Abstract
The relationship between the survival of enteric viral pathogens and their indicators (coliform bacteria and coliphages) is not well understood. We compared the survival rates of feline calicivirus (FCV), Escherichia coli, and a male-specific RNA coliphage MS2 at 4, 25, and 37°C for up to 28 days in dechlorinated water. The survival rates of E. coli and FCV, a surrogate of noroviruses (NV), had a high degree of correlation at 4 and 25°C, while MS2 phage survived significantly longer (P < 0.05) at these two temperatures. At 37°C, the survival rates for all three organisms were highly correlated. Decimal reduction values indicating the number of days needed for 90% reduction in titer (D values) decreased for all three organisms as storage temperatures increased. FCV had the shortest D value among all three organisms at all temperatures investigated. These findings indicate that F-specific RNA phages may be useful indicators of NV in the environment.
Noroviruses (NV), formerly known as Norwalk-like viruses, are estimated to cause 66% of all food-borne illnesses attributable to known causes in the United States (2, 23, 24, 25). NV outbreaks are commonly associated with the consumption of fresh produce and other ready-to-eat foods contaminated by infected food workers or shellfish harvested from waters contaminated by sewage (13-15, 28, 29). The potential for water to serve as the vehicle for NV contamination of fresh produce is an emerging concern for the following reasons: (i) produce is grown and harvested in many areas of the world that lack adequate waste disposal and water treatment facilities; (ii) untreated surface water is often used to irrigate produce; (iii) current water treatment methods may be incapable of removing viruses; (iv) enteric viruses can survive on produce for long periods of time; and (v) no virological criteria exist for water used in produce production (8, 9, 14, 16, 27, 29, 35).
Routine monitoring of water and produce for NV would help improve the safety of fresh produce (3, 4, 7, 16). However, methods for routine detection of NV in the environment are currently not available. To further complicate the situation, fecal-indicator bacteria have been shown to be inadequate indictors of virological risk in the environment (10, 12, 22). In light of these facts, F-specific RNA coliphages have been proposed as alternate indicators of enteric viruses, including NV (10, 17, 20, 31, 39, 40). However, the proposed use of F-specific RNA coliphages as indicators is not without controversy. For example, while a strong correlation has been observed between the seasonal accumulation of F-specific RNA coliphages in oysters and the incidence of NV diseases associated with oysters (6), some investigators have reported that F-specific RNA coliphages are rarely detected in human feces, suggesting that the presence of these coliphages in water does not necessarily indicate human fecal pollution (18, 32).
One of the criteria for an ideal indicator is that it should survive longer than the pathogen itself. Therefore, this study was designed to compare the survival rates of F-specific RNA coliphage (ATCC 15597-B1), feline calicivirus (FCV) strain F9 (ATCC VR-782), and Escherichia coli Famp (ATCC 700891) in dechlorinated water at different temperatures. Since NV cannot be grown under in vitro conditions, we used FCV as a surrogate for NV because FCV is cultivable in feline kidney cells, is easy to detect and titrate in vitro, and has been successfully used as an NV surrogate by other researchers (5, 11, 34).
FCV was propagated in monolayers of Crandell's feline kidney cells. The cells were grown in Eagle's minimal essential medium (Celox, St. Paul, Minn.) supplemented with 8% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), amphotericin B (Fungizone; 1 μg/ml), and lactalbumin hydrolysate (5 mg/ml). Monolayers at 90% confluence were inoculated with FCV and incubated for 1 h at 37°C to allow virus adsorption. Infected cells were then incubated in maintenance medium (Eagle's minimal essential medium without added serum) and examined for cytopathic effects after 96 h. The stock virus was harvested after two freeze-thaw cycles, aliquoted, and stored at −70°C. Cells grown in 96-well plates were used for virus titration, with 3 wells per dilution. Viral titers were calculated by using the method of Reed and Muench (30).
F-specific RNA phage MS2 was grown and titrated according to Environmental Protection Agency method 1601 (37). Briefly, a 1-ml aliquot of phage stock was added to 30 ml of an exponential culture of E. coli Famp grown in tryptic soy broth (TSB) containing ampicillin and streptomycin (Sigma, St. Louis, Mo.). After incubation at 37°C overnight, the culture was centrifuged at 6,000 × g for 15 min to remove bacterial cell debris. The supernatant fluid was then filtered through a 0.45-μm-pore-size cellulose acetate filter (Vanguard International, Neptune, N.J.), and the filtrate was titrated. In brief, serial dilutions of the filtrate were made in TSB, and 1 ml of each dilution was mixed with 200 μl of an exponential culture of E. coli Famp and 3 to 5 ml of 0.75% tryptic soy agar (TSA). This mixture was poured on top of the solidified bottom agar layer (1.5% TSA contained in a petri dish) and allowed to solidify. The plates were then inverted and incubated at 37°C for 24 h, after which plaques were counted and the results were recorded as PFU per milliliter.
An overnight culture of E. coli Famp was prepared by placing 1 ml of a stock culture into 25 ml of 3% TSB, followed by incubation overnight at 37°C on a rotary shaker (Lab-Line Inc, Melrose Park, Ill.). To obtain an exponential culture, 0.5 ml of the overnight culture was added to 50 ml of fresh 3% TSB and incubated for 4 to 6 h at 37°C. Titration was done by the pour plate method (21). In brief, the culture was serially diluted in TSB, and 1 ml of each dilution was added to 20 ml of molten 1% TSA (at approximately 45°C). After thorough mixing, the mixture was poured into petri plates. The media was allowed to solidify, and the plates were incubated in an inverted position at 37°C. After 24 h of incubation, bacterial colonies were counted and recorded as CFU per milliliter.
Tap water was dechlorinated by adding 1 ml of a 3% sodium thiosulfate solution to 1 liter of water. The water was then autoclaved, cooled, and aliquoted in 50-ml amounts in 250-ml screw-cap glass bottles. Each organism was inoculated into three bottles at initial titers of approximately 106 50% tissue culture infective doses/ml for FCV, 109 PFU/ml for MS2 phage, and 109 CFU/ml for E. coli. After being thoroughly mixed for 30 s, a 1-ml sample was withdrawn and assayed to determine the initial titer of the test organisms. The seeded water samples were stored at 4, 25, and 37°C for a total of 28 days. Representative samples (1-ml amounts) were removed on days 1, 2, 3, 7, 14, 21, and 28 and assayed for the appropriate organism. The results were recorded as 50% tissue culture infective doses per milliliter for FCV, PFU per milliliter for MS2 phage, and CFU per milliliter for E. coli.
The mean decimal reduction value indicating the number of days needed for 90% reduction in titer (D value) for three experiments was calculated for each of the test organisms at all three storage temperatures. A one-way nonparametric analysis of variance procedure was used to calculate statistically significant differences in D values. Statistical analysis was performed with Statistical Analysis System software (SAS Institute, Gary, Ind.).
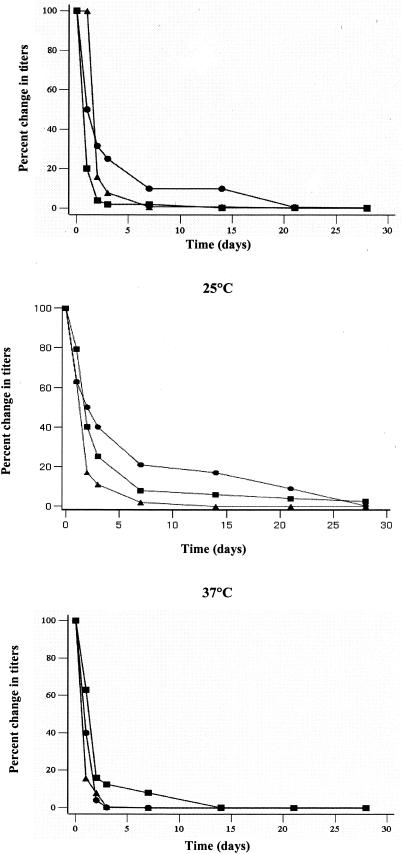
D values for MS2 phage were significantly greater than D values for FCV and E. coli at 4 and 25°C (Table 1). At these temperatures, D values for MS2 exceeded the others by a factor of three. There was no marked difference in D values among the three organisms at 37°C. As expected, D values for all three organisms decreased as the storage temperature increased. However, observed D values for each organism and the relationships between D values remained relatively constant at 4 and 25°C. The percent decrease in titers of each of the three organisms is shown in Fig. 1.
TABLE 1.
D values of three test organisms at different temperaturesa
| Organism | Mean (SD) of D value at:
|
||
|---|---|---|---|
| 4°C | 25°C | 37°C | |
| Escherichia coli | 7.7 (3.8) | 5.7 (4.0) | 3.0 (1.0) |
| FCV | 7.3 (4.7) | 5.2 (2.3) | 2.0 (1.0) |
| F-specific coliphage (MS2) | 25.7 (4.0) | 18.7 (8.1) | 2.7 (1.2) |
D values, decimal reduction values (number of days needed for a 90% reduction in titer).
FIG. 1.
Titers of FCV (▴), F-specific phage MS2 (•), and E. coli (▪) in dechlorinated water stored at 4, 25, or 37°C. Samples were taken at 0, 1, 2, 3, 7, 14, 21, and 28 days. The data points represent the means of the percent change in titers observed over three experimental runs.
At 4°C, MS2 had an average D value of 25.7 days, compared to 7.3 and 7.7 days for FCV and E. coli, respectively. This difference in D values was statistically significant (P < 0.05; Kruskal-Wallis test). At 25°C, the MS2 titer had an average D value of 18.7 days, compared to 5.2 and 5.7 days for FCV and E. coli, respectively. This difference in D values was also statistically significant (P < 0.05; Kruskal-Wallis test). At 37°C, all three organisms had D values ranging from 2 to 3 days, and the differences were not statistically significant (P > 0.05; Kruskal-Wallis test).
Previous research has shown that NV can survive for long periods of time in the environment (1, 26, 38). Thus, a useful indicator of this pathogen must have, among other features, the ability to survive for a long time in the environment. The findings of this study suggest that both E. coli and F-specific phage would survive for as long as, or longer than, NV in clean water free of disinfectants. Since bacterial indicators are generally less resistant to disinfection than enteric viruses, it is possible that in the presence of disinfectants such as chlorine, E. coli survival would differ from that of the two viral organisms (10, 33). Further studies in this regard are warranted.
The finding of a significant difference between the D values of FCV and MS2 at 4 and 25°C needs further evaluation. It has been documented that the relative decay rates of F-specific phages, enteric viruses, and coliform bacteria vary with salinity, pH, temperature, turbidity, and dissolved oxygen concentration (33, 36, 38). However, despite this variability, F-specific phage levels in seafood harvested from sewage-contaminated waters have consistently been higher than those of fecal-indicator bacteria (10, 19). In addition, F-specific coliphages have been reported to survive for up to 30 days in groundwater (40). Our results affirm that F-specific coliphages survive longer than FCV and E. coli, making it a conservative indicator of NV in clean water.
FCV appears to be a reasonably good experimental model for assessing the environmental stability of NV because its physicochemical properties are similar to those of NV and it can be grown and titrated easily in tissue cultures. Thus, FCV allows us to directly measure the rate of virus survival and the ability of the surviving virus to infect cells, which is not possible with NV because the molecular methods available for their detection cannot differentiate between infectious and noninfectious particles.
The levels of F-specific phages in oysters are reported to be the highest when there is a high incidence of viral gastroenteritis among residents of coastal areas (10). This correlation suggests that, under proper environmental conditions, F-specific phages could prove to be a useful indicator of virological risk in food and water. This study appears to be the first that directly compares the survival rate of an F-specific RNA coliphage with the survival rate of an NV surrogate. Additional studies are needed to compare the survival rates of these two organisms under a wider range of environmental conditions to further evaluate F-specific phages as alternate indicators of virological risk in food, water, and the environment.
Acknowledgments
We thank Douglas Wait of the University of North Carolina for providing E. coli and MS2.
REFERENCES
- 1.Abad, F. X., R. M. Pinto, and A. Bosch. 1994. Survival of enteric viruses on environmental fomites. Appl. Environ. Microbiol. 60:3704-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando, T., J. S. Noel, and R. L. Fankhauser. 2000. Genetic classification of “Norwalk-like viruses.” J. Infect. Dis. 181:S336-S348. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, E. C., M. D. Sobsey, C. H. House, and K. D. White. 2001. Microbial indicator removal in onsite constructed wetlands for wastewater treatment in the southeastern U.S. Water Sci. Technol. 44:177-182. [PubMed] [Google Scholar]
- 4.Beuret, C., D. Kohler, A. Baumgartner, and T. M. Luthi. 2002. Norwalk-like virus sequences in mineral waters: one-year monitoring of three brands. Appl. Environ. Microbiol. 68:1925-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bidawid, S., N. Malik, O. Adgburin, S. A. Sattar, and J. M. Farber. 2003. A feline kidney cell line-based plaque assay for feline calicivirus, a surrogate for Norwalk virus. J. Virol. Methods. 107:163-167. [DOI] [PubMed] [Google Scholar]
- 6.Burkhardt, W., III, and K. R. Calci. 2000. Selective accumulation may account for shellfish-associated viral illness. Appl. Environ. Microbiol. 66:1375-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campos, C., A. Guerrero, and M. Cardenas. 2002. Removal of bacterial and viral faecal indicator organisms in a waste stabilization pond system in Choconta, Cundinamarca (Colombia). Water Sci. Technol. 45:61-66. [PubMed] [Google Scholar]
- 8.Croci, L., M. D. De, C. Scalfaro, A. Fiore, and L. Toti. 2002. The survival of hepatitis A virus in fresh produce. Int. J. Food Microbiol. 73:29-34. [DOI] [PubMed] [Google Scholar]
- 9.Deneen, V. C., J. M. Hunt, C. R. Paule, R. I. James, R. G. Johnson, M. J. Raymond, and C. W. Hedberg. 2000. The impact of foodborne calicivirus disease: the Minnesota experience. J. Infect. Dis. 181:S281-S283. [DOI] [PubMed] [Google Scholar]
- 10.Dore, W. J., K. Henshilwood, and D. N. Lees. 2000. Evaluation of F-specific RNA bacteriophage as a candidate human enteric virus indicator for bivalve molluscan shellfish. Appl. Environ. Microbiol. 66:1280-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doultree, J. C., J. D. Druce, C. J. Birch, D. S. Bowden, and J. A. Marshall. 1999. Inactivation of feline calicivirus, a Norwalk virus surrogate. J. Hosp. Infect. 41:51-57. [DOI] [PubMed] [Google Scholar]
- 12.Duran, A. E., M. Muniesa, X. Mendez, F. Valero, F. Lucena, and J. Jofre. 2002. Removal and inactivation of indicator bacteriophages in fresh waters. J. Appl. Microbiol. 92:338-347. [DOI] [PubMed] [Google Scholar]
- 13.Fankhauser, R. L., S. S. Monroe, J. S. Noel, C. D. Humphrey, J. S. Bresee, U. D. Parashar, T. Ando, and R. I. Glass. 2002. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 186:1-7. [DOI] [PubMed] [Google Scholar]
- 14.Glass, R. I., J. Bresee, B. Jiang, J. Gentsch, T. Ando, R. Fankhauser, J. Noel, U. Parashar, B. Rosen, and S. S. Monroe. 2001. Gastroenteritis viruses: an overview. Novartis Found. Symp. 238:5-25. [DOI] [PubMed] [Google Scholar]
- 15.Glass, R. I., J. Noel, T. Ando, R. Fankhauser, G. Belliot, A. Mounts, U. D. Parashar, J. S. Bresee, and S. S. Monroe. 2000. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J. Infect. Dis. 181:S254-S261. [DOI] [PubMed] [Google Scholar]
- 16.Grabow, W. O., M. B. Taylor, and J. C. de Villiers. 2001. New methods for the detection of viruses: call for review of drinking water quality guidelines. Water Sci. Technol. 43:1-8. [PubMed] [Google Scholar]
- 17.Havelaar, A., M. van Olphen, and Y. Drost. 1993. F-specific RNA bacteriophages are adequate model organisms for enteric viruses in fresh water. Appl. Environ. Microbiol. 59:2956-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Havelaar, A. H., W. M. Pot-Hogeboom, K. Furuse, R. Pot, and M. P. Hormann. 1990. F-specific RNA bacteriophages and sensitive host strains in faeces and wastewater of human and animal origin. J. Appl. Bacteriol. 69:30-37. [DOI] [PubMed] [Google Scholar]
- 19.Hernroth, B. E., A. C. Conden-Hansson, A. S. Rehnstam-Holm, R. Girones, and A. K. Allard. 2002. Environmental factors influencing human viral pathogens and their potential indicator organisms in the blue mussel, Mytilus edulis: the first Scandinavian report. Appl. Environ. Microbiol. 68:4523-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu, F. C., Y. S. Shieh, J. van Duin, M. J. Beekwilder, and M. D. Sobsey. 1995. Genotyping male-specific RNA coliphages by hybridization with oligonucleotide probes. Appl. Environ. Microbiol. 61:3960-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson, R. W., K. Osborne, G. Barnes, C. Jolliff, D. Zamani, B. Roll, A. Stillings, D. Herzog, S. Cannon, and S. Loveland. 2000. Multiregional evaluation of the Sim Plate heterotrophic plate count method compared to the standard plate count agar pour plate method in water. Appl. Environ. Microbiol. 66:453-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kator, H., and M. Rhodes. 2001. Elimination of fecal coliforms and F-specific RNA coliphage from oysters (Crassostrea virginica) relaid in floating containers. J. Food Prot. 64:796-801. [DOI] [PubMed] [Google Scholar]
- 23.Koopmans, M., J. Vinje, M. de Wit, I. Leenen, W. van der Poel, and Y. van Duynhoven. 2000. Molecular epidemiology of human enteric caliciviruses in The Netherlands. J. Infect. Dis. 181:S262-S269. [DOI] [PubMed] [Google Scholar]
- 24.Koopmans, M., J. Vinje, E. Duizer, M. de Wit, and Y. van Duijnhoven. 2001. Molecular epidemiology of human enteric caliciviruses in The Netherlands. Novartis Found. Symp. 238:197-218. [DOI] [PubMed] [Google Scholar]
- 25.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mounts, A. W., T. Ando, M. Koopmans, J. S. Bresee, J. Noel, and R. I. Glass. 2000. Cold weather seasonality of gastroenteritis associated with Norwalk-like viruses. J. Infect. Dis. 181:S284-S287. [DOI] [PubMed] [Google Scholar]
- 27.Ooi, P. L., K. T. Goh, K. S. Neo, and C. C. Ngan. 1997. A shipyard outbreak of salmonellosis traced to contaminated fruits and vegetables. Ann. Acad. Med. Singapore 26:539-543. [PubMed] [Google Scholar]
- 28.Parashar, U. D., L. Dow, R. L. Fankhauser, C. D. Humphrey, J. Miller, T. Ando, K. S. Williams, C. R. Eddy, J. S. Noel, T. Ingram, J. S. Bresee, S. S. Monroe, and R. I. Glass. 1998. An outbreak of viral gastroenteritis associated with consumption of sandwiches: implications for the control of transmission by food handlers. Epidemiol. Infect. 121:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parashar, U. D., and S. S. Monroe. 2001. “Norwalk-like viruses” as a cause of foodborne disease outbreaks. Rev. Med. Virol. 11:243-252. [DOI] [PubMed] [Google Scholar]
- 30.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 31.Schaper, M., A. E. Duran, and J. Jofre. 2002. Comparative resistance of phage isolates of four genotypes of F-specific RNA bacteriophages to various inactivation processes. Appl. Environ. Microbiol. 68:3702-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaper, M., J. Jofre, M. Uys, and W. O. Grabow. 2002. Distribution of genotypes of F-specific RNA bacteriophages in human and non-human sources of faecal pollution in South Africa and Spain. J. Appl. Microbiol. 92:657-667. [DOI] [PubMed] [Google Scholar]
- 33.Sinton, L. W., C. H. Hall, P. A. Lynch, and R. J. Davies-Colley. 2002. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 68:1122-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slomka, M. J., and H. Appleton. 1998. Feline calicivirus as a model system for heat inactivation studies of small round structured viruses in shellfish. Epidemiol. Infect. 121:401-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon, E. B., C. J. Potenski, and K. R. Matthews. 2002. Effect of irrigation method on transmission to and persistence of Escherichia coli O157:H7 on lettuce. J. Food Prot. 65:673-676. [DOI] [PubMed] [Google Scholar]
- 36.Stenstrom, T. A., and A. Carlander. 2001. Occurrence and die-off of indicator organisms in the sediment in two constructed wetlands. Water Sci. Technol. 44:223-230. [PubMed] [Google Scholar]
- 37.U.S. Environmental Protection Agency. 2001. Method 1601: male-specific (F +) and somatic coliphage in water by two-step enrichment procedure. [Online.] http://www.epa.gov/microbes/1601ap01.pdf.
- 38.Wait, D. A., and M. D. Sobsey. 2001. Comparative survival of enteric viruses and bacteria in Atlantic Ocean seawater. Water Sci. Technol. 43:139-142. [PubMed] [Google Scholar]
- 39.Woody, M. A., and D. O. Cliver. 1995. Effects of temperature and host cell growth phase on replication of F-specific RNA coliphage Q beta. Appl. Environ. Microbiol. 61:1520-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yates, M. V., C. P. Gerba, and L. M. Kelley. 1985. Virus persistence in groundwater. Appl. Environ. Microbiol. 49:778-781. [DOI] [PMC free article] [PubMed] [Google Scholar]