Abstract
Paralytic shellfish toxins (PSTs) are potent neurotoxins produced by certain dinoflagellate and cyanobacterial species. The autonomous production of PSTs by bacteria remains controversial. In this study, PST production by two bacterial strains, isolated previously from toxic dinoflagellates, was evaluated using biological and analytical methods. Analyses were performed under conditions determined previously to be optimal for toxin production and detection. Our data are inconsistent with autonomous bacterial PST production under these conditions, thereby challenging previous findings for the same strains.
Paralytic shellfish toxins (PSTs) comprise a suite of potent neurotoxins that act by blocking sodium channels in nerve axons (3). These toxins may cause severe human poisoning upon consumption of contaminated shellfish. The production of PSTs by dinoflagellates has been thoroughly documented and confirmed worldwide. Strains of the dinoflagellates Alexandrium lusitanicum Balech and Gymnodinium catenatum Graham isolated from Portuguese waters have been shown by different detection methods to produce PSTs (1, 6, 7, 16).
The potential involvement of dinoflagellate-associated bacteria in PST production, specifically the autonomous synthesis of these toxins, has been addressed using bacterial strains isolated from laboratory dinoflagellate cultures (5, 10, 15) in conjunction with high-performance liquid chromatography (HPLC) (5, 15) and in vitro assay-based toxin detection (10). Two bacterial strains isolated previously by our group from A. lusitanicum and G. catenatum and identified as Pseudomonas stutzeri and Pseudomonas diminuta, respectively, were reported to produce PSTs (5, 7). Nevertheless, the autonomous production of PSTs by bacteria remains a controversial subject, and there have been several recent reports of the incorrect identification of bacterial metabolites as PSTs by HPLC analysis (2, 17, 18, 20, 21).
In the context of such findings and the continuing uncertainty regarding bacterial involvement in PST toxigenesis, the present investigation was undertaken to reevaluate our previous results on the toxin content and profile in P. stutzeri and P. diminuta. Both biological in vitro (mouse neuroblastoma [MNB] assay) and chemical (HPLC) methods were employed to account for sodium channel-blocking (SCB) activity and/or compounds with the same chromatographic behavior as PSTs produced by these two bacterial isolates. Additionally, since nutritional status has been reported to influence bacterial toxicity (4), analyses for intra- and extracellular toxins under both nutrient-replete and phosphorus-limited growth conditions were conducted.
For this study, P. stutzeri and P. diminuta (5, 7) were individually inoculated into 500-ml volumes of two growth media: phosphorus-limiting artificial seawater experimental (ASWE) medium (11) containing 0.5 μM Na2HPO4 as well as 37.5 mM sodium succinate as the sole carbon and energy source, and a complex organic seawater complete medium (SWC) (4, 11) rich in phosphate (5 g of Bacto Tryptone, 3 g of yeast extract, and 6 ml of 50% glycerol per liter of seawater). Cultures were incubated at 19 to 21°C (150 oscillations min−1) under 22-μE m−2 s−1 irradiance until late log phase. At that time, a cell count (in CFU per milliliter) was obtained and cells were separated from growth medium by centrifugation (10,000 × g, 20 min, 8°C).
For toxin extraction, cell pellets were lyophilized after centrifugation and extracted with 1 ml of 0.5 N acetic acid per 100 mg of lyophilized weight. Bacterial cells were mechanically disrupted, and the suspension was centrifuged (2,000 × g, 10 min, room temperature), passed through an ultrafiltration membrane (12,400-molecular-weight cutoff; Ultrafree C3GC; Millipore), and stored at −20°C until analyzed. Aliquots of each extract were then passed over a Sep-Pak C18 cartridge (Millipore Co.) for final cleanup. For extracellular-toxin analysis, 50 ml of each supernatant fraction was stored at −20°C prior to analysis by MNB assay and HPLC. The same volume of a control flask (ASWE or SWC medium only) was used as a negative control in the MNB assay. This assay was carried out according to the method described by Gallacher and Birkbeck (9). The concentrations of ouabain and veratridine required were determined by checkerboard assay (each toxin was titrated against various concentrations of the other to optimize the amount of each; three wells per dilution) using concentrations of ouabain from 0 to 0.3 mM and of veratridine from 0 to 0.07 mM in the absence of saxitoxin (STX). Concentrations causing maximum cell death, as evidenced by absorbance levels below 20% (540 nm), were used in the assays. These levels were optimized for each assay according to the number of subcultures performed. After 12 subcultures with ASWE medium, the assay was not sensitive to veratridine concentrations below 0.05 mM. These results emphasize the importance of monitoring assay performance and minimizing the number of subcultures. For SWC medium, lower concentrations of veratridine could be maintained.
The MNB assay was performed according to the method of Gallacher et al. (10). Briefly, an STX dose-response analysis using a certified reference standard (National Research Council, Halifax, Nova Scotia, Canada) was performed on a 1/10 dilution of each growth medium over the range of 5 to 160 nM. Supernatant fractions and cell extracts, collected before and after cleanup on a Sep-Pak C18 cartridge, were assayed using the following dilutions in 1/10-strength growth medium: 1/8, 1/10, 1/16, 1/32, and 1/64. The same dilutions of uninoculated growth medium served as negative controls.
It is noteworthy that the ASWE medium employed clearly affected the percentage of cell survival and possibly the assay sensitivity, as SCB activity as high as 25% was detected in the 1/10 dilution. Nevertheless, this interference was overcome by using supernatant dilutions higher than 1/16.
For the HPLC analysis, postcolumn oxidation was performed by the method of Oshima (19). Three mixtures of PST standards containing derivatives of the STX group (neoSTX, dcSTX, and STX), gonyautoxins (GTX1 to GTX5), and N-sulfocarbamoyl toxins (C1 to C4), kindly provided by Y. Oshima (Tohoku University, Sendai, Japan), were used for identification purposes. The chromatographic separation was performed on a reversed-phase C8 column (Hypersil; MOS, 5 μm; 4.6 by 150 mm; Supelco).
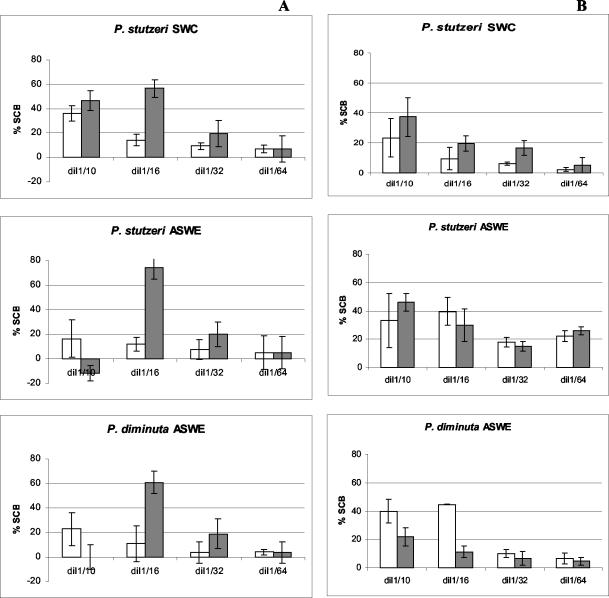
When bacterial extracts (ca. 108 CFU/ml) were analyzed by MNB assay, a low level of cell survival (i.e., direct cell lysis) was observed for dilutions up to the 1/10 (Fig. 1A). SCB activity was detected in cell extracts of P. stutzeri grown in either SWC or ASWE medium. As for P. diminuta, SCB was also detected when the bacterium was grown in ASWE medium but not when this strain was grown in SWC medium (Table 1).
FIG. 1.
Sodium channel-blocking activity of bacterial extracts (shaded bars) and control growth media (white bars) before (A) and after (B) Sep-Pak cleanup (obtained by MNB assay).
TABLE 1.
Quantification of SCB activity in cell extracts
| Strain | Medium | STX concn (nM STX equivalents)
|
|
|---|---|---|---|
| Before cleanup | After cleanupa | ||
| P. diminuta | ASWE | 31.7 | ND |
| P. stutzeri | ASWE | 61.2 | ND |
| SWC | 56.2 | BQ | |
ND, not detected; BQ, below quantification level.
Extracts assayed following C18 Sep-Pak cleanup showed no direct lytic effects on the neuroblastoma cells. Furthermore, sample cleanup caused marked changes in SCB activity. When either bacterial strain was grown in ASWE medium, no significant SCB activity was detected. P. stutzeri grown in SWC medium showed some activity at a dilution of 1/16, although at levels well below quantification with an STX dose-response curve and still lower than those of the controls (Fig. 1B). The low apparent SCB activity for 1/10 dilutions of extracts of ASWE- or SWC-grown P. stutzeri was not ascribed to SCB toxins. The SCB activity of the negative controls at 1/8 and even 1/10 dilutions was highly variable from assay to assay, and the standard deviations were usually above 20% when several assays were considered and always overlapped the error bars for sample extract values (data not shown). Dinoflagellate extracts have shown similar lytic effects (1, 12), which could also be eliminated by sample cleanup with a C18 Sep-Pak cartridge. However, the toxicity values for these extracts remained unaltered after the cleanup, which was not the case for our bacterial extracts. This loss of SCB activity is further proof that PSTs are not responsible for the MNB assay activity in crude bacterial extracts. Since all extracts were passed through a filter with a molecular weight cutoff of 12,400, it can be assumed that the lytic effect was caused by compounds with molecular masses below this cutoff value. The effect of these cleanup procedures on bacterial extracts and supernatant fractions tested in other studies using the MNB assay and yielding positive results (10, 13, 14) was not addressed.
Regardless of the growth medium used, neither compounds with SCB activity nor direct lytic effects were evident upon analysis of P. diminuta and P. stutzeri culture supernatants by MNB assay.
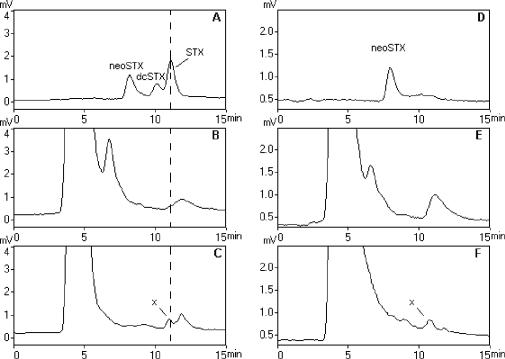
Figure 2 shows the HPLC-FLD (postcolumn fluorescence derivatization) chromatograms for the analysis of the STX group in P. stutzeri and P. diminuta grown in SWC medium. Similar results were obtained for bacteria grown in ASWE medium. One peak with a retention time similar to STX was observed in the P. stutzeri extracts (Fig. 2C). However, unlike the standard toxin, this compound was still detected after the postcolumn oxidation process was deactivated (Fig. 2F) and was therefore considered to represent a compound other than STX.
FIG. 2.
HPLC-FLD analysis for neoSTX, dcSTX, and STX for P. diminuta (B and E) and P. stutzeri (C and F) in SWC medium and for toxin standard mixture (A and D). (A to C) Profile obtained with the oxidizing reagent; (D to F) profile obtained after deactivation of the postcolumn oxidation system. The putative STX peak in bacterial extracts is labeled x.
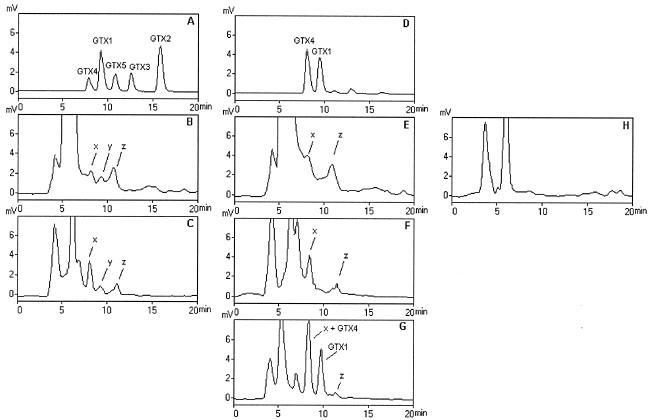
Different compounds, indicated in Fig. 3 as x, y, and z, with retention times similar to GTX4, GTX1, and GTX5, respectively, were detected in both bacterial extracts. Compound x and GTX4 were also both detected in the absence of the oxidizing reagent (Fig. 3F and D, respectively) and coeluted when coinjected with the P. stutzeri extract (Fig. 3G). However, although the level of compound x increased in the absence of the oxidizing reagent, the degree of enhancement was considerably lower than that of the standard treated similarly, and it was therefore considered to be a false peak. A similar finding was reported recently by Baker et al. (2), who did a detailed study of GTX4 imposters produced by the same P. stutzeri strain and another putative PST-producing bacterium. Compound y, unlike GTX1, could no longer be detected after removal of the periodic acid from the postcolumn reaction system (Fig. 3E and F). On the other hand, compound z retained its fluorescence after the postcolumn oxidation process was deactivated, while GTX5 was undetectable. Therefore, compounds y and z were both considered as false peaks and not identified as PSTs.
FIG. 3.
HPLC-FLD analysis of SWC medium cultures of P. diminuta (B and E) and P. stutzeri (C, F, and G) and of toxin standard mixture (A and D). (A to C) Profile obtained with the oxidizing reagent; (D to F) profile obtained after deactivation of the postcolumn oxidation system; (G) profile obtained following addition of standard mixture to P. stutzeri extract in the absence of postcolumn oxidation. The putative GTX4, GTX1, and GTX5 peaks in bacterial extracts are labeled x, y, and z, respectively.
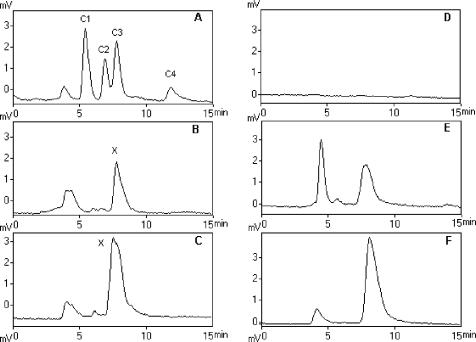
Analysis of both bacterial extracts for toxins C1 to C4 showed a compound (indicated as x in Fig. 4) with a retention time close to that of toxin C3. This compound was not an N-sulfocarbamoyl toxin, since its fluorescence was retained when the oxidizing reagent was replaced by distilled water, unlike the C toxins in the standard mixture (Fig. 4D to F).
FIG. 4.
HPLC-FLD analysis for C toxins in SWC medium cultures of P. diminuta (B and E) and P. stutzeri (C and F) and in toxin standard mixture (A and D). (A to C) Profile obtained with the oxidizing reagent; (D to F) profile obtained after deactivation of the postcolumn oxidation system. The putative C3 peak in bacterial extracts is labeled x.
No compound with the same retention time as a PST was detected in the HPLC analysis of supernatant fractions of either bacterium grown in ASWE or SWC medium.
The analyses of our extracts by postcolumn oxidation HPLC corroborates some of the recent reports (2, 20) of incorrect identification for some PST peaks in bacterial samples. The HPLC profiles obtained were similar to those previously reported for these strains by Franca et al. (5), comprising GTX4, GTX1, and C3, yet discounted in all cases because their chromatographic behavior differed from that of the PST standards. Most PSTs do not exhibit autofluorescence and can be detected as fluorescent compounds only after chemical oxidation with the postcolumn system. However, the bacterial peaks regained their fluorescence even after removal of the oxidizing reagent. As reported by Shimizu et al. (22), the peaks detected by HPLC could be attributed to cellular compounds coextracted by the method used, since neither the growth media by themselves nor the supernatant fractions showed peaks with retention times similar to those of PSTs. Our results were corroborated further by precolumn oxidation HPLC analyses of the same bacterial extracts (data not shown).
In the present study, optimized conditions and approaches for both production and detection of toxins were employed. Specifically, growth conditions were manipulated to yield up to three- to fourfold increases in cellular toxin content (4). Moreover, supernatant fractions that other workers had described as containing toxin levels higher than those found inside the bacterial cells (10) were also assayed in order to more rigorously evaluate our prior results. Nonetheless, using a phosphorous-limited medium with a high salt concentration did not permit detection of PST toxins by the MNB assay, possibly because a high dilution was required to eliminate interference from the medium. In the present study, for the assay with ASWE medium, the use of such high dilutions in high-salt media may have precluded the detection of low toxin levels.
The assay results for the two growth media demonstrate the need to include negative controls when conducting the MNB assay for bacteria (8). In the present case, SCB activity was observed for the ASWE medium by itself; this would yield false-positive results. In fact, the MNB assay is known to be prone to matrix effects (8); this was apparent for the growth media used as controls for both supernatants fractions and extracts in the present study. Arguably, in the latter case, extracts of related bacteria not associated with dinoflagellates might have served as better negative controls; however, centrifugation of cells for extract preparation does not remove all growth medium constituents, and thus certain matrix effects of media and extracts are expected to be similar. Also, the absence of intracellular PSTs, as determined by the MNB assay, was corroborated by HPLC.
In conclusion, our findings are not consistent with previously reported bacterial PST contents determined under similar study conditions, and they add to the growing number of reports that advise caution in the interpretation of HPLC and MNB assay results for PST detection. Regarding the potential for bacterial production of PSTs, further tandem mass spectrometry studies using higher bacterial biomass levels will be required before this possibility can be excluded. Also, the culture conditions may prove to be a determinant in bacterial toxin production. Although phosphorous limitation failed to elicit toxin production, nothing can be concluded regarding the need for dinoflagellates to be present for bacterial toxins to be induced.
Acknowledgments
This work was supported by the POCTI Program (project CTA/1730/95/2001) of the Fundação para a Ciência e Tecnologia (FCT), Portugal.
We appreciate the work of Ana Gago and Jim Lawrence in conducting the precolumn HPLC analyses of the extracts.
The National Ocean Service (NOS) does not approve, recommend, or endorse any product or material mentioned in this publication. No reference shall be made to NOS, or to this publication furnished by NOS, in any advertising or sales promotion which would indicate or imply that NOS approves, recommends, or endorses any product or material mentioned herein or which has as its purpose any intent to cause directly or indirectly the advertised product to be used or purchased because of this NOS publication.
REFERENCES
- 1.Alvito, P., S. Gallacher, A. Gago, J. Lawrence, C. Martins, P. Pereira, and S. Franca. 2000. Application of the mouse neuroblastoma (MNB) assay to the study of PSP toxins from dinoflagellates and cyanobacteria: a comparison of data generated by the MNB assay to pre- and post-column HPLC, p. 257-260. In G. Hallegraeff, S. Blackburn, C. Bolch, and R. Lewis (ed.), Harmful algal blooms. Intergovernmental Oceanographic Commission, UNESCO, Paris, France.
- 2.Baker, T. R., G. J. Doucette, C. L. Powell, G. L. Boyer, and F. G. Plumley. 2003. GTX4 imposters: characterization of fluorescent compounds synthesized by Pseudomonas stutzeri SF/PS and Pseudomonas alteromonas PTB-1, symbionts of saxitoxin-producing Alexandrium spp. Toxicon 41:339-347. [DOI] [PubMed] [Google Scholar]
- 3.Catterall, W. A. 1985. The voltage sensitive sodium channel: a receptor for multiple neurotoxins, p. 329-432. In D. M. Anderson, A. W. White, and D. G. Baden (ed.), Toxic dinoflagellates. Elsevier, New York, N.Y.
- 4.Doucette, G. J., and C. G. Trick. 1995. Characterization of bacteria associated with different isolates of Alexandrium tamarense, p. 33-39. In P. Lassus, G. Arzul, E. Erard, P. Gentien, and C. Marcaillou (ed.), Harmful marine algal blooms, Lavoiser, Intercept Ltd., Paris, France.
- 5.Franca, S., L. Pinto, P. Alvito, I. Sousa, V. Vasconcelos, and G. J. Doucette. 1996. Studies on prokaryotes associated with PSP producing dinoflagellates, p. 347-350. In T. Yasumoto, Y. Oshima, and Y. Fukuyo (ed.), Harmful and toxic algal blooms. Intergovernmental Oceanographic Commission, UNESCO, Paris, France.
- 6.Franca, S., P. Alvito, I. Sousa, and V. Mascarenhas. 1993. The dinoflagellate Gymnodinium catenatum isolated from the coast of Portugal: observations on development, toxicity and ultrastructure, p. 869-874. In T. J. Smayda and Y. Shimizu (ed.), Toxic phytoplankton blooms in the sea. Elsevier Science Publishers B.V., Amsterdam, The Netherlands.
- 7.Franca, S., S. Viegas, V. Mascarenhas, L. Pinto, and G. J. Doucette. 1995. Prokaryotes in association with a toxic Alexandrium lusitanicum in culture, p. 44-51. In P. Lassus, G. Arzul, E. Erard, P. Gentien, and C. Marcaillou (ed.), Harmful marine algal blooms. Lavoiser, Intercept Ltd., Paris, France.
- 8.Gallacher, S., and E. Smith. 1999. Bacteria and paralytic shellfish toxins. Protist 150:245-255. [DOI] [PubMed] [Google Scholar]
- 9.Gallacher, S., and T. H. Birkbeck. 1992. A tissue culture assay for the direct detection of sodium channel blocking toxins in bacterial culture supernatants. FEMS Microbiol. Lett. 92:101-108. [DOI] [PubMed] [Google Scholar]
- 10.Gallacher, S., K. J. Flynn, J. M. Franco, E. E. Brueggemann, and H. B. Hines. 1997. Evidence for production of paralytic shellfish toxins by bacteria associated with Alexandrium spp. (Dinophyta) in culture. Appl. Environ. Microbiol. 63:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haygood, M. G., and K. H. Nealson. 1985. Mechanisms of iron regulation of luminescence in Vibrio fischeri. J. Bacteriol. 162:209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jellet, J. F., L. J. Marks, J. E. Stewart, M. L. Dorey, W. Watson-Wright, and J. F. Lawrence. 1992. Paralytic shellfish poison (saxitoxin family) bioassays: automated endpoint determination and standardization of the in vitro tissue culture bioassay and comparison with the standard mouse bioassay. Toxicon 30:1143-1156. [DOI] [PubMed] [Google Scholar]
- 13.Juntongjin, K., T. Piyakarnchana, K. Kogure, U. Simidu, and K. Ohwada. 1993. Sodium channel blocker-producing bacteria isolated from the Gulf of Thailand. J. Mar. Biotechnol. 1:93-96. [Google Scholar]
- 14.Juntongjin, K., T. Piyakarnchana, M. Kodama, K. Kogure, U. Simidu, and K. Ohwada. 1996. Marine bacteria that produce toxins similar to paralytic shellfish poisons and tetrodotoxins from the sand clam (Asaphis violascens) of the Gulf of Thailand. J. Mar. Biotechnol. 3:268-273. [Google Scholar]
- 15.Lu, Y., T. Chai, and D. Hwang. 2000. Isolation of bacteria from toxic dinoflagellate Alexandrium minutum and their effects on algae toxicity. J. Nat. Toxins 9:409-417. [PubMed] [Google Scholar]
- 16.Mascarenhas, V., P. Alvito, S. Franca, I. Sousa, A. Gago-Martinez, and J. A. Rodriguez-Vazquez. 1995. The dinoflagellate Alexandrium lusitanicum isolated from the coast of Portugal: observations on toxicity and ultrastructure during growth phases, p. 71-76. In P. Lassus, G. Arzul, E. Erard, P. Gentien, and C. Marcaillou (ed.), Harmful marine algal blooms. Lavoiser, Intercept Ltd., Paris, France.
- 17.Negri, A. P., G. J. Jones, S. I. Blackburn, Y. Oshima, and H. Onodera. 1997. Effect of culture and bloom development and of sample storage on paralytic shellfish poisons in the cyanobacterium Anabaena circinalis. J. Phycol. 33:26-35. [Google Scholar]
- 18.Onodera, H., M. Satake, Y. Oshima, T. Yasumoto, and W. Carmichael. 1997. New saxitoxin analogues from the freshwater filamentous cyanobacterium Lyngbya wollei. Nat. Toxins 5:146-151. [DOI] [PubMed] [Google Scholar]
- 19.Oshima, Y. 1995. Post-column derivatization liquid chromatographic method for paralytic shellfish toxins. J. AOAC Int. 78:528-532. [Google Scholar]
- 20.Pereira, P., D. Andrinolo, F. Sam-Bento, P. Alvito, C. Martins, and S. Franca. 2000. Novos resultados sobre estudos de toxicidade PSP em dinoflagelados e bactérias associadas, p. 139-148. In 6th Reunión Ibérica sobre Fitoplancton Tóxico y Biotoxinas, Seville. A. P. D. G. Junta de Andalucia, Seville, Spain.
- 21.Sato, S., and Y. Shimizu. 1998. Purification of a fluorescent product of the bacterium Moraxella sp.: a neosaxitoxin impostor, p. 465-467. In B. Reguera, J. Blanco, M. L. Fernández, and T. Wyatt (ed.), Harmful algae. Xunta de Galicia and UNESCO Intergovernmental Oceanographic Commission, Grafisant, Santiago de Compostela, Spain.
- 22.Shimizu, Y., C. Giorgio, C. Koerting-Walker, and T. Ogata. 1996. Nonconformity of bacterial production of paralytic shellfish poisons—neosaxitoxin production by a bacterium strain from Alexandrium tamarense Ipswich strain and its significance, p. 359-362. In T. Yasumoto, Y. Oshima, and Y. Fukuyo (ed.), Harmful and toxic algal blooms. Intergovernmental Oceanographic Commission, UNESCO, Paris, France.