Abstract
Legume plants have an ability to fix atmospheric nitrogen into nutrients via symbiosis with soil microbes. As the initial event of the symbiosis, legume plants secrete flavonoids into the rhizosphere to attract rhizobia. Secretion of flavonoids is indispensable for the establishment of symbiotic nitrogen fixation, but almost nothing is known about the membrane transport mechanism of flavonoid secretion from legume root cells. In this study, we performed biochemical analyses to characterize the transport mechanism of flavonoid secretion using soybean (Glycine max) in which genistein is a signal flavonoid. Plasma membrane vesicles prepared from soybean roots showed clear transport activity of genistein in an ATP-dependent manner. This transport activity was inhibited by sodium orthovanadate, a typical inhibitor of ATP-binding cassette (ABC) transporters, but was hardly affected by various ionophores, such as gramicidin D, nigericin, or valinomycin, suggesting involvement of an ABC transporter in the secretion of flavonoids from soybean roots. The Km and Vmax values of this transport were calculated to be 158 μm and 322 pmol mg protein−1 min−1, respectively. Competition experiments using various flavonoids of both aglycone and glucoside varieties suggested that this ABC-type transporter recognizes genistein and daidzein, another signaling compound in soybean root exudates, as well as other isoflavonoid aglycones as its substrates. Transport activity was constitutive regardless of the availability of nitrogen nutrition. This is, to our knowledge, the first biochemical characterization of the membrane transport of flavonoid secretion from roots.
Plant root exudates secreted into the soil mediate complex interactions between soil-born microorganisms and plants. These interactions occur in the small region around the plant roots, which is called the rhizosphere, where plants protect root tissues from attack by bacteria and fungi and symbiotic interactions with rhizobia and arbuscular mycorrhiza are also involved in a cooperative context. Examples of root exudates responsible for the protective actions include cyclic hydroxamic acids, such as 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one and the naphthoquinone-derivative shikonin [5,8-dihydroxy-2-(1-hydroxy-4-methylpent-3-en-1-yl)-1,4-naphthoquinone], which were reported in wheat (Triticum aestivum) and Lithospermum erythrorhizon, respectively (Niemeyer, 1988; Brigham et al., 1999). The well-known phenomenon called allelopathy, in which organic compounds in the root exudates or secondary metabolites produced in the aerial part of the plant cause deleterious effects on neighboring plants, thus suppressing the growth of other plants, is an example of interactions between plants in the rhizosphere (Weir et al., 2004). In contrast to the negative interactions between plants and other organisms, positive interactions between plants and microbes also occur in the rhizosphere. Flavonoids in root exudates of legume plants activate nod gene expression of rhizobia (Peters et al., 1986) and strigolactones secreted from various plants induce the hyphal branching of arbuscular mycorrhizal fungi (Akiyama et al., 2005). These two events are not simply advantageous, but indispensable for the establishment of symbiosis to assimilate nitrogen and phosphorus, respectively.
Among rhizosphere interactions, legume-Rhizobium symbiosis is of particular importance in agriculture because, by forming the symbiosis, atmospheric nitrogen can be used to sustain the growth of legume crops, such as soybean (Glycine max), pea (Pisum sativum), and bean (Phaseolus vulgaris), which occupy 12% to 15% of the arable land throughout the world (Graham and Vance, 2003). Moreover, legume-Rhizobium symbiosis is also important for environmental reasons because fixed atmospheric nitrogen can replace nitrogen fertilizers that are used in large quantities, but consume substantial amounts of energy and raw materials in their production, and can result in potential environmental pollution when used on a large scale.
Symbiosis takes place in nodules, which are specifically developed organs in root tissues of legume plants following infection by species-specific bacteria, and the nodulation process starts with the interrecognition of signaling molecules between the plant and rhizobia. Plant roots secrete signaling molecules (e.g. flavonoids, which lead rhizobia to colonize around root tissues of a host legume plant), whereas bacteria produce their own signaling molecules, called Nod factors, which are lipochitooligosaccharides. Nod factors induce formation of a transient subcellular gradient of inorganic ions, such as chloride, potassium, and calcium, as well as a pH gradient in root cells, followed by calcium oscillation (Cardenas et al., 2000). Curled root hairs then entrap rhizobia followed by formation of the infection thread. Rhizobia may enter plant cells via endocytosis, resulting in the formation of symbiosomes in cortex cells, where rhizobia are surrounded by the plasma-membrane-derived peribacteroid membrane (Verma and Hong, 1996).
Recent progress in two genome projects of model legumes, Lotus japonicus and Medicago truncatula, accelerated map-based cloning of the genes responsible for defects in various mutants, which has enabled identification of many polypeptides that function in the recognition of Nod factors and the subsequent signaling pathway leading to the establishment of symbiotic nitrogen fixation (Stacey et al., 2006). Although it has been reported very recently that endogenous flavonoids, such as genistein and daidzein, are essential for the establishment of the symbiotic interaction with rhizobia (Subramanian et al., 2006), almost nothing has been characterized about the membrane transport mechanism of these flavonoid molecules secreted from plant roots, whereas the signal compounds to induce nod genes were identified more than 20 years ago (Firmin et al., 1986; Peters et al., 1986; Redmond et al., 1986; Kosslak et al., 1987). In this article, we used soybean and genistein, which is secreted into the soybean root exudate (Smit et al., 1992) and induces nod genes of Bradyrhizobium japonicum (Kosslak et al., 1987) as a model system to perform biochemical characterization of the secretion mechanism of flavonoids from legume roots.
RESULTS
ATP-Dependent Transport of Genistein by Plasma Membrane Vesicles
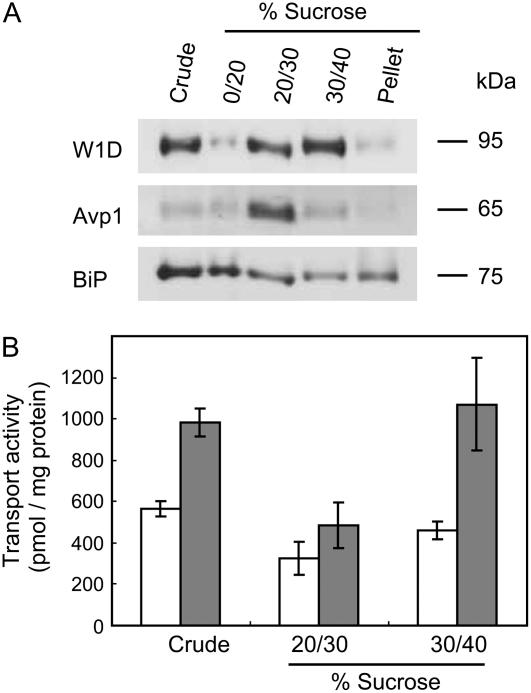
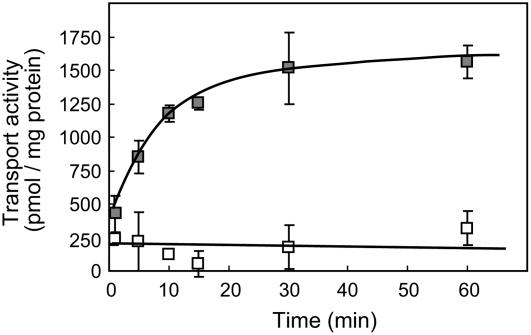
The first event of legume-Rhizobium communication is the secretion of phytochemicals, such as flavonoids, from the root tissues of legumes. To clarify the mechanism of flavonoid secretion from root cells of legumes, we used soybean as a model plant because the signal molecules have been identified as isoflavonoids, such as genistein, whose secretion from soybean roots as well as daidzein was confirmed by liquid chromatography-mass spectrometry analysis of the root exudates (data not shown), and analyzed the transport activity of genistein by plasma membrane vesicles of soybean roots. Plasma membrane vesicles were purified by fractioning microsomes of soybean roots on a discontinuous Suc density gradient. The membrane separation pattern was monitored by western blot with antibodies against plasma membrane H+-ATPase of tobacco (Nicotiana tabacum; W1D), vacuolar pyrophosphatase (Avp1), and luminal binding protein (Bip) of Arabidopsis (Arabidopsis thaliana), which were used as markers for the plasma membrane, tonoplast, and endoplasmic reticulum, respectively. Plasma membrane-enriched vesicles were recovered from the interface between the 30% and 40% Suc layers (Fig. 1A). The plasma membrane fraction showed clear transport activity of genistein in an ATP-dependent manner, whereas vesicles recovered from the 20% to 30% interface where the vacuolar membrane fraction was mainly localized showed no significant ATP-dependent transport of genistein (Fig. 1B). The ATP-dependent genistein transport activity by plasma membrane vesicles of soybean was measured in a time course experiment (Fig. 2). Genistein transport, which was clearly dependent on the presence of MgATP, increased linearly up to 15-min incubation and then reached a plateau at 30 min. The initial rate of genistein transport was calculated as approximately 80 pmol mg protein−1 min−1 in the presence of MgATP, and the genistein content in the membrane vesicles after 60-min incubation was approximately 1.5 nmol mg protein−1. These findings suggested that the plasma membrane of soybean root possesses the ability to secrete genistein in an energy-dependent manner.
Figure 1.
Transport of genistein into plasma membrane vesicles. A, Plasma membrane vesicles were prepared by Suc gradient fractionation. Plasma membrane H+-ATPase (W1D), V-PPase (Avp1), and BiP were immunodetected to confirm the purity of the plasma membrane vesicles. B, Each membrane fraction, as well as a crude microsome fraction, was incubated with 50 μm genistein in the presence (▪) or absence (□) of 5 mm MgATP. Genistein uptake was monitored at 25°C, as described in “Materials and Methods.” Data presented are means ± sd of three replicates.
Figure 2.
Time-dependent transport of genistein into plasma membrane vesicles. Plasma membrane vesicles (30%–40% Suc fraction) were incubated with 50 μm genistein in the presence (▪) or absence (□) of 5 mm MgATP. Data presented are means ± sd of three replicates.
To determine whether the effect of ATP on genistein transport is specific to ATP or not, we used other nucleotides, including GTP, CTP, and TTP, in place of ATP. As shown in Table I, ATP was the most effective nucleotide-triphosphate to drive genistein transport, whereas GTP, CTP, and TTP also increased transport to some extent. However, ATP-γ-S [adenosine 5′-O-(3′-thio)triphosphate], a nonhydrolyzable analog of ATP, AMP, and CMP, did not produce an increase in genistein transport, as seen with ATP, suggesting that the membrane transport of genistein required the energy of ATP hydrolysis, which can be partly substituted by other nucleotide triphosphates.
Table I.
Effects of various nucleotide triphosphates and nucleotide monophosphates on genistein transport
Plasma membrane vesicles were incubated with 100 μm genistein in the presence of 5 mm of the respective compounds listed in the table. Values shown are mean ± sd of three replicates, and asterisks indicate a statistically significant difference compared to the −MgATP control. **, P < 0.01; *, P < 0.05.
| Compound | Genistein Uptake |
|---|---|
| % | |
| −MgATP | 21.8 ± 1.8 |
| +MgGTP | 48.3 ± 12.4* |
| +MgTTP | 55.3 ± 7.3* |
| +MgCTP | 50.4 ± 5.9* |
| +MgATP-γ-S | 28.5 ± 4.2 |
| +MgAMP | 24.2 ± 2.9 |
| +MgCMP | 20.3 ± 3.3 |
| +MgATP (positive control) | 100** |
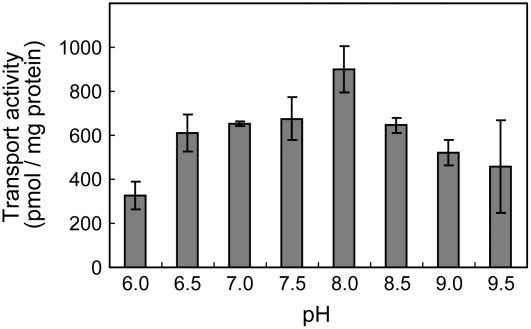
We then examined the pH dependency of genistein transport in the root plasma membrane in which the assay was performed with Tris-MES buffer ranging from pH 6.0 to 9.5. The transport activity had an optimum pH of about 8.0, but appreciable activity was observed within the entire pH range tested (Fig. 3).
Figure 3.
Effect of pH on ATP-dependent genistein transport. The pH was adjusted with Tris-MES buffer. Genistein transport was determined after 10 min of incubation. Data presented are means ± sd of three replicates.
Effects of Inhibitors on Genistein Transport
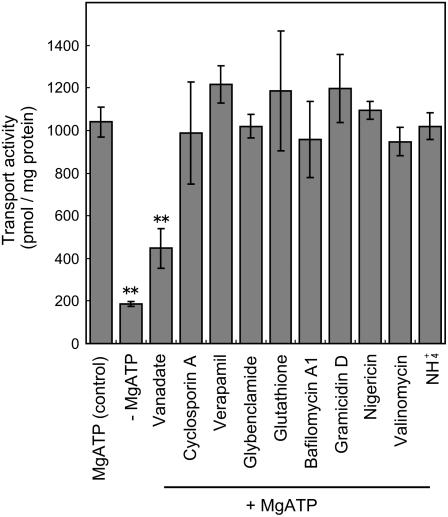
The mechanism of genistein transport across the plasma membrane was further investigated by analyzing the effects of various transport inhibitors. Bafilomycin A1, an inhibitor of the V-type ATPases; gramicidin D, a monovalent-selective ionophore that dissipates both the pH gradient and membrane potential; nigericin, an ionophore that dissipates the pH gradient; valinomycin, a potassium-selective ionophore that dissipates membrane potential, all failed to inhibit the membrane transport activity of genistein (Fig. 4). Similarly, ammonium chloride, which destroys the pH gradient across the membrane, hardly inhibited genistein transport either (Fig. 4). The concentrations of inhibitors we employed were in the conventional range for the purpose of the specific functions mentioned above. These results clearly suggest that pH gradient- or membrane potential-dependent secondary transport does not play an important role in genistein transport across the plasma membrane. In contrast to the negligible effects of the ionophores, sodium orthovanadate, a typical inhibitor of ATP-binding cassette (ABC) transporters acting as a phosphate analog, strongly inhibited (approximately 60%) transport activity (Fig. 4), indicating that an ABC-type transporter is likely to be involved in the membrane transport of genistein. Verapamil and cyclosporine A, which are often used as general inhibitors of ABCB (P-glycoprotein)-type ABC transporters, did not influence transport activity (Fig. 4). We also examined nifedipine and quinidine, which are also inhibitors of ABCB-type ABC transporters (Sakai et al., 2002), but these failed to inhibit genistein transport (data not shown). To assess the possible involvement of ABCC (multidrug resistance-associated protein) family members in genistein transport, glybenclamide, a sulfonylurea derivative that acts as an effective inhibitor of several ABC transporters, especially ABCC-type members, and glutathione, which commonly stimulates ABCC-mediated transport (Deeley and Cole, 2006), were tested, but they did not significantly affect the transport activity in the soybean root membrane assay (Fig. 4).
Figure 4.
Effects of various chemicals on ATP-dependent genistein transport. Plasma membrane vesicles were incubated with 50 μm genistein, where vanadate (1 mm), cyclosporine A (5 μm), verapamil (5 μm), glybenclamide (150 μm), glutathione (1 mm), bafilomycin A1 (0.1 μm), gramicidin D (5 μm), nigericin (2 μm), valinomycin (2 μm), or NH4Cl (5 mm) were added prior to the addition of MgATP. Genistein transport was determined after 10 min of incubation. Data presented are means ± sd of three replicates. Asterisks indicate a statistically significant difference compared to +ATP control (P < 0.01).
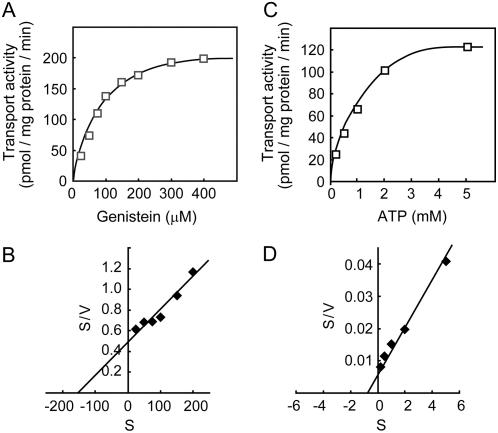
Kinetics of Genistein Transport
Genistein transport by soybean plasma membrane vesicles exhibited Michaelis-Menten-type saturation kinetics. The Km and Vmax values were calculated to be 158 μm and 322 pmol mg protein−1 min−1, respectively (Fig. 5, A and B), and the Km value for ATP was calculated to be 1.15 mm (Fig. 5, C and D). These values are consistent with those observed for other ABC-type transporters involved in the transport of low-Mr organic compounds (Senior et al., 1995; Klein et al., 2000; Frangne et al., 2002; Dean and Mills, 2004; Sauna et al., 2004).
Figure 5.
Effects of genistein concentration on ATP-dependent genistein transport into plasma membrane vesicles. A, Plasma membrane vesicles were incubated with genistein at the concentrations indicated and 5 mm MgATP. Data presented are means of three replicates. B, Hanes-Woolf plot of concentration dependence of genistein transport. C, Plasma membrane vesicles were incubated with 100 μm genistein and MgATP at the concentrations indicated. D, Hanes-Woolf plot of concentration dependence of ATP.
Competitive Inhibition of Genistein Transport
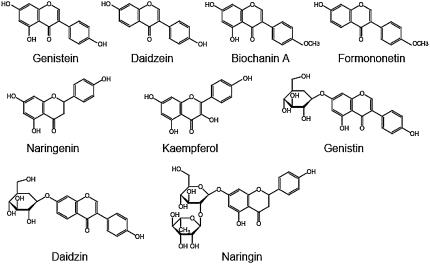
To obtain information about substrate specificity of genistein transport across the soybean root plasma membrane, we monitored transport activity in the presence of other flavonoids of 5-fold excess concentration. As shown in Table II, genistein transport was strongly competed by daidzein, another endogenous flavonoid secreted from soybean roots that also act as a legume-to-Rhizobium signal (Kosslak et al., 1987; Smit et al., 1992). Two other isoflavonoid derivatives, biochanin A, a 4′-O-methyl derivative of genistein, and formononetin, a 4′-O-methyl derivative of daidzein, also clearly inhibited genistein transport (Table II). In contrast, naringenin (flavanone) and its 7′-O-glucoside naringin, as well as kaempferol (flavonol), did not show such competitive inhibition of transport activity when added to the assay mixture (Table II). Interestingly, the 7′-O-glucoside of genistein, which is designated genistin, exhibited an inhibitory effect on the genistein transport that was weaker than the inhibition observed with isoflavone aglycone, whereas daidzin, the 7′-O-glucoside of daidzein, did not significantly inhibit genistein transport (Table II).
Table II.
Effects of various flavonoids on genistein transport
Plasma membrane vesicles were incubated with 50 μm genistein and 5 mm MgATP in the absence (control) or presence of 250 μm of the flavonoids indicated. Values shown are mean ± sd of three replicates, and asterisks indicate a statistically significant difference compared to the +MgATP control. **, P < 0.01; *, P < 0.05.
| Compound | Substance Class | Genistein Uptake |
|---|---|---|
| % | ||
| Control | 100 | |
| Daidzein | Isoflavone | 29.0 ± 6.5** |
| Biochanin A | Isoflavone | 32.1 ± 4.1** |
| Formononetin | Isoflavone | 29.7 ± 6.3** |
| Naringenin | Flavanone | 88.9 ± 13.7 |
| Kaempferol | Flavonol | 74.0 ± 7.7 |
| Genistin | 7-O-glucoside of genistein | 55.9 ± 5.4* |
| Daidzin | 7-O-glucoside of daidzein | 88.4 ± 9.6 |
| Naringin | 7-O-glucoside of naringenin | 89.5 ± 10.7 |
Effect of Nitrogen Nutrition on Transport Activity
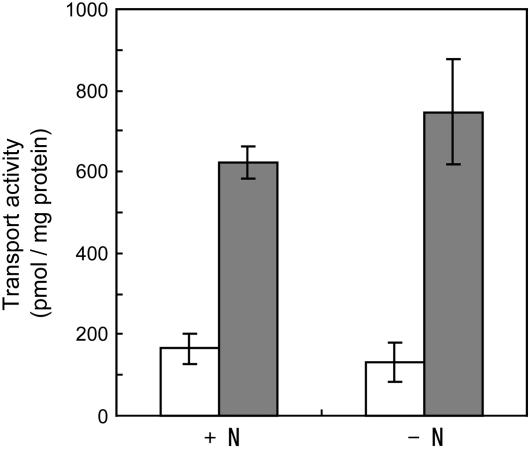
To assess whether or not genistein secretion of soybean roots is induced under nitrogen starvation conditions in which nodule formation is established, we prepared membrane vesicles from roots of soybean grown with supplementary nitrogen in the form of 5 mm KNO3 and compared its genistein transport activity with soybean roots grown without KNO3. Membrane vesicles prepared from plants under nitrogen starvation exhibited a slight, albeit not clearly statistically significant, increase in genistein transport activity (Fig. 6). These findings suggest that the transport machinery is functional regardless of the supply of nitrogen.
Figure 6.
Effects of nitrogen nutrition on ATP-dependent genistein transport into plasma membrane vesicles. Membrane vesicles prepared from soybean roots grown with or without supplementary nitrogen were incubated with 50 μm genistein in the presence (▪) or absence (□) of 5 mm MgATP. Genistein transport was determined after 10 min of incubation. Data presented are means ± sd of three replicates.
DISCUSSION
Plant root exudates play active and important roles in the rhizosphere in interactions with other organisms, such as bacteria, fungi, insects, and other plants. Most of these interactions between plants and other organisms in the rhizosphere are unfavorable and plants secrete both water-soluble and lipid-soluble secondary metabolites from root cells to protect themselves from continuous attacks. Relatively soluble organic compounds, such as glyceollin of soybean and vestitol of L. japonicus, which are classified as phytoalexins, are secreted into the rhizosphere and inhibit the growth of pathogenic microorganisms (Russell et al., 1978; Ebel and Grisebach, 1988), whereas water-insoluble compounds, such as shikonin of L. erythrorhizon, and prenylflavonoids of Sophora flavescens, synthesized in the root epidermis and exodermis are not secreted into the rhizosphere, but accumulate on the surface of the root cells, forming a protective barrier (Yamamoto et al., 1992; Brigham et al., 1999; Yazaki et al., 1999). Plant roots also secrete organic compounds to protect their territory from neighboring plants of different species; for example, cis-dehydromatricaria ester of Solidago altissima was shown to inhibit the growth of other plants (Kobayashi et al., 1980).
In contrast to these negative interactions, some root exudates are also involved in symbiotic interactions between plants and other organisms. The most commonly known symbiotic phenomenon is legume-Rhizobium interactions in symbiotic nitrogen fixation, where root exudates, such as flavonoids from legume plants, are used as the initial legume-to-Rhizobium signal. In this study, we performed a biochemical characterization of flavonoid secretion from soybean roots and experimentally demonstrated the involvement of an ABC-type transporter in the energy-dependent secretion of genistein from soybean roots. Characteristics of the energy-dependent transport of genistein into soybean plasma membrane vesicles were similar to those observed for other plant ABC-type transporters (Klein et al., 1996, 2000, 2001; Dean and Mills, 2004): (1) sodium orthovanadate, a phosphate analog commonly used as an inhibitor of ABC transporters and P-type ATPases, inhibited genistein transport (Fig. 4); (2) ionophores and ammonium ions did not influence genistein transport, excluding the involvement of the pH gradient or membrane potential as a driving force (Fig. 4); (3) other nucleotide triphosphates partially substituted ATP as energy sources (Table I). A vacuolar ATPase inhibitor, bafilomycin A1, did not affect this transport either. The optimal pH of this ABC-type transporter (pH 8.0) was slightly higher than cytosolic pH, which is typically pH 7.2 to 7.5, and similar optimum pH (pH 7.5–8.5) was reported in proton-pumping ATPases, as well as transporters for Arg and berberine (Leigh and Walker, 1980; Churchill and Sze, 1983; Ohsumi and Anraku, 1983; Otani et al., 2005).
ABC proteins constitute a large family with more than 120 members each in Arabidopsis and rice (Oryza sativa; Sanchez-Fernandez et al., 2001; Garcia et al., 2004). We have recently analyzed the ABC protein genes in a model legume, L. japonicus, and found at least 91 members are present in the draft genome sequence of L. japonicus (Sugiyama et al., 2006), which represent almost the same family size as the two model plants, when allowing for the approximately 70% coverage of the L. japonicus genome at this time. ABC proteins can be divided into several subfamilies, depending on membrane topology and amino acid sequence similarity. Thus far, the three major subfamilies, ABCB, ABCC, and pleiotropic drug resistance (PDR), have been reported as functioning in the membrane transport of various organic compounds (Davies and Coleman, 2000; Sanchez-Fernandez et al., 2001; Martinoia et al., 2002). Because potent inhibitors of ABCB-type (verapamil, cyclosporine A, nifedipine, and quinidine) and of ABCC-type ABC (glybenclamide) transporters, as well as glutathione, which often stimulates transport by ABCC members, did not strongly influence genistein transport (Fig. 4), we may speculate that the involvement of an ABC transporter of ABCB or ABCC types in the secretion of flavonoids from soybean roots is unlikely. PDR-type ABC transporters could be possible candidates for the future molecular cloning of the transporter involved. In the soybean EST database at Soybean Gene Index (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=soybean), we found 168 ABC transporter genes, among which 58 genes are expressed in roots. When analyzed in detail, one ABCB-type, one ABCC-type, and 13 PDR-type ABC transporter genes are expressed in roots. This high abundance of PDR-type ABC transporter genes in roots suggests that PDR-type transporters have more extensive functions than other subfamilies in roots, supporting the probability that PDR-type ABC transporters may be strong candidates for the genistein transporter in soybean roots.
Competition experiments indicate that transport of genistein and daidzein through the plasma membrane is mediated by the same transporter (Table II). This is in clear agreement with the equivalent physiological function of these two isoflavones (i.e. both genistein and daidzein are the major flavonoids in soybean root exudates that act as legume-to-Rhizobium signal molecules; Kosslak et al., 1987; Smit et al., 1992). This ABC-type transporter also seems to be specific to isoflavones as transport substrates because all isoflavones tested inhibited genistein transport in the competition assay, whereas flavanone and flavonol do not seem to be recognized by the transporter protein (Table II). In plants, flavonoids often accumulate as the glucoside forms and the transport of flavonoid glucosides into vacuoles has also been reported (Mackenbrock et al., 1992; Klein et al., 1996; Frangne et al., 2002), but the transport of flavonoid aglycones at the plant plasma membrane has not been biochemically characterized thus far, except for this study.
In barley (Hordeum vulgare), the uptake of endogenous flavonoid glucosides into the vacuoles is mediated by a proton antiporter (Klein et al., 1996), whereas the vacuolar transport of the same compounds is conducted by an ABC-type transporter in Arabidopsis (Frangne et al., 2002). We also studied the vacuolar transport of genistin using the vacuolar membrane vesicles of soybean, but we did not detect any energy-dependent transport of genistin (data not shown). The transport of glucosides into vacuoles may not be active at this stage of development in soybean when flavonoid aglycones are actively secreted into the rhizosphere. The substrate specificity of the ABC-type transporter of soybean roots for flavonoid glucosides was not unequivocal: Daidzin and naringin did not inhibit membrane transport of genistein, whereas genistin slightly inhibited this transport (Table II). Also, no glycosidase activity was detected in the membrane vesicles used (data not shown). Although the reason why the difference in the aglycon structure in either genistin or daidzin gave distinctive effects on genistein transport in the competitive assay is not yet clear, we can at least conclude that this transporter has an apparent preference for isoflavonoid aglycones as substrates. Glyceollin could not be tested due to the unavailability of this substance.
Legume plants establish a symbiotic relationship with rhizobia when soil nitrogen is limited, whereas symbiotic nodulation does not occur when soil nitrogen is abundant. It was of interest whether nitrogen nutrition affects the transport activity of genistein; that is, is the transport activity induced by nitrogen starvation? Thus, we compared the genistein transport activities of the root membranes of soybean grown with or without supplementary nitrogen (5 mm KNO3). However, nitrogen nutrition seems to have only a slight effect on transport activity, suggesting that membrane transport activity is not a regulatory factor for flavonoid secretion. The biosynthesis of flavonoids may be the major regulatory factor for flavonoid secretion in conditions of nitrogen starvation (Cho and Harper, 1991).
In some graminaceous plants, iron-chelating substances, such as mugineic acid, which facilitates the uptake of Fe ions, are specifically biosynthesized in roots and secreted out under iron deficiency conditions (Takagi, 1976). The secretion of mugineic acid is mediated by an anion channel using the potassium gradient (Sakaguchi et al., 1999), but this phenomenon also seems to be regulated at the biosynthetic step of mugineic acid (Kobayashi et al., 2005), not at the transport process.
In conclusion, we have shown that flavonoid secretion from soybean roots is mediated by an ABC-type transporter as an initial event of symbiosis formation, and thus defined the ABC-type transporter as an indispensable factor for legume-Rhizobium chemical communication. Although it has been proposed that energy-driven transport processes are involved in the secretion of root exudates (Loyola-Vargas et al., 2007), our results demonstrated by the transport assays using plasma membrane vesicles are, to our knowledge, the first biochemical characterizations of the membrane transport of endogenous organic signal molecules in roots at the plasma membrane. The genome sequencing of soybean as well as global expression analyses by cDNA arrays will enable us to identify the ABC protein gene responsible for flavonoid secretion in soybean, which will provide further breakthroughs to understand the molecular mechanism of the interaction between organisms in the rhizosphere.
MATERIALS AND METHODS
Plant Materials and Chemicals
Seeds of soybean (Glycine max L. Merr.) ‘Fukujishi’ were purchased from Takii Seed Co., Ltd. After imbibition for 24 h, seeds were sown on vermiculite supplemented with one-tenth-strength nitrogen-free medium (Niwa et al., 2001) and germinated at 25°C under continuous light. After 14 d, roots were harvested, frozen in liquid nitrogen, and kept at −80°C until the preparation of membrane vesicles. All chemicals used in this study were purchased from Wako Pure Chemicals, Nacalai Tesque, or Sigma Chemical Co., unless otherwise stated. Root exudate analysis was done by microTOF-Q (Bruker Daltonics) to detect genistein and daidzein with 0.3% formic acid and methanol as the solvent system, using a Cadenza CD-C18 column (3 μm, 2.0 × 250 mm; Imtact Co.).
Preparation of Plasma Membrane Vesicles
Plasma membrane vesicles were prepared from roots of soybean essentially according to the method of Otani et al. (2005). All procedures were performed on ice or at 4°C unless otherwise stated. Roots (approximately 80 g) were homogenized in 160 mL of ice-cold homogenizing buffer containing 10% (v/v) glycerol, 0.5% (w/v) polyvinylpolypyrrolidone, 5 mm EDTA, 100 mm Tris-HCl (pH 8.0), 150 mm KCl, 3.3 mm dithiothreitol (DTT), and 1 mm phenylmethylsulfonyl fluoride. The homogenate was strained through Miracloth (Merck) and centrifuged at 8,000g for 10 min. The supernatant obtained was centrifuged again at 8,000g for 10 min and the subsequent supernatant then centrifuged at 100,000g for 40 min. The pellet was homogenized by 30 strokes of a Dounce homogenizer in a small volume (approximately 3 mL) of resuspension buffer containing 10% (v/v) glycerol, 1 mm EDTA, and 10 mm Tris-HCl (pH 7.6). The suspension was layered over a 20%-30%-40% (w/v) discontinuous Suc gradient in a 13-mL tube that contained, in addition to Suc, 10 mm Tris-HCl buffer (pH 7.6), 1 mm DTT, and 1 mm EDTA, followed by centrifugation at 100,000g for 120 min. Each fraction recovered from the interfaces was resuspended in approximately 35 mL of resuspension buffer to dilute the Suc, and centrifuged at 100,000g for 40 min. Each pellet was homogenized by 30 strokes of a Dounce homogenizer in a small volume (100–200 μL) of resuspension buffer supplemented with 10 μg/mL leupeptin, 2 μg/mL aprotinin, 2 μg/mL pepstatin, 1 mm DTT, and 1 mm phenylmethylsulfonyl fluoride, and stored at −80°C until use. Membrane vesicles prepared with this method are a mixture of inside-out and right-side-out orientation, whereas only inside-out vesicles can hydrolyze ATP due to the outside orientation of the catalytic sites of ATPases that drive membrane transport because ATP cannot permeate the membrane.
Western Blotting
The purity of vesicles was checked by western blotting with antibodies against plasma membrane H+-ATPase (W1D), vacuolar H+-pyrophosphatase (Avp), and endoplasmic reticulum luminal BiP (see “Acknowledgments” for the sources of antibodies). SDS-PAGE, transfer to polyvinylidene difluoride membranes and subsequent immunodetection were performed as described previously (Yazaki et al., 2006).
Measurement of Genistein Transport with Spin Columns
Spin columns for the transport assays were prepared as follows. A small hole was punctured at the bottom of a 1.5-mL plastic tube with a needle (18 G) and another small hole was made at the lid with a soldering iron. Siliconized glass wool was stuffed at the bottom of the prepared tube, which was then placed into a new plastic tube without holes. Sephadex G-50 fine (1,100 μL; GE Healthcare) prepared in 50 mm Tris-MES buffer (pH 7.5) was added to the top tube, followed by centrifugation at 2,000 rpm for 2 min, which resulted in approximately 500 μL of gel bed of Sephadex G-50 in the top tube.
Transport of genistein by membrane vesicles was measured according to the method of Otani et al. (2005) with some modifications. The standard reaction mixture contained, in a total volume of 500 μL, 50 mm Tris-MES buffer (pH 8.0), 100 mm KCl, 5 mm MgATP, 50 μm genistein, and membrane vesicles equivalent to 100 μg protein, unless otherwise specified. The stock solution of genistein was prepared at 10 mm in methanol, and 2.5 μL were added per 500-μL assay mixture. The methanol at this concentration (0.5% v/v) did not affect transport activity. Reactions were initiated by the addition of MgATP, except when studying the specificity of ATP as an energy source, where MgGTP, MgTTP, MgCTP, MgATP-γ-S, MgAMP, or MgCMP was used instead of MgATP. After incubation at 25°C, 130 μL of the reaction mixture were loaded on a Sephadex G-50 fine spin column and centrifuged at 2,000 rpm for 2 min at 4°C. The filtrates were mixed with an equal volume of methanol to extract genistein from the membrane vesicles and centrifuged at 15,000 rpm for 15 min at 4°C. To quantitate genistein, aliquots of the supernatants were injected into an HPLC apparatus (LC-10A, Shimadzu): column, TSKgel ODS-80Ts 4.6 × 250 mm (TOSO); solvent, acetonitrile:water:formic acid (36:64:0.64); flow rate, 0.5 mL/min; detection, 262 nm.
Measurement of Optimal pH
Genistein transport was assayed in Tris-MES buffer (50 mm) with different pH values (from pH 6.0 to 9.5). Reactions were performed at 25°C for 10 min and transported genistein was measured as described above.
Transport Assays with Inhibitors
Each inhibitor was incubated with the membrane vesicles for 2 min before the addition of MgATP. As transport inhibitors, the following compounds were used at the final concentration shown and the solvents used to prepare the stock solution are given in parentheses; 1 mm vanadate (in water), 5 μm cyclosporine A (in dimethyl sulfoxide [DMSO]), 5 μm verapamil (in DMSO), 150 μm glybenclamide (in DMSO), 1 mm glutathione (in water), 0.1 μm bafilomycin A1 (in DMSO), 5 μm gramicidin D (in DMSO), 2 μm nigericin (in DMSO), 2 μm valinomycin (in DMSO), 50 μm nifedipine (in DMSO), 50 μm quinidine (in DMSO), and 5 mm NH4Cl (in water). Sodium vanadate was depolymerized before use according to the method of Goodno (1979). To avoid unexpected side effects, each inhibitor was used at the concentrations shown for its specific inhibitory effect on transporters and pumps, or disrupting membrane potential as well as ΔpH. To the 500-μL assay mixture, 2.5 to 5 μL of each stock solution were added, where the concentration of organic solvent was less than 1% (v/v). For competitive inhibition, daidzein (in methanol), biochanin A (in DMSO), formononetin (in DMSO), naringenin (in methanol), kaempferol (in ethanol), genistin (in DMSO), daidzin (in DMSO), or naringin (in methanol) was added to the reaction mixture at a concentration of 250 μm. After incubation at 25°C for 10 min, transported genistein was measured as described above. DMSO, ethanol, and methanol did not affect the genistein transport at the concentrations used throughout this study. It was also confirmed that membrane vesicles have no glucosidase activity, using genistin as the substrate.
Kinetics of Genistein Transport
The transport assay was performed with different genistein concentrations (from 25–400 μm) and 5 mm MgATP, or with different MgATP concentrations (from 0.2–5 mm) and 100 μm genistein. After incubation at 25°C for 10 min, transported genistein was measured as described above. Hanes-Woolf plots were used to calculate the Km and Vmax values.
Acknowledgments
We thank Dr. Yoshinori Moriyama of Okayama University for helpful technical advice regarding the transport assay. We thank Dr. Marc Boutry, Université Catholique de Louvain, for providing anti-H+-ATPase (W1D) antibodies, Dr. Masahiko Sato, Kyoto Prefectural University, for anti-V-PPase (Avp) antibodies, and Dr. Nozomu Koizumi, Nara Institute of Science and Technology, for anti-BiP antibodies. We also thank Bruker Daltonics, Inc. for assistance in analyzing the root exudates of soybean.
This work was supported by a Grant-in-Aid for Scientific Research (grant nos. 17051018 and 17027016 to K.Y.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by a research fellowship from the Japan Society for the Promotion of Science for Young Scientists (grant no. 183051 to A.S.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Kazufumi Yazaki (yazaki@rish.kyoto-u.ac.jp).
References
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435 824–827 [DOI] [PubMed] [Google Scholar]
- Brigham LA, Michaels PJ, Flores HE (1999) Cell-specific production and antimicrobial activity of naphthoquinones in roots of Lithospermum erythrorhizon. Plant Physiol 119 417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas L, Holdaway-Clarke TL, Sanchez F, Quinto C, Feijo JA, Kunkel JG, Hepler PK (2000) Ion changes in legume root hairs responding to Nod factors. Plant Physiol 123 443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MJ, Harper JE (1991) Effect of inoculation and nitrogen on isoflavonoid concentration in wild-type and nodulation-mutant soybean roots. Plant Physiol 95 435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill KA, Sze H (1983) Anion-sensitive, H+ pumping ATPase in membrane vesicles from oat roots. Plant Physiol 71 610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TGE, Coleman JOD (2000) The Arabidopsis thaliana ATP-binding cassette proteins: an emerging superfamily. Plant Cell Environ 23 431–443 [Google Scholar]
- Dean JV, Mills JD (2004) Uptake of salicylic acid 2-O-β-d-glucose into soybean tonoplast vesicles by an ATP-binding cassette transporter-type mechanism. Physiol Plant 120 603–612 [DOI] [PubMed] [Google Scholar]
- Deeley RG, Cole SP (2006) Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett 580 1103–1111 [DOI] [PubMed] [Google Scholar]
- Ebel J, Grisebach H (1988) Defense strategies of soybean against the fungus Phytophthora megasperma f. sp. glycinea: a molecular analysis. Trends Biochem Sci 13 23–27 [DOI] [PubMed] [Google Scholar]
- Firmin JL, Wilson KE, Rossen L, Johnston AWB (1986) Flavonoid activation of nodulation genes in Rhizobium reversed by other compounds present in plants. Nature 324 90–92 [Google Scholar]
- Frangne N, Eggmann T, Koblischke C, Weissenbock G, Martinoia E, Klein M (2002) Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles: energization occurs by H(+)-antiport and ATP-binding cassette-type mechanisms. Plant Physiol 128 726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia O, Bouige P, Forestier C, Dassa E (2004) Inventory and comparative analysis of rice and Arabidopsis ATP-binding cassette (ABC) systems. J Mol Biol 343 249–265 [DOI] [PubMed] [Google Scholar]
- Goodno CC (1979) Inhibition of myosin ATPase by vanadate ion. Proc Natl Acad Sci USA 76 2620–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham PH, Vance CP (2003) Legumes: importance and constraints to greater use. Plant Physiol 131 872–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Martinoia E, Hoffmann-Thoma G, Weissenbock G (2000) A membrane-potential dependent ABC-like transporter mediates the vacuolar uptake of rye flavone glucuronides: regulation of glucuronide uptake by glutathione and its conjugates. Plant J 21 289–304 [DOI] [PubMed] [Google Scholar]
- Klein M, Martinoia E, Hoffmann-Thoma G, Weissenbock G (2001) The ABC-like vacuolar transporter for rye mesophyll flavone glucuronides is not species-specific. Phytochemistry 56 153–159 [DOI] [PubMed] [Google Scholar]
- Klein M, Weissenbock G, Dufaud A, Gaillard C, Kreuz K, Martinoia E (1996) Different energization mechanisms drive the vacuolar uptake of a flavonoid glucoside and a herbicide glucoside. J Biol Chem 271 29666–29671 [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Morimoto S, Shibata Y, Yamashita K, Numata M (1980) C10-polyacetylenes as allelopathic substances in dominants in early stages of secondary succession. J Chem Ecol 6 119–131 [Google Scholar]
- Kobayashi T, Suzuki M, Inoue H, Itai RN, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2005) Expression of iron-acquisition-related genes in iron-deficient rice is co-ordinately induced by partially conserved iron-deficiency-responsive elements. J Exp Bot 56 1305–1316 [DOI] [PubMed] [Google Scholar]
- Kosslak RM, Bookland R, Barkei J, Paaren HE, Appelbaum ER (1987) Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci USA 84 7428–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh RA, Walker RR (1980) ATPase and acid phosphatase activities associated with vacuoles isolated from storage roots of red beet (Beta vulgaris L.). Planta 150 222–229 [DOI] [PubMed] [Google Scholar]
- Loyola-Vargas VM, Broeckling CD, Badri D, Vivanco JM (2007) Effect of transporters on the secretion of phytochemicals by the roots of Arabidopsis thaliana. Planta 225 301–310 [DOI] [PubMed] [Google Scholar]
- Mackenbrock U, Vogelsang R, Barz W (1992) Isoflavone and pterocarpan malonylglucosides and β-1,3-glucan- and chitin-hydrolases are vacuolar constituents in chickpea (Cicer arietinum L.). Z Naturforsch 47c 815–822 [Google Scholar]
- Martinoia E, Klein M, Geisler M, Bovet L, Forestier C, Kolukisaoglu U, Muller-Rober B, Schulz B (2002) Multifunctionality of plant ABC transporters—more than just detoxifiers. Planta 214 345–355 [DOI] [PubMed] [Google Scholar]
- Niemeyer HM (1988) Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defence chemicals in the gramineae. Phytochemistry 27 3349–3358 [Google Scholar]
- Niwa S, Kawaguchi M, Imazumi-Anraku H, Chechetka SA, Ishizaka M, Ikuta A, Kouchi H (2001) Responses of a model legume Lotus japonicus to lipochitin oligosaccharide nodulation factors purified from Mesorhizobium loti JRL501. Mol Plant Microbe Interact 14 848–856 [DOI] [PubMed] [Google Scholar]
- Ohsumi Y, Anraku Y (1983) Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem 258 5614–5617 [PubMed] [Google Scholar]
- Otani M, Shitan N, Sakai K, Martinoia E, Sato F, Yazaki K (2005) Characterization of vacuolar transport of the endogenous alkaloid berberine in Coptis japonica. Plant Physiol 138 1939–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NK, Frost JW, Long SR (1986) A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233 977–980 [DOI] [PubMed] [Google Scholar]
- Redmond J, Batley M, Djordjevic M, Innes R, Kuempel P, Rolfe B (1986) Flavones induce expression of nodulation genes in Rhizobium. Nature 323 632–635 [Google Scholar]
- Russell GB, Sutherland ORW, Hutchins RFN, Christmas PE (1978) Vestitol: a phytoalexin with insect feeding-deterrent activity. J Chem Ecol 4 571–579 [Google Scholar]
- Sakaguchi T, Nishizawa NK, Nakanishi H, Yoshimura E, Mori S (1999) The role of potassium in the secretion of mugineic acids family phytosiderophores from iron-deficient barley roots. Plant Soil 215 221–227 [Google Scholar]
- Sakai K, Shitan N, Sato F, Ueda K, Yazaki K (2002) Characterization of berberine transport into Coptis japonica cells and the involvement of ABC protein. J Exp Bot 53 1879–1886 [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez R, Davies TG, Coleman JO, Rea PA (2001) The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J Biol Chem 276 30231–30244 [DOI] [PubMed] [Google Scholar]
- Sauna ZE, Nandigama K, Ambudkar SV (2004) Multidrug resistance protein 4 (ABCC4)-mediated ATP hydrolysis: effect of transport substrates and characterization of the post-hydrolysis transition state. J Biol Chem 279 48855–48864 [DOI] [PubMed] [Google Scholar]
- Senior AE, al-Shawi MK, Urbatsch IL (1995) The catalytic cycle of P-glycoprotein. FEBS Lett 377 285–289 [DOI] [PubMed] [Google Scholar]
- Smit G, Puvanesarajah V, Carlson RW, Barbour WM, Stacey G (1992) Bradyrhizobium japonicum nodD1 can be specifically induced by soybean flavonoids that do not induce the nodYABCSUIJ operon. J Biol Chem 267 310–318 [PubMed] [Google Scholar]
- Stacey G, Libault M, Brechenmacher L, Wan J, May GD (2006) Genetics and functional genomics of legume nodulation. Curr Opin Plant Biol 9 110–121 [DOI] [PubMed] [Google Scholar]
- Subramanian S, Stacey G, Yu O (2006) Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant J 48 261–273 [DOI] [PubMed] [Google Scholar]
- Sugiyama A, Shitan N, Sato S, Nakamura Y, Tabata S, Yazaki K (2006) Genome-wide analysis of ATP-binding cassette (ABC) proteins in a model legume plant, Lotus japonicus: comparison with Arabidopsis ABC protein family. DNA Res 13 205–228 [DOI] [PubMed] [Google Scholar]
- Takagi S (1976) Naturally occurring iron-chelating compounds in oat and rice root-washings. Soil Sci Plant Nutr 22 423–433 [Google Scholar]
- Verma D, Hong Z (1996) Biogenesis of the peribacteroid membrane in root nodules. Trends Microbiol 4 364–368 [DOI] [PubMed] [Google Scholar]
- Weir TL, Park SW, Vivanco JM (2004) Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol 7 472–479 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Ichimura M, Ishikawa N, Tanaka T, Iinuma M, Mizuno M (1992) Localization of prenylated flavonoids in Sophora flavescens var. angustifolia plants. Z Naturforsch 47c 535–539 [Google Scholar]
- Yazaki K, Matsuoka H, Ujihara T, Sato F (1999) Shikonin biosynthesis in Lithospermum erythrorhizon: light-induced negative regulation of secondary metabolism. Plant Biotechnol 16 335–342 [Google Scholar]
- Yazaki K, Yamanaka N, Masuno T, Konagai S, Shitan N, Kaneko S, Ueda K, Sato F (2006) Heterologous expression of a mammalian ABC transporter in plant and its application to phytoremediation. Plant Mol Biol 61 491–503 [DOI] [PubMed] [Google Scholar]