Abstract
In this study, we sequenced 18S rRNA genes (rDNA) from 49 fungal strains representing 31 species from 15 genera. Most of these species are common airborne fungi and pathogens that may cause various public health concerns. Sequence analysis revealed distinct divergence between Zygomycota and Ascomycota. Within Ascomycota, several strongly supported clades were identified that facilitate the taxonomic placement of several little-studied fungi. Wallemia appeared as the group most diverged from all the other Ascomycota species. Based on the 18S rDNA sequence variation, 108 oligonucleotide probes were designed for each genus and species included in this study. After homology searches and DNA hybridization evaluations, 33 probes were verified as genus or species specific. The optimal hybridization temperatures to achieve the best specificity for these 33 probes were determined. These new probes can contribute to the molecular diagnostic research for environmental monitoring.
A growing number of research studies and disease investigations show an alarming rate of high concentrations of potentially toxigenic or allergy-producing fungi (mold) found in offices, homes, various factories, and farms (4-6, 17). High levels of mold can cause a spectrum of health effects such as allergy, infections, irritation, or toxic reactions. As reported in a review by Piecková and Jesenská (22) of over 13,000 research publications, the dominant fungi found in indoor environments are species from the genera Aspergillus, Penicillium, and Cladosporium. Other groups, such as Paecilomyces, Mucor, Rhizopus, Trichoderma, Ulocladium, Fusarium, Stachybotrys, and Wallemia, are also frequently detected in air samples from different living and working environments (9, 27, 28).
The conventional methods of detection and identification of fungi have mainly relied on culture isolation and subsequent observations of morphological traits. These methods are time-consuming, laborious, and may require days to weeks for isolation by culture. In addition, not all the fungal species are culturable on a given medium, which leads to analysis that may not accurately reflect the true fungal community in a sample. The nonculturable and/or inviable spores can still be allergenic and cause health problems. For example, the conidia of Stachybotrys chartarum rapidly lose viability upon becoming airborne, without any apparent loss of toxigenicity (11, 16). Thus, rapid detection of fungi in a given environment is essential for monitoring the exposure risk and for developing preventive measures for public health purposes.
Among molecular techniques, PCR-specific amplification is a rapid method used in the direct detection of DNAs and RNAs of microorganisms from clinical and environmental samples to accurately and quantitatively ascribe microorganism compositions. Specific primers are developed for various fungal groups (11, 32, 34, 39). However, due to the immense diversity in fungi, true group- and species-specific detection can be difficult to achieve through selective primer-based PCR amplification alone. A two-step detection strategy, through which a group of target DNAs from the composite samples is selectively amplified followed by more specific examination via probe hybridization to a specific target in the PCR products, can provide better specificity and sensitivity for environmental sample screening (24, 37). In this approach, the first-round selective PCR products are blotted onto nylon membranes and screened with a series of oligonucleotide probes, each designed to be specific to a genus or a species. We have shown in a previous study (37) that, by employing rDNA-based oligonucleotide probes, the detection sensitivity of this approach can reach two to four spores. This level of detection sensitivity is realistic in terms of environmental samples. The sequences of oligonucleotide probes specific for several groups of fungi with different detection scopes have been published (19, 26). However, most of the previously described systems were tested on a narrow range of fungal taxa, and well-established probes for the detection and identification of airborne fungi are still very limited.
In the present study, we aimed at the development of oligonucleotide probes for the detection of common airborne fungi at the genus and species levels. The nuclear small subunit ribosomal DNA (18S rDNA) was selected for several reasons. First, several established universal fungal primers are based on the conserved regions of 18S rDNA, making it possible to obtain the PCR products from most of the fungi for sequencing (36). Second, the large number of 18S rDNA sequences in GenBank makes similarity searches convenient and facilitates the probe specificity evaluation before the start of extensive laboratory screenings. Third, 18S rDNA evolves relatively slowly compared to the rRNA gene (rDNA) internal-transcribed spacers (ITS) and is thus more suitable for finding consensus conserved regions within a group of fungi for developing probes for genus- and group-level detections. Finally, the repetitive nature of rDNA, over 100 copies of which usually exist per fungal genome (18, 30), makes the rDNA-based detection more sensitive than detection systems based on single-copy genes. Based on the patterns of 18S rDNA sequence variation, we also addressed the phylogenetic relationships among the fungal species included in the study.
MATERIALS AND METHODS
Cultures and genomic DNA isolation.
A total of 60 fungal strains representing 36 species from 15 genera of common airborne fungi were included in this study (Table 1). Most of these fungi were obtained from Uppsala University Culture Collection of Fungi (Uppsala, Sweden), Institute for Bioteknologi (Lyngby, Denmark), Centraalbureau voor Schimmelcultures (Utrecht, The Netherlands), and our laboratory culture collection. Genomic DNA was isolated from pure culture of each fungal strain by using procedures described previously (38).
TABLE 1.
Fungal strains included in this study and their 18S rDNA sequences submitted to GenBank
| Codea | Fungal strain | Sequence accession no. |
|---|---|---|
| A1 | Aspergillus flavus UPSC 1768 | AF548060 |
| B1 | Aspergillus fumigatus UPSC 1771 | AF548061 |
| C1 | Aspergillus fumigatus UPSC 2006 | AF548062 |
| D1 | Aspergillus fumigatus ALI 57 | AF548063 |
| E1 | Aspergillus niger UPSC 1769 | AF548064 |
| F1 | Aspergillus ochraceus UPSC 1983 | AF548065 |
| G1 | Aspergillus penicilloides ALI 231 | AF548066 |
| H1 | Eurotium herbariorum ALI216 | AF548072 |
| A2 | Aspergillus silvaticus ALI 234 | AF548067 |
| B2 | Aspergillus versicolor UPSC 2027 | AF548068 |
| C2 | Aspergillus versicolor UPSC 1532 | AF548069 |
| D2 | Aspergillus versicolor IBT 21958 | |
| E2 | Aspergillus versicolor IBT 12382 | |
| F2 | Cladosporium cladosporioides ALI 50 | AF548070 |
| G2 | Cladosporium cladosporioides UPSC 1657 | AF548071 |
| H2 | Fusarium culmorum UPSC 1981 | AF548073 |
| D6 | Gremmeniella abietina ALI 139 | AF548074 |
| E6 | Gremmeniella abietina ALI 148 | AF548075 |
| Gremmeniella abietina ALI 90 | AF548076 | |
| H6 | Microdochium nivale UPSC 3273 | AF548077 |
| A4 | Paecilomyces variotii UPSC 1766 | AF548081 |
| B4 | Paecilomyces variotii UPSC 1651 | AF548080 |
| C4 | Paecilomyces lilacinus UPSC 1722 | AF548079 |
| D4 | Mucor plumbeus UPSC 1492 | AF548078 |
| A3 | Penicillium brevicompactum ALI 318 | AF548082 |
| Penicillium brevicompactum ALI 319 | AF548083 | |
| B3 | Penicillium brevicompactum ALI 320 | AF548084 |
| Penicillium brevicompactum ALI 321 | AF548085 | |
| C3 | Penicillium chrysogenum ALI 229 | AF548086 |
| D3 | Penicillium chrysogenum UPSC 2020 | AF548087 |
| E3 | Penicillium commune IBT 15141 | AF548089 |
| F3 | Penicillium commune CBS 343.51 | AF548088 |
| G3 | Penicillium frequentans ALI 218 | AF548090 |
| H3 | Penicillium italicum UPSC 1577 | AF548091 |
| E4 | Rhizopus microsporus UPSC 1758 | AF548092 |
| F4 | Rhizopus microsporus var. rhizopodiformis CBS 258.79 | AF548093 |
| Stachybotrys bisbyi CBS 317.72 | ||
| Stachybotrys chartarum CBS 251.89 | ||
| Stachybotrys chartarum CBS 330.37 | AF548095 | |
| Stachybotrys chartarum IBT 9291 | ||
| A6 | Stachybotrys chartarum UPSC 2826 | |
| B6 | Stachybotrys chartarum IBT 9299 | AF548097 |
| C6 | Stachybotrys chartarum IBT 7711 | AF548096 |
| Stachybotrys chartarum IBT 9460 | ||
| Stachybotrys cylindrospora CBS 878.68 | ||
| Stachybotrys dichroa CBS 182.80 | AF548098 | |
| Stachybotrys kampalensis CBS 388.73 | AF548099 | |
| Stachybotrys microspora CBS 186.79 | ||
| Stachybotrys oenanthes CBS 252.76 | ||
| Stachybotrys parvispora CBS 253.75 | ||
| A5 | Trichoderma viride ALI 210 | AF548104 |
| B5 | Trichoderma harzianum ALI 232 | AF548100 |
| C5 | Trichoderma pseudokoningii S-38 | AF548101 |
| D5 | Trichoderma reesei RutC-30 | AF548103 |
| E5 | Trichoderma reesei QM 9414 | AF548102 |
| F5 | Ulocladium botrytis CBS 173.82 | AF548105 |
| G5 | Ulocladium botrytis UPSC 3539 | AF548106 |
| H5 | Saccharomyces cerevisiae ALI 308 | AF548094 |
| G4 | Wallemia sebi ALI 158 | AF548107 |
| H4 | Wallemia sebi UPSC 2502 | AF548108 |
Code refers to the sample positions in blots of Fig. 2.
18S rDNA amplification and sequencing.
18S rDNA was amplified from each fungal strain by PCR using the universal fungal primers NS1, NS3, NS4, NS5, NS6, and NS8, as presented by White et al. (36). The optimal conditions for PCR amplification of 18S rDNA segments with these primers were described previously (38). The purified PCR products were used in sequencing reactions with the same set of primers using a BigDye Terminator Cycle Sequencing Ready Reaction Kit version 3.0 (Applied Biosystems, Foster City, Calif.). Sequencing was performed on an ABI 3100 sequencer (Applied Biosystems). All fungal strains were sequenced from both directions. The obtained sequences have been submitted to GenBank (Table 1).
Sequence analysis and probe design.
Sequences were aligned using ClustalX software (29). Sequence divergence was computed in terms of the number of nucleotide differences per site between pairs of sequences according to Kimura's two-parameter model (15) using the program MEGA version 2.1 (Arizona State University, Tempe). Indels were excluded from pairwise sequence comparisons. The distance matrix for all pairwise sequence combinations was analyzed with the neighbor-joining (NJ) method (25) of phylogenetic tree construction with 1,000 bootstrap replicates by using MEGA version 2.1 software.
The polymorphic regions were targeted to develop oligonucleotide probes that were specific for different fungal groups. To increase the detection specificity and to diminish the possible false-negative result due to PCR error-introduced mismatches, the probes were designed to contain at least two to three (in most cases, more than four) base differences over the probe length (18 to 24 bp) from the excluding strains. Due to the limited species coverage for some genera, the probes designed were first screened against the GenBank sequence database to examine their possible homology to other organisms not included in our samples. The probes that showed good specificity at the species or genus level in the homology search were selected and synthesized by Invitrogen Life Technologies (Paisley, United Kingdom). The sequences of the developed probes are shown in Table 2.
TABLE 2.
Probes developed in this study
| No. | Name | Probe sequence (5′-3′) | Length (bp) | Targeta |
|---|---|---|---|---|
| 1 | Asp-3 | CGGAAAGTTGGTCAAACCCG | 20 | NS5/8 |
| 2 | Asp-4 | GATCGGGCGGTGTTTCTAT | 19 | NS1/4 |
| 3 | Aver-1 | AACCCCGACTTCGGGAGG | 18 | NS1/4 |
| 4 | Aver-3 | ATAGCCCGGTCCGCGTCCGCG | 21 | NS5/8 |
| 5 | Aver-5 | TAAAAACCCCGACTTCGGGAG | 21 | NS1/4 |
| 6 | Cla-1 | ACGGTGTTAGTATTTTGACCCGTT | 24 | NS1/4 |
| 7 | Cla-3 | TCATTTCCTTAGCCGAAAGGTTTG | 24 | NS5/8 |
| 8 | Fus-3 | AGTGGTGGGCAACTACCGC | 19 | NS5/8 |
| 9 | Gre-1 | CTTGAACTTGGTTGGTTGGT | 20 | NS1/4 |
| 10 | Gre-2 | CACTGATCCGACCGGGTTT | 19 | NS1/4 |
| 11 | Gre-7 | TCAGGCAGAGTGGCAACACTCCA | 23 | NS5/8 |
| 12 | Mic-4 | CTTCCTTGACAGAAATGTCC | 20 | NS5/8 |
| 13 | Muc-6 | AAACCCTGACTTACGAAAG | 19 | NS1/4 |
| 14 | Muc-9 | TATGCTATGAATAGCTTCGGTT | 22 | NS1/4 |
| 15 | Pen-1 | AAACCCCGACTTCAGGAAGG | 20 | NS1/4 |
| 16 | Pen-10 | TAAAAACCCCGACTTCAGGAA | 21 | NS1/4 |
| 17 | Pen-3 | ACGGGATTCTATAATGACCCGT | 22 | NS1/4 |
| 18 | Pen-4 | ACAGGATTCTATAATGACCTGT | 22 | NS1/4 |
| 19 | Pen-6 | TGGGATTGGCTTAGGAGGGT | 20 | NS5/8 |
| 20 | Pen-7 | CTTTGGGACTGGCTCAGGAG | 20 | NS5/8 |
| 21 | Pen-8 | CGAAAACTTGGTCAAACTCG | 20 | NS5/8 |
| 22 | Pen-9 | CGGAAACTTAGTCAAACTCG | 20 | NS5/8 |
| 23 | Rhi-2 | GTTTATGTTTATCATTTAACTGTG | 24 | NS5/8 |
| 24 | Rhi-5 | TCCTATTTTCGTTGGTTTAG | 20 | NS1/4 |
| 25 | Sta-6 | GTCCTGGGCTGCACGCGCGTTA | 22 | NS5/8 |
| 26 | Tri-1 | ACGATGTTACATTTTTGACGCGTT | 24 | NS1/4 |
| 27 | Tri-2 | TAGTCGAATCGACAGGCCTTGTG | 23 | NS1/4 |
| 28 | Tri-3 | CTCCCTTGGCCGGAAGGCCTG | 21 | NS5/8 |
| 29 | Ulo-4 | GCGTGCTGGGGAATCAGGAC | 20 | NS1/4 |
| 30 | Ulo-8 | GTTCTTCACCTTGTTCGAAAGAAT | 24 | NS5/8 |
| 31 | Wal-1 | TGGATGACGTTATATTATTGACTC | 24 | NS1/4 |
| 32 | Wal-2 | ATTATTGACTCATTCAGCACTCTG | 24 | NS1/4 |
| 33 | Wal-6 | GGTACGACATTTTGTTGTACATT | 23 | NS5/8 |
Probe hybridization target to 18S rDNA region flanked by primer set NS1/4 or NS5/8.
Slot blotting and nonradioactive probe hybridization.
The fungal 18S rDNA can be flanked by two primer sets, NS1/4 and NS5/8 (36). We amplified these two segments from each strain under the PCR conditions previously described (38). In most cases, 5-μl aliquots of the total 25-μl PCR amplification products were blotted onto nylon membranes. In case of difference in the quantity of PCR product among fungal strains, a volume adjustment was applied to ensure even DNA amounts across all strains submitted for blotting. The procedure for slot blotting and hybridization was described previously (37). The probes were nonradioactive 3′-labeled with digoxigenin (DIG)-ddUTP by terminal transferase following the instructions provided by the manufacturer of the DIG Oligonucleotide 3′-End Labeling Kit (Roche Molecular Biochemicals, Mannheim, Germany). The hybridization and detection were carried out by using the DIG Easy Hyb buffer and DIG Luminescent Detection Kit (Roche Molecular Biochemicals) according to the manufacturer's instructions. After hybridization, the membranes were washed twice in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% sodium dodecyl sulfate at 15 to 25°C, each for 5 min, followed by washing twice in 0.5× SSC and 0.1% sodium dodecyl sulfate at the hybridization temperature for 15 min each (37). Kodak X-Omat AR-5 films were exposed to the blots for 1 to 2 h between intensifying screens at 37°C. Various hybridization temperatures were tested for each probe to achieve the best specificity for a given fungal group or species. The optimal hybridization temperature for each probe was evaluated and listed in Table 3.
TABLE 3.
Summary of hybridization results of the 33 probes to 36 fungal speciesa
| Hybridization result for probeb:
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp-3, 62 | Asp-4, 54 | Aver-1, 56 | Aver-3, 64 | Aver-5, 56 | Pen-1, 55 | Pen-10, 55 | Pen-3, 45 | Pen-4, 50 | Pen-6, 58 | Pen-7, 58 | Pen-8, 50 | Pen-9, 52 | |||||||
| A. flavus | + | + | − | − | − | − | − | − | − | − | − | − | − | ||||||
| A. fumigatus | + | + | − | − | − | − | − | − | − | − | − | − | − | ||||||
| A. niger | + | + | − | − | − | − | − | − | − | − | − | − | − | ||||||
| A. ochraceus | + | + | − | − | − | − | − | − | − | − | − | − | − | ||||||
| A. penicilloides | − | + | − | − | − | − | − | − | − | − | − | − | − | ||||||
| A. silvaticus | + | + | + | + | + | − | − | − | − | − | − | − | − | ||||||
| A. versicolor | + | + | + | + | + | − | − | − | − | − | − | − | − | ||||||
| E. herbariorum | + | + | − | − | − | − | − | − | − | − | − | − | − | ||||||
| C. cladosporioides | + | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| F. culmorum | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| G. abietina | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| M. nivale | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| P. lilacinus | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| P. variotii | − | + | − | − | − | − | − | − | − | − | − | − | − | ||||||
| P. brevicompactum | − | − | − | − | − | + | w | − | + | − | + | − | + | ||||||
| P. chrysogenum | − | − | − | − | − | + | w | + | − | + | − | + | − | ||||||
| P. commune | − | − | − | − | − | w | w | + | − | + | − | + | − | ||||||
| P. frequentans | − | − | − | − | − | w | w | f | − | − | + | w | f | ||||||
| P. italicum | − | − | − | − | − | + | w | + | − | + | − | + | − | ||||||
| S. cerevisiae | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| S. bisbyi | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| S. chartarum | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| S. cylindrospora | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| S. dichroa | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| S. kampalensis | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| S. microspora | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| S. oenanthes | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| S. parvispora | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| T. harzianum | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| T. pseudokoningii | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| T. reesei | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| T. viride | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| U. botrytis | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| W. sebi | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| M. plumbeus | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| R. microsporus | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| R. microsporus var. rhizopodiformis | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
| − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | f | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − |
| − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + |
| − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + |
1, positive; 2, negative; w, weak; f, faint. Full binary combinations for all species listed in the table are given in Table 1 or in the text.
For each probe listed, the hybridization temperature (in degrees Celsius) is given following the comma.
Nucleotide sequence accession numbers. The GenBank accession numbers of the sequences that were obtained in this study are listed in Table 1.
RESULTS AND DISCUSSION
Sequence divergence and phylogenetic relationships.
A total of 49 sequences from 31 species of 15 genera were determined, covering about 94% of the 18S rRNA gene, between nucleotide positions 77 and 1769, relative to the yeast Saccharomyces cerevisiae 18S rRNA gene. The sequence lengths ranged between 1,676 (Stachybotrys kampalensis) and 1,724 bp (Mucor plumbeus), and the alignment length for the 49 sequences was 1,744 bp. Different strains of the same species often had identical sequences, e.g., all three Aspergillus fumigatus isolates, the two isolates of Aspergillus versicolor, and three of the four Penicillium brevicompactum isolates shared the same sequences. In three cases, identical sequences were shared by different species of the same genus: A. versicolor and A. silvaticus; Penicillium commune, one P. chrysogenum strain and P. italicum; and Trichoderma viride and T. pseudokoningii. Among the fungal species included in this study, Mucor plumbeus and Rhizopus microsporus are from the Zygomycota, while all the others are from Ascomycota. Sequence analysis revealed significant divergence between the Mucor and Rhizopus genera and the other Ascomycota fungi. The distance between Mucor-Rhizopus and the others ranged between 0.188 and 0.234. The divergence among the Ascomycota genera, excluding Wallemia sebi and yeast, was relatively low and ranged between 0.010 and 0.099 (mean value, 0.059). W. sebi was the most diverged fungus in the Ascomycota group, with sequence divergence from the others ranging from 0.151 to 0.176 (mean value, 0.168). The yeast also showed distinct divergence from the rest of the Ascomycota members, with a mean distance of 0.110.
The phylogenetic relationship of these fungal strains is presented in a distance-based NJ tree (Fig. 1). Stains of the same species that had identical sequences are merged into one entry, but strains of different species that shared the same sequence are kept as separate entries on the NJ tree. In general, the topology recovered in this study agrees well with the established classification supporting the generally accepted idea that Zygomycota is in basal groups within the Fungi (1, 3). In the Ascomycota group, Wallemia is the first to diverge, followed by yeast. Aspergillus (including Eurotium herbariorum, whose anamorph is A. glaucus), Penicillium, and Paecilomyces formed one weakly differentiated clade, suggesting a very close relationship among the three genera. The mean sequence divergence in this clade is 0.013. Cladosporium is the sister group to the Aspergillus-Penicillium-Paecilomyces clade. It is interesting that Paecilomyces lilacinus is far differentiated from Paecilomyces variotii and grouped together with Trichoderma and Fusarium culmorum. The taxonomic status of P. lilacinus deserves further evaluation, and more strains of this fungus and other Paecilomyces spp. should be analyzed in order to verify this result. Stachybotrys is grouped into the same clade as the Sordariomycetes fungi Trichoderma, Fusarium, and Microdochium with strong bootstrap support. Ulocladium botrytis and a forest pathogen, Gremmeniella abietina, formed one weakly supported clade. Few phylogenetic studies in fungi included Wallemia, and the taxonomic position of this monotypic genus is not clear. In the old classification system, Wallemia was placed in the form-order Moniliales, in the form-class Deuteromycetes (35). Based on the morphological and cytological examination, Moore (20) suggested that W. sebi is probably a basidiomycete. Since Basidiomycota species were not included in this study, the exact position of W. sebi remains uncertain. The pronounced sequence divergence between Wallemia and other Ascomycota fungi raises questions as to its position within Ascomycota. If W. sebi is within Ascomycota then it is likely to occupy the basal position.
FIG. 1.
NJ tree showing the phylogenetic relationships among the fungal strains based on 18S rDNA sequences. Pairwise sequence distances were calculated using Kimura’s two-parameter model. Bootstrap values of >50% from a sample of 1,000 replicates are shown on each branch. The tree is rooted with Mucor and Rhizopus. Full binary combinations for all species named in the figure are given in Table 1 or in the text.
Our sampling scheme employed in this study is not aimed at fungus phylogeny, which would require a systematic sampling of the main representative groups of kingdom Fungi. In addition, a single gene tree cannot fully reflect the true species phylogeny. The species included here are mostly common airborne fungi. The obtained sequence information and the phylogenetic relationships provide the basis for developing specific markers for fungal groups and for predicting their detection applicability. Nevertheless, our data provide new information that facilitates the phylogenetic placement of a few little-studied fungi, e.g., Stachybotrys and Wallemia. Our results also revealed certain conflict in the classification of Paecilomyces lilacinus. All these call for detailed investigations in the future.
Probe evaluation.
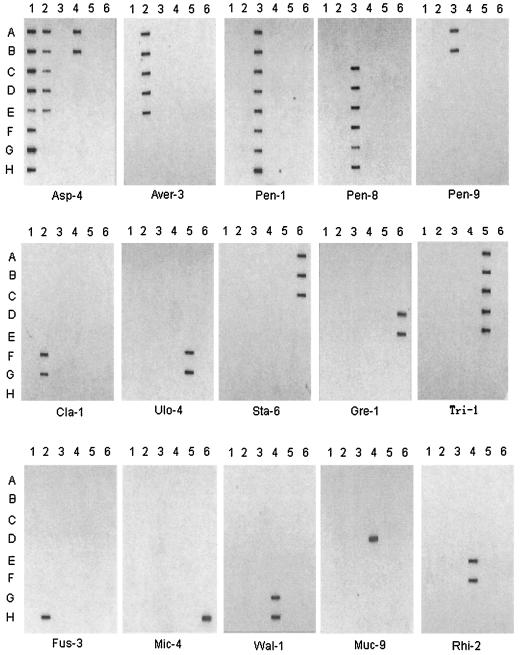
The 18S rDNA sequences were analyzed for polymorphic sites suitable for defining species- or genus-specific probes. Initially, we designed 108 probes for each genus and species included in this study. All these probes were first screened against GenBank for additional homology. After the homology analysis, 33 probes were selected for further evaluation in hybridization assays against 57 fungal strains of 36 species from 15 genera. The specificity and optimal hybridization temperatures for these 33 probes were determined, and the results are summarized in Table 3. A few examples of the detection specificity of the probes are shown in Fig. 2.
FIG. 2.
Examples of hybridization results with probes indicated under each panel. The fungal strains at each grid position (A1 to H6) of the blots are listed in Table 1.
Aspergillus, Penicillium, and Paecilomyces are the most prevalent airborne fungi (22). Some species from these genera are opportunistic pathogens (12, 19). Aspergillus, Penicillium, and Paecilomyces are closely related (Fig. 1) (3, 33). Most of the 18S rDNA sequence variations are shared among the three genera, making the development of genus-specific markers difficult. Two probes, Asp-3 and Asp-4, hybridized to most of the Aspergillus spp. but also cross-hybridized to Cladosporium cladosporioides and Paecilomyces variotii, respectively (Table 3). Probes Aver-1, Aver-3, and Aver-5 were designed to be specific to A. versicolor and A. silvaticus, since both had identical sequences. All three probes gave good specific detection results. Aspergillus versicolor produces carcinogenic mycotoxin sterigmatocystin and 5-methoxy-sterigmatocystin, thus constituting a potential health hazard (7, 8). It has a broad range of favorite substrates and is frequently found in varied environments (8). These three probes will be useful in the detection of the fungus in environmental samples.
Two probes, Pen-1 and Pen-10, can be regarded as Penicillium specific, although at the specified temperature the hybridization signals in some species became weak (Table 3). A decrease in temperature of 2°C resulted in an increase in the intensity of hybridization signals but also faint cross-hybridization to Paecilomyces variotii for Pen-1 and to A. versicolor or A. silvaticus for Pen-10 (data not shown). Three probes, Pen-4, Pen-7, and Pen-9, are specific to Penicillium brevicompactum. Penicillium commune, Penicillium chrysogenum, and Penicillium italicum had identical 18S sequences. Probes Pen-3, Pen-6, and Pen-8 are specific for their detection.
Cladosporium and Ulocladium spp. are important inhalation allergens (8). The hybridization results showed that Cla-1 and Cla-3 were specific to C. cladosporioides (Table 3). Since only one Cladosporium species was included in this study, the probes were further examined against GenBank sequences. Homology search indicated that Cla-3 could also hybridize to Cladosporium herbarum and Cladosporium tenuissimum. Probes Ulo-4 and Ulo-8 were specific to U. botrytis in this study. Homology search indicated that these two probes could potentially hybridize to Alternaria spp. This is in accordance with the close relationship between Ulocladium and Alternaria as suggested by their morphology.
Stachybotrys is commonly found in water-damaged buildings and produces several mycotoxins including satratoxins, trichothecenes, and stachybocins that may cause serious health problems associated with sick-building syndrome (14, 31). The markers for their rapid detection in indoor environments are critical for exposure risk assessment. Probe Sta-6 gave good specificity to Stachybotrys spp. (Table 3) and could be used as a genus marker for this application.
Gremmeniella abietina is a conifer tree pathogen and causes severe damage to the forest in Scandinavia, which is composed mainly of pine and spruce trees (10). The fungus produces large amounts of spores on the tree trunk, branch, and needles. Forest workers handling the cutting and ground clearing are concerned with the spore density in the air. In addition, measures of the spore density can be applied to monitor the disease development in the forest. Specific markers that differentiate G. abietina from other airborne fungi are needed. The three probes Gre-1, Gre-2, and Gre-7 are good markers for this purpose.
Most of the Trichoderma spp. can utilize cellulose and are found in wooden materials. The hybridization results showed that Tri-1 and Tri-2 gave good specificity at 62 and 60°C, respectively (Table 3). Tri-3 gave intense signal for Trichoderma spp. at 54°C, but it also showed faint cross-reaction to Paecilomyces lilacinus (Table 3). This reflects the high sequence similarity between Trichoderma and P. lilacinus as revealed in Fig. 1. Increase of hybridization temperature improved the specificity of Tri-3 but resulted in reduction in signal intensity in Trichoderma spp. (data not shown). Fusarium culmorum potentially produces trichothecene (21, 22). Microdochium nivale (also known as Fusarium nivale) is a pathogen of overwintering cereals (13). For these two species, the probes Fus-3 and Mic-4 showed good specificity in our tests (Table 3). Fus-3 might also detect Gibberella pulicaris and Hypomyces chrysospermus, which share the same type of conidiospore as Fusarium.
Wallemia sebi is a strong xerophilic fungus. Its presence can be detected only by using special media like dichloran 18% glycerol (DG18), DG18 agar supplemented with Triton X-301 (DG18T), or malt agar with 64% sucrose (2, 22). Thus, its role in public health implications has long been underestimated. Recent investigations show that W. sebi is abundant in agricultural environments like cow barns and farms handling hay and is suspected to be another etiological agent of farmer's lung disease (17, 23). Due to its distinct sequence divergence from the other Ascomycota fungi, it is easy to identify regions unique to Wallemia. We listed three probes (Wal-1, Wal-2, and Wal-6) in Table 3; all showed clean and intensive signals that differentiate Wallemia from the others.
Mucor and Rhizopus are from Mucoraceae, belonging to Zygomycetes of Zygomycota, which is distantly related to Ascomycota (Fig. 1) (3). Many regions in the 18S rDNA showed differentiation between Mucor-Rhizopus and the other Ascomycota fungi. Thus, it is easy to find suitable regions to develop probes for differentiation of Mucor-Rhizopus from other common airborne fungi. We listed two probes, Muc-6 and Muc-9, that are specific to M. plumbeus and two probes, Rhi-2 and Rhi-5, for R. microsporus (Table 3). Homology search against GenBank indicated that these probes could potentially hybridize to other species within each genus. Muc-9 has a broader species detection spectrum within Mucor than that of Muc-6 (data not shown) and can thus be used as a marker for Mucor in general. Homology search also indicated that Rhi-2 could hybridize to Rhizopus azygosporus, a species very closely related to R. microsporus (34), and the search indicated that Rhi-5 could hybridize to several species of Rhizopus, including R. stolonifer, R. oryzae, R. azygosporus, and varieties of R. microsporus. Thus, Rhi-5 could be regarded as a marker for common Rhizopus spp.
18S rDNA variation can be exploited for the development of molecular markers for rapid detection and identification of fungal flora in environmental samples. The relatively slow rate of molecular evolution makes the 18S rDNA a good candidate for finding consensus conserved regions suitable for genus or higher taxonomic level detections. Short oligonucleotide probes targeting a specific region of the molecule can also yield species-specific markers to differentiate closely related taxa. The detection procedure, consisting of selective PCR followed by specific oligonucleotide probe hybridization, is sensitive and realistic with regard to environmental samples. The new set of probes developed in this study aims to specifically detect common airborne fungi and could contribute to the diagnostic research for environmental monitoring. These probes can also be applied in the quantification of fungal compositions in environmental samples through real-time PCR detections.
Acknowledgments
This study was supported by grants from the Swedish Council for Forestry and Agricultural Research (SJFR), the Swedish Council for Working Life Research and Social Research (FAS), and the Japan Society for the Promotion of Science (JSPS).
REFERENCES
- 1.Berbee, M. L., and J. W. Taylor. 1999. Fungal phylogeny, p. 21-77. In R. P. Oliver and M. Schweizer (ed.), Molecular fungal biology. Cambridge University Press, Cambridge, United Kingdom.
- 2.Beuchat, L. R., and C. A. Hwang. 1996. Evaluation of modified dichloran 18% glycerol (DG18) agar for enumerating fungi in wheat flour: a collaborative study. Int. J. Food Microbiol. 29:161-166. [DOI] [PubMed] [Google Scholar]
- 3.Bruns, T. D., R. Vilgalys, S. M. Barns, D. Gonzalez, D. S. Hibbett, D. J. Lane, L. Simon, S. Stickel, T. M. Szaro, W. G. Weisburg, and M. L. Sogin. 1992. Evolutionary relationships within the fungi: analyses of nuclear small subunit rRNA sequences. Mol. Phylogenet. Evol. 1:231-241. [DOI] [PubMed] [Google Scholar]
- 4.Chang, C. W., H. Chung, C. F. Huang, and H. J. Su. 2001. Exposure of workers to airborne microorganisms in open-air swine houses. Appl. Environ. Microbiol. 67:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooley, J. D., W. C. Wong, C. A. Jumper, and D. C. Straus. 1998. Correlation between the prevalence of certain fungi and sick building syndrome. Occup. Environ. Med. 55:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eduard, W., P. Sandven, and F. Levy. 1992. Relationships between exposure to spores from Rhizopus microsporus and Paecilomyces variotii and serum IgG antibodies in wood trimmers. Int. Arch. Allergy Immunol. 97:274-282. [DOI] [PubMed] [Google Scholar]
- 7.Engelhart, S., A. Loock, D. Skutlarek, H. Sagunski, A. Lommel, H. Farber, and M. Exner. 2002. Occurrence of toxigenic Aspergillus versicolor isolates and sterigmatocystin in carpet dust from damp indoor environments. Appl. Environ. Microbiol. 68:3886-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravesen, S., P. A. Nielsen, R. Iversen, and K. F. Nielsen. 1999. Microfungal contamination of damp buildings—examples of risk constructions and risk materials. Environ. Health Perspect. 107:505-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanhela, R., K. Louhelainen, and A. L. Pasanen. 1995. Prevalence of microfungi in Finnish cow barns and some aspects of the occurrence of Wallemia sebi and Fusaria. Scand. J. Work Environ. Health 21:223-228. [DOI] [PubMed] [Google Scholar]
- 10.Hansson, P. 1996. Gremmeniella abietina in Northern Sweden. Silvicultural aspects of disease development in the introduced Pinus contorta and Pinus sylvestris. Ph.D. thesis. Swedish University of Agricultural Sciences, Umeå, Sweden.
- 11.Haugland, R. A., and J. L. Heckman. 1998. Identification of putative sequence-specific PCR primers for detection of the toxigenic fungal species Stachybotrys chartarum. Mol. Cell. Probes. 12:387-396. [DOI] [PubMed] [Google Scholar]
- 12.Hendolin, P. H., L. Paulin, P. Koukila-Kahkola, V. J. Anttila, H. Malmberg, M. Richardson, and J. Ylikoski. 2000. Panfungal PCR and multiplex liquid hybridization for detection of fungi in tissue specimens. J. Clin. Microbiol. 38:4186-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiilovaara-Teijo, M., A. Hannukkala, M. Griffith, X. M. Yu, and K. Pihakaski-Maunsbach. 1999. Snow-mold-induced apoplastic proteins in winter rye leaves lack antifreeze activity. Plant Physiol. 121:665-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodgson, M. J., P. Morey, W. Y. Leung, L. Morrow, D. Miller, B. B. Jarvis, H. Robbins, J. F. Halsey, and E. Storey. 1998. Building-associated pulmonary disease from exposure to Stachybotrys chartarum and Aspergillus versicolor. J. Occup. Environ. Med. 40:241-249. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 16.Kozak, P. P. J., J. Gallup, L. H. Cummins, and S. A. Gillman. 1980. Currently available methods for home mold surveys. II. Examples of problem homes surveyed. Ann. Allergy 45:167-176. [PubMed] [Google Scholar]
- 17.Lappalainen, S., A. L. Pasanen, M. Reiman, and P. Kalliokoski. 1998. Serum IgG antibodies against Wallemia sebi and Fusarium species in Finnish farmers. Ann. Allergy Asthma Immunol. 81:585-592. [DOI] [PubMed] [Google Scholar]
- 18.Maicas, S., A. C. Adam, and J. Polaina. 2000. The ribosomal DNA of the zygomycete Mucor miehei. Curr. Genet. 37:412-419. [DOI] [PubMed] [Google Scholar]
- 19.Martin, C., D. Roberts, M. van Der Weide, R. Rossau, G. Jannes, T. Smith, and M. Maher. 2000. Development of a PCR-based line probe assay for identification of fungal pathogens. J. Clin. Microbiol. 38:3735-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore, R. T. 1986. A note on Wallemia sebi. Antonie Leeuwenhoek 52:183-187. [DOI] [PubMed] [Google Scholar]
- 21.Niessen, M. L., and R. F. Vogel. 1998. Group-specific PCR detection of potential trichothecene-producing Fusarium species in pure cultures and cereal samples. Syst. Appl. Microbiol. 21:618-631. [DOI] [PubMed] [Google Scholar]
- 22.Piecková, E., and Z. Jesenská. 1999. Microscopic fungi in dwellings and their health implications in humans. Ann. Agric. Environ. Med. 6:1-11. [PubMed] [Google Scholar]
- 23.Reboux, G., R. Piarroux, F. Mauny, A. Madroszyk, L. Millon, K. Bardonnet, and J. C. Dalphin. 2001. Role of molds in farmer's lung disease in Eastern France. Am. J. Respir. Crit. Care Med. 163:1534-1539. [DOI] [PubMed] [Google Scholar]
- 24.Roe, J. D., R. A. Haugland, S. J. Vesper, and L. J. Wymer. 2001. Quantification of Stachybotrys chartarum conidia in indoor dust using real-time, fluorescent probe-based detection of PCR products. J. Expo. Anal. Environ. Epidemiol. 11:12-20. [DOI] [PubMed] [Google Scholar]
- 25.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 26.Sandhu, G. S., B. C. Kline, L. Stockman, and G. D. Roberts. 1995. Molecular probes for diagnosis of fungal infections. J. Clin. Microbiol. 33:2913-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shelton, B. G., K. H. Kirkland, W. D. Flanders, and G. K. Morris. 2002. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl. Environ. Microbiol. 68:1743-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi, T. 1997. Airborne fungal colony-forming units in outdoor and indoor environments in Yokohama, Japan. Mycopathologia 139:23-33. [DOI] [PubMed] [Google Scholar]
- 29.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuchiya, D., and M. Taga. 2001. Application of fibre-FISH (fluorescence in situ hybridization) to filamentous fungi: visualization of the rRNA gene cluster of the ascomycete Cochliobolus heterostrophus. Microbiology 147:1183-1187. [DOI] [PubMed] [Google Scholar]
- 31.Tuomi, T., K. Reijula, T. Johnsson, K. Hemminki, E. L. Hintikka, O. Lindroos, S. Kalso, P. Koukila-Kahkola, H. Mussalo-Rauhamaa, and T. Haahtela. 2000. Mycotoxins in crude building materials from water-damaged buildings. Appl. Environ. Microbiol. 66:1899-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turin, L., F. Riva, G. Galbiati, and T. Cainelli. 2000. Fast, simple, and highly sensitive double-rounded polymerase chain reaction assay to detect medically relevant fungi in dermatological specimens. Eur. J. Clin. Investig. 30:511-518. [DOI] [PubMed] [Google Scholar]
- 33.Verweij, P. E., J. F. Meis, P. van den Hurk, J. Zoll, R. A. Samson, and W. J. Melchers. 1995. Phylogenetic relationships of five species of Aspergillus and related taxa as deduced by comparison of sequences of small subunit ribosomal RNA. J. Med. Vet. Mycol. 33:185-190. [DOI] [PubMed] [Google Scholar]
- 34.Voigt, K., E. Cigelnik, and K. O'Donnell. 1999. Phylogeny and PCR identification of clinically important Zygomycetes based on nuclear ribosomal-DNA sequence data. J. Clin. Microbiol. 37:3957-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Arx, J. A. 1981. The genera of fungi sporulating in pure culture. J. Cramer, Vaduz, Switzerland.
- 36.White, T. J., T. Bruns, S. Lee, and J. Talor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, San Diego, Calif.
- 37.Wu, Z., G. Blomquist, S.-O. Westermark, and X.-R. Wang. 2002. Application of PCR and probe hybridization techniques in detection of airborne fungal spores in environmental samples. J. Environ. Monit. 4:673-678. [DOI] [PubMed] [Google Scholar]
- 38.Wu, Z., X.-R. Wang, and G. Blomquist. 2002. Evaluation of PCR primers and PCR conditions for specific detection of common airborne fungi. J. Environ. Monit. 4:377-382. [DOI] [PubMed] [Google Scholar]
- 39.Zhou, G., W.-Z. Whong, T. Ong, and B. Chen. 2000. Development of a fungus-specific PCR assay for detecting low-level fungi in an indoor environment. Mol. Cell. Probes 14:339-348. [DOI] [PubMed] [Google Scholar]