Abstract
Volatile aroma-active esters are responsible for the fruity character of fermented alcoholic beverages such as beer and wine. Esters are produced by fermenting yeast cells in an enzyme-catalyzed intracellular reaction. In order to investigate and compare the roles of the known Saccharomyces cerevisiae alcohol acetyltransferases, Atf1p, Atf2p and Lg-Atf1p, in volatile ester production, the respective genes were either deleted or overexpressed in a laboratory strain and a commercial brewing strain. Subsequently, the ester formation of the transformants was monitored by headspace gas chromatography and gas chromatography combined with mass spectroscopy (GC-MS). Analysis of the fermentation products confirmed that the expression levels of ATF1 and ATF2 greatly affect the production of ethyl acetate and isoamyl acetate. GC-MS analysis revealed that Atf1p and Atf2p are also responsible for the formation of a broad range of less volatile esters, such as propyl acetate, isobutyl acetate, pentyl acetate, hexyl acetate, heptyl acetate, octyl acetate, and phenyl ethyl acetate. With respect to the esters analyzed in this study, Atf2p seemed to play only a minor role compared to Atf1p. The atf1Δ atf2Δ double deletion strain did not form any isoamyl acetate, showing that together, Atf1p and Atf2p are responsible for the total cellular isoamyl alcohol acetyltransferase activity. However, the double deletion strain still produced considerable amounts of certain other esters, such as ethyl acetate (50% of the wild-type strain), propyl acetate (50%), and isobutyl acetate (40%), which provides evidence for the existence of additional, as-yet-unknown ester synthases in the yeast proteome. Interestingly, overexpression of different alleles of ATF1 and ATF2 led to different ester production rates, indicating that differences in the aroma profiles of yeast strains may be partially due to mutations in their ATF genes.
During fermentation processes, yeast cells produce a broad range of aroma-active substances which greatly affect the complex flavor of fermented alcoholic beverages. While these secondary metabolites are often formed only in trace amounts, their concentrations determine the distinct aroma of these beverages. Flavor-active substances produced by fermenting yeast cells can be divided into five main groups: sulfur-containing molecules, organic acids, higher alcohols, carbonyl compounds, and volatile esters (32, 39, 56, 57, 66). Of these categories, volatile esters represent the largest and most important group. They are responsible for the highly desired fruity character of beer and, to a lesser extent, other alcoholic beverages, such as wine.
The major flavor-active esters in beer are acetate esters such as ethyl acetate (solvent-like aroma), isoamyl acetate (banana flavor), and phenylethyl acetate (flowery, rose aroma). In addition, C6-C10 medium-chain fatty acid ethyl esters such as ethyl hexanoate (ethyl caproate) and ethyl octanoate (ethyl caprylate), which have “sour apple” aromas, are also important for the overall bouquet (46-48).
The means of controlling ester synthesis during industrial beer fermentations are very limited (for reviews, see references 18, 19, 77, and 78). It is well known that ester formation is highly dependent on the yeast strain used (58, 63) and on certain fermentation parameters, such as temperature (20, 69), pitching rate (16, 45), and top pressure (40, 68). In addition, the concentrations of assimilable nitrogen compounds (12, 32, 69), carbon sources (65, 81, 87, 88), dissolved oxygen (3, 4, 7, 69), and fatty acids (75, 76) have a profound impact on ester production rates. However, these factors allow only minor adjustments to the final ester concentrations of the produced beverages, so that the overall ester balance after fermentation is often suboptimal, resulting in an inferior end product. The lack of control over ester production is a particular problem in modern high-gravity beer production, as the use of high-gravity worts leads to disproportionate amounts of ethyl acetate and isoamyl acetate (2, 32, 46, 65, 81). In addition, the use of today's large-scale cylindroconical fermentation vessels causes a dramatic drop in ester production, resulting in an even greater imbalance in the ester profile (46). In order to obtain better control over ester synthesis, much research has been focused on the elucidation of the biochemical mechanisms of ester synthesis as well as on the factors influencing ester synthesis rates.
Esters are formed intracellularly in an enzyme-catalyzed condensation reaction between two cosubstrates, a higher alcohol and an activated acyl-coenzyme A (acyl-CoA) molecule (53-55). Hence, the ester production rate is influenced by two primary factors, namely, the concentrations of the two cosubstrates and the total ester synthase activity. The relative impact of these two primary factors, however, remains unclear.
Early research on ester production focused on substrate availability as the main determining factor for ester synthesis. As some studies showed a certain degree of correlation between substrate concentrations and corresponding ester formation, it was generally accepted that substrate concentrations control ester formation (12, 75, 87). However, this model fails to explain some experimental data (78, 79, 85). More recent studies have therefore focused on the impact of ester synthase activities on the ester formation rates (42, 78, 79).
The best-known enzymes involved in ester synthesis are the so-called alcohol acetyltransferases (AATases; EC 2.3.1.84). These enzymes catalyze the formation of acetate esters from the two substrates: an alcohol and acetyl-CoA. It was shown that, during fermentation, acetate ester production rates follow a pattern corresponding to the AATase activity (42). Furthermore, cellular AATase activity is repressed by the presence of unsaturated fatty acids in the medium, a factor known to decrease acetate ester production (8, 9, 21). In addition, Alvarez et al. (1) found a clear correlation between the concentrations of ethyl acetate and isoamyl acetate in beer, indicating that these esters may be synthesized by the same rate-limiting enzyme.
Purification of the acetate ester-synthesizing enzymes has led to the identification of three distinct AATases: AATase I, its closely related homologue Lg-AATase I, and AATase II. These AATases are encoded by ATF1, the ATF1 homologue Lg-ATF1, and ATF2, respectively (22-24, 43, 49, 83, 84, 86). While ATF1 and ATF2 are present in both Saccharomyces cerevisiae (ale) and Saccharomyces bayanus (lager) strains, Lg-ATF1 is found only in S. bayanus strains (10, 19, 38, 84). Homology-based searches of the S. cerevisiae genome have not revealed other genes with homology to ATF1 and/or ATF2. However, Malcorps and Dufour (43) speculated that apart from Atf1p, Lg-Atf1p, and Atf2p, at least one other, as-yet-unidentified enzyme with AATase activity is present in the yeast proteome. In addition to the three known AATases, a possible alcohol acyltransferase, Eht1p (ethanol hexanoyl transferase), has recently been described (5, 44). This ester synthase is involved in the bioformation of medium-chain (C6-C10) fatty acid esters (17, 18).
The only ester-synthesizing enzymes that have already been studied in any detail are Atf1p and its closely related homologue Lg-Atf1p. In haploid S. cerevisiae atf1Δ strains, isoamyl acetate transferase and ethanol acetate transferase activities were 80 and 20% lower, respectively, than in the wild-type strain. This resulted in an 80% decrease in isoamyl acetate production and a 30% decrease in ethyl acetate production compared to rates in wild-type cells (24). In another study, overexpression of ATF1 derived from an industrial lager brewer's yeast strain resulted in a 27-fold increase in isoamyl acetate production and a 9-fold increase in ethyl acetate production compared to rates in empty-vector transformants (22). Similarly, overexpression of Lg-ATF1 showed a sevenfold increase in isoamyl acetate and a twofold increase in ethyl acetate concentrations (22).
However, the strains, fermentation medium, and set-up used for these studies were significantly different from industrial conditions, so that it is unclear how meaningful the results are for industrial applications. Other researchers have therefore performed semi-industrial fermentations using grape must or barley malt wort as the fermentation medium and industrial strains overexpressing ATF1. Lilly et al. (41) showed that overexpression of ATF1 in the commercial wine yeasts VIN7 and VIN13 leads to a 3- to 10-fold increase in ethyl acetate concentrations and a 4- to 12-fold increase in isoamyl acetate concentrations in the produced wines. Similarly, Verstrepen et al. (79) have demonstrated that overexpression of ATF1 in a commercial brewer's strain leads to significantly increased concentrations of isoamyl acetate and ethyl acetate in the beers produced. These results indicate that the expression level of ATF1 is an important limiting factor for ester synthesis under industrial conditions, confirming the hypothesis of Malcorps et al. (42).
However, as all different research groups have used different yeast strains and different fermentation conditions for the study of ATF1, it is difficult to compare the results. Furthermore, most overexpression studies were based on multicopy plasmids, so some differences between strains may in fact be due to differences in plasmid copy number. In addition, little is known about the contribution of other enzymes to the total ester-synthesizing activity in fermenting yeast cells. Nagasawa et al. (49) and Yoshimoto et al. (83) suggested that Atf2p might be responsible for the remaining 20% of isoamyl acetate production in a atf1Δ disruptant, but this remains to be confirmed. In addition, it remains unclear whether the Atf proteins are also responsible for the formation of other esters besides isoamyl acetate and ethyl acetate.
The aim of this study was to investigate and compare the effects on volatile ester production of deletion and overexpression of ATF1 and the less-studied genes Lg-ATF1 and ATF2 in the same genetic background. Apart from clearly identifying the contribution of each AATase to the production of the well-known aroma compounds such as ethyl acetate and isoamyl acetate, the effect on the production of a broad range of other volatile compounds was also evaluated through gas chromatography combined with mass spectrometry (GC-MS). The results provide insight into the controlling role of ATF gene expression in the production of a wide variety of volatile compounds. In addition, we demonstrate the existence of other, as-yet-unidentified ester-synthesizing enzymes in the S. cerevisiae proteome. The observations can lead to the development of new production strategies and/or new yeast strains, allowing further optimization of beer flavor profiles, as well as to a better understanding of the physiological role of ester synthesis.
MATERIALS AND METHODS
Microbial strains, plasmids, media, and culturing conditions.
All plasmids and bacterial and yeast strains used in this study are listed in Table 1. Yeast cultures were routinely grown at 28°C in YPGluc medium (4% [wt/vol] glucose [Merck], 2% peptone [Difco], and 1% yeast extract [Difco]) (71). Cultures were shaken with an orbital shaker at 50 rpm for test tubes or a horizontal shaker at 150 rpm for Erlenmeyer flasks. For selection of yeast transformants, minimal synthetic defined (SD) medium was used, containing 0.67% yeast nitrogen base without amino acids (Difco) and 2% glucose (Merck), supplemented with 60 mg of sulfometuron methyl (SMM; E. I. Du Pont de Nemours) liter−1 (13, 71). Escherichia coli was grown in Luria-Bertani medium containing 1% Bacto tryptone (Difco), 1% NaCl, and 0.5% yeast extract (Difco).
TABLE 1.
Microbial strains and plasmids used
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| S. cerevisiae | ||
| BY4742 (wild type) | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | ResGen/Invitrogen Belgium |
| BY4742 (atf1Δ) | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 atf1Δ::KANr | ResGen/Invitrogen Belgium |
| BY4742 (atf2Δ) | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 atf2Δ::KANr | ResGen/Invitrogen Belgium |
| BY4742 (atf1Δ atf2Δ) | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 atf1Δ::KANr atf2Δ::KANr | This study |
| CMBS33 | Commercial lager strain | KU Leuvena |
| CMBS212 | Commercial ale strain | KU Leuven |
| CMBS33 ps | Commercial lager strain; PGK1p-PGK1T SMR1-410 (empty vector; control strain) | This study; two independent strains were constructed |
| CMBS33 pATF1 (CMBS33)s | Commercial lager strain; PGK1p-ATF1(CMBS33)-PGK1T SMR1-410 | This study; two independent strains were constructed |
| CMBS33 pATF1 (CMBS212)s | Commercial lager strain; PGK1p-ATF1(CMBS212)-PGK1T SMR1-410 | This study; two independent strains were constructed |
| CMBS33 pATF2 (CMBS33)s | Commercial lager strain; PGK1p-ATF2(CMBS33)-PGK1T SMR1-410 | This study; two independent strains were constructed |
| CMBS33 pATF2 (CMBS212)s | Commercial lager strain; PGK1p-ATF2(CMBS212)-PGK1T SMR1-410 | This study; two independent strains were constructed |
| CMBS33 pLg-ATF1 (CMBS33)s | Commercial lager strain; PGK1p-Lg-ATF1(CMBS33)-PGK1T SMR1-410 | This study; two independent strains were constructed |
| E. coli DH5α | F′ end A1 hsdR17 supE44 thi-1 recA gyrA relA1 Δ(lacZYA-argF) U169 deoR [φ80dlac DE(lacZ)M15] | GIBCO-BRL/Life Technologies |
| Plasmids | ||
| ps | bla LEU2 SMR1-410 PGK1p-PGK1T (empty vector, YIp) | 41 |
| pATF1(CMBS33)s | bla LEU2 SMR1-410 PGK1p-ATF1(CMBS33)-PGK1T | This study, two independent plasmids were constructed |
| pATF1(CMBS212)s | bla LEU2 SMR1-410 PGK1p-ATF1(CMBS212)-PGK1T | This study; two independent plasmids were constructed |
| pATF2(CMBS33)s | bla LEU2 SMR1-410 PGK1p-ATF2(CMBS33)-PGK1T | This study; two independent plasmids were constructed |
| pATF2(CMBS212)s | bla LEU2 SMR1-410 PGK1p-ATF2(CMBS212)-PGK1T | This study; two independent plasmids were constructed |
K.U. Leuven, Centre for Malting and Brewing Collection, Centre for Malting and Brewing, Louvain, Belgium.
DNA manipulations.
Standard procedures for the isolation and manipulation of DNA were used (6). Restriction enzymes, T4 DNA ligase, and Expand high-fidelity DNA polymerase (Boehringer Mannheim) were used for enzymatic DNA manipulations as recommended by the supplier. The following primers were used for the amplification of DNA fragments by PCR: for the ATF1 open reading frame (ORF), XhoI-ATF1-ORF-F (TTGCCTCGAGATGAATGAAATCGATGAGAAAAATC; the XhoI restriction site is underlined) and XhoI-ATF1-ORF-R (TTGCCTCGAGCTAAGGGCCTAAAAGGAGAGC) (the XhoI restriction site is underlined); for the ATF2 ORF, BglII-SalI-ATF2-ORF-F (CCCAGATCTGAGTCGACATGGAAGATATAGAAGGATACG; the BglII restriction site is underlined, and the SalI restriction site is in italics) and BglII-SalI-ATF2-ORF-R (CCCAGATCTGACTCGAGTTAAAGCGACGCAAATTCGCCG; the BglII site is underlined, and the SalI restriction site is in italics); for the Lg-ATF1 ORF, XhoI-LgATF1-ORF-F (TAGTTGCCTCGAGGACATGGAAACAGAAGAAAGCC; the XhoI restriction site is underlined) and XhoI-LgATF1-ORF-R (TAGTTGCCTCGAGTCAGGGATTTAAAAGCAGAGCC; the XhoI restriction site is underlined).
The plasmids pATF1s, pLg-ATF1s, and pATF2s were constructed by insertion of the respective ORFs into the XhoI restriction site in the PGK1 overexpression cassette of the ps vector (the ATF1 and Lg-ATF1 PCR products were cut with XhoI; the ATF2 PCR product was cut with SalI). Before transformation, the vectors were linearized in the LEU2 gene with EcoRV. Yeast transformation was carried out using the lithium acetate method (31). After transformation, the cells were plated on SD medium containing 60 mg of SMM liter−1 (13). SMM-resistant colonies were further analyzed by PCR and subsequent restriction analysis to confirm the integration of the respective PGK overexpression constructs into the genomic LEU2 gene.
Sequencing of vector inserts and genomic copies of ATF1, ATF2, and Lg-ATF1 was performed by the dideoxy chain-termination method with an Applied Biosystems (Foster City, Calif.) model 3100 Avant sequencer according to the supplier's instructions. All sequencing reactions were performed at least twice. Sequences were analyzed with ABI Prisma and vector NTI Advance (Informax/Invitrogen, Merelbeke, Belgium) software.
The single-deletion strains BY4742 (atf1Δ) and BY4742 (atf2Δ) were obtained from Invitrogen. A double atf1Δ atf2Δ deletion strain, designated BY4742 (atf1Δ atf2Δ), was constructed by crossing of two single-deletion strains and subsequent sporulation. The double deletion was confirmed by PCR and sequence analysis, and the haploidy was confirmed by PCR of the MAT loci.
Fermentation experiments.
Yeast precultures were shaken overnight at 28°C in test tubes containing 5 ml of YPGluc medium. After 16 h of growth, 1 ml of the preculture was used to inoculate 100 ml of YPGluc medium in 350-ml Erlenmeyer flasks, and this second preculture was shaken at 28°C until mid-logarithmic growth phase (optical density at 600 nm = 1) was reached. Cells were washed with sterile, distilled water, counted, and used to inoculate 100 ml of fresh, prewarmed (28°C) YPGluc medium containing 8% glucose to a cell concentration of 107 cells ml−1. Cultures were shaken (150 rpm) at 28°C in order to maximize ester production. For the deletion strains, water locks were placed on each flask in order to create semianaerobic conditions, allowing maximal expression of the ATF genes in the wild-type cells. Samples for chromatographic analysis were taken after 36 h of fermentation and immediately cooled on ice in an airtight container. After sampling for headspace analysis, the cell concentrations of the cultures were determined by using a counting chamber. The sugar consumption of the cultures was analyzed by measuring the specific gravity of the fermentation medium using a DSA-48 density and sound analyzer with an SP-1 autosampler (A. Paar, Graz, Austria).
Northern blot analysis.
For Northern blot analysis, 20-ml cell culture samples were taken after 12, 24, and 36 h of fermentation. Samples were added to 40 ml of ice-cold water and rapidly further cooled on ice. The cells were pelleted and washed once with ice-cold water and then stored at −70°C. Isolation and quantification of total RNA was performed as described previously (64, 71). Probes were labeled with [α-32P]dCTP (Amersham-Pharmacia Biotech) by using a High Prime kit from Boehringer Mannheim. Northern blots were made by separation of total RNA (18 μg of total RNA/lane) in gels containing 1% agarose in 50 mM boric acid, 1 mM sodium citrate, 5 mM NaOH (pH 7.5), and 1% formaldehyde. RNA was transferred by capillary blotting to a Hybond-N membrane (Amersham) by using 10× SSC buffer (1.5 M NaCl-0.15 M sodium citrate, pH 7.0). These blots were hybridized with labeled probes of the coding region of ATF1, ATF2, Lg-ATF1, and ACT1. The blots were analyzed with a Fuji BAS-1000 phosphorimager and PCBAS 2.0 software. As the sampling reduced the volume of the fermentation medium by 60%, which could affect the production and/or evaporation of volatiles, it was decided not to use the samples of these fermentations for the systematic measurement of volatile production.
HS-GC.
Headspace gas chromatography (HS-GC) was used for the measurement of n-propanol, isobutanol, isoamyl alcohol, ethyl acetate, isoamyl acetate, ethyl hexanoate, ethyl octanoate, acetaldehyde, and dimethyl sulfide (DMS). Samples of 5 ml were collected in 15-ml precooled glass tubes, which were immediately closed and cooled on ice. Samples were then analyzed with a calibrated Autosystem XL gas chromatograph with a headspace sampler (HS40; Perkin-Elmer, Wellesley, Mass.) and equipped with a CP-Wax 52 CB column (length, 50 m; internal diameter, 0.32 mm; layer thickness, 1.2 μm; Chrompack; Varian, Palo Alto, Calif.). Samples were heated for 16 min at 60°C in the headspace autosampler. The injection block and flame ionization detector temperatures were kept constant at 180 and 250°C, respectively; helium was used as the carrier gas. The oven temperature was 75°C held for 6 min and then increased to 110°C at 25°C min−1 and held at 100°C for 3.5 min. Results were analyzed with Perkin-Elmer Turbochrom Navigator software.
Dynamic HS-GC-MS analysis.
Samples (25 ml) were collected in airtight tubes and centrifuged (5 min; 5,000 × g; 2°C). The supernatant was poured into precooled 25-ml airtight tubes, and 100 μl of a 10% antifoam reagent (Sigma Aldrich-Fluka; St. Louis, Mo.) was added to the sample. In addition, 100 μl of a 250-mg liter−1 solution of 2-ethyl hexanal (Sigma Aldrich) in distilled water was added as an internal standard. Five milliliters of this sample was transferred into a Tekmar Dohrman 3000 (Emerson, Mason, Ohio) purge-and-trap sampler unit with the following characteristics: helium carrier gas; 10-min purge at 120°C; 15-min dry purge; cold trap temperature, −100°C; 6-min desorption at 250°C. A Fisons GC 8000 + MFA 815 cold-trap/control unit (ThermoFinnigan, San Jose, Calif.) contained a Chrompack CP-WAX-52-CB column (length, 50 m; internal diameter, 0.32 mm; film thickness, 1.2 μm; Varian). The oven program was as follows: 1 min at 50°C, 4°C min−1 to 120°C, 2.5°C min−1 to 165°C, 15°C min−1 to 240°C, and 5 min at 240°C. Total ion mass chromatograms were obtained in a Fisons MD 800 apparatus and analyzed with the Masslab software program in order to identify unknown compounds.
RESULTS
Construction of ATF deletion and overexpression strains and experimental set-up.
The haploid BY4742 strain was used to evaluate the effect of deletion of the ATF genes, as it is difficult to delete all copies of these genes in aneuploid industrial strains that are genetically not well characterized. However, for the overexpression studies, the industrial brewer's lager yeast strain CMBS33 was used to better evaluate the possible implications for real-scale industrial fermentation processes. It was decided to use two different genetic backgrounds for the deletion and the overexpression studies, as the studies serve different purposes. The deletion studies reveal the relative contribution of each of the AATases to the synthesis of the different acetate esters. The overexpression studies can confirm the results obtained with the deletion strains, but more importantly, they show to what extent the synthesis of the different esters in brewer's yeast depends on the transcription rates of the respective ATF genes.
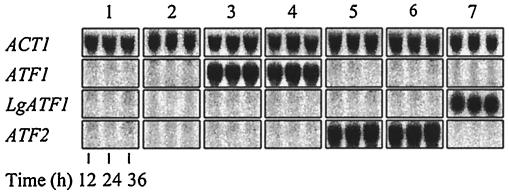
In order to obtain constitutive overexpression of ATF1, ATF2, and Lg-ATF1 throughout fermentation, these genes were cloned into the previously described ps vector (41). This integrating vector contains a PGK1 overexpression cassette and the dominant SMR1-410 selection marker, allowing selection on SMM-containing medium (13). Two different alleles of ATF1 and ATF2 (one from the industrial lager strain CMBS33 and one from the industrial ale strain CMBS212) were cloned into the ps vector. As Lg-ATF1 is not present in ale strains, in this case only the allele found in the CMBS33 lager strain was cloned into the ps vector. To obtain stable single-copy overexpression strains, the overexpression plasmids were linearized in LEU2 and subsequently integrated in the genomic LEU2 gene of the commercial lager strain CMBS33. Smr transformants were selected on minimal SD medium supplemented with SMM. Resistant colonies were further checked by PCR to ensure correct insertion of the vector into the genome, while overexpression of the respective genes was confirmed through Northern blotting (Fig. 1). In order to exclude possible cloning artifacts (e.g., due to PCR errors or secondary mutations), each overexpression construct was made twice, with independent, separate PCRs and transformations. To identify possible cloning artifacts, all inserts were sequenced. The constructs were then both used for yeast transformation, yielding two independent overexpression strains for each respective overexpressed gene. For the same reason, two analogous deletion strains were used in every trial (commercially obtained MATa and MATα strains). Each fermentation trial and the subsequent analysis procedures were repeated three times for each of the two analogous strains. The mean of the three results obtained with one strain was statistically compared to the results obtained for the second strain (t test, α = 0.05; results not shown). Since the means did not differ significantly for any of the pairs tested, it was decided to use the mean of the six fermentations for further interpretation. In other words, the results shown are always the results of six fermentations, of which three were conducted with one specific overexpression or deletion strain and the other three were conducted with an analogous strain obtained through a completely separate cloning and transformation procedure. In addition, all plasmid inserts were sequenced and compared to the genomic sequences to identify possible PCR cloning artifacts.
FIG. 1.
Northern analysis of the CMBS33 overexpression transformants during aerobic fermentation. Lane 1, wild-type CMBS33; lane 2, CMBS33 ps (empty vector control strain); lane 3, CMBS33 pATF1(CMBS33)s; lane 4, CMBS33 pATF1(CMBS212)s; lane 5, CMBS33 pATF2(CMBS33)s; lane 6, CMBS33 pATF2(CMBS212)s; lane 7, CMBS33 pLg-ATF1(CMBS33)s.
Effect of deletion and overexpression of ATF1 and ATF2 on the production of volatile flavor compounds measured by HS-GC.
BY4742 (atf1Δ), BY4742 (atf2Δ), BY4742 (atf1Δ atf2Δ), and the five ATF overexpression strains derived from the industrial lager yeast CMBS33 (see above) were used for a series of fermentations. During these fermentations, each strain's production of volatile compounds was analyzed by HS-GC and compared to that of the respective wild-type and empty vector control strains. Fermentations were performed as described in Materials and Methods. The results of the HS-GC analysis are given in Tables 2 and 3. As mentioned before, each value is the mean for six independent experiments. In some experiments, additional samples were taken after 24 h of fermentation. The results obtained after 24 h show very similar trends, but the measured concentrations were lower (results not shown). In addition to the concentration of volatile flavor compounds, the growth rates and sugar consumption were also measured. It was found that all strains showed completely similar growth patterns and sugar consumption rates. After 36 h of growth, virtually all sugar had been fermented (results not shown).
TABLE 2.
HS-GC measurement of volatile compounds produced by ATF deletion strains after 36 h of fermentation
| Compound | Concn (mg liter−1)a
|
|||
|---|---|---|---|---|
| BY4742 (atf1Δ) | BY4742 (atf2Δ) | BY4742 (atf1Δ atf2Δ) | BY4742 (wild type) | |
| Ethyl acetate | 13.0 (0.62) | 18.2 (0.87) | 10.8 (0.52) | 20.8 |
| Isoamyl acetate | 0.22 (0.16) | 1.13 (0.82) | 0.05 (0.04) | 1.37 |
| Ethyl hexanoate | 1.17 (1.05) | 1.13 (1.01) | 1.12 (1.00) | 1.12 |
| Ethyl octanoate | 0.38 (1.01) | 0.34 (0.92) | 0.37 (0.98) | 0.38 |
| Isoamyl alcohol | 112 (1.06) | 104 (0.98) | 109 (1.03) | 106 |
| Isobutanol | 22.9 (0.92) | 23.4 (0.94) | 22.7 (0.91) | 25.0 |
| Propanol | 27.1 (0.98) | 27.4 (0.99) | 25.5 (0.92) | 27.7 |
| Acetaldehyde | 30.9 (0.91) | 31.9 (0.94) | 35.5 (1.05) | 34.0 |
| DMS | 0.27 (1.14) | 0.24 (0.99) | 0.27 (1.12) | 0.24 |
Results are averages from six independent fermentations. Values in parentheses show the value relative to that for the wild type. Values that are significantly (α = 0.05) different from those for the wild-type strain are in bold. Standard deviations were typically about 10% of the values and did not exceed 20%.
TABLE 3.
HS-GC measurement of volatile compounds produced by ATF overexpression strains after 36 h of fermentation
| Compound | Concn (mg liter−1)a
|
||||||
|---|---|---|---|---|---|---|---|
| CMBS33 pATF1(CMBS33)s | CMBS33 pATF1(CMBS212)s | CMBS33 pATF2(CMBS33)s | CMBS33 pATF2(CMBS212)s | CMBS33 pLg- ATF1(CMBS33)s | CMBS33 ps | CMBS33 (wild type) | |
| Ethyl acetate | 273 (30.9) | 219 (24.8) | 28.4 (3.20) | 19.7 (2.23) | 16.7 (1.89) | 8.59 (0.97) | 8.86 |
| Isoamyl acetate | 49.2 (185) | 48.7 (183) | 6.94 (26.1) | 3.20 (12.0) | 1.22 (4.60) | 0.22 (0.80) | 0.27 |
| Ethyl hexanoate | 0.06 (1.10) | 0.05 (1.01) | 0.05 (0.90) | 0.07 (1.32) | 0.06 (1.07) | 0.07 (1.27) | 0.05 |
| Ethyl octanoate | 0.02 (0.46) | 0.07 (1.28) | 0.04 (0.79) | 0.07 (1.25) | 0.05 (0.88) | 0.03 (0.60) | 0.05 |
| Isoamyl alcohol | 138 (0.54) | 174 (0.69) | 224 (0.89) | 232 (0.92) | 204 (0.81) | 222 (0.88) | 253 |
| Isobutanol | 90.6 (1.11) | 78.1 (0.96) | 95.8 (1.18) | 74.8 (0.92) | 94.8 (1.17) | 85.7 (1.05) | 81.3 |
| Propanol | 45.7 (0.95) | 47.5 (0.98) | 47.2 (0.98) | 48.4 (1.00) | 48.4 (1.00) | 48.7 (1.01) | 48.3 |
| Acetaldehyde | 5.16 (0.85) | 6.34 (1.04) | 6.36 (1.05) | 6.43 (1.06) | 6.70 (1.10) | 5.95 (0.98) | 6.08 |
| DMS | 0.04 (1.06) | 0.04 (1.10) | 0.05 (1.34) | 0.05 (1.35) | 0.06 (1.38) | 0.07 (1.84) | 0.04 |
Results are the averages for six independent fermentations. Values in parentheses show the value relative to that for the wild type. Values that are significantly (α = 0.05) different from those for the wild-type strain are in bold. The standard deviations for large values (>1.5) were typically around 10% of the value and did not exceed 20% for any value. For small values (<1.5), the standard deviations were typically around 0.1, and did not exceed 0.4.
Table 2 shows that the levels of isoamyl acetate produced during fermentation with BY4742 (atf1Δ) were reduced to about 16% of those produced by the wild-type strain. Deletion of ATF2 resulted in an 18% decrease in isoamyl acetate formation. The double deletion strain BY4742 (atf1Δ atf2Δ) produced almost no isoamyl acetate (4% of the wild-type value), indicating that Atf1p and Atf2p are responsible for most of the cellular isoamyl acetyltransferase activity. Deletion of ATF1 caused a 40% reduction of ethyl acetate formation compared to that in the wild-type cells. Compared to the atf1Δ mutation, deletion of ATF2 resulted in a much smaller decrease in ethyl acetate formation (13%), demonstrating that Atf2p plays only a minor role in ethyl acetate formation. Interestingly, the atf1Δ atf2Δ strain still produced about 50% of the ethyl acetate concentration obtained with wild-type cells. This indicates that Atf1p and Atf2p are not the only enzymes involved in the biosynthesis of this important ester.
The results given in Tables 2 and 3 indicate that other major flavor compounds, such as acetaldehyde, the higher alcohols n-propanol, isoamyl alcohol, and isobutanol, the ethyl esters ethyl hexanoate and ethyl octanoate, and the sulfur compound DMS, were not significantly affected by the deletion or overexpression of ATF1 or ATF2. This indicates that the ATF genes are not involved in the synthesis of these flavor substances. Moreover, it also implies that, apart from the differences in acetate ester formation, the different strains show very similar flavor profiles. As the formation of flavors is closely related to cell growth and metabolism, this observation also confirms the similar growth patterns and sugar utilization profiles of the different strains.
The results obtained with the different overexpression strains (Table 3) confirm those obtained with the deletion strains (Table 2). A first important conclusion that can be drawn from the results shown in Table 3 is that the ester production of the cells transformed with the empty vector was virtually the same as that of the untransformed wild-type cells. This indicates that all effects in the overexpression strains were indeed the result of the overexpression of ATF genes, and were not due to a nonspecific vector effect. Overexpression of ATF1 caused a 180-fold increase in isoamyl acetate production and a 30-fold increase in ethyl acetate production compared to wild-type cells. Depending on the allele, the strains overexpressing ATF2 produced 10 to 25 times more isoamyl acetate and two to three times more ethyl acetate than the wild-type strains. Lg-ATF1 overexpression resulted in a 4.5-fold increase in isoamyl acetate production, while ethyl acetate levels were nearly doubled. Furthermore, while there was no significant effect of the deletion of ATF genes on higher alcohol production, the ATF1 overexpression strains showed a clear and statistically significant reduction in isoamyl alcohol production. Isoamyl alcohol concentrations were reduced to 70% of the concentrations obtained with the control strains. Interestingly, the absolute decrease in isoamyl alcohol production of the ATF1 overexpression strains was more than 110 mg liter−1, while the increase in isoamyl acetate concentration was only 50 mg liter−1, indicating that a significant portion of the latter may have evaporated during fermentation. The ATF2 and Lg-ATF1 overexpression strains also showed a slight reduction in isoamyl alcohol production, but the variance between the different samples was too large to statistically confirm these differences.
As shown in Table 3, there were no significant differences in ester production between the strains that overexpressed different alleles of ATF1. Sequence analysis revealed that the ATF1 alleles of CMBS33 (GenBank accession number AY242062) and CMBS212 (GenBank accession number AY242063) differed in 9 out of 1,578 bp, resulting in six different amino acids. Remarkably, the strains overexpressing the ATF2 allele derived from CMBS33 produced significantly greater amounts of both ethyl acetate and isoamyl acetate than the strains overexpressing the CMBS212 ATF2 allele. In the case of the ATF2 alleles, 5 out of 1,608 nucleotides were different between the ATF2 alleles of CMBS33 (GenBank accession number AY242064) and CMBS212 (GenBank accession number AY242065). This results in two different amino acids: one at position 11, where in Atf2p of CMBS33 leucine takes the place of isoleucine found in the Atf2p of CMBS212 and S. cerevisiae S288C, and a second at position 298, where the CMBS33 Atf2p has a serine instead of a leucine, as in CMBS212 and S288C. None of the sequenced ATF1 and ATF2 alleles or the sequenced Lg-ATF1 allele (GenBank accession number AY242066) showed a mutation in the conserved WRLICLP region, and it is unclear why the minor differences in the sequences of the ATF2 alleles result in significant changes in activity.
Effect of overexpression of ATF1, Lg-ATF1, and ATF2 on the production of volatile compounds measured with GC-MS.
In order to identify other volatile compounds formed by AATase I and AATase II, fermentation products (samples taken at 36 h) obtained with the different ATF deletion and overexpression (only the CMBS33 alleles) strains were analyzed by GC-MS. In order to increase sensitivity, purge-and-trap sampling was used. This enabled the analysis of components which were produced in very low concentrations or which are only moderately volatile. The results of the GC-MS analysis are given in Table 4. This analysis revealed only relative changes in the production of certain volatiles between the different strains because the apparatus was not calibrated for all the different components, and absolute concentrations therefore could not be determined.
TABLE 4.
GC-MS measurement of volatile compounds produced by ATF deletion and overexpression strains after 36 h of fermentation
| Compound | Relative concna in:
|
||||||
|---|---|---|---|---|---|---|---|
| Deletion strains
|
Overexpression strains
|
||||||
| BY4742 (atf1Δ) | BY4742 (atf2Δ) | BY4742 (atf1Δ atf2Δ) | CMBS33 pATF1(CMBS33)s | CMBS33 pATF2(CMBS33)s | CMBS33 pLgATF1(CMBS33)s | CMBS33 ps | |
| Propyl acetate | 0.69 | 0.81 | 0.53 | 20.4 | 8.8 | 2.77 | 1.22 |
| Isobutyl acetate | 0.75 | 0.82 | 0.38 | 24.7 | 15.7 | 2.15 | 1.08 |
| Pentyl acetate | 0.39 | 0.65 | 0.19 | 51.5 | 8.05 | 1.42 | 1.13 |
| Hexyl acetate | 0.38 | 0.85 | 0.12 | 53.5 | 6.17 | 3.92 | 1.30 |
| Heptyl acetate | 0.31 | 0.74 | 0.15 | 81.2 | 6.15 | 1.53 | 1.32 |
| Octyl acetate | 0.10 | 0.69 | 0.05 | 38.0 | 8.45 | 1.02 | 0.88 |
| Phenylethyl acetate | 0.25 | 0.66 | 0.11 | 8.28 | 5.13 | 1.15 | 1.45 |
| Ethyl hexanoate | 1.15 | 1.02 | 1.24 | 0.86 | 0.78 | 1.07 | 1.17 |
| Ethyl heptanoate | 1.11 | 1.00 | 1.16 | 1.06 | 0.78 | 1.11 | 1.19 |
| Ethyl nonaoate | 1.05 | 0.98 | 1.02 | 0.91 | 0.92 | 0.84 | 1.01 |
| Ethyl decanoate | 1.04 | 1.01 | 1.17 | 1.01 | 0.85 | 0.79 | 1.09 |
| 1-Pentanol | 1.18 | 1.08 | 1.36 | 0.66 | 0.82 | 0.83 | 1.12 |
| 1-Hexanol | 2.18 | 1.38 | 2.21 | 0.69 | 0.85 | 0.97 | 1.10 |
| 1-Heptanol | 1.15 | 1.02 | 1.23 | 0.58 | 0.59 | 0.71 | 1.05 |
| Phenyl ethanol | 1.18 | 1.06 | 1.06 | 0.74 | 0.91 | 0.91 | 1.06 |
Concentration relative to that in the respective wild-type (BY4742 or CMBS33) strain. Results are the averages for six independent fermentations. Values that are significantly (α = 0.05) different from the wild-type strain are in bold. The standard deviations for large values (>1.5) were typically around 10% of the value, and did not exceed 25% for any of the values. For small values (<1.5), the standard deviations were typically around 0.15, and did never exceed 0.45.
The results indicate that deletion of ATF1 reduced the production of all acetate esters that were analyzed, while overexpression of ATF1 caused a clear 10- to 80-fold increase in the production of all measured acetate esters. The same was true for the ATF2 gene, although the differences between the wild-type and modified strains were smaller than for the ATF1 gene. The double-deletion strain, BY4742 (atf1Δ atf2Δ), confirmed the results for the respective single deletion strains. Moreover, deletion of both ATF genes resulted in almost complete abolition of acetate ester formation from alcohols of relatively long chain length (pentyl acetate, hexyl acetate, heptyl acetate, and octyl acetate). The concentration of these esters was strongly increased in the ATF overexpression strains: ATF1 overexpression caused a 40- to 80-fold increase, and ATF2 overexpression resulted in a 6- to 8-fold increase. In contrast, the production of acetate esters formed from shorter alcohol residues (e.g., ethyl acetate, propyl acetate and isobutyl acetate) was only partially abolished in the single- and double-deletion strains. This corresponds to the smaller increase in the production of these esters measured in the respective overexpression strains. The ATF1 overexpression strain produced about 20 times more of these short-alcohol esters than the wild-type cells, while production in the ATF2 overexpression strain was increased 10 to 15 times. This indicates that Atf1p is most active towards long-chain alcohols, while Atf2p activity does not seem to differ much for different alcohol substrates. Another possible explanation might be that due to the very high AATase activity in the ATF1 overexpression strain, some of the alcohol substrates were depleted, while the higher concentration of other alcohols allowed a higher production of the respective esters. However, this hypothesis seems unlikely, since the concentrations of propanol and isobutanol in the ATF1 overexpression strain were almost equal to those produced by fermentation with the wild-type strain (Table 3).
There were no major changes in higher alcohol production by the different ATF2 and Lg-ATF1 deletion and overexpression strains in comparison to the control strains, except for a 40% decrease in heptanol production by the ATF2 overexpression strain. Overexpression of ATF1 on the other hand caused a significant decrease in the production of all monitored higher alcohols (except for phenyl ethanol, where the decrease could not be confirmed statistically). Accordingly, deletion of ATF1 led to a slight increase in the production of these alcohols. While statistical analysis showed that, except for hexanol, the latter observation was not significant, the observed changes agree with the monitored differences in corresponding ester production. Tables 3 and 4 also indicate that ethyl esters, such as ethyl hexanoate, ethyl heptanoate, ethyl octanoate, ethyl nonaoate, and ethyl decanoate, were produced in virtually equal quantities in the different deletion and overexpression strains and the respective wild-type strains. Thus, the Atf enzymes are not involved in the biosynthesis of any of these medium-chain fatty acid ethyl esters.
DISCUSSION
The HS-GC and GC-MS analyses of the fermentation products obtained with the different ATF deletion and overexpression strains clearly indicate that the expression level of ATF genes is an important factor controlling volatile acetate ester production. Atf1p seems to be responsible for the majority of all acetate esters that were analyzed in this study. For most acetate esters, including the important flavor component isoamyl acetate, deletion of the ATF1 gene caused a reduction of 60 to 90% compared to the level in wild-type cells. The monitored effects of ATF1 deletion and overexpression on isoamyl acetate formation agree with results obtained in earlier research (24, 41). The data obtained with the Lg-ATF1 overexpression strain indicate that Lg-Atf1p has only a very limited role in the synthesis of volatile esters. This observation corresponds with the results obtained by Fujii et al. (22), although an Lg-ATF1 deletion strain would be needed in order to confirm this hypothesis. As the C terminus and middle part of Lg-Atf1p are highly (80%) homologous to those Atf1p, the low activity of Lg-Atf1p may be due to its N-terminal extension. This extension may cause a lower activity, altered substrate specificity, or a different subcellular localization.
Compared to ATF1, deletion of ATF2 resulted in only minor (10 to 35%) decreases in the formation of most esters. Similarly, overexpression of ATF2 caused smaller increases in ester formation than overexpression of ATF1. The reason for this smaller contribution of Atf2p to ester synthesis (roughly 10 to 50% of the contribution of Atf1p for both deletion and overexpression) is unclear. However, the smaller effect of ATF2 and Lg-ATF1 overexpression on ester production may be an advantage for the industrial use of these overexpression strains, since the dramatic effects of ATF1 overexpression could be undesirable in industrial applications.
Atf1p and Atf2p seem to be the only enzymes involved in the synthesis of acetate esters from long-chain alcohols (C5 or longer). Indeed, the atf1Δ atf2Δ strain showed virtually no residual synthesis of isoamyl acetate, pentyl acetate, hexyl acetate, heptyl acetate, octyl acetate, or phenyl ethyl acetate. Remarkably, the production of ethyl acetate, propyl acetate, and isobutyl acetate was reduced by only 50% in the atf1Δ atf2Δ strain. This demonstrates that there is at least one other, as-yet-unidentified enzyme with AATase activity, as was previously suggested by Malcorps et al. (42, 43). This enzymatic activity is of great economical interest, as the main problem associated with high-gravity brewing is the disproportionate production of ethyl acetate, which results in an undesirable solvent-like aroma. Elucidation of the mechanism underlying the production of residual ethyl acetate could be a first step toward obtaining better control over ester production during high-gravity fermentations. Moreover, since isoamyl acetate formation depends only on Atf1p and Atf2p, identification of the enzyme involved in Atf1p- and Atf2p-independent ethyl acetate formation could allow the specific management of ethyl acetate. Furthermore, the atf1Δ atf2Δ strain still produced virtually the same concentrations of medium-chain fatty acid ethyl esters as the wild-type cells. This indicates that Atf1p and Atf2p are not involved in the synthesis of these esters and that other enzymes, such as Eht1p, must be responsible for their formation.
In addition to ester synthases, esterases (EC 3.1.1.1.) also play a role in ester accumulation. Esterases represent a diverse group of hydrolases catalyzing the cleavage, but in some cases also the formation, of ester bonds. They are widely distributed in animals, plants, and microorganisms (11). As esterases are responsible for the breakdown of esters, it is clear that their activity may affect the concentration of certain volatile esters. In S. cerevisiae, for example, it has been shown that the balance between ester-synthesizing enzymes and esterases, such as Iah1p (also known as Est2p), is important for the net rate of ester accumulation (27-30). Residual esterase activity in finalized beer may even be responsible for the decrease in ester concentration that often occurs during storage. It has been suggested that ester hydrolysis occurs mainly as a consequence of yeast autolysis followed by the liberation of certain esterases in the medium (52).
Moreover, certain esterases may be capable of synthesizing ethyl acetate from ethanol and acetic acid in a simple buffered solution without any cofactor (59, 60, 61, 62, 70). This indicates that esterases may also play a role in the formation of certain esters (42, 62, 70). In Acetobacter pasteurianus, for example, deletion of the esterase-encoding gene est1 causes the abolishment of ethyl acetate and isoamyl acetate production (15, 36, 37, 50). Another example is the sake yeast Hansenula mrakii, for which an isoamyl acetate-synthesizing esterase has been described (35). However, firm evidence for the ester-synthesizing capacity of yeast esterases is lacking. Deletion of the esterase-encoding gene IAH1 results in an increase in the production of ethyl acetate and isoamyl acetate, showing that Iah1p mainly hydrolyzes these esters and is not responsible for a major part of their production (29, 30). However, the effect of Iah1p on other esters, such as the group of ethyl esters, has not yet been thoroughly studied. In addition, partial purification of esterase activities in S. cerevisiae has shown that at least two different fractions with esterase activity exists, indicating that the proteome may contain more, as-yet-unidentified esterases (28, 33, 59, 60, 70, 73, 82). Hence, a possible role for these additional esterases in ester synthesis in brewer's yeast cannot be ruled out.
Different yeast strains usually show distinct ester production patterns (51, 58, 67). Whereas the activities resulting from the two overexpressed alleles of Atf1p seemed not to differ very much, this was not the case for the Atf2p alleles. While the two alleles differed in only two amino acids, overexpression of the CMBS33 ATF2 allele resulted in 50 to 100% more ester production than overexpression of the CMBS212 allele. It therefore is possible that differences in acetate ester production between yeast strains are also caused by differences in the properties of the Atf proteins, in addition to differences in the expression levels of the ATF genes. Hence, it may be possible to predict the flavor production by determining the sequence of the ATF gene promoter and ORF. Interestingly, wild-type cells of CMBS33 produce slightly (about 20%; results not shown) more acetate esters than CMBS212 when a fermentation at 28°C in glucose medium is carried out. However, as CMBS33 is a lager strain, it is normally used at lower fermentation temperatures than the ale strain CMBS212, leading to a reduction in ester formation.
The most striking observation in this study is the very broad substrate specificity of the Atf proteins towards the alcohol cosubstrates: all acetate esters that could be monitored with HS-GC and GC-MS seem to be at least partially synthesized by Atf1p and Atf2p. This clearly shows that Atf1p and Atf2p are able to transfer an activated acetate group to a wide variety of substrates with an alcohol group. Hence, volatile aroma-active esters may only be by-products of physiologically more relevant reactions. Furthermore, the difference in the apparent activities of Atf1p and Atf2p with respect to different substrates may indicate that these enzymes have distinct physiological functions, as suggested by Cauet et al. (14), who proposed that Atf2p is involved in the detoxification of hydroxysteroids and possibly certain phytochemicals.
The physiological role of Atf1p remains uncertain, but a few hypotheses can be proposed. One possibility is that yeast cells may esterify free hydroxyl groups of certain membrane components in order to optimize membrane properties under stressful (e.g., anaerobic) conditions. This hypothesis agrees with the coregulation of ATF1 and the Δ9 desaturase-encoding gene, OLE1, by oxygen and unsaturated fatty acids (21, 25, 26). In addition, recent studies have revealed that Atf1p is located in the cellular lipid particles (K. J. Verstrepen, S. D. M. Van Laere, J.-P. Dufour, I. S. Pretorius, J. Winderickx, J. M. Thevelein, and F. R. Delvaux, submitted for publication), which is again in agreement with its possible role in fatty acid metabolism. Alternatively, the low alcohol substrate specificity and the high specificity towards acetyl-CoA might indicate that the main role of ester synthesis is the recycling of free CoA. In other words, yeast cells might produce esters in order to regenerate free CoA under conditions in which normal regeneration of acetyl-CoA through the tricarboxylic acid cycle or lipid synthesis is hampered. Another hypothesis is that esterification might be a detoxification mechanism for the medium-chain fatty acids that can be released during fatty acid synthesis under anaerobic conditions. Upon release from the fatty acid synthesis complex, these toxic medium-chain fatty acids rapidly dissociate and are thus unable to cross cellular membranes (1, 18, 34, 72, 74, 80). Esterification allows (partial) diffusion of the fatty acid residues and could thus serve as a strategy to remove these toxic intermediates of fatty acid synthesis. However, as the present results indicate that Atf1p and Atf2p do not play a role in the formation of medium-chain fatty acid ethyl esters, such as ethyl hexanoate and ethyl octanoate, it seems unlikely that Atf enzymes would be involved in this kind of detoxification process. Although this study did not fully elucidate the intriguing cellular role of ester formation, the findings presented in this paper do provide a sound basis for further research, which will eventually lead to the improvement of beer flavor.
Acknowledgments
We thank Birgit Janssens for excellent scientific help with gas chromatographic analysis.
K. J. Verstrepen is a Research Assistant of the Fund for Scientific Research—Flanders (Belgium) (FWO-Vlaanderen) and greatly acknowledges the FWO-Vlaanderen for the financial support of his research. This work was supported by grants from the FWO-Vlaanderen (FWO project G.0082.03) and the research fund of Katholieke Universiteit Leuven (O.T. project 03/40 and Flanders-South Africa project BIL02/34 to J. M. Thevelein and F. R. Delvaux).
REFERENCES
- 1.Alvarez, P., P. Malcorps, A. Sa Almeida, A. Ferreira, A. M. Meyer, and J. P. Dufour. 1994. Analysis of free fatty acids, fusel alcohols and esters in beer: an alternative to CS2 extraction. J. Am. Soc. Brew. Chem. 52:127-134. [Google Scholar]
- 2.Anderson, R. G., and B. H. Kirsop. 1974. The control of volatile ester synthesis during the fermentation of wort of high specific gravity. J. Inst. Brew. 80:48-55. [Google Scholar]
- 3.Anderson, R. G., and B. H. Kirsop. 1975. Oxygen as a regulator of ester accumulation during the fermentation of worts of high specific gravity. J. Inst. Brew. 81:111-115. [Google Scholar]
- 4.Anderson, R. G., and B. H. Kirsop. 1975. Quantitative aspects of the control by oxygenation of acetate ester formation of worts of high specific gravity. J. Inst. Brew. 81:296-301. [Google Scholar]
- 5.Athenstaedt, K., D. Zweytick, A. Jandrositz, S. D. Kohlwein, and G. Daum. 1999. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J. Bacteriol. 181:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 7.Avhenainen, J., and V. Mäkinen. 1989. The effect of pitching yeast aeration on fermentation and beer flavor. Proc. Congr. Eur. Brew. Conv. 22:517-519. [Google Scholar]
- 8.Äyräpää, T., and I. Lindström. 1977. Aspects of the influence of exogenous fatty acids on the fatty acid metabolism of yeast. Proc. Congr. Eur. Brew. Conv. 16:507-517. [Google Scholar]
- 9.Äyräpää, T., and I. Lindström. 1973. Influence of long-chain fatty acids on the formation of esters by brewer's yeast. Proc. Congr. Eur. Brew. Conv. 14:271-283. [Google Scholar]
- 10.Barnett, J. A., R. W. Payne, and D. Yarrow. 2000. Yeasts, characteristics and identification, 2nd ed. Cambridge University Press, Cambridge, United Kingdom.
- 11.Bornscheuer, U. T. 2002. Microbial carboxyl esterases: classification, properties and applications in biocatalysis. FEMS Microbiol. Rev. 26:73-81. [DOI] [PubMed] [Google Scholar]
- 12.Calderbank, J., and J. R. M. Hammond. 1994. Influence of higher alcohol availability on ester formation by yeast. J. Am. Soc. Brew. Chem. 52:84-90. [Google Scholar]
- 13.Casey, G. P., W. Xiao, and G. H. Rank. 1988. A convenient dominant selection marker for gene transfer in industrial strains of Saccharomyces cerevisiae: SMR1 encoded resistance to the herbicide sulfometuron methyl. J. Inst. Brew. 94:93-97. [Google Scholar]
- 14.Cauet, G., E. Degryse, C. Ledoux, R. Spagnoli, and T. Achstetter. 1999. Pregnenolone esterification in Saccharomyces cerevisiae. A potential detoxification mechanism. Eur. J. Biochem. 261:317-324. [DOI] [PubMed] [Google Scholar]
- 15.Cristiani, G., and V. Monnet. 2001. Food micro-organisms and aromatic ester synthesis. Sci. Aliments 21:211-230. [Google Scholar]
- 16.D'Amore, T., G. Celotto, and G. G. Stewart. 1991. Advances in the fermentation of high gravity wort. Proc. Congr. Eur. Brew. Conv. 23:337-344. [Google Scholar]
- 17.Dufour, J.-P., B. Llorente, B. Dujon, S. Kumara, K. J. Verstrepen, and G. Derdelinckx. 2002. A three member gene family is responsible for the synthesis of short chain fatty acid esters during fermentation. Brew. Dig. 77:36-37. [Google Scholar]
- 18.Dufour, J. P., P. Malcorps, and P. Silcock. 2003. Control of ester synthesis during brewery fermentation, p. 213-233. In K. Smart (ed.), Brewing yeast fermentation performance, 2nd ed., vol. 2. Blackwell Science, Oxford, United Kingdom.
- 19.Dufour, J.-P., K. J. Verstrepen, and G. Derdelinckx. 2002. Brewing yeasts, p. 347-388. In T. Boekhout and V. Robert (ed.), Yeasts in food. Behr's Verlag Gmbh & Co., Hamburg, Germany.
- 20.Engan, S., and O. Aubert. 1977. Relations between fermentation temperature and the formation of some flavour components. Proc. Congr. Eur. Brew. Conv. 16:591-607. [Google Scholar]
- 21.Fujii, T., O. Kobayashi, H. Yoshimoto, S. Furukawa, and Y. Tamai. 1997. Effect of aeration and unsaturated fatty acids on expression of the Saccharomyces cerevisiae alcohol acetyltransferase gene. Appl. Environ. Microbiol. 63:910-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujii, T., N. Nagasawa, A. Iwamatsu, T. Bogaki, Y. Tamai, and M. Hamachi. 1994. Molecular cloning, sequence analysis and expression of the yeast alcohol acetyltransferase gene. Appl. Environ. Microbiol. 60:2786-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii, T., H. Yoshimoto, N. Nagasawa, T. Bogaki, Y. Tamai, and M. Hamachi. 1996. Nucleotide sequence of alcohol acetyltransferase genes from lager brewing yeast, Saccharomyces carlsbergensis. Yeast 12:593-598. [DOI] [PubMed] [Google Scholar]
- 24.Fujii, T., H. Yoshimoto, and T. Tamai. 1996. Acetate ester production by Saccharomyces cerevisiae lacking the ATF1 gene encoding the alcohol acetyltransferase. J. Ferment. Bioeng. 81:538-542. [Google Scholar]
- 25.Fujiwara, D., O. Kobayashi, H. Yoshimoto, S. Harashima, and Y. Tamai. 1999. Molecular mechanisms of the multiple regulation of the Saccharomyces cerevisiae ATF1 gene encoding alcohol acetyltransferase. Yeast 15:1183-1197. [DOI] [PubMed] [Google Scholar]
- 26.Fujiwara, D., H. Yoshimoto, H. Sone, S. Harashima, and Y. Tamai. 1998. Transcriptional co-regulation of Saccharomyces cerevisiae alcohol acetyltransferase gene ATF1 and D-9 fatty acid desaturase gene OLE1 by unsaturated fatty acids. Yeast 14:711-721. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda, K., Y. Kiyokawa, T. Yanagiuchi, Y. Wakai, K. Kitamoto, Y. Inoue, and A. Kimura. 2000. Purification and characterization of isoamyl acetate-hydrolyzing esterase encoded by the IAH1 gene of Saccharomyces cerevisiae from a recombinant Escherichia coli. Appl. Environ. Microbiol. 53:596-600. [DOI] [PubMed] [Google Scholar]
- 28.Fukuda, K., O. Kuwahata, Y. Kiyokawa, T. Yanagiuchi, Y. Wakai, K. Kitamoto, Y. Inoue, and A. Kimura. 1996. Molecular cloning and nucleotide sequence of the isoamyl acetate-hydrolyzing esterase gene (EST2) from Saccharomyces cerevisiae. J. Ferment. Bioeng. 82:8-15. [Google Scholar]
- 29.Fukuda, K., N. Yamamoto, Y. Kiyokawa, T. Yanagiuchi, Y. Wakai, K. Kitamoto, Y. Inoue, and A. Kimura. 1998. Brewing properties of sake yeast whose EST2 gene encoding isoamyl acetate-hydrolyzing esterase was disrupted. J. Ferment. Bioeng. 85:101-106. [Google Scholar]
- 30.Fukuda, K., N. Yamamoto, Y. Kiyokawa, T. Yanagiuchi, J. Wakai, K. Kitamoto, Y. Inoue, and A. Kimura. 1998. Balance of activities of alcohol acetyltransferase and esterase in Saccharomyces cerevisiae is important for production of isoamyl acetate. Appl. Environ. Microbiol. 64:4076-4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gietz, R. D., and R. H. Schiestl. 1995. Transforming yeast with DNA. Methods Mol. Cell. Biol. 5:255-269. [Google Scholar]
- 32.Hammond, J. R. M. 1993. Brewer's yeast, p. 7-67. In H. A. Rose and J. S. Harrison (ed.), The yeasts, vol. 5. Academic Press, London, United Kingdom.
- 33.Horsted, M. W., E. S. Dey, S. Holmberg, and M. C. Kielland-Brandt. 1998. A novel esterase from Saccharomyces carlsbergensis, a possible function for the yeast TIP1 gene. Yeast 14:793-803. [DOI] [PubMed] [Google Scholar]
- 34.Hunkova, Z., and Z. Fencl. 1977. Toxic effects of fatty acids on yeast cells: dependence of inhibitory effects on fatty acid concentration. Biotechnol. Bioeng. 19:1623-1641. [DOI] [PubMed] [Google Scholar]
- 35.Inoue, Y., S. Trevanichi, K. Fukuda, S. Izawa, Y. Wakai, and A. Kimura. 1997. Roles of esterase and alcohol acetyl transferase on production of isoamyl acetate in Hansenula mrakii. J. Agric. Food. Chem. 45:644-649. [Google Scholar]
- 36.Kashima, Y., M. Iijima, T. Nakano, K. Tayama, W. Koizumi, S. Udaka, and F. Yanagida. 2000. Role of intracellular esterases in the production of esters by Acetobacter pasteurianus. J. Biosci. Bioeng. 89:81-83. [DOI] [PubMed] [Google Scholar]
- 37.Kashima, Y., M. Iijima, A. Okamoto, Y. Koizumi, S. Udaka, and F. Yanagida. 1998. Purification and characterization of intracellular esterases related to ethylacetate formation in Acetobacter pasteurianus. J. Ferment. Bioeng. 85:584-588. [Google Scholar]
- 38.Kurtzman, C. P., and J. W. Fell. 1998. The yeasts—a taxonomic study, 4th ed. Elsevier, Amsterdam, The Netherlands.
- 39.Lambrechts, M. G., and I. S. Pretorius. 2000. Yeast and its importance to wine aroma: a review. S. Afr. J. Enol. Vitic. 21:97-129. [Google Scholar]
- 40.Landaud, S., E. Latrille, and G. Corrieu. 2001. Top pressure and fermentation control the fusel alcohol/ester ratio through yeast growth in beer fermentation. J. Inst. Brew. 107:107-117. [Google Scholar]
- 41.Lilly, M., M. G. Lambrechts, and I. S. Pretorius. 2000. Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Appl. Environ. Microbiol. 66:744-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malcorps, P., J. M. Cheval, S. Jamil, and J.-P. Dufour. 1991. A new model for the regulation of ester synthesis by alcohol acetyltransferase in Saccharomyces cerevisiae. J. Am. Soc. Brew. Chem. 49:47-53. [Google Scholar]
- 43.Malcorps, P., and J.-P. Dufour. 1992. Short-chain and medium chain aliphatic ester synthesis in Saccharomyces cerevisiae. Eur. J. Biochem. 210:1015-1022. [DOI] [PubMed] [Google Scholar]
- 44.Mason, B., and J.-P. Dufour. 2000. Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast 16:1287-1298. [DOI] [PubMed] [Google Scholar]
- 45.Maule, D. R. J. 1967. Rapid gas chromatographic examination of beer flavour. J. Inst. Brew. 73:351-361. [Google Scholar]
- 46.Meilgaard, M. C. 2001. Effects on flavour of innovations in brewery equipment and processing: a review. J. Inst. Brew. 107:271-286. [Google Scholar]
- 47.Meilgaard, M. C. 1975. Flavor chemistry of beer. Flavor and threshold of 239 aroma volatiles. MBAA Tech. Q. 12:151-168. [Google Scholar]
- 48.Meilgaard, M. C. 1975. Flavor chemistry of beer. Flavor interaction between principal volatiles. MBAA Tech. Q. 12:107-117. [Google Scholar]
- 49.Nagasawa, N., T. Bogaki, A. Iwamatsu, M. Hamachi, and C. Kumagai. 1998. Cloning and nucleotide sequence of the alcohol acetyltransferase II gene (ATF2) from Saccharomyces cerevisiae Kyokai No. 7. Biosci. Biotechnol. Biochem. 62:1852-1857. [DOI] [PubMed] [Google Scholar]
- 50.Nardi, M., C. Fiez-Vandal, P. Tailliez, and V. Monnet. 2002. The EstA esterase is responsible for the main capacity of Lactococcus lactis to synthesize short chain fatty acid esters in vitro. J. Appl. Microbiol. 93:994-1002. [DOI] [PubMed] [Google Scholar]
- 51.Narziss, L., H. Miedaner, and A. Gresser. 1983. Yeast strain and beer quality-the formation of esters during fermentation. Brauwelt 123:2024-2034. [Google Scholar]
- 52.Neven, H., F. R. Delvaux, and G. Derdelinckx. 1997. Flavor evolution of top fermented beers. MBAA Tech. Q. 34:115-118. [Google Scholar]
- 53.Nordström, K. 1964. Formation of esters from alcohols by brewer's yeast. J. Inst. Brew. 70:328-336. [Google Scholar]
- 54.Nordström, K. 1962. Formation of ethyl acetate in fermentation with brewer's yeast III: participation of coenzyme A. J. Inst. Brew. 68:398-407. [Google Scholar]
- 55.Nordström, K. 1963. Formation of ethyl acetate in fermentation with brewer's yeast IV: metabolism of acetyl coenzyme A. J. Inst. Brew. 69:142-153. [Google Scholar]
- 56.Nykänen, I., and H. Suomalainen. 1983. Aroma of beer, wine and distilled alcoholic beverages. Reidel Publishing Company, Dordrecht, The Netherlands.
- 57.Nykänen, L. 1986. Formation and occurrence of flavor compounds in wine and distilled alcoholic beverages. Am. J. Enol. Vitic. 37:84-96. [Google Scholar]
- 58.Nykänen, L., and I. Nykänen. 1977. Production of esters by different yeast strains in sugar fermentations. J. Inst. Brew. 83:30-31. [Google Scholar]
- 59.Parkkinen, E. 1980. Multiple forms of carboxylesterases in bakers yeast. Cell. Mol. Biol. 26:147-154. [PubMed] [Google Scholar]
- 60.Parkkinen, E., E. Oura, and H. Suomalainen. 1978. Esterases of bakers-yeast. 1. Activity and localization in yeast cell. J. Inst. Brew. 84:5-8. [Google Scholar]
- 61.Parkkinen, E., and H. Suomalainen. 1982. Esterases of bakers-yeast. 2. Substrate specificities towards esters formed during sugar fermentations. J. Inst. Brew. 88:34-38. [Google Scholar]
- 62.Parkkinen, E., and H. Suomalainen. 1982. Esterases of baker's yeast. 3. The ester/acid ratio in model solutions. J. Inst. Brew. 88:98-101. [Google Scholar]
- 63.Peddie, H. A. B. 1990. Ester formation in brewery fermentations. J. Inst. Brew. 96:327-331. [Google Scholar]
- 64.Pernambuco, M. B., J. Winderickx, M. Crauwels, G. Griffioen, W. H. Mager, and J. M. Thevelein. 1996. Differential requirement for sugar phosphorylation in cells of the yeast Saccharomyces cerevisiae grown on glucose or grown on non-fermentable carbon sources for glucose-triggered signalling phenomena. Microbiology 142:1775-1882. [DOI] [PubMed] [Google Scholar]
- 65.Pfisterer, E., and G. G. Stewart. 1975. Some aspects on the fermentation of high gravity worts. Proc. Congr. Eur. Brew. Conv. 15:255-267. [Google Scholar]
- 66.Pisarnitskii, A. F. 2001. Formation of wine aroma: Tones and imperfections caused by minor components (review). Appl. Biochem. Microbiol. 37:552-560. [PubMed] [Google Scholar]
- 67.Ramos-Jeunehomme, C., R. Laub, and C. A. Masschelein. 1991. Why is ester formation in brewery fermentations yeast strain dependent? Proc. Congr. Eur. Brew. Conv. 23:257-264. [Google Scholar]
- 68.Renger, R. S., S. H. Van Hateren, and K. C. A. M. Luyben. 1992. The formation of esters and higher alcohols during brewery fermentation—the effect of carbon dioxide pressure. J. Inst. Brew. 98:509-513. [Google Scholar]
- 69.Sablayrolles, J. M., and C. B. Ball. 1995. Fermentation kinetics and the production of volatiles during alcoholic fermentation. J. Am. Soc. Brew. Chem. 53:71-78. [Google Scholar]
- 70.Schermers, F. H., J. H. Duffus, and A. M. MacLeod. 1976. Studies on yeast esterase. J. Inst. Brew. 82:170-174. [Google Scholar]
- 71.Sherman, F., G. R. Fink, and J. Hicks. 1991. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 72.Sumper, M. 1974. Control of fatty acid biosynthesis by long-chain acylCoAs and by lipid membranes. Eur. J. Biochem. 49:469-475. [DOI] [PubMed] [Google Scholar]
- 73.Suomalainen, H. 1981. Yeast esterases and aroma esters in alcoholic beverages. J. Inst. Brew. 87:296-300. [Google Scholar]
- 74.Taylor, G. T., and B. H. Kirsop. 1977. The origin of medium chain length fatty acids present in beer. J. Inst. Brew. 83:241-243. [Google Scholar]
- 75.Thurston, P. A., D. E. Quain, and R. S. Tubb. 1982. Lipid metabolism and the regulation of volatile synthesis in Saccharomyces cerevisiae. J. Inst. Brew. 88:90-94. [Google Scholar]
- 76.Thurston, P. A., R. Taylor, and J. Avhenainen. 1981. Effects of linoleic acid supplements on the synthesis by yeast of lipids and acetate esters. J. Inst. Brew. 87:92-95. [Google Scholar]
- 77.Verstrepen, K. J., F. F. Bauer, J. Winderickx, G. Derdelinckx, J.-P. Dufour, J. M. Thevelein, I. S. Pretorius, and F. R. Delvaux. 2001. Genetic modification of Saccharomyces cerevisiae: fitting the modern brewer's needs. Cerevisia 26:89-97. [Google Scholar]
- 78.Verstrepen, K. J., G. Derdelinckx, J.-P. Dufour, J. Winderickx, J. M. Thevelein, I. S. Pretorius, and F. R. Delvaux. Flavour-active esters: adding fruitiness to beer. J. Biosci. Bioeng., in press. [PubMed]
- 79.Verstrepen, K. J., N. Moonjai, G. Derdelinckx, J.-P. Dufour, J. Winderickx, J. M. Thevelein, I. S. Pretorius, and F. R. Delvaux. 2003. Genetic regulation of ester synthesis in brewer's yeast: new facts, insights and implications for the brewer, p. 234-248. In K. Smart (ed.), Brewing yeast fermentation performance, 2nd ed., vol. 2. Blackwell Science, Oxford, United Kingdom.
- 80.Wakil, S. J., J. K. Stoops, and V. Joshi. 1983. Fatty acid synthesis and its regulation. Annu. Rev. Biochem. 52:537-579. [DOI] [PubMed] [Google Scholar]
- 81.White, F. H., and A. D. Portno. 1979. The influence of wort composition on beer ester levels. Proc. Congr. Eur. Brew. Conv. 17:447-460. [Google Scholar]
- 82.Wohrmann, K., and P. Lange. 1980. The polymorphism of esterases in yeast. J. Inst. Brew. 86:174-175. [Google Scholar]
- 83.Yoshimoto, H., D. Fujiwara, T. Momma, K. Tanaka, H. Sone, N. Nagasawa, and T. Tamai. 1999. Isolation and characterization of the ATF2 gene encoding alcohol acetyl transferase II in the bottom fermenting yeast Saccharomyces pastorianus. Yeast 15:409-417. [DOI] [PubMed] [Google Scholar]
- 84.Yoshimoto, H., T. Momma, D. Fujiwara, H. Sone, Y. Kaneko, and T. Tamai. 1998. Characterization of the ATF1 and Lg-ATF1 genes encoding alcohol acetyltransferases in the bottom fermenting yeast Saccharomyces pastorianus. J. Ferment. Bioeng. 86:15-20. [Google Scholar]
- 85.Yoshioka, K., and N. Hashimoto. 1984. Acetyl-CoA of brewer's yeast and formation of acetate esters. Agric. Biol. Chem. 48:207-209. [Google Scholar]
- 86.Yoshioka, K., and N. Hashimoto. 1981. Ester formation by alcohol acetyl transferase from brewer's yeast. Agric. Biol. Chem. 45:2183-2190. [Google Scholar]
- 87.Younis, O. S., and G. G. Stewart. 2000. The effect of wort maltose content on volatile production and fermentation performance in brewing yeast, p. 170-176. In K. Smart (ed.), Brewing yeast fermentation performance, vol. 1. Blackwell Science, Oxford, United Kingdom.
- 88.Younis, O. S., and G. G. Stewart. 1998. Sugar uptake and subsequent ester and higher alcohol production by Saccharomyces cerevisiae. J. Inst. Brew. 104:255-264. [Google Scholar]