankton could not be ascertained from this study. The most common genera of green algae observed in pond water samples during the three efficacy tests of anthraquinone-59 were Actinastrum, Ankistrodesmus, Closterium, Coelastrum, Crucigenia, Kirchneriella, Dictyosphaerium, Oocystis, Pediastrum, Scenedesmus, Schroederia, Snowella, and Staurastrum.
TABLE 2.
Results of the first efficacy study with anthraquinone-59
| Measured variable | Value for limnocorral treatment (μM) (n = 3) ofa:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
0.1
|
0.3
|
1.0
|
|||||||||
| 0 h | 16 h | % Changeb | 0 h | 16 h | % Changeb | 0 h | 16 h | % Changeb | 0 h | 16 h | % Changeb | |
| Chlorophyll a levels (μg/liter) | 102.5 (a) | 111.3 (a) | +8.6 | 97.2 (a) | 98.6 (a) | +1.4 | 99.9 (a) | 54.1 (b) | −45.8 | 95.5 (a) | 46.2 (c) | −51.6 |
| No. of O. perornata filaments/ml | 2,595 (a) | 3,056 (a) | +17.8 | 2,941 (a) | 2,076 (b) | −29.4 | 3,056 (a) | 1,442 (b) | −52.8 | 2,076 (a) | 1,326 (b) | −36.1 |
| No. of A. circinalis filaments/ml | 980 (a) | 1,038 (a) | +5.9 | 807 (a) | 865 (a) | +7.2 | 1,211 (a) | 634 (a) | −47.7 | 1,384 (a) | 0 (b) | −100 |
| Amt of green algae (natural units/ml) | 1,326 (a) | 1,788 (a) | +34.8 | 1,442 (a) | 2,018 (a) | +39.9 | 1,326 (a) | 1,384 (a) | +4.4 | 923 (a) | 1,269 (a) | +37.5 |
| MIB levels (ng/liter) | 2,422 (a) | 1,568 (a) | −35.3 | 2,249 (a) | 1,402 (a) | −37.7 | 2,581 (a) | 535 (b) | −79.3 | 2,470 (a) | 892 (c) | −63.9 |
| Geosmin levels (ng/liter) | 258 (a) | 80 (a) | −69.0 | 208 (a) | 101 (b) | −51.4 | 317 (a) | 77 (a) | −75.7 | 286 (a) | 44 (c) | −84.6 |
For each group of measured data, means (n = 3) on the same day with the same letter are not significantly different (P ≤ 0.05) based upon LSD values.
Change is from time 0 to 16 h.
Second and third efficacy studies of anthraquinone-59.
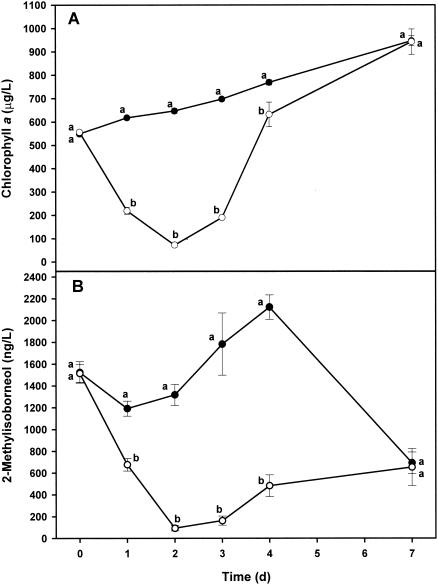
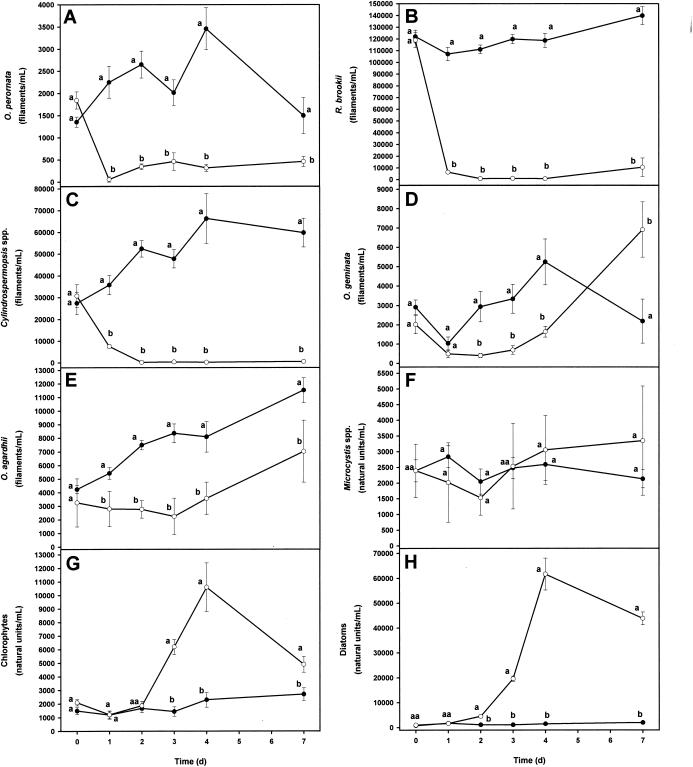
The results from both of these efficacy studies were similar, and therefore, only the results from the third efficacy study are presented. Chlorophyll a levels within treatment limnocorrals were significantly reduced 24 h after application of 0.3 μM anthraquinone-59 (Fig. 3A). Levels of MIB (Fig. 3B) and the abundance of O. perornata (Fig. 4A) were significantly reduced within 24 h after application of 0.3 μM anthraquinone-59. Filaments of Raphidiopsis brookii and Cylindrospermopsis spp. were also significantly reduced 1 day after application of 0.3 μM anthraquinone-59 (Fig. 4B and C, respectively). The abundance of Oscillatoria geminata remained lower than in the controls for 4 days after application and was significantly lower than in controls from 2 days through 4 days after application (Fig. 4D). Filaments of Oscillatoria agardhii were significantly reduced by application of 0.3 μM anthraquinone-59, while Microcystis spp. were not greatly affected compared to the control limnocorrals (Fig. 4E and F, respectively). Off-flavor compound production has not been linked to Microcystis spp., O. geminata, and O. agardhii. Numbers of green algae and diatoms began to increase dramatically in treatment limnocorrals 3 days after application and were significantly higher than numbers in the controls (Fig. 4G and H, respectively).
FIG. 3.
Third efficacy study of the effect of anthraquinone-59 on chlorophyll a levels (A) and 2-methylisoborneol levels (B) in pond water. Each point is the mean ± standard deviation of the mean of measurements in six replicate limnocorrals. Means on the same day with the same letter are not significantly different (P ≤ 0.05) based upon LSD values. Symbols: •, controls; ○, 0.3 μM anthraquinone-59.
FIG. 4.
Third efficacy study of the effect of anthraquinone-59 on the abundance of O. perornata (A), R. brookii (B), Cylindrospermopsis spp. (C), O. geminata (D), O. agardhii (E), Microcystis spp. (F), green algae (G), and diatoms (H) in pond water. Each point is the mean ± standard deviation of the mean of measurements in six replicate limnocorrals. Means on the same day with the same letter are not significantly different (P ≤ 0.05) based upon LSD values. Symbols: •, controls; ○, 0.3 μM anthraquinone-59.
Dissipation rate of anthraquinone analogs in pond water column.
Attempts to develop a reproducible analytical method to determine the levels of anthraquinone-19 in pond water were unsuccessful. Anthraquinone-19 is believed to bind quickly to suspended soil particles due to the double positively charged nature of the 2-methylamino chain, and this is made evident by the lack of detection of anthraquinone-19 in water 30 min after its application. Since the levels of suspended soil particles in each water sample varied and an accurate measurement of the soil particles in each sample was not possible, consistent recoveries of anthraquinone-19 from the particulate portions of water samples could not be achieved. In addition, anthraquinone-19 could not be detected in the soluble fractions of water samples collected at 30 min and 24 h after application of anthraquinone-19 to water within the limnocorrals.
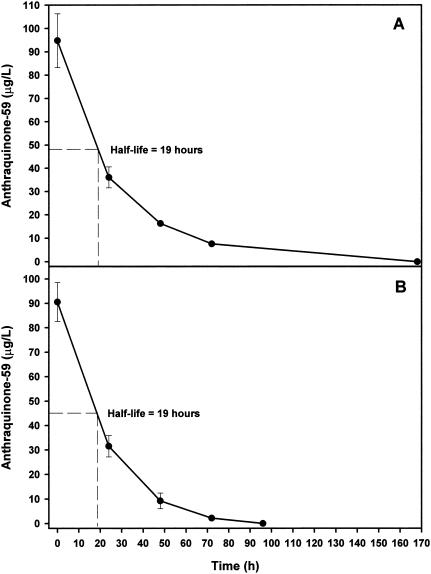
Anthraquinone-59 quickly dissipated from the pond water column and was found to have a half-life of 19 h as determined from the graphed data in each of the second and third efficacy studies (Fig. 5A and B, respectively). Levels of anthraquinone-59 dropped below 10 μg/liter in the water column within 2 days after application of 125 μg/liter (0.3 μM), and the compound could not be detected in the pond water 4 days after application (Fig. 5B).
FIG. 5.
Second (A) and third (B) efficacy study of anthraquinone-59 with determination of dissipation rate and half-life in pond water. Each point is the mean ± standard deviation of the mean of measurements in three replicate limnocorrals.
DISCUSSION
Anthraquinone-59 is the most promising of the analogs tested in this study for further research and development as a selective algicide for use in catfish aquaculture. The reduction in numbers of O. perornata and MIB levels by application of 0.3 μM anthraquinone-59 in limnocorral studies confirms its potential for alleviating musty off-flavor problems in catfish production. Additionally, anthraquinone-59 reduces the abundance of several other prevalent types of cyanobacteria found in Mississippi catfish ponds, such as R. brookii, Cylindrospermopsis spp., O. geminata, and, to a lesser extent, O. agardhii. However, anthraquinone-59 cannot be designated as a broad-spectrum cyanobacteriocide at application rates of 0.3 μM, since the abundance of Microcystis spp. was unaffected. This lack of toxicity towards Microcystis spp. is unfortunate, since members in this genera are known to produce toxins, such as microcystin (4, 5), and the mortality of farm-raised catfish has been attributed to Microcystis aeruginosa (36).
The reduced abundance of filamentous cyanobacteria after treatment correlates directly with an increase in the abundance of green algae and diatoms. These groups of algae were predominant in the phytoplankton community several days after application of anthraquinone-59. In our studies, the increase in numbers of green algae and diatoms may be attributed to the removal of several types of prevalent planktonic cyanobacteria that have unique physiological attributes that allow them to outcompete other types of phytoplankton (19). Cyanobacteria are usually the dominant type of phytoplankton in catfish ponds (3). Additionally, green algae and diatoms are the first types of phytoplankton to colonize “new” aquatic habitats and are eventually replaced by colonial and filamentous cyanobacteria as a pattern of succession after algicide treatment (20, 21). Although the primary target is the removal of O. perornata from catfish ponds, application of anthraquinone-59 may have additional benefits for its use by catfish farmers due to its elimination or reduction in the abundance of other filamentous cyanobacteria. Because cyanobacteria have low growth rates, they are poor oxygenators of the water compared to most eukaryotic species of phytoplankton (19). Also, bloom-forming cyanobacteria are prone to producing surface scums that can reduce the net input of dissolved oxygen into the pond (19). Sudden die-offs of cyanobacterial blooms can cause dramatic reductions of dissolved oxygen levels in ponds that can stress catfish and potentially even lead to their death. Mid-water blooms of eukaryotic algae are less susceptible to massive die-offs than cyanobacterial surface blooms (2). Essentially, eukaryotic algae provide a more reliable and stable type of aquatic ecosystem that is preferred in aquaculture. The establishment and maintenance of eukaryotic algae as the dominant type of phytoplankton in catfish ponds may enhance fish production through reductions in fish loss from poor water quality (e.g., low dissolved oxygen levels) and higher stocking rates.
Although results from the dose-response study were limited, anthraquinone-59 also appears to be effective in reducing the abundance of A. circinalis and levels of geosmin in the pond water, though at a higher application rate (≥0.3 μM) than required for reducing the abundance of O. perornata (≥0.1 μM). Additional studies are needed to confirm that anthraquinone-59 would also be useful in reducing geosmin-related off-flavor problems in catfish.
The results from our study show that anthraquinone-59 is more effective in reducing the abundance of O. perornata at lower application rates than anthraquinone-19. Anthraquinone-19 is double-positively charged compared to anthraquinone-59, and therefore, anthraquinone-19 is more likely to bind to suspended sediment particles than anthraquinone-59, thereby reducing its effectiveness towards O. perornata. In fact, anthraquinone-59 was synthesized with the specific intent that it would have less of a positive charge on the methylamino portion of the compound.
In comparison with copper-based products and diuron, anthraquinone-59 offers greater selective toxicity towards cyanobacteria than other phytoplankton. Anthraquinone-59 is much more selective towards O. perornata than the preferred types of phytoplankton, such as green algae. Another potential advantage of anthraquinone-59 involves the public's negative perception of the use of herbicides such as diuron in food fish production ponds. Anthraquinone-59 is derived from the natural compound 9,10-anthraquinone, which is found in certain plants (22). Also, anthraquinone-59 has much lower persistence in pond water (half-life of 19 h) than diuron, which can persist for weeks in the water column after application to catfish aquaculture ponds (half-life of 2 weeks in pond water; C. S. Tucker, unpublished data). Environmental safety issues also persist on the use of copper sulfate in catfish ponds, since the copper accumulates in the pond sediments and long-term applications may adversely affect microbial activity in the pond sediments (7).
Results from our study indicate that one or two applications of anthraquinone-59 at a 0.3 μM concentration (125 μg/liter) may be adequate in reducing MIB levels in pond water of commercial catfish ponds to levels sufficient to permit off-flavor catfish to lose their musty taint. This application regime for anthraquinone-59 would be much less intensive than the management approach involving weekly applications of low doses of copper sulfate to catfish aquaculture ponds (33) or the management approach using nine applications of diuron at 10 μg/liter (label-recommended rate; EPA registration, #19713-274) used by Zimba et al. (37) to effectively control MIB levels. Because anthraquinone-59 has a much shorter half-life in the water column than diuron, future experiments using entire ponds will need to determine if one or two applications of anthraquinone-59 will be adequate to reduce MIB levels for a sufficient amount of time to permit depuration of MIB from the catfish flesh.
In order to further evaluate anthraquinone-59 for use as a commercial, selective algicide in catfish aquaculture, the following studies need to be performed: (i) determine the environmental fate of anthraquinone-59, i.e., breakdown products, in catfish ponds; (ii) determine if anthraquinone-59 and its degradation products accumulate in the flesh of channel catfish; (iii) determine the lowest-observed-effect concentration and LC50 of anthraquinone-59 toward channel catfish; and (iv) conduct additional toxicological testing to evaluate anthraquinone-59 for antimicrobial activity, cytotoxic activity, and mutagenic activity. Eventually, efficacy testing of anthraquinone-59 using entire catfish ponds stocked with channel catfish needs to be performed to fully evaluate application rates required to produce on-flavor, acceptable catfish. Such studies would also help with comparisons of the economic costs to producers of using anthraquinone-59 versus diuron or copper-based products to help manage musty off-flavor problems in commercially cultured channel catfish.
Acknowledgments
The technical assistance of Ramona Pace, Dewayne Harries, Yarda Tables, Susan Kingsbury, Margaret Dennis, and Darren Austin is greatly appreciated. We thank Deborah Boykin (U.S. Department of Agriculture, Agricultural Research Service, Jamie Whitten Delta States Research Center, Stoneville, Miss.) for assistance with performing statistical analyses.
This research was supported in part by the Southern Regional Aquaculture Center, Stoneville, Miss., through grants 97-38500-4124 and 98-38500-5865 from the U.S. Department of Agriculture Cooperative States Research, Education, and Extension Service.
REFERENCES
- 1.American Public Health Association, American Water Works Association, and Water Pollution Control Federation. 1992. Standard methods for the examination of water and wastewater, 18th ed. American Public Health Association, Washington, D.C.
- 2.Boyd, C. E. 1990. Water quality in ponds for aquaculture. Auburn University/Alabama Agricultural Experiment Station, Auburn, Ala.
- 3.Boyd, C. E., and C. S. Tucker. 1998. Pond aquaculture water quality management. Kluwer, Norwell, Mass.
- 4.Carmichael, W. W. 1992. Cyanobacteria secondary metabolites—the cyanotoxins. J. Appl. Bacteriol. 72:445-459. [DOI] [PubMed] [Google Scholar]
- 5.Codd, G. A. 1995. Cyanobacterial toxins: occurrence, properties and biological significance. Water Sci. Technol. 32:149-156. [Google Scholar]
- 6.Gerber, N. N. 1979. Volatile substances from actinomycetes: their role in the odor pollution of water. Crit. Rev. Microbiol. 7:191-194. [DOI] [PubMed] [Google Scholar]
- 7.Han, F. X., J. A. Hargreaves, W. L. Kingery, D. B. Huggett, and D. K. Schlenk. 2001. Accumulation, distribution, and toxicity of copper sulfate in sediments of catfish ponds receiving periodic copper sulfate applications. J. Environ. Qual. 30:912-919. [DOI] [PubMed] [Google Scholar]
- 8.Izaguirre, G., and W. D. Taylor. 1998. A Pseudanabaena species from Castaic Lake, California, that produces 2-methylisoborneol. Water Res. 32:1673-1677. [Google Scholar]
- 9.Izaguirre, G., C. J. Hwang, S. W. Krasner, and M. J. McGuire. 1982. Geosmin and 2-methylisoborneol from cyanobacteria in three water supply systems. Appl. Environ. Microbiol. 43:708-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelen, H., and E. Wasowicz. 1998. Volatile fungal metabolites and their relation to the spoilage of agricultural commodities. Food Rev. Int. 14:391-426. [Google Scholar]
- 11.Johnsen, P. B., and C. A. Kelly. 1990. A technique for the quantitative sensory evaluation of farm raised catfish. J. Sens. Stud. 4:189-199. [Google Scholar]
- 12.Johnsen, P. B., and S. W. Lloyd. 1992. Influence of fat content on uptake and depuration of the off-flavor 2-methylisoborneol by channel catfish (Ictalurus punctatus). Can. J. Fish. Aquat. Sci. 49:2406-2411. [Google Scholar]
- 13.Johnsen, P. B., S. W. Lloyd, B. T. Vinyard, and C. P. Dionigi. 1996. Effects of temperature on the uptake and depuration of 2-methylisoborneol (MIB) in channel catfish Ictalurus punctatus. J. World Aquacult. Soc. 27:15-20. [Google Scholar]
- 14.Littell, R. C., G. A. Milliken, W. W. Stroup, and R. D. Russell. 1996. SAS system for mixed models. SAS Institute, Inc., Cary, N.C.
- 15.Lloyd, S. W., and C. S. Tucker. 1998. Comparison of three solvent systems for extraction of chlorophyll a from fish pond phytoplankton communities. J. World Aquacult. Soc. 19:36-40. [Google Scholar]
- 16.Lloyd, S. W., J. M. Lea, P. V. Zimba, and C. C. Grimm. 1998. Rapid analysis of geosmin and 2-methylisoborneol in water using solid phase micro extraction procedures. Water Res. 32:2140-2146. [Google Scholar]
- 17.Martin, J. F., L. W. Bennett, and W. H. Graham. 1988. Off-flavor in channel catfish (Ictalurus punctatus) due to 2-methylisoborneol and its dehydration products. Water Sci. Technol. 20:99-105.
- 18.Martin, J. F., G. Izaguirre, and P. Waterstrat. 1991. A planktonic Oscillatoria species from Mississippi catfish ponds that produces the off-flavor compound 2-methylisborneol. Water Res. 25:1447-1451. [Google Scholar]
- 19.Paerl, H. W., and C. S. Tucker. 1995. Ecology of blue-green algae in aquaculture ponds. J. World Aquacult. Soc. 26:109-131. [Google Scholar]
- 20.Reynolds, C. S. 1984. The ecology of phytoplankton. Cambridge University Press, Cambridge, United Kingdom.
- 21.Reynolds, C. S. 1997. Vegetation processes in the pelagic: a model for ecosystem theory. Ecology Institute, Oldendorf/Luhe, Germany.
- 22.Robinson, T. 1967. The constituents of higher plants. Burgess, Minneapolis, Minn.
- 23.Saadoun, I., K. K. Schrader, and W. T. Blevins. 1997. Identification of 2-methylisoborneol (MIB) and geosmin as volatile metabolites of Streptomyces violaceusniger. Actinomycetes 8:37-41. [Google Scholar]
- 24.Saadoun, I., K. K. Schrader, and W. T. Blevins. 2001. Identification of geosmin as a volatile metabolite of Anabaena sp. J. Basic Microbiol. 41:51-55. [DOI] [PubMed] [Google Scholar]
- 25.Schrader, K. K., and W. T. Blevins. 1993. Geosmin-producing species of Streptomyces and Lyngbya from aquaculture ponds. Can. J. Microbiol. 39:834-840. [Google Scholar]
- 26.Schrader, K. K., and M. D. Harries. 2001. Compounds with selective toxicity toward the musty-odor cyanobacterium Oscillatoria perornata. Bull. Environ. Contam. Toxicol. 66:801-807. [DOI] [PubMed] [Google Scholar]
- 27.Schrader, K. K., F. E. Dayan, S. N. Allen, M. Q. de Regt, C. S. Tucker, and R. N. Paul, Jr. 2000. 9,10-Anthraquinone reduces the photosynthetic efficiency of Oscillatoria perornata and modifies cellular inclusions. Int. J. Plant Sci. 161:265-270. [DOI] [PubMed] [Google Scholar]
- 28.Schrader, K. K., M. Q. de Regt, P. R. Tidwell, C. S. Tucker, and S. O. Duke. 1998. Selective growth inhibition of the musty-odor producing cyanobacterium Oscillatoria cf. chalybea by natural compounds. Bull. Environ. Contam. Toxicol. 60:651-658. [DOI] [PubMed] [Google Scholar]
- 29.Schrader, K. K., M. Q. de Regt, P. D. Tidwell, C. S. Tucker, and S. O. Duke. 1998. Compounds with selective toxicity towards the off-flavor metabolite-producing cyanobacterium Oscillatoria cf. chalybea. Aquaculture 163:85-99. [Google Scholar]
- 30.Schrader, K. K., M. Q. de Regt, C. S. Tucker, and S. O. Duke. 1997. A rapid bioassay for selective algicides. Weed Technol. 11:767-774. [Google Scholar]
- 31.Schrader, K. K., C. S. Tucker, M. Q. de Regt, and S. K. Kingsbury. 2000. Evaluation of limnocorrals for studying the effects of phytotoxic compounds on plankton and water chemistry in aquaculture ponds. J. World Aquacult. Soc. 31:403-415. [Google Scholar]
- 32.Tucker, C. S. 2000. Off-flavor problems in aquaculture. Rev. Fish. Sci. 8:45-88. [Google Scholar]
- 33.Tucker, C. S., T. R. Hanson, and S. K. Kingsbury. 2001. Management of off-flavors in pond-cultured channel catfish with weekly applications of copper sulfate. N. Am. J. Aquacult. 63:118-130. [Google Scholar]
- 34.van der Ploeg, M., and C. S. Tucker. 1993. Seasonal trends in flavor quality of channel catfish, Ictalurus punctatus, from commercial ponds in Mississippi. J. Appl. Aquacult. 3:121-140. [Google Scholar]
- 35.van der Ploeg, M., C. S. Tucker, and C. E. Boyd. 1992. Geosmin and 2-methylisoborneol production by cyanobacteria in fish ponds in the southeastern United States. Water Sci. Technol. 25:283-290. [Google Scholar]
- 36.Zimba, P. V., L. Khoo, P. S. Gaunt, S. Brittain, and W. W. Carmichael. 2001. Confirmation of catfish, Ictalurus punctatus (Rafinesque), mortality from Microcystis toxins. J. Fish Dis. 24:41-47. [Google Scholar]
- 37.Zimba, P. V., C. S. Tucker, C. C. Mischke, and C. C. Grimm. 2002. Short-term effect of diuron on catfish pond ecology. N. Am. J. Aquacult. 64:16-23. [Google Scholar]