Abstract
In most streptococci, glucose is transported by the phosphoenolpyruvate (PEP):glucose/mannose phosphotransferase system (PTS) via HPr and IIABMan, two proteins involved in regulatory mechanisms. While most strains of Streptococcus thermophilus do not or poorly metabolize glucose, compelling evidence suggests that S. thermophilus possesses the genes that encode the glucose/mannose general and specific PTS proteins. The purposes of this study were to determine (i) whether these PTS genes are expressed, (ii) whether the PTS proteins encoded by these genes are able to transfer a phosphate group from PEP to glucose/mannose PTS substrates, and (iii) whether these proteins catalyze sugar transport. The pts operon is made up of the genes encoding HPr (ptsH) and enzyme I (EI) (ptsI), which are transcribed into a 0.6-kb ptsH mRNA and a 2.3-kb ptsHI mRNA. The specific glucose/mannose PTS proteins, IIABMan, IICMan, IIDMan, and the ManO protein, are encoded by manL, manM, manN, and manO, respectively, which make up the man operon. The man operon is transcribed into a single 3.5-kb mRNA. To assess the phosphotransfer competence of these PTS proteins, in vitro PEP-dependent phosphorylation experiments were conducted with purified HPr, EI, and IIABMan as well as membrane fragments containing IICMan and IIDMan. These PTS components efficiently transferred a phosphate group from PEP to glucose, mannose, 2-deoxyglucose, and (to a lesser extent) fructose, which are common streptococcal glucose/mannose PTS substrates. Whole cells were unable to catalyze the uptake of mannose and 2-deoxyglucose, demonstrating the inability of the S. thermophilus PTS proteins to operate as a proficient transport system. This inability to transport mannose and 2-deoxyglucose may be due to a defective IIC domain. We propose that in S. thermophilus, the general and specific glucose/mannose PTS proteins are not involved in glucose transport but might have regulatory functions associated with the phosphotransfer properties of HPr and IIABMan.
Streptococcus thermophilus is a gram-positive bacterium widely used in the dairy industry to produce fermented products such as Swiss cheese and yogurt. The ability of S. thermophilus to ferment milk is related to its capacity to rapidly take up the milk sugar lactose (43, 46). The importance of this process to the dairy industry has stimulated a tremendous amount of research aimed at understanding lactose transport mechanisms with the ultimate goal of constructing improved strains (10, 22). In contrast, very few studies have been conducted on glucose transport, most likely because most strains of S. thermophilus do not or poorly metabolize glucose (43, 55). However, studies on sugar metabolism conducted with other streptococci, including the phylogenetically related bacterium Streptococcus salivarius (4, 51), as well as other lactic bacteria (5, 7, 16, 38, 53, 56) have demonstrated that some phosphoenolpyruvate (PEP)-sugar phosphotransferase system (PTS) proteins involved in glucose transport are also involved in several regulatory mechanisms.
In a number of streptococci, glucose is transported by the glucose/mannose PTS, a multienzymatic system that sequentially catalyzes the transport and phosphorylation of glucose, mannose, the nonmetabolizable glucose and mannose analog 2-deoxyglucose (2-DG), and (to a lesser extent) fructose (26, 35, 51). It is composed of the general proteins HPr and enzyme I (EI) and three sugar-specific proteins called IIABMan, IICMan, and IIDMan (3, 29, 37, 39, 42). EI catalyzes the transfer of a phosphate group from PEP to histidine 15 of HPr, generating HPr(His∼P). HPr(His∼P) transfers the phosphate group to a histidine residue of the EIIA domain, which in turn transfers it to a histidine residue of the IIB domain. IIB∼P then phosphorylates the incoming sugar, which is transported by the permease made up of the membrane proteins IIC and IID.
Previous studies indicated that S. thermophilus possesses the genes encoding the general and specific glucose/mannose PTS proteins. HPr was first detected by Western blotting experiments using anti-S. salivarius HPr antibody (40) and was later purified from S. thermophilus ST11 (17). S. thermophilus ATCC 19258 was shown to possess the pts operon (49; A. Cochu, C. Vadeboncoeur, and M. Frenette, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. K88, 1999), confirming the presence of ptsI, the gene encoding EI. Pelletier et al. (37) found a protein in S. thermophilus ATCC 19258 that reacted with rabbit polyclonal antibodies directed against S. salivarius IIILMan (formerly IIABMan), a protein involved in the uptake of the glucose/mannose PTS substrates (3). In addition, data obtained from the S. thermophilus LMG18311 genome sequence project confirmed the presence of the complete pts and man operons (Université Catholique de Louvain [UCL]; contigs 110 and 63). However, it has yet to be determined how transcription of these genes is controlled and whether the proteins they encode are functional with respect to phosphate transfer from PEP and the uptake of the common glucose/mannose PTS substrates.
In this paper, we report (i) the characterization of the pts and man operons of S. thermophilus ATCC 19258, (ii) the overproduction and purification of S. thermophilus EI, HPr, and EIIABMan, (iii) in vitro PEP-dependent phosphorylation experiments using PTS purified proteins, and (iv) transport experiments using mannose and 2-DG, two specific substrates of the glucose/mannose PTS.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains used in this study are listed in Table 1. S. thermophilus was grown at 42°C in M17 (Difco Laboratories) (45), Hogg-Jago (31), or Elliker (11) broth supplemented with 0.5% (wt/vol) of the relevant sugar. S. salivarius ATCC 25975 was grown at 37°C in Hogg-Jago broth supplemented with 0.5% (wt/vol) of the relevant sugar. The sugar solutions were sterilized by filtration and added aseptically to autoclaved media. Escherichia coli DH5α, XL1 Blue, and BL21(DE3) were cultured aerobically in Luria-Bertani medium at 37°C with agitation, while E. coli LMG194 was grown under the same conditions in RM medium (per liter: 2% [wt/vol] Casamino Acids, 0.2% [wt/vol] glucose, 1 mM MgCl2, 6 g of Na2HPO4, 3 g of KH2PO4, 0.5 g of NaCl, 1 g of NH4Cl; adjusted to pH 7.4 with NaOH). When necessary, 50 μg of ampicillin/ml, 20 μg of tetracycline/ml, and/or 30 μg of kanamycin/ml was added.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant genotype or phenotype(s) | Reference or sourcea |
|---|---|---|
| Strains | ||
| S. thermophilus | ||
| ATCC 19258 | Glu+ Lac+ Man− | ATCC |
| SMQ-119 | Glu+ Lac+ Man− | 33 |
| SMQ-173 | Glu− Lac+ Man− | 33 |
| SMQ-301 | Glu− Lac+ Man− | 47 |
| S. salivarius | ||
| ATCC 25975 | Glu+ Lac+ Man+ | ATCC |
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 18 |
| XL1 Blue | supE44 hsdR17 recA1 endA1 gyrA46 thi relA1 lac− F′[proAB+lacIq lacZΔM15 Tn10 (Tetr)] | Stratagene |
| BL21(DE3) | F−ompT hsdSB(rB−mB−) gal dcm (DE3) | Novagen |
| LMG194 | F− ΔlacX74 galE thi rpsL ΔphoA (PvuII) Δara714 leu::Tn10 | Invitrogen |
| Plasmids | ||
| pBAD-HisB | Expression vector, Ampr | Invitrogen |
| pET28a | Expression vector, Kanr | Novagen |
| pST118 | S. thermophilus His6-HPr fusion in pBAD-HisB | This work |
| pST119 | S. thermophilus His6-IIABMan fusion in pET28a | This work |
| pST121 | S. thermophilus His6-EI fusion in pET28a | This work |
ATCC, American Type Culture Collection.
DNA manipulations.
S. thermophilus DNA was isolated using the method previously described for S. salivarius (28). Unless otherwise mentioned, all DNA manipulations were performed using standard procedures (2). Using appropriate oligonucleotides, DNA sequencing was completed on both strands by the DNA sequencing service of Université Laval. Computer-assisted DNA and protein analyses were performed using a Genetics Computer Group sequence analysis software package, version 9.1 (9). The data from the S. thermophilus LMG18311 genome sequence project were retrieved from the UCL website (http://www.biol.ucl.ac.be/gene/genome/). The references for other bacterial genome sequencing projects or bacterial genes are mentioned in the text.
Sequencing of the S. thermophilus pts operon.
A 3,991-bp region of S. thermophilus DNA comprising the pts operon and flanking genes was sequenced using two PCRs conducted with Vent-DNA polymerase (New England Biolabs) and a DNA Thermal Cycler 480 apparatus (Perkin-Elmer) (Fig. 1A). The 5′ end of the pts operon (2.9 kb) was amplified using forward F1 (5′-ATCTTTGCTATGAGTCG-3′) and reverse R1 (5′-GGGTTCATTTCTTTAAGGA-3′) primers with the following temperature-time profile: 94°C for 1 min, 51°C for 1 min, and 72°C for 3 min. The 3′ end (1.3 kb) was amplified using forward F2 (5′-GTTGGTCTTTATCGTAC-3′) and reverse R2 (5′-ATCTGCTGCTTTATGAA-3′) primers with the following temperature-time profile: 94°C for 1 min, 47°C for 1 min, and 72°C for 2 min. The sequences of the F1, R1, and F2 primers were complementary to regions of the S. salivarius icd gene and pts operon (13, 14) (GenBank accession number L14780). The R2 primer was a degenerated oligonucleotide with conserved S. salivarius and Streptococcus mutans gapN nucleotide sequences (28). Amplicons from four independent PCRs were pooled and used as sequencing templates.
FIG. 1.
Schematic representation of the pts locus and Northern blot analysis. (A) Genes are illustrated as white arrows oriented to indicate the direction of transcription. The black arrows above the white arrows indicate the positions of the primers used to amplify the pts operon and flanking genes. The promoters (P) are indicated by arrows upstream from ptsH and icd, and the T0 and T1 terminators are indicated by stem-loop structures. The two thick lines below the white arrows indicate the probes that were used in Northern blot experiments. The two thin lines represent the pts transcripts. (B) Northern blot analysis of total RNA isolated from S. thermophilus with ptsH- and ptsI-specific DNA probes. Cells were grown at 42°C in Hogg-Jago medium supplemented with 0.5% lactose until the OD660 reached 0.45. S. thermophilus RNA (5 μg) was hybridized with probes PTS1 (specific to ptsH) (lane 1) and PTS2 (specific to ptsI) (lane 2).
Sequencing of the S. thermophilus man operon.
The promoter, the full-length coding region, and the contiguous regions of the man operon of S. thermophilus ATCC 19258 were amplified by PCR with Vent-DNA polymerase and the following pairs of primers (see Fig. 3): primer pair MAN-L-13F (5′-CGACCCATTTTTTGGATTGC-3′) and MAN-L-AR (5′-ACTAATGGATCCGAATGAAGATTATTGA-3′) (temperature-time profile: 94°C for 1 min, 54°C for 1 min, and 72°C for 2 min), primer pair MAN-L-424F (5′-AAAAGGAGGAGAACACGAATG-3′) and MAN-O-3546R (5′-CTCAAAGTGACGACTAACCC-3′) (94°C for 1 min, 55°C for 1 min, and 72°C for 3.5 min), and primer pair MAN-O-AF (5′-CGGGTGTTTCCTACATG-3′) and SER-S-5290R (5′-AGCTAGAAAGTGGACGAAAAG-3′) (94°C for 1 min, 52°C for 1 min, and 72°C for 2 min). MAN-L-13F was deduced from the S. salivarius man operon sequence (29). All the other primer sequences were inferred from the genome sequence of S. thermophilus LMG18311 (UCL|C063, gl1, and gl4). Amplicons from four independent PCRs were pooled and used as sequencing templates.
FIG. 3.
Schematic representation of the man locus and Northern blot analysis. (A) Genes are illustrated as white arrows oriented to indicate the direction of transcription. The black arrows above the white arrows indicate the positions of the primers used to amplify the man operon and flanking genes. The putative promoters (P) are indicated by arrows upstream from manL, manO, and serS and downstream from hcp, and the putative terminator is indicated by a stem-loop structure. The thick lines below the white arrows indicate the probes that were used in Northern blot experiments. The thin lines represent the transcripts. (B) Northern blot analysis of total RNA isolated from S. thermophilus and S. salivarius. Cells were grown in Hogg-Jago medium supplemented with 0.5% lactose or glucose until the OD660 reached 0.45. Lanes 1 to 4: RNA was hybridized with the probe MAN-L. Lane 1, 5 μg of total S. thermophilus RNA isolated from lactose-grown cells; lane 2, 5 μg of total S. thermophilus RNA isolated from glucose-grown cells; lane 3, 5 μg of total S. salivarius RNA isolated from lactose-grown cells; lane 4, 5 μg of total S. salivarius RNA isolated from glucose-grown cells; lane 5, 5 μg of total S. thermophilus RNA isolated from glucose-grown cells hybridized with probe MANO-SER; lane 6, 5 μg of total S. thermophilus RNA isolated from glucose-grown cells hybridized with probe serS.
Cloning of the S. thermophilus ptsH, ptsI, and manL genes into expression vectors.
S. thermophilus ptsH was PCR amplified (94°C for 1 min, 51°C for 1 min, and 72°C for 1 min) using primers PTSH-135 (5′-GGAGACTCGAGTATGGCTTCTAAAGAT-3′) and PTSH-416R (5′-CATTTTTGTCGAATTCTTATGCCAATCCTT-3′), which contained engineered XhoI and EcoRI restriction sites (underlined), respectively. The 281-bp amplicon was digested with XhoI and EcoRI and ligated into pBAD-HisB digested with the same restriction enzymes, generating pST118. This plasmid was used to transform E. coli LMG194. S. thermophilus ptsI was PCR amplified (94°C for 1 min, 44°C for 1 min, and 72°C for 2 min) using primers PTSI-1822 (5′-GGCATAAACATATGACAAAAATGCT-3′) and PTSI-3558R (5′-AGCCTCGAGTTTTTTTGATTAATCTAC-3′), which contained engineered NdeI and XhoI restriction sites (underlined), respectively. The 1,736-bp amplicon was digested with NdeI and XhoI and ligated into pET28a digested with the same restriction enzymes, producing pST121. S. thermophilus manL was PCR amplified (94°C for 1 min, 48°C for 1 min, and 72°C for 2 min) using the primers MANL-44 (5′-GGAGAACACATATGGGTATCGGTATTAT-3′) and MANL-1041R (5′-ACTAATGGATCCGAATGAAGATTATTGA-3′), which contained engineered NdeI and BamHI restriction sites (underlined), respectively. The 1,024-bp amplicon was digested by NdeI and BamHI and ligated into pET28a, producing pST119. Plasmids pST121 and pST119 were used to transform E. coli strain XL1 Blue. All primers were designed from S. thermophilus ATCC 19258 pts and man nucleotide sequences. All PCRs were performed with Vent DNA polymerase. DNA sequencing on both strands was performed on pST118, pST119, and pST121 to confirm the constructions.
Purification of His6-HPr, His6-EI, and His6-IIABMan.
E. coli LMG194 bearing pST118 was used to inoculate 500 ml of RM medium supplemented with tetracycline and ampicillin. When the optical density at 660 nm (OD660) reached 0.37, l-arabinose was added to achieve a final concentration of 0.002% (wt/vol) and the incubation was continued overnight. E. coli BL21(DE3) bearing pST119 or pST121 was used to inoculate 500 ml of LB medium supplemented with kanamycin. When the OD660 reached 0.7, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to achieve a final concentration of 1 mM and the incubation was continued for 3 h. After induction of the recombinant proteins, cells were collected by centrifugation (12,000 × g, 10 min, 4°C), resuspended in 18 ml of lysis buffer (50 mM potassium phosphate [pH 8.0], 300 mM NaCl, 10 mM imidazole, 5 mM benzamidine, 1 mg of lysozyme/ml), and placed on ice for 1 h before sonication (Branson Sonic Power Co.) (4 × 30 s; 50% duty cycle; power level, 150 W). The soluble fraction of the disrupted cells was recovered after centrifugation (22,000 × g, 60 min, 4°C) and ultracentrifugation (100,000 × g, 90 min, 4°C). The His6 fusion proteins were then purified on Ni-nitrilotriacetic acid His.Bind resin (Qiagen) according to the manufacturer's instructions. Recombinant His6-EI and His6-IIABMan were quantified by spectrophotometry at 280 nm, using their extinction coefficients of 30,640 cm−1 M−1 and 15,250 cm−1 M−1, respectively. Recombinant His6-HPr was quantified by loading various amounts of HPr on a 15% polyacrylamide gel containing sodium dodecyl sulfate (23). After 1 h of electrophoresis at 200 V, the gel was stained for 3 h in a 0.04% (wt/vol) Coomassie brilliant blue-40% (vol/vol) ethanol-5% (vol/vol) glacial acetic acid solution and destained for 18 h in a 15% (vol/vol) acetic acid-10% (vol/vol) methanol solution. Prestained broad-range (6 to 175 kDa) protein markers (P7708S; New England Biolabs) were used to estimate the molecular masses of the proteins. The gel image was digitalized (RS-170/CCIR camera; Bio-Rad) and analyzed using Quantity One software (Bio-Rad). Known amounts of S. salivarius His6-HPr quantified by the Centre protéomique de l'Est du Québec on the basis of the amino acid composition were used as standards.
RNA manipulations.
S. thermophilus and S. salivarius cells were grown to the mid-log phase in Hogg-Jago or tryptone-yeast extract (TYE) medium supplemented with 0.5% (wt/vol) lactose or glucose. Total RNA was extracted using the RNeasy kit (Qiagen) according to the manufacturer's protocol with the modifications reported by Vaillancourt et al. (52). Denaturing formaldehyde agarose gels (1% [wt/vol]), buffers, and samples were prepared according to the method of Ausubel et al. (2). Using a PosiBlot pressure blotter (Stratagene) according to the manufacturer's instructions, total RNA (5 μg) from S. thermophilus and S. salivarius was transferred to positively charged nylon membranes (Roche). RNA was fixed to the membrane by UV cross-linking. RNA molecular weight markers (Promega) were used to determine the size of the transcripts. Probes used for Northern blot analyses (Fig. 1A; also see Fig. 3) were prepared from S. thermophilus DNA as follows. Probe PTS1 (specific to ptsH) was a 292-bp amplicon generated using oligonucleotides PTSH-135 and PTSH-416R. Probe PTS2 (specific to ptsI) was a 1,518-bp amplicon generated using oligonucleotides PTSI-1978F (5′-CGGGATCCCAAGACGAGCTTTCTG-3′) and PTSI-3496R (5′-CGGGTACCATATTCTTCCATTTTA-3′) with the following temperature-time profile: 94°C for 1 min, 52°C for 1 min, and 72°C for 2 min.
Probe MAN-L (specific to manL) was a 787-bp amplicon obtained using oligonucleotides 458 (5′-CACCAATCAGGCTCTATG-3′) and 1245R (5′-TTACCAGTTGAGTGAGC-3′) with the following temperature-time profile: 94°C for 1 min, 51°C for 1 min, and 72°C for 1 min. Probe MAN-M (specific to manM) was a 706-bp amplicon obtained using oligonucleotides MAN-M-AF (5′-CCAATTCCAATTCCACCAACCAC-3′) and MAN-M-AR (5′-ATGAAGTTCCGCCACCATTTCC-3′) with the following temperature-time profile: 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Probe MAN-N (specific to manN) was a 695-bp amplicon obtained using oligonucleotides MAN-N-AF (5′-ATTCTTCAACACTCACCCTTAC-3′) and MAN-N-AR (5′-GCTTGCAACAATACCAACTAC-3′) with the following temperature-time profile: 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Probe MAN-NO was a 1,155-bp amplicon obtained using oligonucleotides MAN-N-AF and MAN-O-AR (5′-GAATGAGAGTTGGTAGCTTGA-3′) with the following temperature-time profile: 94°C for 1 min, 53°C for 1 min, and 72°C for 2 min. This probe encompassed the 3′ end of manN and the 5′ end of manO.
Probe MANO-SER was a 768-bp amplicon obtained using oligonucleotides MAN-O-AF and SERS-R (5′-TGGCTGCAATTTGTTCTGA-3′) with the following temperature-time profile: 94°C for 1 min, 52°C for 1 min, and 72°C for 2 min. It encompassed the 3′ end of manO and the 5′ end of serS. Probe SERS (specific to serS) was a 1,240-bp amplicon obtained using oligonucleotides SERS-F (5′-CGACTTTGATGCCCTTTC-3′) and SERS-R with the following temperature-time profile: 94°C for 1 min, 54°C for 1 min, and 72°C for 2 min. Using a random primer labeling technique, all probes were labeled with [α-32P]dATP (Amersham Pharmacia Biotech Inc.). Northern blotting membranes were stained with Coomassie brilliant blue (0.25 g in 90 ml of methanol:H2O [1:1 vol/vol] and 10 ml of glacial acetic acid) to detect 16S and 23S rRNAs, and autoradiograms (Kodak X-Omat) were digitalized as described above. Relative amounts of pts and man transcripts were determined using Quantity One quantification software (Bio-Rad), and the values were corrected for the total amount of RNA loaded, which was determined by quantification of 16S and 23S rRNAs. Each quantification experiment was performed two to three times, as specified.
Primer extension.
Primer extension analysis was performed using a PE-R primer (5′-AACAAGCAAAGTAGCTGG-3′) labeled with T4 polynucleotide kinase and [γ-32P]ATP, as described by Ausubel et al. (2). The radiolabeled oligonucleotide (10 ng) was hybridized with 20 μg of S. thermophilus total RNA, and the extension was performed using 200 U of murine leukemia virus reverse transcriptase (Gibco-BRL) for 1 h at 42°C. The extended product was denatured and analyzed by electrophoresis on a 6.5% (wt/vol) polyacrylamide gel containing 7 M urea. The DNA sequence of the relevant portion of the pts operon was determined using the same oligonucleotide as a primer.
Preparation of the membrane fractions.
S. thermophilus cells cultivated in Hogg-Jago medium supplemented with 0.5% (wt/vol) glucose were broken by grinding with alumina (Sigma) in the presence of 10 mM potassium phosphate buffer (pH 7.5) containing 0.1 mM phenylmethylsulfonyl fluoride, 14 mM 2-mercaptoethanol (2-ME), and 1 mM EDTA (48). Membrane fractions obtained by differential centrifugation as previously described (3) were washed twice with 10 mM potassium phosphate buffer (pH 7.5) containing 0.5 M KCl and 14 mM 2-ME and resuspended in 10 mM potassium phosphate buffer (pH 7.5).
PEP-dependent phosphorylation of sugars.
The reaction mixture (600 μl) contained 50 mM potassium phosphate buffer (pH 7.0), 4 mM MgCl2, 2 mM PEP or ATP, 5 mM 2-mercaptoethanol (2-ME), 10 mM NaF, 100 μg of His6-HPr, 1 μg of His6-EI, and 1 to 10 μg of His6-IIABMan as well as membrane fragments (400 μg of protein) as a source of IICMan and IIDMan. After 30 min of preincubation at 37°C, 60 μl of the 14C-labeled sugar (10 mM [0.1 μCi/μmol]) alone or with 30 μl of a solution of a putative competitive unlabeled sugar (200 mM) was added. The reaction was allowed to continue for 20 min at 37°C and was then stopped by the addition of 6 ml of 0.03 M BaBr2. Under these conditions, the reaction rate measured with S. thermophilus PTS recombinant proteins with glucose as the substrate was linear over time for at least 40 min. The amount of phosphorylated sugar produced was determined as previously described (48). Results were calculated from two to three independent experiments.
Uptake experiments.
The uptake of [14C]mannose and [14C]2-DG was performed as previously described (50), with the following modifications. The uptake experiments were carried out at 37°C with cells grown in Hogg-Jago, M17, or Elliker medium. Uptake was initiated by adding 350 μl of a cell suspension (75 mg [dry weight] of cells/ml) to a buffer (10 ml) containing 50 mM potassium phosphate buffer (pH 7.0), 40 mM MgCl2, 0.5 mM 2-ME with 0.005, 0.01, or 0.02 mM unlabeled lactose (added 5 min before the labeled sugar) or with no lactose added, and 0.05 mM [14C]-labeled sugar (1 μCi/μmol). At various times, 1.0-ml samples were passed through a Millipore filter (type HA; pore size, 0.45 μm) and washed with 5 ml of cold potassium phosphate buffer (pH 7.0). The radioactivity retained on the filters was measured using an LS6500 Beckman Coulter scintillation counter.
Nucleotide sequence accession numbers.
The GenBank accession numbers assigned to the nucleotide sequences of the S. thermophilus ATCC 19258 pts operon comprising ptsH and ptsI and the man operon comprising manL, manM, manN, and manO are AY253327 and AY253328, respectively.
RESULTS
Analysis of the pts operon.
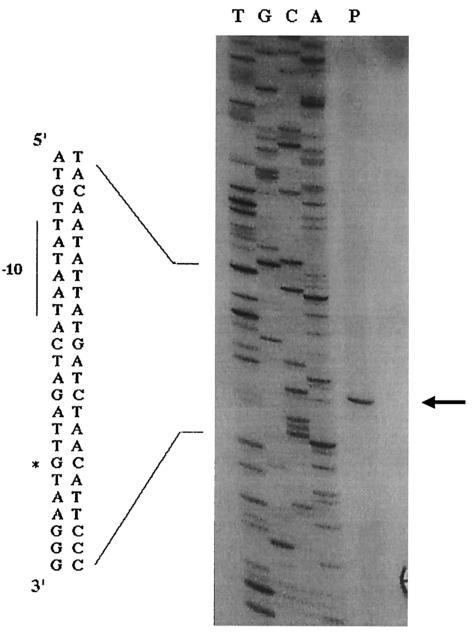
Sequence analysis of the amplicons generated with primers F1-R1 and F2-R2 allowed the identification of four open reading frames (ORFs) in the following order: (i) a 1,176-bp ORF corresponding to the icd gene possibly encoding an isocitrate dehydrogenase, (ii) a 261-bp ORF identified as the ptsH gene encoding HPr, (iii) a 1,734-bp ORF corresponding to the ptsI gene encoding EI, and (iv) an incomplete ORF corresponding to the first 207 bp of the gapN gene most likely encoding glyceraldehyde-3-phosphate dehydrogenase (Fig. 1A). The ribosome binding sites for S. thermophilus ptsH (AGGAGA) and ptsI (AGAAGG) were located seven and 14 bp, respectively, upstream from the start codon of the corresponding genes. A consensus promoter (TTGATA-N17-TATAAT) with a TRTG motif (−16 region) (54) was identified upstream from ptsH. Primer extension analyses showed that transcription of the pts operon was initiated at a G nucleotide located 9 bp downstream from the putative −10 box (Fig. 2). A putative catabolite-responsive element (cre) sequence bearing only one mismatch (C→G) with the consensus sequence WWTGNAARCGNWWWCAWW (32) (mismatch underlined) overlapped the transcriptional start site of the S. thermophilus pts operon.
FIG. 2.
Mapping of the 5′ ends of the ptsH and ptsHI transcripts by primer extension analysis. For lane P, oligonucleotide PE-R was annealed to 20 μg of S. thermophilus RNA and extended using murine leukemia virus reverse transcriptase. The nucleotide sequence was determined with an amplicon encompassing the DNA region of interest by using the same oligonucleotide as a primer (lanes T, G, C, and A). The arrow and asterisk indicate the G residue corresponding to the transcriptional start point.
Northern blot experiments using ptsH- and ptsI-specific probes with total RNA isolated from lactose-grown S. thermophilus cells allowed the detection of two transcripts originating from the same promoter: (i) a ptsH-specific 0.6-kb transcript terminating at T0 located 125 bp downstream from a ptsH stop codon and (ii) a 2.3-kb transcript covering ptsH and ptsI terminating at T1 located 4 bp downstream from ptsI (Fig. 1). The transcript amounts and the 0.6-kb/2.3-kb transcript ratios (2.6 ± 0.25) were the same in glucose- and lactose-grown cells (data not shown), suggesting that the nature of the energy source did not affect transcription of the pts operon.
S. thermophilus HPr, an 87-amino-acid protein (molecular mass, 8.9 kDa), possessed the His15 and the Ser46 phosphorylation sites common to all HPr proteins from gram-positive bacteria (data not shown). The amino acid sequence shared 94 to 96% identity with streptococcal HPrs and 38 to 93% identity with other bacterial orthologs. A proline was present at position 68 of S. thermophilus HPr, whereas other known HPrs have an alanine, a serine, or an aspartate at this position. Pro68 was located two residues upstream from an aspartate that N caps the third α-helix (21). A Pro68 was also found in the HPrs of four other S. thermophilus strains, namely, SMQ119, SMQ173, SMQ301 (this work), and LMG18311 (UCL|C063:g14). The 582-amino-acid sequence of S. thermophilus EI (molecular mass, 63.1 kDa) shared 87 to 97% identity with streptococcal orthologs. S. thermophilus EI possessed the conserved phosphorylation site (His191) and PEP-binding site (residues 334 to 489) (data not shown).
Analysis of the man operon.
Sequence analysis of three amplicons generated as described in Materials and Methods allowed the identification of six ORFs. Three of the ORFs shared high levels of identity with proteins of the mannose PTS family. The six ORFs were arranged in the following order: (i) the beginning of the 5′ end of the hcp gene encoding a hypothetical conserved protein, (ii) the 992-bp manL encoding IIABMan, (iii) the 827-bp manM encoding IICMan, (iv) the 911-bp manN encoding IIDMan, (v) the 374-bp manO encoding a protein of unknown function, and (vi) the serS gene most likely encoding a seryl-tRNA synthetase that shared 96% identity with the S. salivarius ortholog (Fig. 3A). Analysis of the sequence of the man operon revealed the presence of −35 and −10 boxes (Pman) upstream from manL that had only one mismatch (−35; TTGATA [mismatch underlined]) with the consensus sequence of E. coli σ70- and Bacillus subtilis σA-dependent promoters (27, 34). A second putative promoter (PmanO) (TTAGCA-N22-TATAAT) that was identical to and located at the same position as the functional S. salivarius manO promoter (29) was also identified.
Northern blot analyses (using a manL intragenic probe) of total RNA isolated from S. thermophilus grown in Hogg-Jago medium supplemented with lactose or glucose revealed the presence of a single 3.5-kb mRNA transcript (Fig. 3B, lanes 1 and 2). The same results were obtained using manM, manN, and manN-manO-specific probes (data not shown). Two mRNAs (3.5 kb and 1.6 kb) were detected with the manO-ser probe (Fig. 3B, lane 5), while only the 1.6-kb mRNA was detected with the serS-specific probe (Fig. 3B, lane 6). These results indicated that (i) Pman allowed the transcription of manL, manM, manN, and manO in the form of a tetracistronic mRNA, (ii) serS was transcribed independently of the man operon, and (iii) PmanO was not functional under the conditions tested. The expression of the man operon of S. thermophilus, a species that is unable to metabolize mannose, was compared with that of S. salivarius, which is able to metabolize mannose. Northern blot analyses of RNA isolated from S. salivarius grown on Hogg-Jago medium supplemented with lactose or glucose also revealed the presence of a single transcript covering the entire man operon (Fig. 3B, lanes 3 and 4). S. salivarius grown in glucose-supplemented TYE medium possessed two transcripts, one that hybridized with a manO-specific probe (a 0.7-kb mRNA specific to manO), suggesting that PmanO is functional in TYE-grown cells, and a second 3.6-kb mRNA transcript initiated from Pman (29). As S. thermophilus did not grow in TYE medium, we were unable to test whether S. thermophilus PmanO could be activated during growth in TYE.
The estimated molecular masses of the IIABMan, IICMan, IIDMan, and ManO proteins of S. thermophilus were 36, 28, 33, and 14 kDa, respectively. They shared 72 to 96% identity with other streptococcal orthologs and 25 to 96% identity with other bacterial orthologs. Alignment of mannose PTS family members IIAMan and IIBMan revealed that the two phosphorylated residues (His10 and His181) were conserved in S. thermophilus IIABMan (data not shown). Comparison of the S. thermophilus IICMan domain with orthologs revealed that the proposed signature (GX3G[DNH]X3G[LIVM]2XG2[STL][LT][EQ]) was also conserved (29). However, in the S. thermophilus IICMan protein, the Ala61, His145 and Gly232 residues, which are strictly conserved in other IICMan, were replaced by threonine, tyrosine, and serine or cysteine, respectively (Fig. 4). Moreover, among streptococcal orthologs, only the IICMan protein of S. thermophilus possessed a four-amino-acid duplication (GGGN) at the carboxy terminal of the protein. These characteristics were also found in the IICMan protein of S. thermophilus LMG18311 (UCL| C063:gl4).
FIG. 4.
Multiple alignments of bacterial IICMan. Only regions in which S. thermophilus exhibited nonconserved residues are shown. Residues are numbered according to the S. thermophilus sequence. The nonconserved amino acids in S. thermophilus IICMan are shaded in gray. Pileup and Pretty Genetics Computer Group programs were used for optimal alignments. Accession numbers or references are as follows: St1, S. thermophilus ATCC 19258 (this work); St2, S. thermophilus LMG18311 (UCL|C063:gl4); Ss, S. salivarius (Af130465); Sm, S. mutans (OUACGT|Contig2:1771358-1772172); Spn, Streptococcus pneumoniae (AAK34483); Spyo, Streptococcus pyogenes (Sanger Institute|pyoBAC15A5Pf05.s1c); Sg, Streptococcus gordonii (TIGR|bvs_699:2646-1939); Se, Streptococcus equi (Sanger Institute|equi389h03.p1c); Ll, Lactococcus lactis (AE006401); Lc, Lactobacillus curvatus (U28163); Bs, B. subtilis (P26381); Lm, Listeria monocytogenes (TIGR|Contig760:1:334293-246257); Ca, Clostridium acetobutylicum (AAK76813); Sty, Salmonella enterica serovar Typhimurium (Sanger Institute|STY1960); and Ec, E. coli (J02699).
In vitro phosphorylation of sugars by the mannose PTS.
Recombinant S. thermophilus His6-HPr, His6-EI, and His6-IIABMan were overproduced and purified as described in Materials and Methods. The purity of the proteins was over 98%, as estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). The purified proteins were mixed with IICMan/IIDMan-containing membranes isolated from S. thermophilus glucose-grown cells to determine whether they could catalyze the transfer of a phosphate group from PEP to glucose, mannose, 2-DG, and fructose, the common substrates of the glucose/mannose streptococcal PTS (51). Glucose, mannose, 2-DG, and (to a lesser extent) fructose, but not galactose, were phosphorylated to various degrees in the presence of membranes, EI, HPr, and PEP (Table 2). The phosphorylation rate was similar to that previously reported with S. salivarius PTS components (3). Little phosphorylation of glucose was detected in the absence of PEP or EI and HPr. The phosphorylation rate of glucose/mannose PTS substrates but not of galactose was enhanced in the presence of IIABMan. The rate increased with increasing amounts of IIABMan, demonstrating the involvement of IIABMan in the phosphorylation process. The specificity of the IICMan/IIDMan domains, which are involved in substrate recognition, was assessed by glucose phosphorylation competition experiments. A 10-fold mannose excess reduced the rate of glucose phosphorylation by approximately 2-fold, while a 25% reduction was observed in the presence of 2-DG (Table 3). Fructose did not inhibit the reaction under the conditions tested, which is consistent with the weak activity reported in Table 2. This suggested that fructose was a poor substrate for the S. thermophilus glucose/mannose PTS. As expected, galactose did not interfere with glucose phosphorylation.
TABLE 2.
In vitro PEP-dependent phosphorylation of various sugarsa
| Addition to the reaction mixture | PTS activity (nmol/mg/min)b for:
|
||||
|---|---|---|---|---|---|
| Glucose | Mannose | 2-DG | Fructose | Galactose | |
| Membd + PEP | 0.15 | NDc | 0.35 | ND | ND |
| Memb + PEP + EI + IIABMan | 0.60 | ND | ND | ND | ND |
| Memb + EI + HPr + IIABMan | 0.24 | ND | ND | ND | ND |
| Memb + PEP + EI + HPr | 5.44 | 4.87 | 3.11 | 1.85 | 0.38 |
| Memb + PEP + EI + HPr + IIABMan (1 μg) | 5.96 | ND | ND | ND | ND |
| Memb + PEP + EI + HPr + IIABMan (5 μg) | 9.33 | ND | ND | ND | ND |
| Memb + PEP + EI + HPr + IIABMan (10 μg) | 11.32 | 8.89 | 4.93 | 2.38 | 0.14 |
A total of 400 μg of membrane proteins, 2 mM PEP, 1 μg of His6-EI, 100 μg of His6-HPr, 1 to 10 μg of His6-IIABMan, and 10 mM 14C-labeled sugar (0.1 μCi/μmol) were mixed together and incubated for 20 min at 37°C.
The activities are expressed as nanomoles of phosphorylated product per milligram of membrane protein per minute. Results are from at least two independent experiments. The standard errors are less than 17%.
ND, not determined.
Memb, membrane.
TABLE 3.
Inhibition of glucose PEP-dependent phosphorylation by various sugarsa
| Inhibitor sugar | Glucose PTS activity (nmol/mg/min ± SE)b | % Inhibition |
|---|---|---|
| None | 5.96 ± 1.03 | 0 |
| Galactose | 5.77 ± 0.10 | 3.18 |
| Fructose | 5.81 ± 0.45 | 2.51 |
| 2-DG | 4.46 ± 0.56 | 25.16 |
| Mannose | 3.52 ± 0.43 | 40.94 |
A total of 400 μg of membrane proteins, 2 mM PEP, 1 μg of His6-EI, 100 μg of His6-HPr, 1 to 10 μg of His6-IIABMan, 10 mM 14C-labeled sugar (0.1 μCi/μmol), and 200 mM unlabeled sugar were mixed together and incubated for 20 min at 37°C.
The activities are expressed as nanomoles of phosphorylated product per milligram of membrane protein per minute. Results are from at least two independent experiments.
Uptake of sugars.
The phosphorylation experiments confirmed the capacity of the general and glucose/mannose-specific PTS proteins to transfer a phosphate group from PEP to a sugar substrate. To determine whether this system was operational in vivo, we measured the transport of 2-DG (Fig. 5) and mannose (data not shown) by whole S. thermophilus cells after growth in Hogg-Jago (Fig. 5), M17, or Elliker medium (data not shown). Surprisingly, no uptake was detected in all cases. In contrast, when 2-DG uptake was studied using S. salivarius cells grown in Hogg-Jago medium, 2-DG was readily taken up and detected as a phosphorylated product inside the cell (Fig. 5), as previously reported (50). To determine whether the lack of mannose and 2-DG uptake by S. thermophilus resulted from a shortage of PEP, we measured the uptake with cells that had first been incubated with 0.02 mM lactose, a readily metabolizable substrate that should increase the PEP potential. Even under these conditions, S. thermophilus cells were unable to take up 2-DG and mannose (data not shown).
FIG. 5.
Uptake of 2-DG by S. thermophilus (open symbols) and S. salivarius (closed symbols). Cells were cultured at 37°C (S. salivarius) or 42°C (S. thermophilus) in Hogg-Jago medium supplemented with 0.5% glucose and harvested at mid-log phase. Uptake experiments were carried out at 37°C in 50 mM potassium phosphate buffer (pH 7.0) containing 40 mM MgCl2, 0.5 mM 2-ME, and 0.05 mM [14C]-labeled sugar (1 μCi μmol−1). Results were obtained from two independent experiments, and the standard deviation is less than 0.3 nmol/mg of protein. Circles indicate accumulations of total 2-DG; squares indicate accumulations of 2-DG phosphate.
DISCUSSION
In a number of streptococcal species, the glucose/mannose PTS is able to readily transport and phosphorylate glucose, mannose, 2-DG, and (to a lesser extent) fructose (51). Glucose transport by the PTS has a significant impact on cell physiology, as it inactivates some sugar permeases and metabolic enzymes and stimulates or decreases the transcription of a number of genes (8, 41, 44). These regulatory functions are accomplished by HPr (5, 16, 51) and possibly IIABMan, a protein specifically involved in the transport of glucose/mannose PTS substrates (4, 7, 38, 51, 53, 56). The genes encoding the PTS proteins involved in glucose transport were identified on the S. thermophilus chromosome, and biochemical studies showed that HPr and IIABMan were present. However, no study has yet demonstrated the functionality of these proteins as a multienzymatic PEP-dependent sugar kinase or their involvement in the transport of glucose/mannose PTS substrates.
S. thermophilus HPr and EI proteins were encoded by two contiguous genes, ptsH and ptsI, which made up the pts operon. Northern blot experiments showed that this operon was efficiently transcribed. The cis elements controlling the transcription of the S. thermophilus pts operon consisted of (i) a strong consensus promoter, (ii) a rho-dependent terminator at the 5′ end of ptsI, and (iii) an intrinsic terminator downstream from the 3′ end of ptsI. These regulatory elements are similar to those found in S. salivarius and several other gram-positive bacteria (for a review, see reference 49). A cre sequence overlapping the transcription initiation site was identified upstream from S. thermophilus ptsH. This cre sequence is present in all streptococcal pts operons but not in other bacterial species (49). We do not know whether this sequence, which is the target of the transcriptional regulator CcpA (19, 41, 44), regulates transcription of the pts operon. It is noteworthy, however, that transcription of the S. thermophilus pts operon was not affected by the nature of the growth sugar (glucose or lactose), unlike the pts operons in Streptococcus bovis (1) and S. salivarius, which are upregulated by glucose (49).
S. thermophilus possessed an operon comprising all the genes encoding the specific proteins of the glucose/mannose PTS, i.e., IIABMan, IICMan, and IIDMan (24, 29), which were encoded by manL, manM, and manN, respectively. These genes (together with manO, which coded for a protein of unknown function) made up the man operon. The man operon was efficiently transcribed in glucose- and lactose-grown cells into a single mRNA that encompassed the four man genes. The structure of the S. thermophilus man operon was similar to that of S. salivarius (29), a mannose-positive streptococcus, and the gene products shared high identity with the S. salivarius orthologs (96% for IIABMan, 92% for IICMan, and 96% for IIDMan).
The high level of identity observed with the general and specific proteins of the glucose/mannose PTS of other streptococci that are able to metabolize mannose suggested that the S. thermophilus proteins were functional. Indeed, in vitro PEP-dependent sugar phosphorylation experiments showed that glucose, mannose, 2-DG, and (to a lesser extent) fructose were phosphorylated by these proteins. This result indicated that (i) a phosphate group could be efficiently transferred from PEP to the IIB domain via EI∼P, HPr(His∼P), and IIA∼P, (ii) the S. thermophilus general PTS proteins EI and HPr were efficient with respect to their phosphotransfer function, and (iii) the Pro68 in S. thermophilus HPr, which is not found in other HPrs, did not interfere with the phosphorylation of His15 by EI. Our results also indicated that in an open system, the IICD complex was able to specifically recognize and bind glucose/mannose PTS substrates and present them to domain IIB in the proper orientation for phosphorylation.
However, whole S. thermophilus cells, unlike S. salivarius cells, were unable to take up mannose and 2-DG, two specific substrates of the glucose/mannose streptococcal PTS. The in vitro PEP-dependent phosphorylation experiments ruled out the possibility that the lack of uptake was caused by a faulty EI and/or HPr. These experiments also indicated that IIABMan, which shared 96% identity with S. salivarius IIABLMan, can be phosphorylated and can transfer its phosphate group to the sugar bound to the IICD complex, suggesting that the lack of uptake was not the result of a defective IIABMan. Therefore, the inability to transport sugar may be due to a defective IICDMan complex that conserved its phosphorylation activity but lost its transport activity. This is consistent with several studies that demonstrated that transport and phosphorylation catalyzed by the PTS can occur independently when specific mutations are introduced into the glucose and mannose PTS IIC domains (6, 12, 30, 36). The inability of S. thermophilus to take up the glucose/mannose PTS substrates can thus be explained in at least two ways: (i) the IIC and/or IID proteins were not properly assembled in the membrane and/or (ii) the configuration of the transporter was “locked” in a state that prevented binding and/or translocation of extracellular substrates.
Consistent with these hypotheses, S. thermophilus IICMan had three amino acid substitutions at strictly conserved positions and a 4-amino-acid duplication at the cytoplasmic C-terminal end, a region of the protein that presumably interacts with the N-terminal extremity of the IID domain (20, 24). These modifications might have resulted from the adaptation of S. thermophilus to the milk environment. Bacterial cells accumulate subtle amino acid substitutions that can alter transporter specificities for substrates to expand the repertoire of substances that can be used as nutrients (25). However, the selective pressure from the predominance of lactose with respect to other carbon sources in milk might have led to the accumulation of mutations disabling the transport functionality of the IICDMan permease. We thus propose that in S. thermophilus, the general and specific glucose/mannose PTS proteins are not involved in glucose transport but can accomplish (via HPr and IIABMan) regulatory functions associated with the phosphotransfer properties of these proteins, such as those described for HPr(His∼P) and/or HPr(Ser-P) in catabolic repression via phosphorylation of PRD domains or in association with CcpA (5, 8, 16, 38, 51), those involving IIABMan in the control of lactose, galactose, and sorbose transport and metabolism in Lactobacillus casei (15, 53, 56), and those involved in fructose transport (7) and catabolite repression of the xpkA gene in Lactobacillus pentosus (38).
Acknowledgments
We thank the following organizations for their financial support: Conseil des Recherches en Pêche et en Agroalimentaire du Québec (CORPAQ), Action Concertée Fonds FCAR-NOVALAIT-MAPAQ, and Fonds pour la Formation de Chercheurs et l'Aide à la Recherche (FCAR). A.C. is a recipient of an FCAR studentship.
We also thank Pascal Hols from the UCL for providing the sequence of the S. thermophilus LMG18311 man operon, Denis Roy for helping with the 32P-[PEP] synthesis, and Gene Bourgeau for providing editorial assistance.
REFERENCES
- 1.Asanuma, N., and T. Hino. 2003. Molecular characterization of HPr and related enzymes, and regulation of HPr phosphorylation in the ruminal bacterium Streptococcus bovis. Arch. Microbiol. 179:205-213. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. A., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1990. Current protocols in molecular biology. Greene Publishing/Wiley-Interscience, New York, N.Y.
- 3.Bourassa, S., L. Gauthier, R. Giguère, and C. Vadeboncoeur. 1990. A IIIman protein is involved in the transport of glucose, mannose and fructose by oral streptococci. Oral Microbiol. Immunol. 5:288-297. [DOI] [PubMed] [Google Scholar]
- 4.Brochu, D., L. Trahan, M. Jacques, M. C. Lavoie, M. Frenette, and C. Vadeboncoeur. 1993. Alterations in the cellular envelope of spontaneous IIIManL-defective mutants of Streptococcus salivarius. J. Gen. Microbiol. 139:1291-1300. [DOI] [PubMed] [Google Scholar]
- 5.Brückner, R., and F. Titgemeyer. 2002. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 209:141-148. [DOI] [PubMed] [Google Scholar]
- 6.Buhr, A., G. A. Daniels, and B. Erni. 1992. The glucose transporter of Escherichia coli. Mutants with impaired translocation activity that retain phosphorylation activity. J. Biol. Chem. 267:3847-3851. [PubMed] [Google Scholar]
- 7.Chaillou, S., P. W. Postma, and P. H. Pouwels. 2001. Contribution of the phosphoenolpyruvate:mannose phosphotransferase system to carbon catabolite repression in Lactobacillus pentosus. Microbiology 147:671-679. [DOI] [PubMed] [Google Scholar]
- 8.Deutscher, J., A. Galinier, and I. Martin-Verstraete. 2002. Carbohydrate uptake and metabolism, p. 129-150. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 9.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vos, W. M. 1996. Metabolic engineering of sugar catabolism in lactic acid bacteria. Antonie Leeuwenhoek 70:223-242. [DOI] [PubMed] [Google Scholar]
- 11.Elliker, P. R., A. W. Anderson, and G. Hannesson. 1956. An agar culture medium for lactic acid streptococci and lactobacilli. J. Dairy Sci. 39:1611-1612. [Google Scholar]
- 12.Erni, B., B. Zanolari, and H. P. Kocher. 1987. The mannose permease of Escherichia coli consists of three different proteins. Amino acid sequence and function in sugar transport, sugar phosphorylation, and penetration of phage λ DNA. J. Biol. Chem. 262:5238-5247. [PubMed] [Google Scholar]
- 13.Gagnon, G., C. Vadeboncoeur, and M. Frenette. 1993. Phosphotransferase system of Streptococcus salivarius: characterization of the ptsH gene and its product. Gene 136:27-34. [DOI] [PubMed] [Google Scholar]
- 14.Gagnon, G., C. Vadeboncoeur, R. C. Levesque, and M. Frenette. 1992. Cloning, sequencing and expression in Escherichia coli of the ptsI gene encoding enzyme I of the phosphoenolpyruvate:sugar phosphotransferase transport system from Streptococcus salivarius. Gene 121:71-78. [DOI] [PubMed] [Google Scholar]
- 15.Gosalbes, M. J., V. Monedero, C. A. Alpert, and G. Perez-Martinez. 1997. Establishing a model to study the regulation of the lactose operon in Lactobacillus casei. FEMS Microbiol. Lett. 148:83-89. [DOI] [PubMed] [Google Scholar]
- 16.Gunnewijk, M. G., P. T. van den Bogaard, L. M. Veenhoff, E. H. Heuberger, W. M. de Vos, M. Kleerebezem, O. P. Kuipers, and B. Poolman. 2001. Hierarchical control versus autoregulation of carbohydrate utilization in bacteria. J. Mol. Microbiol. Biotechnol. 3:401-413. [PubMed] [Google Scholar]
- 17.Gunnewijk, M. G. W., P. W. Postma, and B. Poolman. 1999. Phosphorylation and functional properties of the IIA domain of the lactose transport protein of Streptococcus thermophilus. J. Bacteriol. 181:632-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 19.Henkin, T. N. 1996. The role of the CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis. FEMS Microbiol. Lett. 135:9-15. [DOI] [PubMed] [Google Scholar]
- 20.Huber, F., and B. Erni. 1996. Membrane topology of the mannose transporter of Escherichia coli K12. Eur. J. Biochem. 239:810-817. [DOI] [PubMed] [Google Scholar]
- 21.Jia, Z., J. W. Quail, L. T. J. Delbaere, and E. B. Waygood. 1994. Structural comparison of the histidine-containing phosphocarrier protein HPr. Biochem. Cell Biol. 72:202-217. [DOI] [PubMed] [Google Scholar]
- 22.Kleerebezem, M., I. C. Boels, M. Nierop Groot, I. Mierau, W. Sybesma, and J. Hugenholtz. 2002. Metabolic engineering of Lactococcus lactis: the impact of genomics and metabolic modelling. J. Biotechnol. 98:199-213. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Lengeler, J. W., K. Jahreis, and U. F. Wehmeier. 1994. Enzymes II of the phosphoenolpyruvate-dependent phosphotransferase systems: their structure and function in carbohydrate transport. Biochim. Biophys. Acta 1188:1-28. [DOI] [PubMed] [Google Scholar]
- 25.Liao, M. K., S. Gort, and S. Maloy. 1997. A cryptic proline permease in Salmonella typhimurium. Microbiology 143:2903-2911. [DOI] [PubMed] [Google Scholar]
- 26.Liberman, E. S., and A. S. Bleiweis. 1984. Transport of glucose and mannose by a common phosphoenolpyruvate-dependent phosphotransferase system in Streptococcus mutans GS5. Infect. Immun. 43:1106-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lisser, S., and H. Margalit. 1993. Compilation of E. coli mRNA promoter sequences. Nucleic Acids Res. 21:1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lortie, L.-A., G. Gagnon, and M. Frenette. 1994. IS1139 from Streptococcus salivarius: identification and characterization of an insertion sequence-like element related to mobile DNA elements from Gram-negative bacteria. Plasmid 32:1-9. [DOI] [PubMed] [Google Scholar]
- 29.Lortie, L.-A., M. Pelletier, C. Vadeboncoeur, and M. Frenette. 2000. The gene encoding IIABLMan in Streptococcus salivarius is part of a tetracistronic operon encoding a phosphoenolpyruvate:mannose/glucose phosphotransferase system. Microbiology 146:677-685. [DOI] [PubMed] [Google Scholar]
- 30.Manayan, R., G. Tenn, H. B. Yee, J. D. Desai, M. Yamada, and M. H. Saier, Jr. 1988. Genetic analyses of the mannitol permease of Escherichia coli: isolation and characterization of a transport-deficient mutant which retains phosphorylation activity. J. Bacteriol. 170:1290-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marciset, O., and B. Mollet. 1994. Multifactorial experimental designs for optimizing transformation: electroporation of Streptococcus thermophilus. Biotechnol. Bioeng. 43:490-496. [DOI] [PubMed] [Google Scholar]
- 32.Miwa, Y., A. Nakata, A. Ogiwara, M. Yamamoto, and Y. Fujita. 2000. Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis. Nucleic Acids Res. 28:1206-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moineau, S., S. Walker, A., B. J. Holler, E. R. Vedamuthu, and P. A. Vandenbergh. 1995. Expression of a Lactococcus lactis phage resistance mechanism by Streptococcus thermophilus. Appl. Environ. Microbiol. 61:2461-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran, C. P. J., N. Lang, S. F. LeGrice, G. Lee, M. Stephens, A. L. Sonenshein, J. Pero, and R. Losick. 1982. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol. Gen. Genet. 186:339-346. [DOI] [PubMed] [Google Scholar]
- 35.Néron, S., and C. Vadeboncoeur. 1987. Two functionally different glucose phosphotransferase transport systems in Streptococcus mutans and Streptococcus sobrinus. Oral Microbiol. Immunol. 2:171-177. [DOI] [PubMed] [Google Scholar]
- 36.Nuoffer, C., B. Zanolari, and B. Erni. 1988. Glucose permease of Escherichia coli. The effect of cysteine to serine mutations on the function, stability, and regulation of transport and phosphorylation. J. Biol. Chem. 263:6647-6655. [PubMed] [Google Scholar]
- 37.Pelletier, M., M. Frenette, and C. Vadeboncoeur. 1995. Distribution of proteins similar to IIIHMan and IIILMan of the Streptococcus salivarius phosphoenolpyruvate:mannose-glucose phosphotransferase system among oral and nonoral bacteria. J. Bacteriol. 177:2270-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Posthuma, C. C., R. Bader, R. Engelmann, P. W. Postma, W. Hengstenberg, and P. H. Pouwels. 2002. Expression of the xylulose 5-phosphate phosphoketolase gene, xpkA, from Lactobacillus pentosus MD363 is induced by sugars that are fermented via the phosphoketolase pathway and is repressed by glucose mediated by CcpA and the mannose phosphoenol pyruvate phosphotransferase system. Appl. Environ. Microbiol. 68:831-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robitaille, D., L. Gauthier, and C. Vadeboncoeur. 1991. The presence of two forms of the phosphocarrier protein HPr of the phosphoenolpyruvate:sugar phosphotransferase system in streptococci. Biochimie 73:573-581. [DOI] [PubMed] [Google Scholar]
- 41.Saier, M. H., Jr., S. Chauvaux, G. M. Cook, J. Deutscher, I. T. Paulsen, J. Reizer, and J. J. Ye. 1996. Catabolite repression and inducer control in Gram-positive bacteria. Microbiology 142:217-230. [DOI] [PubMed] [Google Scholar]
- 42.Saier, M. H., Jr., and J. Reizer. 1992. Proposed uniform nomenclature for the proteins and protein domains of the bacterial phosphoenopyruvate:sugar phosphotransferase system. J. Bacteriol. 174:1433-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Somkuti, G. A., and D. H. Steinberg. 1979. Adaptability of Streptococcus thermophilus to lactose, glucose and galactose. J. Food Prot. 11:885-887. [DOI] [PubMed] [Google Scholar]
- 44.Stulke, J., and W. Hillen. 2000. Regulation of carbon catabolism in Bacillus species. Annu. Rev. Microbiol. 54:849-880. [DOI] [PubMed] [Google Scholar]
- 45.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas, T. D., and V. L. Crow. 1983. Lactose and sucrose utilization by Streptococcus thermophilus. FEMS Microbiol. Lett. 17:13-17. [Google Scholar]
- 47.Tremblay, D. M., and S. Moineau. 1999. Complete genomic sequence of the lytic bacteriophage DT1 of Streptococcus thermophilus. Virology 255:63-76. [DOI] [PubMed] [Google Scholar]
- 48.Vadeboncoeur, C. 1984. Structure and properties of the phosphoenolpyruvate:glucose phosphotransferase system of oral streptococci. Can. J. Microbiol. 30:495-502. [DOI] [PubMed] [Google Scholar]
- 49.Vadeboncoeur, C., M. Frenette, and L.-A. Lortie. 2000. Regulation of the pts operon in low G+C Gram-positive bacteria. J. Mol. Microbiol. Biotechnol. 2:483-490. [PubMed] [Google Scholar]
- 50.Vadeboncoeur, C., and L. Trahan. 1982. Glucose transport in Streptococcus salivarius. Evidence for the presence of a distinct phosphoenolpyruvate:glucose phosphotransferase system which catalyses the phosphorylation of a α-methyl glucoside. Can. J. Microbiol. 28:190-199. [DOI] [PubMed] [Google Scholar]
- 51.Vadeboncoeur, C., and M. Pelletier. 1997. The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol. Rev. 19:187-207. [DOI] [PubMed] [Google Scholar]
- 52.Vaillancourt, K., S. Moineau, M. Frenette, C. Lessard, and C. Vadeboncoeur. 2002. Galactose and lactose genes from the galactose-positive bacterium Streptococcus salivarius and the phylogenetically related galactose-negative bacterium Streptococcus thermophilus: organization, sequence, transcription, and activity of the gal gene products. J. Bacteriol. 184:785-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veyrat, A., V. Monedero, and G. Pérez-Martinez. 1994. Glucose transport by the phosphoenolpyruvate:mannose phosphotransferase system in Lactobacillus casei ATCC 393 and its role in carbon catabolite repression. Microbiology 140:1141-1149. [DOI] [PubMed] [Google Scholar]
- 54.Voskuil, M. I., and G. H. Chambliss. 1998. The −16 region of Bacillus subtilis and other gram-positive bacterial promoters. Nucleic Acids Res. 26:3584-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright, H. D. 1936. Direct fermentation of disaccharides and valorisation in sugar utilization by Streptococcus thermophilus. J. Pathol. Bacteriol. 43:487-501. [Google Scholar]
- 56.Yebra, M. J., A. Veyrat, M. A. Santos, and G. Pérez-Martínez. 2000. Genetics of l-sorbose transport and metabolism in Lactobacillus casei. J. Bacteriol. 182:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]