Abstract
Improgan, a congener of the H2 antagonist cimetidine, produces non-opioid antinociception which is blocked by the CB1 antagonist rimonabant, implying a cannabinoid mechanism of action. Since cannabinoids produce hypothermia as well as antinociception in rodents, the present study investigated the pharmacological activity of improgan on core body temperature and nociceptive (tail flick) responses. Improgan (60, 100, and 140 μg, intraventricular [ivt]) elicited significant decreases in core temperature 3–30 min following injection with a maximal hypothermic effect of −1.3°C. Pretreatment with rimonabant (50 μg, ivt) produced a statistically significant, but incomplete (29% – 42%) antagonism of improgan hypothermia. In control experiments, the CB1 agonist CP-55, 940 (37.9 μg, ivt) induced significant decreases in core temperature (−1.8°C) 3–30 min following injection. However, unlike the case with improgan, pretreatment with rimonabant completely blocked CP-55,940 hypothermia. Furthermore, CP-55,940 and improgan elicited maximal antinociception over the same time course and dose ranges, and both effects were attenuated by rimonabant. These results show that, like cannabinoid agonists in the rat, improgan produces antinociception and hypothermia which is blocked by a CB1 antagonist. Unlike cannabinoid agonists, however, improgan does not produce locomotor inhibition at antinociceptive doses. Additional experiments were performed to determine the effect of CC12, a recently-discovered improgan antagonist which lacks affinity at CB1 receptors. Pretreatment with CC12 (183 μg, ivt) produced complete inhibition of both the antinociception and the hypothermia produced by improgan, suggesting the possible role of an unknown improgan receptor in both of these effects.
1. Introduction
Improgan (N-cyano-N’-[3-(imidazole-4-yl)propyl]-N”-methyl-guanidine), a derivative of the H2 receptor antagonist cimetidine, is a member of a new class of non-opioid analgesics chemically related to histamine. Direct administration of improgan into the central nervous system via the lateral ventricle produces a robust antinociceptive effect as measured by thermal and mechanical nociceptive tests (Li et al., 1997a). However, in contrast to morphine, daily dosing with improgan does not result in tolerance (Bannoura et al., 1998). Thus, improgan seems to have a favorable clinical profile as an analgesic agent that lacks the aversive side-effects often associated with current clinically used analgesics such as morphine (Hough et al., 2000). However, improgan’s mechanism remains unknown. In vitro and in vivo studies have shown that improgan does not activate known histamine (Izadi et al., 2003; Hough et al., 2004), opioid, (Li et al., 1997b; Hough et al., 2000), serotonergic (Nalwalk et al., 2005) or adrenergic receptors, as well as over 50 other known G-protein coupled receptors (Hough et al., 2000).
A possible breakthrough in understanding improgan action identified a potential link between improgan and cannabinoids. It was shown that pretreatment with the CB1 antagonist rimonabant (SR141716A) completely blocked improgan antinociception (Hough et al., 2002), suggesting a role for cannabinoid modulation in improgan action. However, radioligand binding studies showed that improgan possesses little or no affinity for known cannabinoid receptors in either rat or mouse preparations, as well as in recombinant cell lines containing the human CB1 receptor (Hough et al., 2002). Furthermore, it was recently shown that development of tolerance to ∆ 9-tetrahydrocannabinol (THC) was accompanied by cross-tolerance to improgan (Nalwalk et al., 2006). Taken together, these findings suggest that improgan elicits its antinociceptive effect either indirectly by a CB1 -mediated endocannabinoid mechanism, or possibly by action at an unknown cannabinoid receptor (Nalwalk et al., 2006).
In addition to antinociception, cannabinoids are known to produce a variety of pharmacological effects including hypomobility, catalepsy, (Lichtman et al., 1996; Lichtman and Martin, 1991, 1997) and the hallmark, hypothermia (Schmeling and Hosko, 1980; Lichtman et al., 1996; Malone and Taylor, 1998). If improgan activates cannabinoid mechanisms, then this drug may also possess other non-antinociceptive properties shared by cannabinoids. In contrast with well-documented cannabinoid actions in rats, improgan does not reduce spontaneous locomotor activity nor impair motor coordination at maximal antinociceptive doses in this species (Li et al., 1997a). However, the effects of improgan on body temperature have not been reported. The present study assessed the effect of improgan on core body temperature, and whether these changes were modulated by the cannabinoid antagonist rimonabant and/or by the recently-discovered putative improgan antagonist CC12 (Hough et al., 2007).
2. Results
Administration of improgan to rats decreased core body temperature (Fig. 1A) and increased nociceptive tail flick latencies (Fig. 1B). On core temperature, ANOVA (between groups: dose of improgan, within groups [repeated measures]: time) showed significant main effects of dose (F=7.53, DF=3, P < 0.01) and time (F=10.86, DF=4, P < 0.001), and a signficant dose by time interaction term (F=3.33, DF=12, P<0.001). Lower doses of improgan (60 μg and 100 μg) induced maximal hypothermic effects of −0.85ºC at 5-min and −1.3ºC at 10-min post-injection, respectively, compared to vehicle at the same time. The highest dose of improgan (140 μg) induced a delayed hypothermic effect (−1.3ºC) that was maximal at the 30 min post-injection interval (Fig. 1A). Additional experiments found that improgan-induced hypothermia (100 μg) was maximal at 10- and 30-min post-injection and returned to baseline levels 90 min later (data not shown).
Figure 1.
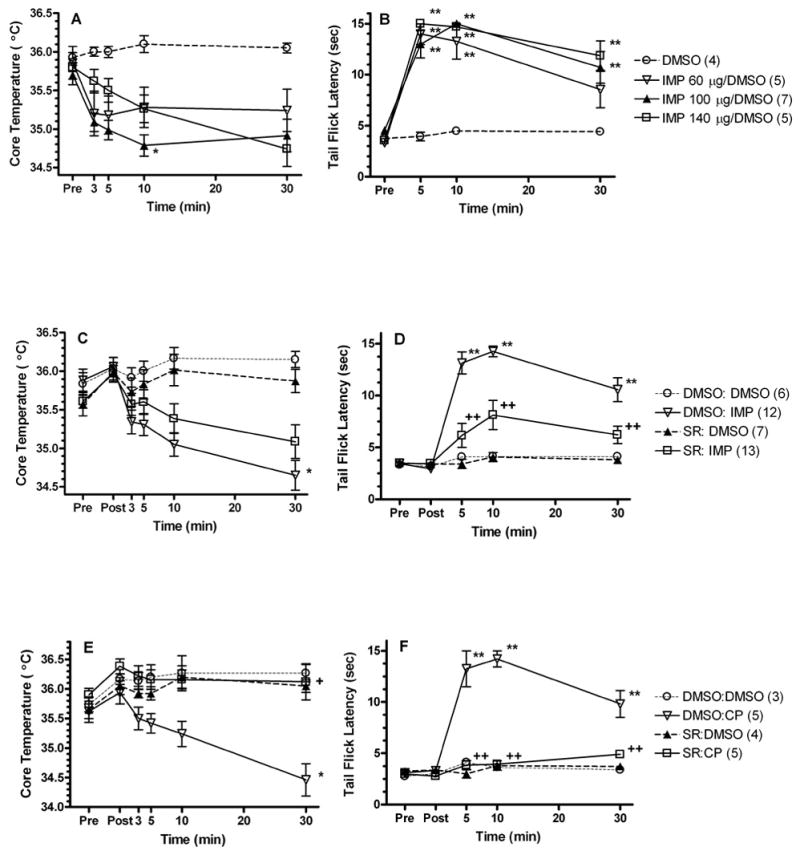
Effects of improgan (A–D) and the cannabinoid agonist CP-55,940 (E, F) on core temperature (left, A, C, E) and nociceptive responses (right, B, D, F). The effects of the CB1 antagonist rimonabant on these responses are also shown (C–F). Top (A,B): Each animal was tested for baseline (Pre) responses prior to a single ivt injection (5 μL over 5 min) of either vehicle (60% DMSO) or improgan (60, 100, or 140 μg). Animals were then re-tested at the times indicated on the x-axis. Data shown at the 30 min point were pooled from subjects tested at 30- and 35-min. Each group represents the mean ± SEM for the number of animals given in parentheses. *,**P < 0.05, 0.01, respectively compared to DMSO vehicle at the same time. Middle and Bottom (C–F): Following baseline testing (Pre), subjects received a single injection (2 μL, ivt) over 1 min of either vehicle (100% DMSO) or rimonabant (SR, 50 μg) and then were re-tested (Post). Animals then received a second injection (5 μL, ivt) over 5 min of vehicle (100% DMSO, C–F), improgan (IMP, 100 μg, C,D) or CP-55,940 (CP, 37.9 μg, E,F) and were re-tested at the times indicated on the x-axis. Each group represents the mean ± SEM, for the number of animals given in parentheses. The control groups (DMSO:DMSO and SR:DMSO) are the same data in the improgan (C,D) and CP-55,940 (E,F) experiments. *,**P <0.05, 0.01, respectively compared to DMSO: DMSO at the same time. +, ++P <0.05, 0.01, respectively, compared to DMSO:IMP (C,D) or DMSO:CP (E,F) at the same time.
On tail flick latencies, ANOVA (between groups: dose of improgan, within groups [repeated measures]: time) showed significant main effects of dose (F=22.16, DF=3, P < 0.0001) and time (F=61.38, DF=3, P < 0.0001), and a signficant dose by time interaction (F=5.82, DF=9, P<0.0001) . All three doses of improgan increased tail flick latencies to near- maximal or maximal nociceptive thresholds (Fig. 1B). Since improgan (100 μg, ivt) induced both a maximal hypothermic and antinociceptive effect 10 min after administration, this dose of improgan was chosen for subsequent experiments.
Because improgan decreases core temperature and because previous studies reported that the CB1 antagonist rimonabant blocks improgan antinociception (Hough et al., 2002), it was of interest to determine the effects of rimonabant on both improgan-induced antinociception and hypothermia. Similar to the findings in Figs. 1A and 1B (performed with a single ivt injection), data from Figs. 1C and 1D (with two ivt injections) confirmed that improgan produced both hypothermia and antinociception. Rimonabant pretreatment signficantly reduced both effects. On core temperature (Fig. 1C), ANOVA (between groups #1: rimonabant pretreatment, between groups #2: improgan treatment, within groups [repeated measures]: time) showed significant main effects of improgan (F=9.9, DF=1, P< 0.01) and time (F=14.0, DF=5, P<0.0001), with signficant improgan by time (F=22.7, DF=5, P<0.0001), and rimonabant by improgan by time (F=2.3, DF=5, P<0.05 ) interactions. The three-way interaction term indicates that rimonabant significantly reduced improgan hypothermia, although post-hoc testing did not identify specific time points at which this occurred.
Rimonabant reduced mean values of improgan-induced hypothermia (calculated by comparing with control values at each time) by 40%, 42%, 30% and 29% at the 3, 5, 10 and 30-min intervals, respectively. Rimonabant also inhibited improgan antinociception (Fig. 1D). ANOVA (between groups #1: rimonabant pretreatment, between groups #2: improgan treatment, within groups [repeated measures]: time) found highly significant main effects of rimonabant (F=14.3, DF=1, P<0.001), improgan (F=56.4, DF=1, P<0.001) and time (F=25.4, DF=4, P<0.001). All interaction terms were also highly signficant (P<0.001; improgan by rimonabant: F= 12.4, DF=1; rimonabant by time: F=5.8, DF=4; improgan by time: F=17.8, DF=4; rimonabant by improgan by time: F=4.0, DF=4). Rimonabant reduced improgan antinociception by 77%, 60% and 67% at 5, 10 and 30 min, respectively. All of these effects were significant by post-hoc testing (Fig. 1D).
In order to properly assess the role of cannabinoid receptors in improgan hypothermia, control experiments documenting cannabinoid hypothermia and its reversal by rimonabant were required (Figs. 1E). ANOVA (between groups #1: rimonabant pretreatment, between groups #2: CP-55,940 treatment, within groups [repeated measures]: time) found a significant main effect of time (F=7.0, DF=5, P<0001), with signficant rimonabant by CP-55,940 (F=9.1, DF=1, P<0.01), rimonabant by time (F=3.7, DF=5, P<0.01), CP-55,940 by time (F=9.3, DF=5, P<0.001) and rimonabant by CP-55,940 by time (F=4.9, DF=5, P<0.001) interactions. CP-55,940 produced a maximal decrease in core temperature (−1.8º) at 30-min post-injection (Fig. 1E). Additional studies found that CP-55,940 hypothermia was maximal at 30- and 45-min post-injection and returned toward baseline levels 90 min later (data not shown). Rimonabant appeared to completely attenuate cannabinoid hypothermia at all time points. Post-hoc testing confirmed the cannabinoid hypothermia and its reversal by rimonabant at the 30 min time point (Fig. 1E).
As expected, CP-55,940 produced robust antinociception which was blocked by rimonabant (Fig. 1F). ANOVA (between groups #1: rimonabant pretreatment, between groups #2: CP-55,940 treatment, within groups [repeated measures]: time) found all terms to be highly significant (P<0.001: main effects of rimonabant [F=53.5, DF=1], CP-55,940 [F=67.8, DF=1], and time [F=22.7, DF=4]; interactions were rimonabant by CP-55,940 [F=55.5, DF=1], rimonabant by time [F=14.7, DF=4], CP-55,940 by time [F=15.5, DF=4], and rimonabant by CP-55,940 by time [F=10.4, DF=4]). Antagonism of CP-55,940 antinociception by rimonabant exceeded 95% at all times (Fig. 1F). Rimonabant alone had no significant effect on either core temperature (Fig. 1C, 1E) or nociceptive latency (Fig. 1D, 1F).
To further explore the mechanism by which improgan produces hypothermia, the effects of the putative improgan antagonist CC12 were studied (Fig. 2). Similar to earlier observations, improgan (100 μg) elicited a maximal decrease in core temperature (−1.25°C) 10-min post-injection (Fig. 2A). CC12 pretreatment effectively blocked improgan hypothermia (89% – 119%) over the time course of the experiment (3 – 30 min). ANOVA (between groups #1: CC12 pretreatment, between groups #2: improgan treatment, within groups [repeated measures]: time) found a significant main effect of improgan (F=4.6, DF=1, P<0.05) and significant CC12 by improgan (F=12.7, DF=1, P<0.05), CC12 by time (F=7.3, DF=5, P<0.001), and CC12 by improgan by time (F=6.0, DF=5, P<0.001) interactions. The latter term confirms the significant antagonism by CC12. No differences were observed at individual time points by post-hoc testing (Fig. 2A).
Figure 2.
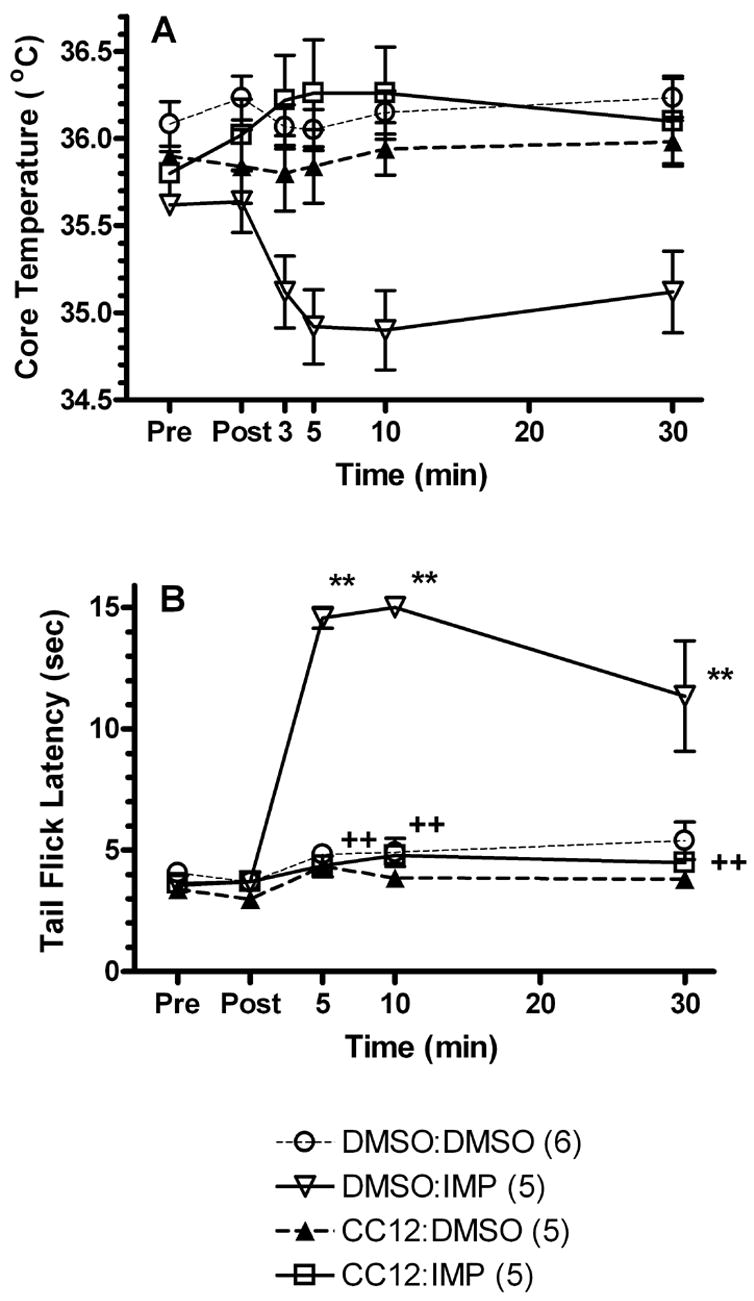
Effect of CC12 pretreatment on improgan-induced changes in core temperature (A) and nociceptive responses (B). Each animal was tested for baseline (Pre) responses, received a single injection (5 μL, ivt) over 5 min of either vehicle (100% DMSO) or CC12 (183 μg) and were re-tested (Post) 15 min following the end of the injection. Animals then immediately received a second injection (5 μL, ivt) over 5 min of vehicle (60% DMSO) or improgan (IMP, 100 μg) and were re-tested at the times indicated on the x-axis. Each group represents the mean ± SEM for the number of animals shown in parentheses. ** P < 0.01 compared to DMSO:DMSO vehicle at the same time. ++ P < 0.01 compared to DMSO: IMP at the same time.
On nociceptive responses (Fig. 2B), ANOVA (between groups #1: CC12 pretreatment, between groups #2: improgan treatment, within groups [repeated measures]: time) found all terms to be highly significant (P<0.001: main effects of CC12 [F=77.7, DF=1], improgan [F=60.2, DF=1], and time [F=43.5, DF=4]; interactions were CC12 by improgan [F=39.8, DF=1], CC12 by time [F=23.8, DF=4], improgan by time [F=22.4, DF=4], and CC12 by improgan by time [F=22.0, DF=4]). CC12 alone had no effect on core temperature or nociceptive thresholds.
3. Discussion
The effects of improgan on body temperature (not previously reported) are of interest because of the possible cannabinoid mechanism of action of this drug. Pilot studies with a maximal analgesic dose of improgan (100 μg) showed a decrease in core temperature of approximately −1ºC. To examine the dose-response relationship of this effect, two other doses were studied. Since all three doses (60, 100 and 140 μg) resulted in approximately the same decrease in core temperature (Fig. 1A), the observed temperature change of −1.3 ºC is likely to be the maximal hypothermic effect, suggesting that the hypothermic EC50 dose of improgan is less than 60 μg. The hypothermic potency (Fig. 1A) of improgan may be similar to that of its antinociceptive potency (Fig. 1B), in that for both antinociception and hypothermia the EC50 dose of improgan seems to be less than 60 μg.
Previous results established that improgan produces dose-dependent antinociceptive responses (Li et al., 1996). The present results (Fig. 1B), which suggest that improgan’s antinociceptive EC50 is less than 60 μg, are in contrast to those of an earlier study which reported the EC50 dose of improgan to be 64.5 μg (Li et al., 1996). This difference most likely results from the vehicle used in the respective studies. In the Li et al. (1996) study, improgan was dissolved in saline, whereas, in the present study the drug was dissolved in 60% DMSO. Due to DMSO’s well-known ability to increase drug permeability, improgan most likely had a greater diffusion gradient in the present study. In an earlier cannabinoid-improgan study (Hough et al., 2002), improgan (dissolved in 60% DMSO) showed an ivt antinociceptive EC50 of approximately 40 μg, consistent with present results (Fig. 1B). Although DMSO had no antinociceptive effects alone, it is possible that this vehicle produces antinociceptive potentiation of improgan responses due to a pharmacodynamic action on the brain, rather than a pharmacokinetic effect on drug permeability. Although DMSO is known to modify nociceptive responses when given systemically due to actions on peripheral neurons (Dajani et al., 1999), potentiation of analgesic effects by an action on the brain has not been reported.
The finding that improgan decreases core body temperature seems consistent with (but not proof of) the hypothesis that improgan acts through cannabinoid CB1 receptors. Earlier results suggest that either WIN-55,212-2 or CP-55,940 might serve as a suitable cannabinoid control drug for inducing hypothermia. (Lichtman et al., 1996; Rawls et al., 2002). However, pilot studies with ivt WIN 55,212-2 (20 μg) failed to elicit significant hypothermia between 3–30 min following drug administration (data not shown). The lack of hypothermia after ivt WIN 55,212-2 was not a result of contamination or inactive drug, because it produced a robust hypothermia following systemic dosing (5 mg/kg, i.m., data not shown). However, the present study, which found a maximal hypothermic effect by CP-55,940 (36.9 μg, ivt) of −1.8 º C (Fig. 1E), confirms the findings of Lichtman et al. (1996), and shows that this drug serves as an appropriate positive control. As expected, the present study found that ivt rimonabant completely blocked ivt CP-55,940-induced hypothermia (Fig. 1E), which supports a brain CB1-mediated mechanism for this effect (Pertwee, 2001). Systemic dosing with rimonabant has been shown to block hypothermia induced by either systemic, ivt or intracerebral cannabinoid administration (Rinaldi-Carmona et al., 1994; Rawls et al., 2002), but we are unaware of any previous studies documenting the ability of ivt rimonabant to block hypothermia produced by ivt cannabinoids.
Since both improgan and cannabinoids elicit antinociceptive and hypothermic effects (Fig. 1), and rimonabant blocks both of the antinociceptive actions (Figs. 1D, 1F), the effect of rimonabant on improgan hypothermia was of considerable interest. Although improgan hypothermia was not completely reversed by rimonabant (as was the case with cannabinoid hypothermia, Fig. 1E), the incomplete (29% – 42%) antagonism of improgan hypothermia by the CB1 antagonist was statistically sound (Fig. 1C). At face value, this finding suggests that both of improgan’s effects (antinociception and hypothermia) may utilize a cannabinoid mechanism. If CB1 or other unknown cannabinoid receptors contribute to improgan hypothermia, then the incomplete inhibitory effects of rimonabant observed presently suggest that additional receptors may also be involved.
Recently, CC12, an imidazole derivative discovered from binding assays, was reported to be a competitive antagonist of improgan antinociception which lacks CB1 receptor-blocking properties (Hough et al., 2007). Although the mechanism of CC12’s antagonism of improgan remains unknown, the possibility that CC12 might act as an improgan receptor antagonist was suggested (Hough et al., 2007). If so, then CC12 might be expected to block all improgan-induced effects, including improgan hypothermia. The present results, showing that CC12 blocked both improgan hypothermia (Fig. 2A) and improgan antinociception (Fig. 2B), support this hypothesis, and suggests that these two distinct effects may be activated by the same unknown improgan receptor.
The present demonstration of a hypothermic effect of improgan is the first non-analgesic action to be described for this drug. The magnitude of the maximal hypothermic effect is smaller than observed with cannabinoids. Statistically significant (but incomplete) antagonism of this effect by the CB1 antagonist rimonabant suggests that both the antinociceptive and the hypothermic effects of improgan may utilize a cannabinoid mechanism. Complete blockade of improgan hypothermia and antinociception by the improgan antagonist CC12 further supports the hypothesis that a novel improgan receptor may underlie both of improgan’s actions. Further studies must be performed to elucidate these mechanisms.
4. Experimental Procedures
Animals
Male Sprague-Dawley rats (150–300g, from Taconic Farms, Germantown, NY) were maintained on a twelve-hr light/dark cycle (lights on from 0700–1900). Animals were provided with food and water ad libitum. They were housed in pairs prior to surgery and individually thereafter. Each subject was used in a single experiment. All animal experiments were approved by the Institutional Animal Care and Use Committee of Albany Medical College.
Drugs and solutions
Rimonabant (base) was kindly provided by the National Institute on Drug Abuse. CC12 (4(5)-((4-iodobenzylthio) methyl)-1H-imidazole hydrochloride), dosed as salt, was synthesized by Dr. James Phillips (Curraugh Chemicals Inc., Cleveland, OH; Hough et al., 2007). CC12, CP-55,940 (Tocris, Ellisville, MO) and rimonabant were dissolved in 100% DMSO. Improgan (base), provided by Dr. Rob Leurs, Vrie University, Amsterdam, Netherlands, was dissolved in 60% aqueous DMSO. The identities of all solutions were blinded to the investigator.
Surgery
Animals were anesthetized with Nembutal (50 mg/kg, i.p.) and supplemented with isoflurane. Chronic guide cannulas (17mm in length) were stereotaxically implanted into the left lateral ventricle at coordinates with respect to bregma (mm) (Paxinos and Watson, 1986): −0.8 anterior/posterior, +1.5 medial/lateral, −3.3 dorsal/ventral. Cannulas were anchored to the skull with three stainless steel screws and cranioplast dental cement (Crane and Glick, 1979). Following surgery, animals were allowed to recover for 5–7 days before testing.
Nociceptive testing
Drug-induced nociceptive responses were measured by the tail flick test (D'Amour and Smith, 1941). A randomly selected portion of the ventral surface of the tail (2–5 cm from the tip) was exposed to a radiant heat source. Three tail flick latencies were measured at one min intervals with the third measurement serving as the baseline measurement. The heat source was set so that baseline latencies were generally 3 and 4s with a 15s cutoff. The heat source was not adjusted for individual animals.
Temperature testing
Core (rectal) temperature was measured by inserting a probe (Digi-Sense, Model 8523-00) approximately 2.5 cm into the rectum. Skin temperature was measured by securing a probe (Physitemp, Model BAT-12) to the dorsal surface near the base of the tail. For both measurements, the rats were gently immobilized by a laboratory pad and probes were allowed to equilibrate for 30s prior to recording temperature readings. Skin temperatures showed high variability and are not presently reported. Room temperature was maintained between 23 °C and 24 °C.
Testing procedure
Animals were tested for baseline nociceptive responses followed by baseline core temperature testing. They were then gently secured and wrapped in a laboratory pad, the stylet removed, and an injection cannula was inserted. The injection cannula extended 1 mm beyond the guide cannula into the left lateral ventricle. An intraventricular (ivt) injection (5 μl) was performed manually over a specified time interval. One min following the end of the infusion, the injection cannula was cut to seal off the injection cannula approximately 2 mm above the juncture where the guide and injection cannulas meet. A core temperature measurement was made without tail flick testing 3 min after the end of the infusion. Nociceptive responses, followed immediately by temperature measurements, were taken at 5, 10 and 30 min following the end of the ivt injection. In some experiments, animals received a second ivt injection. Unless indicated otherwise, temperature measurements always immediately followed nociceptive testing at each time point.
Successful ivt injections were confirmed by following the movement of an air bubble in the tubing between the syringe and injection cannula and by the absence of leakage at the juncture between the guide and injection cannulas. Following the completion of the experiments the animals received an injection of pentobarbital sodium (100 mg/kg, i.p.) and India ink (5 μL, ivt). Proper placement of the injection cannula into the lateral ventricle was confirmed by verifying the distribution of the India ink in the cerebroventricular system. Data from animals with unsuccessful injections or poor cannula placements were excluded.
Data analysis
Results are expressed as mean ± SEM latencies (s) or core temperatures (ºC). Analyses of variance (ANOVA) with repeated measures were used to analyze the data. Bonferroni post-hoc analyses were performed to determine significant differences between groups. Statistical analyses were performed with either Graphpad Prism 4.0 (San Diego, CA) or Statistica (Statsoft, Tulsa, OK).
Acknowledgments
This work was supported by grants from National Institute on Drug Abuse (DA-003816, DA-015915). We thank Prof. R. Leurs (Vrije University, Amsterdam), and Dr. James Phillips (Curragh Chemistries, Cleveland, OH) for providing improgan and CC12, respectively. We also thank Konstantina Svokos for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bannoura MD, Nalwalk JW, Tang Y, Carlile M, Leurs R, Menge WM, Timmerman H, Hough LB. Absence of antinociceptive tolerance to improgan, a cimetidine analog, in rats. Brain Res. 1998;814:218–221. doi: 10.1016/s0006-8993(98)01024-5. [DOI] [PubMed] [Google Scholar]
- Crane LA, Glick SD. Simple cannula for repeated intracerebral drug administration in rats. Pharmacol Biochem Behav. 1979;10:799–800. doi: 10.1016/0091-3057(79)90336-8. [DOI] [PubMed] [Google Scholar]
- Dajani EZ, Larsen KR, Taylor J, Dajani NE, Shahwan TG, Neeleman SD, Taylor MS, Dayton MT, Mir GN. 1',1'-Dimethylheptyl-delta-8-tetrahydrocannabinol-11-oic acid: a novel, orally effective cannabinoid with analgesic and anti-inflammatory properties. Journal of Pharmacology and Experimental Therapeutics. 1999;291:31–38. [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. The Journal of Pharmacology and Experimental Therapeutics. 1941;72:74–79. [Google Scholar]
- Hough LB, Nalwalk JW, Barnes WG, Leurs R, Menge WM, Timmerman H, Wentland M. A third life for burimamide. Discovery and characterization of a novel class of non-opioid analgesics derived from histamine antagonists. Ann NY Acad Sci. 2000;909:25–40. doi: 10.1111/j.1749-6632.2000.tb06674.x. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Stadel R, Timmerman H, Leurs R, Paria BC, Wang X, Dey SK. Inhibition of improgan antinociception by the cannabinoid (CB)(1) antagonist N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-p yrazole-3-carboxamide (SR141716A): lack of obligatory role for endocannabinoids acting at CB(1) receptors. J Pharmacol Exp Ther. 2002;303:314–322. doi: 10.1124/jpet.102.036251. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Svokos K, Leurs R, Timmermann H. Pain-relieving drugs and the brain histaminergic system: multiple analgesic mechanisms from histamine, improgan and cimetidine. Inflamm Res. 2004;53(Suppl 1):S43–S44. doi: 10.1007/s00011-003-0320-7. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Phillips JG, Kern B, Shan Z, Wentland MP, de Esch IJP, Janssen E, Barr T, Stadel R. CC12, a high affinity ligand for 3H-cimetidine binding, is an improgan antagonist. Neuropharmacology. 2007 doi: 10.1016/j.neuropharm.2007.01.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadi MJ, Nalwalk JW, Watanabe T, Sakurada S, Hoffman M, Leurs R, Timmerman H, Silos-Santiago I, Yanai K, Hough LB. Improgan antinociception does not require neuronal histamine or histamine receptors. Brain Res. 2003;974:146–152. doi: 10.1016/s0006-8993(03)02572-1. [DOI] [PubMed] [Google Scholar]
- Li BY, Nalwalk JW, Barker LA, Cumming P, Parsons ME, Hough LB. Characterization of the antinociceptive properties of cimetidine and a structural analog. J Pharmacol Exp Ther. 1996;276:500–508. [PubMed] [Google Scholar]
- Li BY, Nalwalk JW, Finkell JM, Glick SD, Hough LB. SKF92374, a cimetidine analogue, produces mechanical and thermal antinociception in the absence of motor impairment. Analgesia. 1997a;3:15–20. [Google Scholar]
- Li BY, Nalwalk JW, Hough LB. Effects of naltrexone and histamine antagonists on the antinociceptive activity of the cimetidine analog SKF92374 in rats. Brain Res. 1997b;748:168–174. doi: 10.1016/s0006-8993(96)01288-7. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Cannabinoid-induced antinociception is mediated by a spinal alpha 2-noradrenergic mechanism. Brain Res. 1991;559:309–314. doi: 10.1016/0006-8993(91)90017-p. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. The selective cannabinoid antagonist SR 141716A blocks cannabinoid-induced antinociception in rats. Pharmacol Biochem Behav. 1997;57:7–12. doi: 10.1016/s0091-3057(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. Modulation of delta9-tetrahydrocannabinol-induced hypothermia by fluoxetine in the rat. Br J Pharmacol. 1998;124:1419–1424. doi: 10.1038/sj.bjp.0701980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Hough LB. Cannabinoid-improgan cross tolerance: improgan is a cannabinomimetic analgesic lacking affinity at the CB1 receptor. Eur J Pharmacol. 2006;549:79–83. doi: 10.1016/j.ejphar.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Leurs R, Hough LB. Absence of 5-HT3 and cholinergic mechanisms in improgan antinociception. Pharmacol Biochem Behav. 2005;80 :505–510. doi: 10.1016/j.pbb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney: 1986. [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Cabassa J, Geller EB, Adler MW. CB1 Receptors in the Preoptic Anterior Hypothalamus Regulate WIN 55212-2 [(4,5-Dihyro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl-)6H-pyrrolo{3,2,1ij]quinolin-6-one]-Induced Hypothermia. The Journal of Pharmacology and Experimental Therapeutics. 2002;301:963–968. doi: 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Schmeling WT, Hosko MJ. Hypothermic effects of intraventricular and intravenous administration of cannabinoids in intact and brainstem transected cats. Neuropharmacology. 1980;19:567–573. doi: 10.1016/0028-3908(80)90028-3. [DOI] [PubMed] [Google Scholar]


