Abstract
Artemisinin loses its antimalarial activity on prolonged exposure to erythrocytes, especially α-thalassemic erythrocytes. In this report, we show that the major artemisinin-inactivating factor in cytosol of normal erythrocytes was heat-labile but a heat-stable factor from α-thalassemic cells also played a significant role in reducing artemisinin effectiveness, which was shown to be heme released from hemoglobin (Hb). Studies of fractionated lysate from genetically normal erythrocytes revealed that the protein fraction with molecular weight greater than 100 kDa was capable of reducing artemisinin effectiveness more readily than lower molecular weight fraction. Catalase and Hb A, but not selenoprotein glutathione peroxidase, were capable of reducing artemisinin effectiveness. Hemin (ferriprotoporphyrin IX) also reduced artemisinin effectiveness in a concentration- and time-dependent manner. It is concluded that heme and heme-containing proteins in erythrocyte are largely responsible for reducing artemisinin effectiveness and may contribute to resistance of P. falciparum infecting α-thalassemic erythrocytes observed in vitro.
Keywords: malaria, Plasmodium falciparum, artemisinin, heme, catalase, thalassemia
1. Introduction
Plasmodium falciparum malaria remains one of the most lethal and widespread diseases in tropical regions due to the resistance of the parasite to most available antimalarial drugs [1]. Artemisinin or qinghaosu, a sesquiterpene lactone containing an endoperoxide bridge, and its derivatives are effective antimalarials that act rapidly to clear parasites in multidrug resistant malaria with no serious side effects. They form the prominent class of drugs for treatment of falciparum malaria [2, 3]. Several successful regimens have been developed with artemisinin derivatives in combination with other antimalarials that have more prolonged drug action [4].
Host-dependent artemisinin resistance has been shown in P. falciparum infecting α-thalassemic erythrocytes, including those containing hemoglobin (Hb) H alone (α-thal1/α-thal2) or Hb H with Hb Constant Spring (CS) (Hb H/CS or α-thal1/Hb CS) [5–8]. The resistance is associated with the fact that these genetically variant erythrocytes are capable of taking up a large portion of artemisinin drugs [6] due to a higher binding affinity of the drugs to Hb H in α-thalassemic erythrocytes than to Hb A [7]. In addition, both α-thalassemic and normal erythrocytes are capable of interacting with artemisinin, thereby reducing artemisinin effectiveness. Factors responsible for reducing artemisinin effectiveness are found in both cytosolic and, to a lesser extent, membrane fractions. Purified Hb H and membrane heme have been reported to markedly reduce artemisinin effectiveness [8, 9]. Since the effect in modifying artemisinin effectiveness is more pronounced in the cytosolic fraction, both in normal and Hb H erythrocytes, and that Hb H is present in limited amounts, we hypothesize that in addition to Hb H and membrane heme, other cytosolic components present in both normal and thalassemic cytosol may also play a major role in reducing artemisinin effectiveness.
In this report, we investigated cytosolic fractions of normal and α-thalassemic erythrocytes that are responsible for reducing artemisinin effectiveness, and determined that in addition to Hb H and membrane heme, free heme and hemoproteins in the cytosol, such as catalase and Hb A, are also responsible for reducing artemisinin effectiveness.
2. Methods and Materials
2.1 Materials
Artemisinin was purchased from the National Center of Natural Science and Technology, Hanoi, Vietnam, and recrystallized as white needles from a dichloromethane/hexane mixture (m.p. 154–156 °C). Radioactive 15-[14C]-artemisinin (26.1 Ci/mol) was a gift from the Research Triangle Institute (NC, USA). 8-[3H]-hypoxanthine (27.1 Ci/mmol) was purchased from Moravek Biochemicals (CA, USA). Bovine liver catalase, human glutathione peroxidase (GSH-Px), human Hb A and hemin were purchased from the Sigma Chemical Co. (MO, U.S.A.). Deferiprone (1,2-dimethyl-3-hydroxypyrid-4-one) was a generous gift from Dr. Kovit Pattanapanyasat, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. Microcon® filters and Centriprep® tubes were obtained from Amicon (MA, USA).
2.2 Subjects
Blood samples, in citrate-phosphate-dextrose solution, were obtained from the Thalassemia Research Center, Institute of Science and Technology for Research and Development, Mahidol University, Thailand. All thalassemic subjects (α-thal1/α-thal2 and α-thal1/Hb CS) were non-splenectomized and had received no blood transfusion for at least 3 months before the collection of blood. Samples from normal individuals were obtained voluntarily and were examined for their hematological parameters. No subjects had a previous history of malaria infection.
2.3 In vitro culture of P. falciparum
P. falciparum K1 strain (chloroquine- and pyrimethamine-resistant), originally isolated from a malaria patient in Kanchanaburi province, Thailand [10], was maintained using the ‘candle jar’ culture technique as described by Trager and Jensen [11]. Parasites were grown in human erythrocytes (blood group O) using RPMI 1640 medium supplemented with 25 mM HEPES, pH 7.4, 0.2% NaHCO3, 40 μg/mL of gentamycin and 10% human serum. For assay of antimalarial activity, parasite growth was synchronized at the ring stage by 5% sorbitol treatment [12] and growth monitored by measuring incorporation of [3H]-hypoxanthine into parasites (see below).
2.4 Preparation of membrane-free erythrocyte cytosol
One mL of 50% (v/v) erythrocyte suspension was lysed by addition of 4.5 mL of hypotonic solution containing 10 mM sodium phosphate buffer, pH 7.4, by a cycle of freeze-thawing in liquid nitrogen to obtain complete lysis. The lysed erythrocyte suspension was centrifuged at 10,000 g (Mikro 24-48R centrifuge, Hettich, Tuttlingen, Germany) for 10 min at 4°C to sediment membrane. The membrane pellet was discarded and the supernatant used as membrane-free lysate (cytosolic fraction) in further experiments [8].
In some experiments, membrane-free lysates from normal and α-thalassemic individuals (equivalent to 10% (v/v) erythrocytes) were heated in boiling water for 5 min. Following centrifugation at 10,000 g for 5 min to remove precipitated proteins, the supernatant (heated lysate) was further filtered through Microcon-10® filter to remove protein remnants. These fractions were tested for their effects on artemisinin effectiveness.
2.5 Fractionation of cytosol
To investigate components responsible for artemisinin inactivation, 15 mL aliquot of the membrane-free lysate was filtered by centrifugation through a Centriprep-10® tube at 1,366 g until 9 mL of the filtrate (designated as F0, MW less than 10 kDa) were collected. A 3 mL aliquot of retentate (L, MW higher than 10 kDa) was further filtered through a Centricon-100® tube at 1,300 g until the retentate was reduced to 20% of its initial volume. To the retentate (R100, MW higher than 100 kDa) were added 4 volumes of F0 (F0 did not inactivate artemisinin) and the resulting solution was filtered as described above to remove any remaining small molecules with MW less than 100 kDa. This procedure was repeated once more and the retentate (R100) was adjusted to its original volume (3 mL) with F0. The first filtrate and the other filtrates (MW less than 100 kDa) were combined and fractionated through Centricon-50® tube to give R50 (50 kDa < MW < 100 kDa) and then through a Centricon-10® tube, to give R10 (10 kDa < MW < 50 kDa). Aliquots of 270 μL from each fraction (L, R10, R50 and R100) were then used to determine their effects on artemisinin activity.
2.6 Determination of effect of cytosolic fractions on artemisinin activity
Aliquots (270 μL) of the membrane-free cytosol, cytosolic fractions, or proteins equivalent to 10% (v/v) erythrocytes, were incubated at 37°C with 30 μL of 10 μM [14C]-artemisinin, for 0, 2, 6, 12 and 24 h. The final concentration of the radiolabeled drug was 1 μM. As control, phosphate-buffered saline was mixed with [14C]-artemisinin and treated under the same conditions. In some experiments, the lysate was pre-treated with 5 μM deferiprone for 30 min at 37°C prior to incubating with artemisinin. After incubation, the mixture was filtered through 10 kDa cut-off membrane (Microcon-10®) by centrifuging at 22,620 g for 15 min at 4°C to separate free drug from the mixture. The concentrations of the free drug in the filtrates were determined from radioactivity counting in a liquid scintillation counter (Packard, IL, USA). These filtrates were then tested for their effects on artemisinin effectiveness.
Artemisisnin effectiveness of the each sample was determined by the [3H]-hypoxanthine incorporation method [13]. In brief, the filtrate was 4-fold serially diluted with culture medium. Aliquots of 25 μL of diluted samples were placed in a 96-well plate together with 200 μL of a 1.5 % parasite-infected erythrocyte suspension, 1% parasitemia at the early ring stages. The cell suspensions were incubated in a candle jar at 37°C for 24 hr, and then an aliquot of 25 μL (0.25 μCi) of [3H]-hypoxanthine was added to each well. The suspensions were incubated under the candle-jar condition for a further 18–24 h. Parasites were harvested onto glass filters (Unifilter®, Packard, IL, USA), which were then dried and radioactivity measured in a liquid scintillation counter (Packard, IL, USA). Less than 1% of the radioactivity was from the [14C]-artemisinin in the diluted filtrate. The IC50 value was determined from the sigmoid curve of percent [3H]-hypoxanthine incorporation versus log of artemisinin concentration. Drug effectiveness index was defined as IC50 of control/IC50 of test sample [8].
2.7 Statistical analysis
Statistical analysis was performed using Mann-Whitney U-test for data from independent random samples and Sign-Rank test for data from dependent samples.
3. Results
3.1 Effect of erythrocyte lysate and membrane-free lysate on artemisinin effectiveness
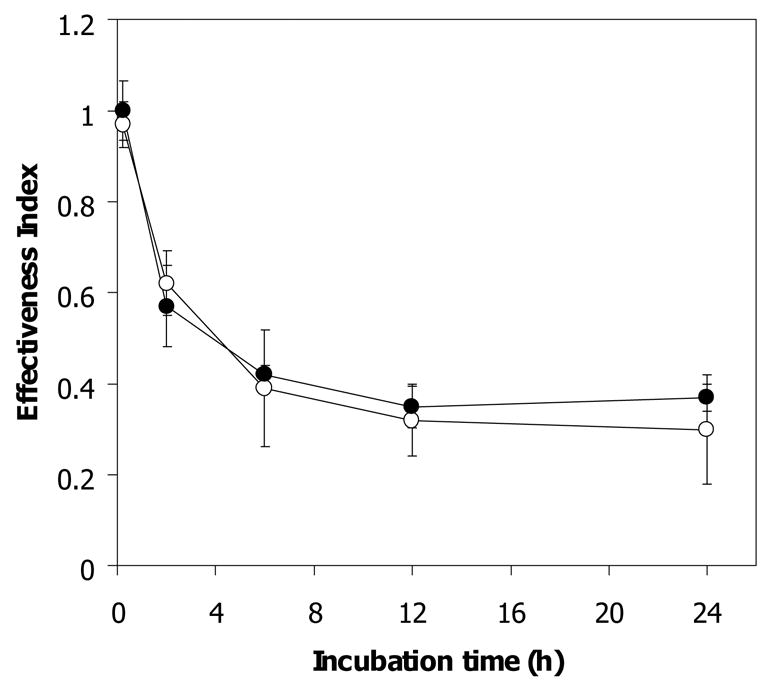
Reduction of antimalarial activity of artemisinin by cytosolic and membrane fractions of normal and both types of α-thalassemic (Hb H and Hb H/CS) erythrocytes has been reported previously [8]. The effect of membrane fraction on limiting artemisinin effectiveness correlates with membrane heme content of these cells [9]. We investigated factors other than Hb H in the membrane-free lysates of normal and Hb H-containing erythrocytes that could reduce artemisinin effectiveness. As shown in Fig 1, upon 2 h exposure of membrane-free lysates of normal, Hb H and Hb H/HbCS erythrocytes to 1 μM [14C]-artemisinin, the effectiveness index of artemisinin of membrane free lysates was significantly decreased from 1.0 ± 0.12 in control buffer to 0.67 ± 0.06 for normal (n = 5, p-value ≤ 0.01), 0.57 ± 0.01 for Hb H (n = 5, p-value ≤ 0.01) and 0.37 ± 0.01 for Hb H/Hb CS cells (n = 5, p-value ≤ 0.02). After 12 h of incubation, drug effectiveness index was further decreased to 0.35 ± 0.1, 0.27 ± 0.07 and 0.25 ± 0.06 for normal, Hb H and Hb H/CS lysate, respectively, and remained unchanged for up to 24 h of incubation of [14C]-artemisinin with these membrane-free lysates. These data confirmed previous observation [8] that membrane-free lysates from normal, Hb H, and Hb H/CS erythrocytes have the ability to reduce artemisinin effectiveness and that the inactivation was more pronounced in lysates from Hb H-containing red cells.
Figure 1.
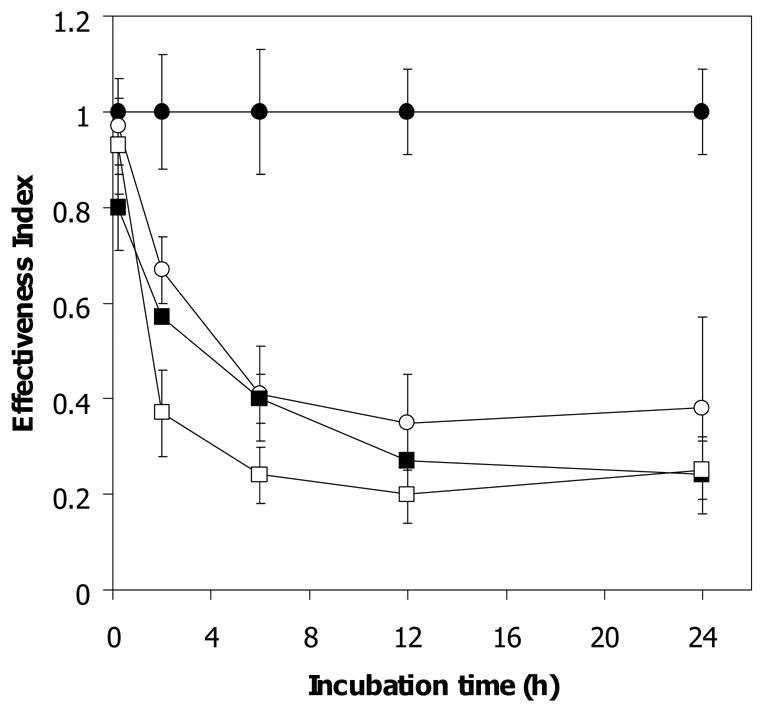
Antimalarial effectiveness of free [14C]-artemisinin exposed to membrane-free lysate of normal and α-thalassemic lysate. A 270 μl aliquot of lysate sample was incubated at 37 °C with 1 μM [14C]-artemisinin for 0, 2, 6, 12, and 24 h. After incubation, free drug was separated by ultracentrifugation through 10 kDa cut-off membrane and its effectiveness index was determined. PB, phosphate buffer control (●); N, normal (○); H, Hb H lysate (■); H/CS, Hb H/Hb CS lysate (□). Mean and s.e.m. of 5 subjects are shown.
3.2 Effect of heated erythrocyte lysate on artemisinin effectiveness
We hypothesized that proteins in the cytosol may be responsible for reducing artemisinin effectiveness. In order to demonstrate this, lysates were heated in boiling water for 5 min and centrifuged to sediment any precipitated protein. The supernatants were then subjected to artemisinin effectiveness assay. As summarized in Table 1, heated lysate from normal erythrocytes lost its ability to reduce artemisinin effectiveness during the first 6 h of incubation compared to unheated lysate (0.90 ± 0.10 vs 0.41 ± 0.10, p-value ≤ 0.02). However, after 24 h exposure, the effect of heated lysate on reducing artemisinin effectiveness was similar to that of unheated lysate (0.29 ± 0.13 vs 0.38 ± 0.19). This suggests that factors responsible for reducing artemisinin effectiveness in normal erythrocyte lysate are heat-labile components, presumably proteins, but heat stable components were also present either at low concentrations and/or with low capability to reduce artemisinin effectiveness.
Table 1.
Comparison between antimalarial effectiveness of artemisin following exposure to membrane-free unheated and heated hemolysates. A 270 μl aliquot of lysate sample was incubated 1 μM [14C]-artemisinin at 37°C for 0, 6, 12, and 24 h. Free drug was separated and effectiveness index determined after incubation. PB, phosphate buffer; lysate, unheated lysate; heated L, heated lysate. Numbers of samples in the tests were 5 for control and unhetaed lysate, and 4 for heated lysate. The results are presented as mean and s.e.m.
| Type of Lysate | [14C]-Artemisinin effectiveness index | ||
|---|---|---|---|
| Normal | Hb H | Hb H/Hb CS | |
| Lysate (6 h) | 0.41 ± 0.10 | 0.4 ± 0.05 | 0.24 ± 0.06 |
| Lysate (24 h) | 0.38 ± 0.19 | 0.24 ± 0.08 | 0.25 ± 0.06 |
| Heated lysate (6 h) | 0.90 ± 0.10 | 0.35 ± 0.06 | 0.26 ± 0.05 |
| Heated lysate (24 h) | 0.29 ± 0.13 | 0.22 ± 0.04 | 0.26 ± 0.08 |
| Filtrate of lysate (24 h) | 0.98 ± 0.04 | 1.08 ± 0.07 | 0.97 ± 0.04 |
| Filtrate of heated lysate (24 h) | 0.91 ± 0.13 | 1.08 ± 0.11 | 1.06 ± 0.06 |
In contrast to the results from heated normal erythrocyte lysate, heated lysates of Hb H-containing erythrocytes retained their ability to reduce artemisinin effectiveness to a similar extent as their corresponding unheated lysate following 6 h and 24 h incubation (Table 1). As SDS-PAGE of these heated lysates indicated the presence of only small amounts of proteins (data not shown), these samples (heated and non heated) were filtered through a 10 kDa cut-off membrane to eliminate the proteins. Surprisingly, the filtrate from both the heated lysate of normal and Hb H-containing erythrocytes exhibited no significant effect on artemisinin effectiveness for up to 24 h of incubation
3.3 Effect of heme in heated thalassemic erythrocyte lysate on artemisinin effectiveness
From the above observations we hypothesized that the supernatant of heated lysate from Hb H-containing erythrocytes contain heme, thus causing the reduction in artemisinin effectiveness. Spectrophotometric properties of these lysates and their filtrate fractions, both before and after heating, were examined at wavelengths between 250 to 650 nm. Heme and hemin solutions in 10 mM phosphate-buffered saline, pH 7.2, containing 1% SDS (to maintain heme and hemin in monomeric form) were used as reference spectra. Hemin and heme possess absorption peaks around 350–450 nm with their maximum absorption at approximately 415 and 407 nm respectively (Fig 2). The absorption peak of hemin was removed by filtration, suggesting that hemin was unable to pass through the membrane. Likewise, lysate and heated lysate also showed absorption peaks in the same region with their maximum absorption at about 415 and 407 nm respectively. These absorption peaks of lysate and heated lysate were not observed in their filtrate fractions, indicating the presence of heme/hemin in the heated lysates, presumably released from Hb H.
Figure 2.
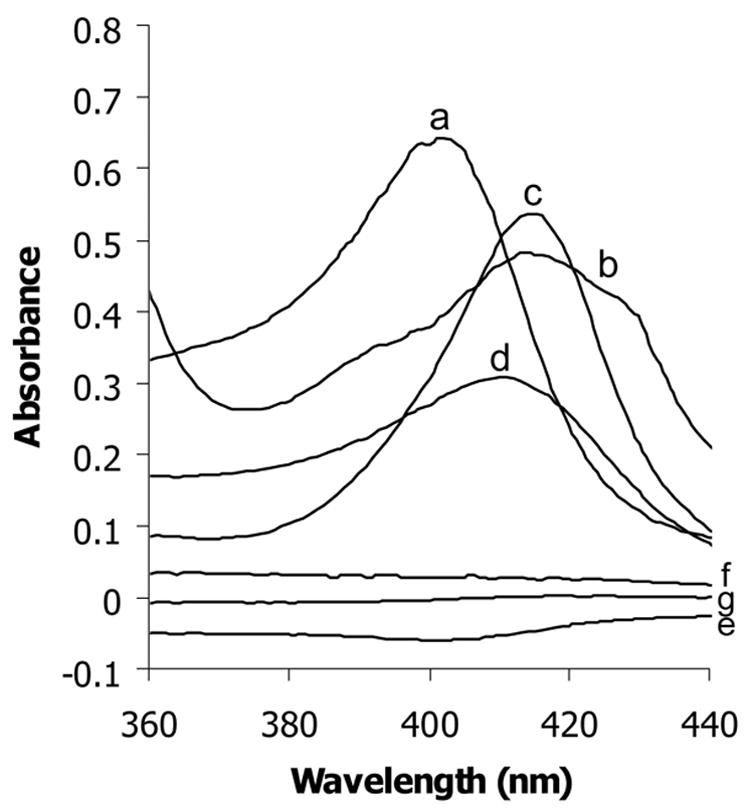
Spectra of heme (a), hemin (b), lysate (c) and heated lysate (d) and filtrates of hemin (e), lysate (f) and heated lysate (g).
3.4 Effect of heme on artemisinin effectiveness
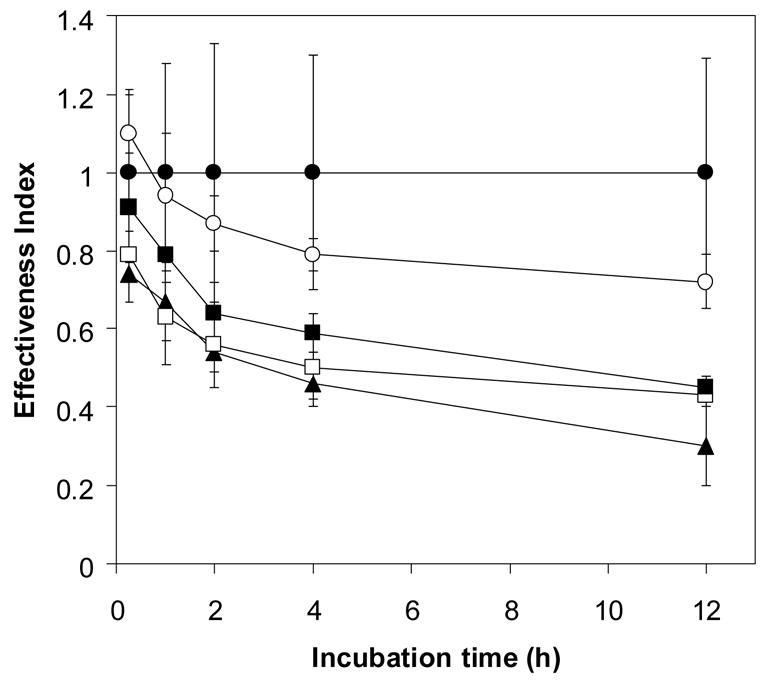
Artemisinin activity was found to decrease following incubation with hemin in a concentration- and time-dependent manner (Fig 3). It is worth noting that the concentration of hemin needed to exert an artemisinin inactivation effect equivalent to 10% thalassemic erythrocyte lysate was greater than 0.2 μM. It has been reported that both heme- and non-heme iron are found at higher levels in α-thalassemic than normal erythrocytes [14, 15]. It is possible that such iron-containing compounds may lead to a faster rate of drug inactivation by Hb H-containing lysate than that by normal lysate. To study the possible role of non-heme iron in the hemolysate in inactivating artemisinin, deferiprone (L1), an effective oral iron chelator used in treatment of thalassemia [16], was added to chelate non-heme iron. The effectiveness index (0.57 ± 0.09) of artemisinin following 2 h exposure to deferiprone-treated Hb H lysate was not significantly different from untreated Hb H lysate (0.62 ± 0.07) (Fig 4B). Similar effect was obtained with deferiprone-treated normal erythrocyte lysate (effectiveness index of 0.84 ± 0.1 vs 0.81 ± 0.05 for untreated hemolysate) (Fig 4A). The effectiveness index of artemisinin continued to decrease up to 24 h of incubation with no significant differences between deferiprone-treated and non-treated lysates from normal (0.47 ± 0.05 vs 0.43 ± 0.06) and Hb H (0.3 ± 0.12 vs 0.37 ± 0.03) erythrocytes. Altogether, these results indicate that chelatable non-heme iron in the hemolysates is not responsible for reducing artemisinin effectiveness.
Figure 3.
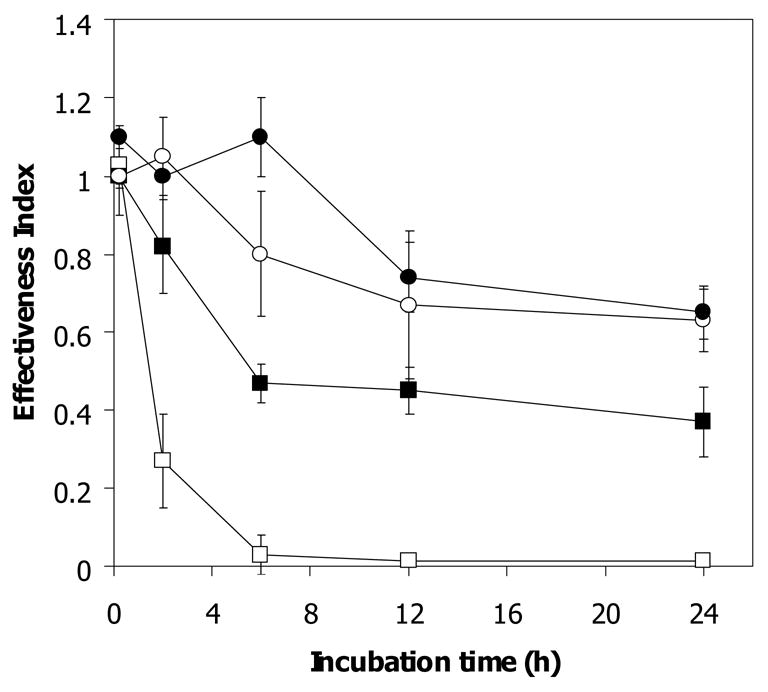
Antimalarial effectiveness index of [14C]-artemisinin (1 μM) following exposure to various hemin concentrations at 37°C. 0.002 μM (●), 0.02 μM (○), 0.2 μM (■) and 1 μM (□).
Figure 4.
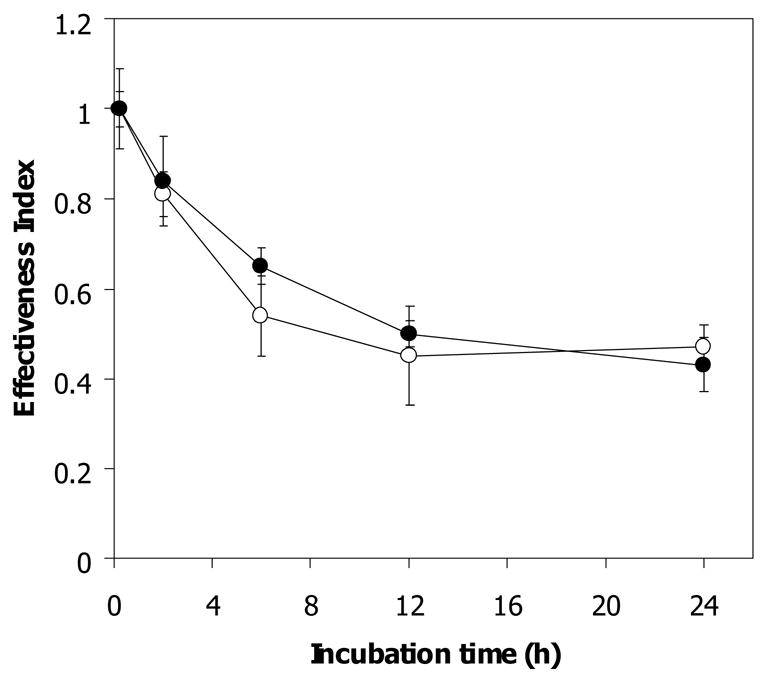
Ineffectiveness of deferipone (L1) (5 μM) in reversing inactivation of artemisinin by normal (4A) and Hb H (4B) lysate. ●; without L1, ○; with L1.
3.5 Identification of proteins in normal hemolysate that interact with artemisinin
In order to identify proteins responsible for reducing artemisinin effectiveness, lysate (L) from normal erythrocytes were was fractionated using filter membranes with different MW cut-off levels to obtain fractions R10, R50, and R100 containing proteins with molecular weight less that 50 kDa, between 50 and 100 kDa, and higher than 100 kDa, respectively. An aliquot of each fraction was subjected to artemisinin effectiveness assay by incubating with [14C]-artemisinin for 15 min and 6 h. As shown in Fig 5, the effectiveness index of artemisinin after 15 min incubation with fraction L, R10, R50 and R100 was 0.7 ± 0.05, 0.84 ± 0.11, 0.9 ± 0.09 and 0.58 ± 0.11, respectively, suggesting that fraction R100 with proteins of greater than 100 kDa contained the major artemisinin-inactivating factors, while fractions R50 and R10 had only minimal effects on artemisinin effectiveness at this short period of treatment. After 6 h of incubation, the effectiveness index was further reduced to 0.57 ± 0.06, 0.50 ± 0.07, 0.34 ± 0.12 and 0.37 ± 0.11 for L, R10, R50 and R100, repectively, indicating that, given sufficient time, all the fractions were capable of reducing artemisinin effectiveness, albeit to different extents.
Figure 5.
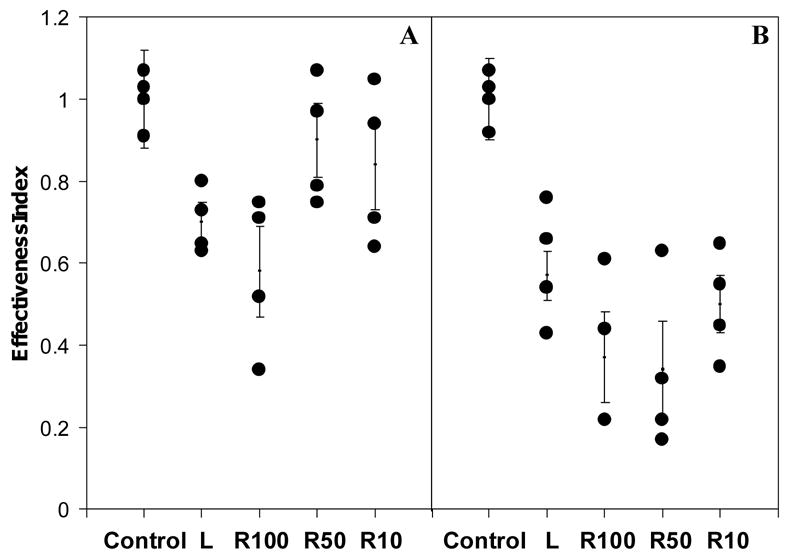
Artemisinin effectiveness indices of cytosolic (L) and its fractions with molcular weight higher than100 kDa (R100), 100-50 kDa (R50) and 50-10 kDa (R10). An aliquot of 270 μl from each fraction was incubated with 1 μM [14C]-artemisinin at 37°C for 15 min (A) and for 6 h (B). Phosphate buffer served as a control.
3.6 Effect of antioxidant enzymes and hemoproteins on artemisinin effectiveness
The above observation indicates that fraction R100 has a more pronounced role in affecting artemisinin effectiveness than fraction R50. This led us to investigate possible cytosolic proteins with molecular weight higher than 100 kDa and between 50 and 100 kDa on artemisinin effectiveness. On SDS-PAGE, the majority of proteins stained with Coomassie blue in lysate, R100 and R50 was hemoglobin. In fraction R50 and R10, protein band of molecular weight of 32 kDa was also observed. Immunoblot of the gel on nitrocellulose membrane with anti-Hb antibody revealed approximately equal amounts of hemoglobin. Although the band of catalase was not observed on Coomassie blue-stained gel in these fractions, it was detected by anti-catalase antibody in fraction R100 and R50, with higher amount in R100 fraction (data not shown).
Based on the detection of catalase and hemoglobin in fractions R100 and R50, which are capable of reducing artemisinin effectiveness, catalase, glutathione peroxidase (GSH-Px) and Hb were therefore selected for artemisinin effectiveness assay. Interestingly, artemisinin effectiveness decreased following exposure to 1,080–10,800 units/ml (0.4–4 μM) of catalase (Fig 6A) and 0.1–200 μM Hb A (Fig 6B) in a time- and dose-dependent manner whereas drug effectiveness was not affected following exposure to 0.01–1.0 U/ml of GSH-Px (in the presence of 2 mM glutathione), remaining at 0.98–1.0 for up to 12 h of incubation. These results suggest that Hb and catalase may be the major proteins in the erythrocytes responsible for reducing artemisinin effectiveness.
Figure 6.
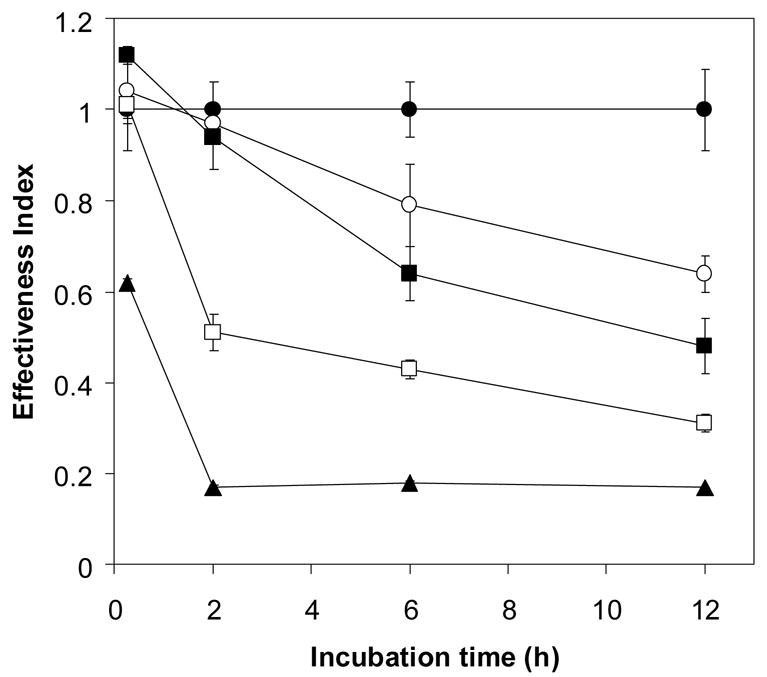
The effect of catalase (A) and Hb A (B) on reducing antimalarial effectiveness of artemisinin. One μM [14C]-artemisinin was incubated with various concentrations of the hemoproteins in 10 mM phosphate buffer pH 7.4 at 37 °C for 0, 1, 2, 4 and 12 h. control (●), 1,080 (○), 2,700 (■), 5,400 (□) and 10,800 (▲) unit/ml of catalase (A) and control (●), 0.1 μM (○),1 μM (■), 10 μM (□) and 200 μM (▲) of Hb A (B). Free drug was separated from drug-enzyme mixture by ultracentrifugation through a 10 kDa cut-off membrane. The filtrate fractions were then tested for the drug effectiveness against P. falciparum.
4 Discussion
Artemisinin-resistant parasites are found in vitro when α-thalassemic erythrocytes, both Hb H and Hb H/Hb CS, are used for cultivation [5, 6]. The reduced artemisinin effectiveness against P. falciparum-infecting thalassemic erythrocytes is due partly to competition of the host cell components for binding with the drug [6, 7], and partly due to an inactivation of the drug by cell components in both cytosolic and membrane fractions [8, 9]. The cytosolic fraction is mostly responsible for the reduction in drug effectiveness, but the membrane fraction also has a significant effect. Our previous studies showed that Hb H in the cytosol and heme in the membrane could reduce artemisinin effectiveness [8, 9]. In this study, such effects on reducing artemisinin effectiveness were confirmed in membrane-free lysate of normal, Hb H and Hb H/CS erythrocytes. We also demonstrated that hemoprotein (catalase), free heme and non chelatable iron in the lysate were the major factors responsible for reducing artemisinin effectiveness.
In membrane-free lysate prepared from normal and α-thalassemic erythrocytes, protein components played a major role in reducing artemisinin effectiveness. In normal hemolysate, the effect on reducing artemisinin effectiveness was abolished upon heating, as well as by filtration to remove the protein content. On the other hand, both heated and unheated lysate of Hb H-containing samples readily inhibited artemisinin, probably due to their high content of accessible heme, especially in the heated lysate. However, the inactivating effects of heated and unheated Hb H lysates on artemisinin effectiveness were abolished upon filtration when both protein and heme (released from Hb H) were filtered out. In the presence of cyanide, a strong pi-binding ligand which forms a 6-coordinated low spin stable complex with heme as characterized by the shift of absorption peak from 403 to 419 and 550 nm [17], the filtrate of the cyanide-treated heated-HbH lysate could resume its capability of reducing artemisinin effectiveness, with an effectiveness index of 0.61 ± 0.06 after 6 h of incubation, thus confirming the presence of heme in the heated lysate. It was estimated from measurements of the absorption spectra that heated Hb H-containing lysate contained 1.5 times higher level of released heme than normal heated lysate.
In screening fractions of normal lysate for proteins that may be capable of reducing artemisinin effectiveness, the potency of each fraction was found in the rank order of R100 > lysate > R50 > R10. The presence of catalase in higher amounts in fraction R100 as detected by immunoblot (data not shown) makes it a more likely candidate than Hb A in reducing artemisinin effectiveness. However, Hb A at 200 μM, a concentration lower than that present in 10% lysate (approximately 500 μM), was much more potent in reducing artemisinin effectiveness than the amount of catalase present in 10% lysate (approximately 7,000 units/ml). This discrepancy may be due to the presence of other factors that can protect artemisinin from interacting with Hb A. The effect of catalase in reducing artemisinin effectiveness is in fact in line with previous observation where addition of catalase to culture media could diminish artemisinin antimalarial activity in vitro [18].
Although the role of catalase in reducing the effectiveness of artemisinin was investigated due to its presence in erythrocyte, other heme-containing proteins, such as cytochrome C, readily inactivated artemisinin following a brief exposure (data not shown). Detoxification of artemisinin by hemoproteins in various tissues and organs may provide an explanation for the rapid clearance of the drug from the human body [2].
Iron chelators have been reported to antagonize the antimalarial activity of artemsinin, and thus chelatable iron was postulated to play a role in the mode of action of artemisinin [19, 20]. However, addition of deferiprone, an iron chelator, to the hemolysate did not prevent artemisinin from inactivation, in line with a previous report [9]. This may possibly be due to low amount of chelatable iron in normal erythrocyte cytosol [21], thus ruling out the role of chelatable iron in this inactivation phenomenon in erythrocyte lysate. The ability of catalase and cytochrome C, but not glutathione peroxidase (a selenoprotein), to detoxify artemisinin emphasizes the role of the heme prosthetic group in this process.
It has been reported that interaction of catalase with t-butylhydroperoxide leads not only to detoxification of peroxides but also to destruction of the heme moiety of catalase in the absence of hydrogen donor such as NADPH [22]. Similarly, it is possible that interaction of artemisinin with catalase in the malaria parasite where reducing power is diminished [23] may lead to inactivation of catalase, thus promoting oxidative killing of the parasite both by the drug and from cellular metabolism. If this is the case, catalase may serve as one of the targets of artemisinin, and being of host origin, resistance of P. falciparum to this drug should not be easily developed.
The reduced artemisinin effectiveness described here is due in part to the interaction of artemisinin with erythrocyte lysate. It was observed that upon interaction with erythrocyte lysate, approximately 30–40% of the radiolabelled artemisinin were retained in the retentate fractions of normal and thalassemic lysates, presumable covalently attached to proteins [24] and as nonfilterable heme [25], while 60–70% of the radioactive drug were recovered in the filtrate fraction as free drug with reduced antimalarial effectiveness. Thus after activation, in addition to the known alkylation of artemisinin to nearby biological molecules, the drug may become inactivated and released in forms whose structures have yet to be elucidated. The phenomenon is not a random chemical activity as it has been shown that in the presence of human serum proteins, artemisinin is not inactivated but is protected from inactivation by intact normal erythrocytes [8]. Other non-heme protein such as glutathione peroxidase, a selenium-containing protein, could not reduce artemisinin effectiveness.
It is still unclear whether artemisinin derivatives exert their antimalarial activities through reaction with heme [2, 26, 27]. Among other mechanisms, it was proposed that reductive scission of the peroxide by ferroprotoporphyrin IX gives rise to an active intermediate which can alkylate both heme and essential parasite proteins leading to parasite death [27, 28, 29]. However, a number of 10-deoxo artemisinin derivatives, which are active antimalarials, have been reported not to react with ferroprotoporphyrin IX [30]. While this result is still a matter of controversy [31, 32], the ability of artemisinin to interact with heme, as reported earlier [24], and to interact with a number of heme-containing proteins, resulting in reduction in effectiveness of the drug, emphasize the role of heme in the mechanism of inactivation as well as action of this drug. A number of other host components outside the erythrocytes may also interact with artemisinin and its derivatives, thereby reducing its effectiveness.
Acknowledgments
We are grateful to Professor Suthat Fucharoen for kindly supplying the blood samples used throughout the project. The work was supported by National Institutes of Health International Collaborations in Infectious Disease Research Grant U01-AI35827 to Y.Y., a Senior Research Fellowship from The Thailand Research Fund to P.W., and National Science and Technology Development Agency/Thailand Graduate Institute of Science and Technology (NSTDA/TGIST) and UNICEF/World Bank/World Health Organization/Special Program in Tropical Diseases to SK, who is also an international research scholar of Howard Hughes Medical Institute (HHMI), USA.
Abbreviations
- Hb H
α-thalassemia1/α-thalassemia2
- Hb H/Hb CS
α-thalassemia1/hemoglobin Constant Spring
- Hb A
hemoglobin A
- GSH-Px
glutathione peroxidase
- IC50
50% inhibitory concentration
- MW
molecular weight
- kDa
kilodalton
- p
p-value
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olliaro PL, Yuthavong Y. An overview of chemotherapeutic targets for antimalarial drug discovery. Pharmacol Ther. 1999;81:91–110. doi: 10.1016/s0163-7258(98)00036-9. [DOI] [PubMed] [Google Scholar]
- 2.Meshnick SR, Taylor TE, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: From herbal remedy to targeted chemotherapy. Microbiol Rev. 1996;60:301–15. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price RN. Artemisinin drugs: novel antimalarial agents. Exp Opin Investig Drugs. 2000;9:1815–27. doi: 10.1517/13543784.9.8.1815. [DOI] [PubMed] [Google Scholar]
- 4.Nwaka S, Ridley RG. Virtual drug discovery and development for neglected diseases through public-private partnerships. Nat Rev Drug Discov. 2003;2:919–28. doi: 10.1038/nrd1230. [DOI] [PubMed] [Google Scholar]
- 5.Yuthavong Y, Butthep P, Bunyaratvej A, Fucharoen S. Decreased sensitivity to artesunate and chloroquine of Plasmodium falciparum infecting hemoglobin H and/or hemoglobin Constant Spring erythrocytes. J Clin Invest. 1989;83:502–5. doi: 10.1172/JCI113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamchonwongpaisan S, Chandra-ngam G, Avery MA, Yuthavong Y. Resistance to artemisinin of malaria parasite (Plasmodium falciparum) infecting α–thalassemic erythrocytes: Competition in drug accumulation with uninfected erythrocytes. J Clin Invest. 1994;93:467–73. doi: 10.1172/JCI116994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vattanaviboon P, Wilairat P, Yuthavong Y. Binding of dihydroartemisinin to hemoglobin H: role in drug accumulation and host-induced antimalarial ineffectiveness of α–thalassemic erythrocytes. Mol Pharmacol. 1998;53:492–6. doi: 10.1124/mol.53.3.492. [DOI] [PubMed] [Google Scholar]
- 8.Charoenteeraboon J, Kamchonwongpaisan S, Wilairat P, Vattanaviboon P, Yuthavong Y. Inactivation of artemsinin by thalassemic erythrocytes. Biochem Pharmacol. 2000;59:1337–44. doi: 10.1016/s0006-2952(00)00271-9. [DOI] [PubMed] [Google Scholar]
- 9.Vattanaviboon P, Siritanaratkul N, Ketpirune J, Wilairat P, Yuthavong Y. Membrane heme as a host factor in reducing effectiveness of dihydroartemisinin. Biochem Pharmacol. 2002;64:91–8. doi: 10.1016/s0006-2952(02)01060-2. [DOI] [PubMed] [Google Scholar]
- 10.Thaithong S, Beale GH, Chutmongkonkul M. Susceptibility of Plasmodium falciparum to five drugs: An in vitro study of isolates mainly from Thailand. Trans R Soc Trop Med Hyg. 1983;77:228–31. doi: 10.1016/0035-9203(83)90080-9. [DOI] [PubMed] [Google Scholar]
- 11.Trager W, Jensen JB. Human malarial parasites in continuous culture. Science. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 12.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–20. [PubMed] [Google Scholar]
- 13.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–8. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrier SL. Thalassemia: pathophysiology of red cell changes. Ann Rev Med. 1994;45:211–8. doi: 10.1146/annurev.med.45.1.211. [DOI] [PubMed] [Google Scholar]
- 15.Shinar E, Rachmilewitz EA. Oxidative denaturation of red blood cells in thalassemia. Semin Hematol. 1990;27:70–82. [PubMed] [Google Scholar]
- 16.Rombos Y, Tzanetea R, Konstantopoulos K, Simitzis S, Zervas C, Kyriaki P, et al. Chelation therapy in patients with thalassemia using the orally active iron chelator deferiprone (L1) Haematologica. 2000;85:115–7. [PubMed] [Google Scholar]
- 17.Rothgeb TM, Gurd FRN. Physical methods for the study of myoglobin. Methods Enzymol. 1978;52:473–86. doi: 10.1016/s0076-6879(78)52052-1. [DOI] [PubMed] [Google Scholar]
- 18.Krungkrai SR, Yuthavong Y. The antimalarial action on Plasmodium falciparum of qinghaosu and artesunate in combination with agents which modulate oxidant stress. Trans R Soc Trop Med Hyg. 1987;81:710–4. doi: 10.1016/0035-9203(87)90003-4. [DOI] [PubMed] [Google Scholar]
- 19.Kamchonwongpaisan S, Vanitcharoen N, Yuthavong Y. The mechanism of antimalarial action of artemisinin (qinghaosu) In: Ong ASH, Packer L, editors. Lipid-Soluble Antioxidants: Biochemistry and Clinical Applications. Basel: Birkhuser-Verlag; 1992. pp. 363–72. [Google Scholar]
- 20.Meshnick SR, Yang Y-Z, Lima V, Kuypers F, Kamchonwongpaisan S, Yuthavong Y. Iron-dependent free radicals generation from the antimalarial agent artemisinin (qinghaosu) Antimicrob Agents Chemother. 1993;37:1108–14. doi: 10.1128/aac.37.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cazzola M, Dezza L, Bergamaschi G, Barosi G, Bellotti V, Caldera D, et al. Biologic and clinical significance of red cell ferritin. Blood. 1983;62:1078–87. [PubMed] [Google Scholar]
- 22.Pichorner H, Jessner G, Ebermann R. tBOOH acts as a suicide substrate for catalase. Arch Biochem Biophys. 1993;300:258–64. doi: 10.1006/abbi.1993.1036. [DOI] [PubMed] [Google Scholar]
- 23.Ittarat W, Sreepian A, Srisarin A, Pathepchotivong K. Effect of dihydroartemisinin on the antioxidant capacity of P. falciparum-infected erythrocytes Southeast Asian J Trop Med Public Health. 2003;34:744–50. [PubMed] [Google Scholar]
- 24.Asawamahasakda W, Ittarat I, Pu YM, Ziffer H, Meshnick SR. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob Agents Chemother. 1994;38:1854–8. doi: 10.1128/aac.38.8.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong YL, Yang YZ, Meshnick SR. The interaction of artemisinin with malarial hemozoin. Mol Biochem Parasitol. 1994;63:121–8. doi: 10.1016/0166-6851(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 26.Olliaro PL, Haynes RK, Meunier B, Yuthavong Y. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 2001;17:122–6. doi: 10.1016/s1471-4922(00)01838-9. [DOI] [PubMed] [Google Scholar]
- 27.O’Neill PM, Posner GH. A medicinal chemistry perspective on artemisinin and related endoperoxides. J Med Chem. 2004;47:2945–64. doi: 10.1021/jm030571c. [DOI] [PubMed] [Google Scholar]
- 28.Paitayatat S, Tarnchompoo B, Thebtaranonth Y, Yuthavong Y. Correlation of antimalarial activity of artemisinin derivatives with binding affinity with ferroprotoporphyrin IX. J Med Chem. 1997;40:633–8. doi: 10.1021/jm960767v. [DOI] [PubMed] [Google Scholar]
- 29.Robert A, Benoit-Vical F, Claparols C, Meunier B. The antimalarial drug artemisinin alkylates heme in infected mice. Proc Natl Acad Sci USA. 2005;102:13676–80. doi: 10.1073/pnas.0500972102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haynes RK, Ho W-Y, Chan H-W, Fugmann B, Stetter J, Croft SL, et al. Highly antimalaria-active artemisinin derivatives: biological activity does not correlate with chemical reactivity. Angew Chem Int Ed. 2004;43:1381–5. doi: 10.1002/anie.200352343. [DOI] [PubMed] [Google Scholar]
- 31.Laurent SAL, Robert A, Meunier B. C10-modified artemisinin derivatives: efficient heme – alkylating agents. Angew Chem Int Ed. 2005;44:2060–3. doi: 10.1002/anie.200462556. [DOI] [PubMed] [Google Scholar]
- 32.Haynes RK. Reply to comments on “Highly antimalaria-active artemisinin derivatives: Biological activity does not correlate with chemical reactivity”. Angew Chem Int Ed. 2005;44:2064–5. doi: 10.1002/anie.200352343. [DOI] [PubMed] [Google Scholar]


