Abstract
Plant defense mechanisms against necrotrophic pathogens, such as Botrytis cinerea, are considered to be complex and to differ from those that are effective against biotrophs. In the abscisic acid-deficient sitiens tomato (Solanum lycopersicum) mutant, which is highly resistant to B. cinerea, accumulation of hydrogen peroxide (H2O2) was earlier and stronger than in the susceptible wild type at the site of infection. In sitiens, H2O2 accumulation was observed from 4 h postinoculation (hpi), specifically in the leaf epidermal cell walls, where it caused modification by protein cross-linking and incorporation of phenolic compounds. In wild-type tomato plants, H2O2 started to accumulate 24 hpi in the mesophyll layer and was associated with spreading cell death. Transcript-profiling analysis using TOM1 microarrays revealed that defense-related transcript accumulation prior to infection was higher in sitiens than in wild type. Moreover, further elevation of sitiens defense gene expression was stronger than in wild type 8 hpi both in number of genes and in their expression levels and confirmed a role for cell wall modification in the resistant reaction. Although, in general, plant defense-related reactive oxygen species formation facilitates necrotrophic colonization, these data indicate that timely hyperinduction of H2O2-dependent defenses in the epidermal cell wall can effectively block early development of B. cinerea.
Botrytis cinerea causes gray mold diseases in a broad range of plant species and is one of the most comprehensively studied necrotrophic plant pathogens. Necrotrophs kill their host cells by secreting toxic compounds or lytic enzymes and, in addition, produce an array of pathogenicity factors that can subdue host defenses (for review, see van Kan, 2006). Despite elaborate research studies, the biochemical and genetic basis of resistance to Botrytis is still not fully understood. The ability of the fungus to kill cells was proposed as a major determinant in host specificity of different Botrytis species (Mansfield and Hutson, 1980) and, similarly, plant resistance to Botrytis is supposed to depend on the balance between cell death and survival (van Baarlen et al., 2007). In addition, constitutive and inducible plant secondary metabolites determine host specificity of different Botrytis species and fungal colonization in compatible interactions (for review, see van Baarlen et al., 2004). Structural barriers and cell wall fortifications are also considered to be involved in arresting Botrytis, although the actual contribution to the effective inhibition of infection is often unclear (van Baarlen et al., 2004).
One of the most important plant defense responses to pathogens is the production of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), during oxidative burst (Mehdy, 1994). ROS can function in cell wall modification, defense signaling, the hypersensitive response (HR), or be directly toxic to pathogens (Lamb and Dixon, 1997). There is evidence, however, that generation of ROS assists the colonization of plant tissue by necrotrophic pathogens: B. cinerea infection can be suppressed by spraying antioxidants on plants (Elad, 1992) and H2O2 is produced during common bean (Phaseolus vulgaris) and Arabidopsis (Arabidopsis thaliana) colonization (von Tiedemann, 1997; Govrin and Levine, 2000). Therefore, defense reactions effective against biotrophic pathogens are believed to increase vulnerability toward necrotrophs (for review, see Lyon et al., 2004). Nevertheless, the role of ROS in defense against B. cinerea remains controversial. Some studies suggest a positive effect of ROS on plant resistance: Chemical induction of oxidative burst in tomato (Solanum lycopersicon) with O-hydroxyethylorutin resulted in resistance (Malolepsza and Urbanek, 2002) and a biphasic oxidative burst has been reported in bean cells attacked by B. cinerea (Unger et al., 2005). The secondary oxidative burst was much stronger in infections with a nonaggressive rather than with an aggressive strain, which led to the conclusion that ROS-mediated HR-like cell death was able to block B. cinerea.
Four Botrytis-susceptible loci (BOS1, BOS2, BOS3, and BOS4) that affect Arabidopsis susceptibility to B. cinerea were recently identified. The BOS1 locus has been shown to encode a MYB transcription factor that interacts with the jasmonate signaling pathway mediated by ROS (Mengiste et al., 2003; Veronese et al., 2004). Within the complex transcriptional response of Arabidopsis during infection, subsets of the Botrytis-induced genes depend on functional jasmonate, ethylene, and salicylic acid (SA) signaling pathways and three Botrytis-induced transcription factors are sensitive to abscisic acid (ABA; AbuQamar et al., 2006). These results demonstrate that the outcome of interactions between plants and necrotrophs is, as with biotrophs, determined by complex interplay between different plant hormones regulating defense gene expression and disease resistance (Thomma et al., 1998; Glazebrook, 2005).
Recently, it has become clear that there is an important overlap between biotic and abiotic stress signaling involving ABA (Xiong and Yang, 2003; Anderson et al., 2004; Fujita et al., 2006). Some exceptions notwithstanding, high or basal ABA levels contribute to a susceptible response to the pathogen (Henfling et al., 1980; Mohr and Cahill, 2001; Mauch-Mani and Mauch, 2005). During the plant's defense to pathogens, interactions of ABA with SA, jasmonate, or ethylene are mostly antagonistic, as is the case in several plant developmental processes. However, besides the interaction of ABA with other hormones in defense signaling, there is little or no knowledge of the primary mechanisms of ABA-induced disease susceptibility (Mauch-Mani and Mauch, 2005). During abiotic stress, ABA-derived signal transduction often involves accumulation of H2O2 (Kwak et al., 2006). On the other hand, in physiological processes, such as seed germination, the release of H2O2 is inhibited by ABA (Schopfer et al., 2001).
The tomato ABA-deficient sitiens mutant is highly resistant to B. cinerea and displays a stronger SA-dependent defense response than the wild type (Audenaert et al., 2002). In addition, it is less susceptible to the biotrophic fungal pathogen Oidium neolycopersici (Achuo et al., 2006), the hemibiotrophic bacterium Pseudomonas syringae pv tomato (Thaler and Bostock, 2004), and the necrotrophs Sclerotinia sclerotiorum and Erwinia chrysanthemi (B. Asselbergh, unpublished data). Here, we show that rapid H2O2 accumulation is essential within the resistance mechanism of sitiens to B. cinerea. H2O2 accumulated rapidly in the sitiens epidermal cells upon pathogen inoculation, causing increased protein cross-linking and peroxidative incorporation of phenolic compounds in the cell wall, reactions that were not present in the wild type. This fast cell wall-related defense response was also reflected at the transcriptome level with increased defense-related transcript levels. Our data suggest that timing, quantity, and localization of ROS determine the outcome of the B. cinerea-tomato interaction.
RESULTS
B. cinerea Is Blocked in the Early Steps of the Infection Process in sitiens
The first symptoms on tomato leaf tissue artificially infected with a B. cinerea conidial suspension appear as necrotic spots beneath the inoculation droplet between 24 and 48 h postinoculation (hpi). In a susceptible reaction, these primary necrotic spots develop to water-soaked, macerated lesions within 96 hpi (Benito et al., 1998). In a resistant reaction, fungal development is restricted to a few dark-brown spots (nonspreading lesions). After conidia inoculation, the ABA-deficient sitiens mutant shows primary necrotic spots, but displays strong reduction in the percentage of spreading lesions compared to wild-type plants (Audenaert et al., 2002). In this study, we specifically assessed the differences between wild type and sitiens during the early stages of the infection process. We modified the drop inoculation method described by Audenaert et al. (2002) by adding a pregermination step to synchronize B. cinerea conidia germination and by using 1-cm-diameter leaf discs floating on water. This procedure leads to a more uniform and synchronized infection without compromising the resistance response in sitiens (Fig. 1A).
Figure 1.
Disease symptoms on wild-type and sitiens tomato leaf discs after infection with two 5-μL drops of a B. cinerea conidial suspension. A, Macroscopic disease symptoms. In wild-type leaf discs, spreading gray disintegration of the host tissue is visible and in sitiens discs necrotic spots at the site of the infection droplet are observed. B, Close up of macroscopic disease symptoms. In sitiens, primary necrotic lesions contain a high number of distinct dark spots at 48 and 72 hpi. In wild type, primary necrotic spots at 48 hpi are typically larger, less numerous, have a paler shade of brown, and more often develop into spreading water-soaked lesions.
Until 24 hpi, infection events were very similar in wild-type and sitiens leaf discs. Conidial attachment and germination occurred within 4 hpi, followed by normal hyphal growth, accompanied by appressorium-mediated and hyphal tip penetration attempts (data not shown). The first differences were observed during primary necrosis events between 24 and 48 hpi (Fig. 1B): In sitiens, spots were more abundant and appeared as dry, dark-brown, round-shaped dots, whereas in wild-type leaf tissue, necrotic spots were larger, irregularly shaped, and pale brown. These observations suggest that, in sitiens, resistance mechanisms are already activated prior to, or at latest during, the onset of primary necrosis.
Resistance in sitiens Is Associated with Rapid Accumulation of H2O2 in Epidermal Cells
Because ROS production is one of the earliest defense responses in plant-pathogen interactions, we compared H2O2 accumulation at the inoculation site by using 3,3′-diaminobenzidine (DAB) staining. In this protocol, brown precipitates are formed at the sites of H2O2 accumulation (Thordal-Christensen et al., 1997). Wild-type and sitiens leaf discs were inoculated with a droplet of a B. cinerea conidial suspension and floated for 3 h in a solution containing 1 mg/mL DAB before sampling 4, 8, 12, 24, 48, and 72 hpi. In mock-infected leaf discs, no DAB accumulation was observed. In wild type, DAB staining was macroscopically detectable at 48 hpi and was associated with lesion progression. In sitiens, staining became macroscopically visible from 8 hpi and further intensified at later time points, but remained restricted to the area covered by the infection droplet (Fig. 2). When the DAB solution was supplemented with ascorbic acid, staining was abolished, indicating that staining was due to H2O2 accumulation (data not shown).
Figure 2.
Temporal evolution of H2O2 accumulation in wild-type and sitiens tomato after infection with B. cinerea. DAB staining of leaf discs infected with two 5-μL drops of a conidial suspension was performed at different time points post inoculation (4, 8, 12, 16, 20, 24, 48, and 72 hpi). One representative disc out of three replicates is shown for each time point. [See online article for color version of this figure.]
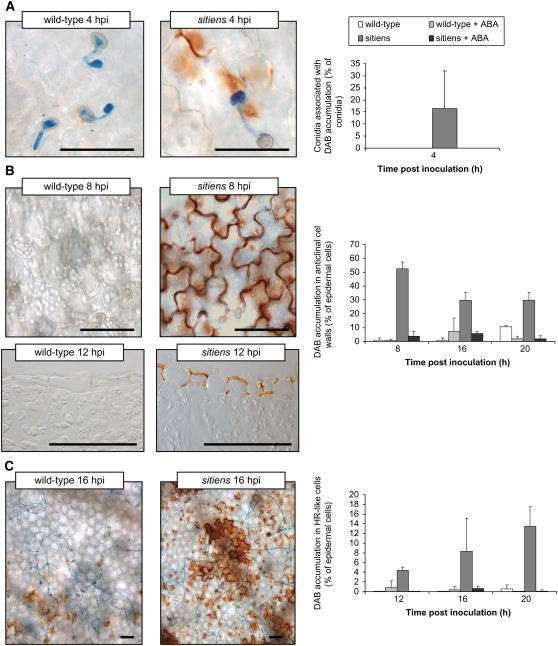
Microscopic observations revealed that, in sitiens, DAB staining was detectable as early as 4 hpi in epidermal cell walls in close contact to the fungal germ tubes (Fig. 3A). Between 4 and 8 hpi, H2O2 accumulation was detected also in the entire anticlinal wall of epidermal cells in contact with the fungus and in neighboring epidermal cells. The spreading of H2O2 accumulation from the sites of fungal contact resulted in general DAB staining of mainly the anticlinal walls of the majority of epidermal cells beneath the infection droplet at 8 hpi (Fig. 3B). At later time points, extracellular H2O2 accumulation gradually decreased, but from 12 hpi, H2O2 was also clearly visible inside multiple epidermal cells in sitiens (Fig. 3C). Epidermal cells with intracellular H2O2 accumulation displayed autofluorescence due to accumulation of phenolic compounds and cytoplasmic aggregation (Fig. 4), two features that are considered as hallmarks of a HR (Heath, 2000). None of these reactions were visible in wild-type cells at these early time points. Instead, the developing lesion showed intense DAB staining in the mesophyll layer that started between 24 and 48 hpi (Fig. 3; data not shown).
Figure 3.
Effect of ABA on H2O2 accumulation in epidermal cells after infection with B. cinerea. A, Association of DAB accumulation with B. cinerea conidia. In sitiens, H2O2 was located at the site of penetration and in some parts of the anticlinal wall of the penetrated cell, whereas in wild type and in sitiens supplemented with ABA, no H2O2 accumulation was detected. Germinating conidia were classified in two groups based on the presence or absence of associated DAB accumulation in the epidermal cells, whose percentage is shown. B, DAB accumulation in epidermal cell walls. In sitiens, DAB staining was general in the entire outline of the anticlinal cell wall, whereas in wild type and sitiens supplemented with ABA, no H2O2 accumulation was detected at 8 hpi (top). The restriction of sitiens DAB accumulation to the epidermal layer was confirmed on cross sections (bottom). Epidermal cells were classified in two groups based on the presence or absence of DAB accumulation in the anticlinal walls, whose percentage is shown in the anticlinal walls at 8, 16, and 20 hpi. C, Intracellular DAB accumulation in epidermal cells showing an HR-like reaction. At 16 hpi, wild-type and ABA-supplemented sitiens epidermal cells did not accumulate intracellular DAB, whereas in sitiens, groups of HR-like cells with intracellular DAB accumulation were present near the site of fungal penetration. Epidermal cells were classified in two groups based on the presence or absence of intracellular DAB accumulation and the percentage is shown at 12, 16, and 20 hpi. In all graphs, bars represent the means and the sds of data from six inoculation droplets originating from three plants. In each inoculation droplet, at least 50 conidia (A) or 300 epidermal cells (B and C) originating from representative zones within each inoculation droplet were counted. Data from one experiment is presented. The experiment was repeated with similar results. Scale bar = 50 μm.
Figure 4.
HR-like reaction of sitiens epidermal cells at 12 hpi. Cells near the site of fungal penetration with this reaction showed intracellular DAB accumulation (A), cytoplasmic aggregation (B), and green to yellow autofluorescence after UV excitation (C). Scale bar = 50 μm. [See online article for color version of this figure.]
Nitroblue tetrazolium (NBT) was used to visualize accumulation of superoxide (Doke, 1983) in wild-type and sitiens leaf tissue after inoculation with B. cinerea. NBT accumulated only at the leaf disc border and was not detected at the infection site at any of the examined time points (Supplemental Fig. S1).
H2O2 Burst Is Necessary for sitiens Resistance
To address whether the rapid H2O2 burst in sitiens is directly inferred from ABA deficiency, exogenous ABA (100 μm) was applied before infection and DAB staining. Petiole feeding with exogenous ABA 24 h before infection was shown to restore B. cinerea susceptibility of sitiens to wild-type levels (Audenaert et al., 2002). Repetitive spraying of ABA had the same effect (data not shown; see also Achuo et al., 2006) and suppressed H2O2 accumulation (Fig. 5A). Microscopic assessment during the early stages of infection confirmed that ABA supplementation decreased the number of DAB-positive epidermal cells toward the amount detectable in wild-type tissue (Fig. 3).
Figure 5.
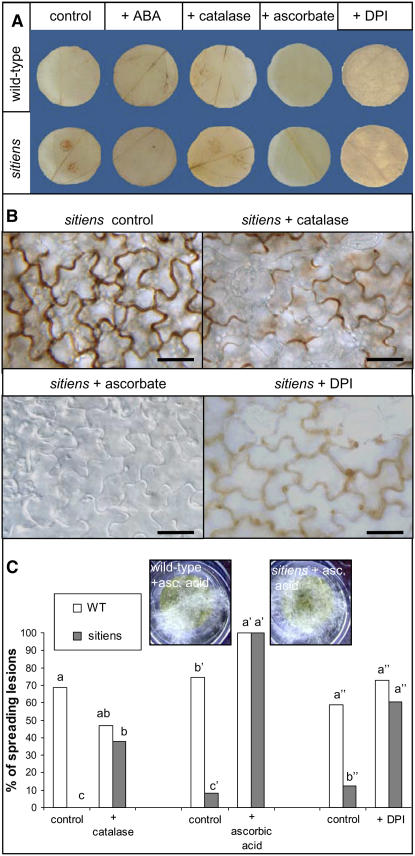
Effect of ABA, ascorbate, catalase, and DPI treatment on H2O2 accumulation and symptom development in wild-type and sitiens tomato leaf discs after inoculation with two 5-μL droplets of B. cinerea conidial suspension. A, DAB staining at 20 hpi. One representative disc out of three replicates is presented. B, Micrograph of sitiens H2O2 accumulation at 8 hpi. C, B. cinerea symptom development. For each treatment, at least 18 leaf discs from at least five plants were infected and the number of spreading lesions was evaluated at 4 dpi. Data were statistically analyzed using binary logistic regression. Bars with different letters are significantly different at P < 0.05. Profuse pathogen development is visible on discs treated with ascorbic acid at 4 dpi. All experiments were repeated with similar results.
Artificial impairment of the rapid oxidative burst in sitiens also resulted in restoration of susceptibility. Floating of sitiens leaf discs on a solution supplemented with 1,100 units/mL catalase or 5 mm ascorbate during conidia inoculation significantly increased or totally reestablished susceptibility, respectively (Fig. 5C). Whereas catalase treatment did not significantly affect susceptibility of the wild type, ascorbate provoked pathogen lesions to spread earlier than in untreated leaf discs and led to abundant superficial fungal growth and accelerated sporulation (Fig. 5C). Interestingly, only treatment with ascorbate completely eliminated all detectable H2O2 from the sitiens leaf tissue. The partial nature of enzymatic H2O2 removal with catalase was observed both macroscopically (Fig. 5A) and microscopically: Catalase treatment resulted in faint DAB staining that covered only part of the epidermal anticlinal walls (Fig. 5B) without altering the number of cells that displayed extracellular or intracellular H2O2 accumulation (data not shown). Application of 50 μm diphenilene iodonium (DPI), an inhibitor of the ROS-generating enzyme NADPH oxidase, restored B. cinerea susceptibility to wild-type levels (Fig. 5C). DPI inhibited the specific accumulation of H2O2 underneath the inoculation droplet, but also caused nonspecific background staining covering the entire leaf disc surface (Fig. 5, A and B).
H2O2 Accumulation in sitiens Coincides with Elevated Levels of Extracellular Peroxidase and Modification of the Epidermal Anticlinal Cell Walls
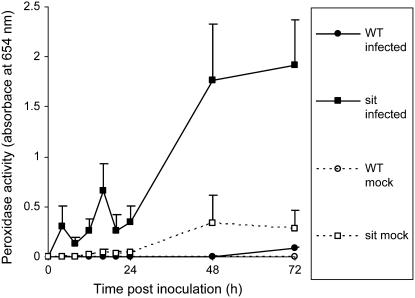
Because H2O2 production after pathogen attack can result from increased peroxidase activity and peroxidases mediate many H2O2-related defense responses, we examined extracellular peroxidase activity with the tetramethylbenzoidine (TMB) assay described by Ros Barceló (1998). Leaf discs inoculated with B. cinerea were fixed in ethanol and incubated in a solution of TMB and H2O2. Peroxidation of the TMB molecule resulted in blue discoloration of both leaf tissue and incubation solution. The latter was used to quantify the activity of extracellular peroxidases (Lucena et al., 2003). In wild-type leaf discs, a minor increase was detected 72 hpi, whereas in sitiens peroxidase activity increased significantly between 4 and 24 hpi, followed by a drastic and sustained increase at 24 hpi (Fig. 6). In mock-infected sitiens leaf discs, peroxidase levels also increased at 48 and 72 hpi, but levels remained lower than in infected sitiens discs.
Figure 6.
Extracellular peroxidase activity in wild-type and sitiens leaf discs infected with B. cinerea. Leaf discs were inoculated with two 5-μL droplets of B. cinerea (infected) or with the control (mock) solution and fixed in ethanol at the different time points. Peroxidase activity was measured at 654 nm after addition of TMB and 0.03% H2O2. The mean and se of the absorbance of the incubation solution from three discs of different plants are presented.
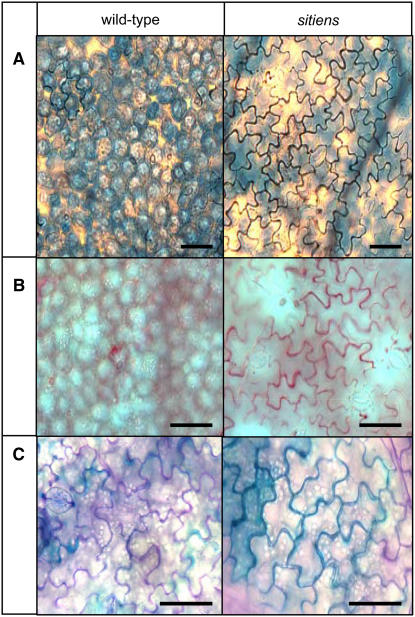
Because peroxidase-dependent defense responses often use H2O2 as a substrate to cross-link cell wall components, we performed different staining procedures to visualize changes in the cell wall. Cross-linking of cell wall proteins was detected with Coomassie Blue subsequent to protein denaturation and free protein removal (Mellersh et al., 2002; Fig. 7A). In addition, we used safranin-O and toluidine blue to detect the peroxidative incorporation of phenolic compounds in the cell wall, a fortification mechanism important during lignification and suberization (Mellersh et al., 2002; Lucena et al., 2003; Fig. 7, B and C). For both genotypes, no staining was visible outside the infection droplets. Cell wall modification was more abundant and appeared earlier in sitiens than in the wild type. Moreover, accumulation of all three stains clearly coincided in timing and location with the presence of extracellular H2O2: Starting from 8 hpi, the anticlinal cell walls of sitiens epidermal cells stained intensely during the time course (12, 16, 20, 24, 48, and 72 hpi; data not shown), whereas in wild-type tissue, only limited zones of the anticlinal cell walls of a few cells were positive and only after 12 hpi (Fig. 7). Underneath the inoculation droplet, the number of epidermal cells displaying clear cell wall modification in the anticlinal walls ranged between 40% and 80% in sitiens, but was never higher than 20% in wild-type cell walls (data not shown).
Figure 7.
Cell wall modifications in wild-type and sitiens tomato inoculated with B. cinerea. In sitiens, cell wall modifications were first detected at the sites of H2O2 accumulation and were generally present from 8 hpi beneath the inoculation droplet in the epidermal anticlinal cell walls, with an increase in staining intensity at subsequent time points. In wild-type tomato, only few cells had limited cell wall modifications. Cell wall modifications were visualized with Coomassie Blue staining after SDS denaturation to detect protein cross-linking (dark blue; A) with safranin-O (red-pink; B) and with toluidine blue to detect phenolic compounds (turquoise), whereas pectic fragments stain purple (C). For each time point and stain, infection sites on at least three leaf discs from independent sitiens and wild-type plants were examined and gave the same pattern of cell wall fortification. Scale bar = 50 μm.
H2O2 dependency of the cell wall modification in sitiens was further confirmed on leaf discs treated with antioxidants. Whereas ascorbate treatment removed all sitiens cell wall modifications, treatment with catalase resulted in lower staining intensity (Fig. 8).
Figure 8.
Effect of ascorbate and catalase treatment on epidermal cell wall modification in sitiens tomato inoculated with B. cinerea. The sitiens leaf discs that were floated in a solution of ascorbate or catalase and infected with B. cinerea were fixed in ethanol at 16 hpi and stained with safranin-O. Ascorbate treatment completely removed the cell wall staining, whereas catalase treatment resulted in fainter accumulation of the stain compared to the control treatment. For each treatment, at least three discs were examined and gave the same pattern of cell wall modification. Scale bar = 50 μm.
Transcriptome Profiling of the Tomato Response to B. cinerea
To assess changes in gene expression, we compared the transcriptome of wild-type and sitiens plants infected with B. cinerea. Detached leaves were spray infected with a conidial suspension to ensure a standardized sampling procedure because of a uniform infection covering the entire leaf area. Disease scoring showed that symptom development was similar to that after drop inoculation, with sitiens being more resistant than the wild type (Supplemental Fig. S2). We performed a genome-wide transcriptome analysis with TOM1 tomato cDNA arrays containing 12,899 ESTs, representing approximately 8,600 unigenes (Alba et al., 2004). A loop design was constructed, consisting of seven replicated dye-swap experiments in which two independent pools of four leaflets harvested from 5-week-old mock-sprayed and spray-infected plants at 0 and 8 hpi were compared in an equal treatment replication structure on a total of 14 TOM1 cDNA microarrays (Supplemental Fig. S3). We chose to sample at 8 hpi because, at that stage, no necrosis is visible yet, despite obvious fungal penetration attempts and H2O2 accumulation. In addition, time-course analyses with cDNA-amplified fragment length polymorphism indicated that gene expression induced by B. cinerea was first detectable between 4 and 8 hpi (data not shown). Similar to previous studies (Vuylsteke et al., 2005), we used a mixed-model approach to produce estimates of the gene-specific genotype, treatment, and genotype × treatment effects along with appropriate ses. Briefly, a linear mixed normalization model was used to estimate the global variation of the collection of the 12,899 cDNA fragments in the form of random array effects, random channel effects, and random error. In a second step, 12,899 gene-specific models were applied to partition gene-specific variation into fixed dye effects, fixed treatment (e.g. genotype, infection, time) effects, random spot effects, and random error. In a third and last step, for each transcript, expression differences were estimated for contrasts among treatments and were tested by t tests. To make tables within the article not too exhaustive, we used a conservative P-value cutoff of 0.001, whereas results with a cutoff of 0.01 are given as Supplemental Table S1. Microarray results were confirmed with quantitative real-time (RT)-PCR with gene-specific primers (see “Materials and Methods”) for six genes selected based on the following analyses (Supplemental Fig. S4).
First, we assessed differential gene expression between wild type and sitiens prior to pathogen infection. Differential expression (P < 0.001 and >2-fold change [FC]) was evident for 56 ESTs representing 40 unigenes (Table I). Within the unigenes up-regulated in sitiens compared to wild type (28), there was clear enrichment for proteins involved in defense responses. The strongest up-regulated genes (>5 FC) were almost all associated with plant-pathogen interactions: pathogenesis-related (PR) protein PR1, osmotin-like proteins, glucan endo-1,3-β-glucosidase B, and thaumatin-like proteins (van Loon and van Strien, 1999), suggesting that sitiens plants are already in a prealerted state of defense before pathogen infection. Interestingly, expression of the PR transcriptional activator Pti5 was also higher in sitiens prior to inoculation.
Table I.
Nonredundant list of genes significantly differentially expressed in wild-type and sitiens plants prior to inoculation with B. cinerea (P < 0.001)
When more than one EST for the same gene is present in the data, the EST with the highest FC is presented. Unigene ID, Sol Genomics Network Unigene identifier; FC, fold change of expression in sitiens compared to wild type; GO, gene ontology based on Urbanczyk-Wochniak et al. (2006).
| Unigene ID | Gene Description | ESTs | FC | GO Annotation |
|---|---|---|---|---|
| Genes with higher expression in sitiens than in wild type | ||||
| SGN-U143838 | PR protein PR-1 | 4 | 11.14 | Biotic stress |
| SGN-U144488 | Osmotin-like protein (PA15) | 1 | 10.67 | Biotic stress |
| SGN-U150295 | Expressed protein | 1 | 9.64 | No ontology |
| SGN-U143416 | Glucan endo-1,3-β-glucosidase B | 3 | 7.93 | Miscellaneous β-1,3-glucan hydrolases |
| SGN-U160528 | Thaumatin homolog NP24 | 1 | 7.23 | Biotic stress |
| SGN-U147967 | No hits found | 1 | 5.99 | Unknown |
| SGN-U143414 | NP24 protein (P23) | 7 | 5.61 | Biotic stress |
| SGN-U145000 | Wound-induced protein | 3 | 4.40 | Abiotic stress |
| SGN-U143337 | Endochitinase 3 | 1 | 3.97 | Biotic stress |
| SGN-U149296 | Protease inhibitor/seed storage/lipid transfer protein family | 1 | 3.54 | Miscellaneous protease inhibitor |
| SGN-U155388 | Activator-like transposable element | 1 | 3.34 | DNA synthesis |
| SGN-U144273 | Expressed protein | 1 | 3.33 | No ontology |
| SGN-U146585 | Putative membrane protein | 1 | 3.32 | Hormone metabolism |
| SGN-U145477 | Peroxidase | 1 | 2.98 | Miscellaneous peroxidases |
| SGN-U147667 | Expressed protein | 1 | 2.72 | Unknown |
| SGN-U145711 | Disease resistance response protein related/dirigent protein related | 2 | 2.66 | Biotic stress |
| SGN-U147854 | PR transcriptional activator Pti5 | 1 | 2.61 | Hormone metabolism |
| SGN-U143283 | Putative glutathione S-transferase | 1 | 2.61 | Miscellaneous glutathione S-transferases |
| SGN-U148968 | Expressed protein | 1 | 2.54 | Signaling |
| SGN-U145988 | AAA-type ATPase family | 2 | 2.49 | Protein degradation |
| SGN-U146340 | 9-cis-Epoxycarotenoid dioxygenase 4 | 1 | 2.37 | Hormone metabolism |
| SGN-U143678 | Actin | 1 | 2.32 | Cellular organization |
| SGN-U144386 | 14-3-3 protein 7 | 1 | 2.14 | Signaling |
| SGN-U151205 | Expressed protein | 1 | 2.12 | Unknown |
| SGN-U146043 | Putative PR protein | 1 | 2.09 | Biotic stress |
| SGN-U157113 | No hits found | 1 | 2.04 | Unknown |
| SGN-U154156 | Hypothetical protein | 1 | 2.04 | Unknown |
| SGN-U147469 | Acetyl-CoA C-acyltransferase | 1 | 2.01 | Lipid metabolism |
| Genes with lower expression in sitiens than in wild type | ||||
| SGN-U143179 | Lipid transfer protein 2 | 2 | −3.47 | Lipid metabolism |
| SGN-U144375 | Monooxygenase | 1 | −3.19 | Miscellaneous oxidases |
| SGN-U151742 | Small blue copper protein Bcp1 | 1 | −3.01 | Miscellaneous plastocyanin like |
| SGN-U145371 | Anthocyanidin 3-O-glucosyltransferase | 1 | −2.60 | Miscellaneous UDP glucosyl and glucuronyl transferases |
| SGN-U144440 | β-Amyrin synthase | 1 | −2.27 | Secondary metabolism isoprenoids |
| SGN-U144270 | Probable cytochrome P450 | 1 | −2.16 | Miscellaneous cytochrome P450 |
| SGN-U143901 | Cytochrome P450 family | 1 | −2.16 | Miscellaneous cytochrome P450 |
| SGN-U148216 | Receptor protein kinase-like protein | 1 | −2.15 | Posttranslational modification |
| SGN-U144995 | No hits found | 1 | −2.11 | Unknown |
| SGN-U151022 | Chlorophyllase | 1 | −2.03 | Tetrapyrrole synthesis |
| SGN-U144679 | Annexin related | 1 | −2.02 | Cellular organization |
| SGN-U147643 | Ca2+/H+-exchanging protein | 1 | −2.01 | Transport |
Next, we filtered the genes that were significantly differentially expressed between mock-inoculated and pathogen-inoculated samples. In wild type, 11 genes were significantly differentially regulated 8 hpi upon pathogen infection (P < 0.001), whereas in sitiens 41 were. In wild type, two extensins and a cell wall protein were significantly modulated by the B. cinerea infection with a FC of at least 1.5 compared to 26 unigenes in sitiens (Table II; Supplemental Table S1; P < 0.01), indicating a stronger response to infection in sitiens than in wild type at 8 hpi. Most prominent inductions were observed for PR-related genes, including PR-1A1, PR1, an extensin, a protease inhibitor, and a lipoxygenase. Because of the involvement of cell wall-related defense mechanisms in sitiens resistance as indicated by the previous experiments, we assessed expression patterns of cell wall-related genes upon infection. Supplemental Table S2 presents the gene ontology-annotated cell wall genes together with five genes that we manually annotated as cell wall-related genes based on literature surveys, which were deregulated upon pathogen infection in sitiens with a FC of at least 1.5. Most of these cell wall genes are involved in cross-linking, supporting the evidence for rapid cell wall modification in the resistance of sitiens.
Table II.
Nonredundant list of genes significantly differentially expressed in wild-type or sitiens plants after infection with B. cinerea (P < 0.001) with FC of at least 1.5
When more than one EST for the same gene is present in the data, the EST with the highest FC is presented. Genes are ranked according to the largest difference in expression at 8 hpi between mock and pathogen infection. Unigene ID, Sol Genomics Network Unigene identifier; FC, fold change of expression 8 hpi compared to 0 hpi; GO, gene ontology based on Urbanczyk-Wochniak et al. (2006).
| Unigene ID | Gene Description | ESTs | FC Pathogen | FC Mock | GO Annotation |
|---|---|---|---|---|---|
| Genes differentially expressed in wild type after infection with B. cinerea | |||||
| SGN-U143866 | Pro-rich protein EIG-I30, extensin | 1 | 2.33 | −1.18 | Cell wall related |
| SGN-U161846 | Gly-rich protein, extensin | 1 | 4.64 | 1.87 | Cell wall related |
| SGN-U147913 | Cell wall protein | 1 | 3.27 | 1.6 | Cell wall related |
| Genes differentially expressed in sitiens after infection with B. cinerea | |||||
| SGN-U143332 | Proteinase inhibitor type II CEVI57 precursor | 1 | 8.1 | 1.3 | No ontology |
| SGN-U144237 | Hyp-rich glycoprotein | 1 | 2.8 | −1.7 | Cell wall related |
| SGN-U147362 | Ser-rich protein | 1 | 4.9 | 1.2 | No ontology |
| SGN-U143866 | Pro-rich protein EIG-I30, extensin | 2 | 12.7 | 3.8 | Cell wall related |
| SGN-U144656 | PR protein 1A1 | 3 | 32 | 10.2 | Biotic stress |
| SGN-U143838 | PR protein PR-1 | 3 | 17.9 | 5.8 | Biotic stress |
| SGN-U143303 | Lipoxygenase A | 1 | 4.5 | 1.6 | Hormone metabolism |
| SGN-U145531 | Expressed protein | 3 | 3.5 | 1.2 | Hormone metabolism |
| SGN-U144553 | Miraculin homolog | 3 | 4.4 | 1.8 | Biotic stress |
| SGN-U154970 | DnaJ domain-containing protein | 1 | 4.9 | 2.1 | Abiotic stress |
| SGN-U144826 | PR protein STH-2 | 1 | 2 | −1.2 | Biotic stress |
| SGN-U143809 | Cinnamic acid 4-hydroxylase | 1 | 2.1 | −1.1 | Secondary metabolism, lignin biosynthesis |
| SGN-U144200 | Cytochrome P450 76A2 | 1 | 3 | 1.3 | Miscellaneous cytochrome P450 |
| SGN-U143841 | Putative peroxidase | 1 | 2.1 | 1 | Miscellaneous peroxidases |
| SGN-U146275 | Putative protein kinase | 1 | 1.7 | −1 | Posttranslational modification |
| SGN-U149410 | GTP-binding protein Rab11e | 1 | 1.5 | −1 | Signaling |
| SGN-U144528 | Ethylene-responsive protein related | 1 | 2 | 1.1 | Hormone metabolism |
| SGN-U145664 | Suberization-associated anionic peroxidase 2 | 1 | 1.6 | −1.1 | Miscellaneous peroxidases |
| SGN-U143930 | Bifunctional Lys-ketoglutarate reductase/saccharopine dehydrogenase | 2 | 3.7 | 2.2 | Amino acid metabolism |
| SGN-U144114 | Expressed protein | 1 | 2.7 | 1.7 | No ontology |
| SGN-U151083 | Unknown | 1 | 1.6 | 1 | Transcription regulation |
| SGN-U148425 | bHLH protein | 1 | 1.8 | 1.2 | Transcription regulation |
| SGN-U156084 | Phospholipid/glycerol acyltransferase | 1 | 1.7 | 1.2 | No ontology |
| SGN-U144589 | Ser/Thr specific protein phosphatase 2A B regulatory subunit β | 1 | 1.6 | 1.2 | Protein degradation |
| SGN-U146465 | Hsr201 protein | 1 | −2.5 | −1.4 | Biotic stress |
| SGN-U145272 | Wound-induced protein Sn | 1 | −3.1 | −1.3 | Abiotic stress |
DISCUSSION
Here, we show that the very high level of resistance to B. cinerea in ABA-deficient sitiens tomato plants coincided with a prompt localized accumulation of H2O2 that could be prevented by exogenous ABA application. H2O2 accumulation in sitiens was accompanied by an increase of extracellular peroxidase activities and was located in the epidermal cell layer, where it caused both cell wall modification and an HR-like response. Although ROS have a dual role after pathogen attack, acting as key defense compounds against biotrophic pathogens on the one hand, but serving as the molecules by which necrotrophs exploit these responses on the other hand, we demonstrate that timely hyperinduction of H2O2-dependent defenses can be effective in arresting the necrotroph B. cinerea.
Transcript-profiling analysis revealed that defense-related transcript accumulation prior to infection is higher in sitiens than in wild type. Moreover, further elevation of defense gene expression is stronger after B. cinerea attack, both in number of genes and their expression levels. Our results indicate that lower basal ABA levels result in a prealerted state of defense in sitiens, allowing the mutant to respond earlier and more strongly to pathogen challenge. Recently, Mohr and Cahill (2007) reported that addition of exogenous ABA induced susceptibility in a normally incompatible interaction between Arabidopsis and P. syringae pv tomato by suppression of lignin and SA accumulation. Moreover, treatment with ABA suppressed the expression of many defense-related genes (Mohr and Cahill, 2007). Anderson et al. (2004) similarly observed higher basal transcript levels of defense-related genes in ABA-deficient Arabidopsis mutants. Moreover, the abi2-1 mutant was more resistant against the necrotrophic fungus Fusarium oxysporum. An obvious genetic or biochemical link between ABA and the higher resistance level is not available. Pti5, a tomato pathogen-inducible ethylene response element-binding protein-like transcription factor, whose expression is higher in sitiens than in wild type prior to infection, can provide a link between ABA deficiency and pathogen-induced gene activation. Pti5 is expressed specifically during biotic but not abiotic or hormonal stresses, suggesting a specific role for Pti5 in plant defense against pathogens (Thara et al., 1999). Overexpression of Pti5 in tomato enhances resistance against P. syringae pv tomato and primes for expression of osmotin, β-glucanase, and catalase (He et al., 2001). Interestingly, an osmotin-like protein (SGN-U144488) and β-glucanase (SGN-U143416; Table I) are also highly expressed in sitiens prior to infection. In Arabidopsis, SA-regulated PR protein genes were regulated by Pti5 (Gu et al., 2002). Hence, high expression levels of PR proteins in sitiens before and after B. cinerea infection might be a result of Pti5-mediated regulation. Otherwise, the fact that the sitiens mutant can be considered to permanently suffer from drought stress because of the lack of ABA-mediated stomatal regulation (Nagel et al., 1994) might explain the higher basal expression of defense-related genes in the mutant because abiotic stress can cause priming for pathogen defense (Conrath et al., 2002).
Consistent with Audenaert et al. (2002), we found some genes transcriptionally activated by B. cinerea that are involved in SA-dependent signaling (e.g. PR1 protein) or the phenylpropanoid pathway (e.g. cinnamic acid 4-hydroxylase; Table II; Supplemental Table S1). It was shown that NahG tomato plants that are unable to accumulate SA are slightly more susceptible to B. cinerea. Moreover, Phe ammonia lyase activity, implicated in SA biosynthesis, was higher upon infection in sitiens than in wild-type plants (Audenaert et al., 2002). Opposing results to these were found in Arabidopsis, where NahG mutants showed no susceptibility to B. cinerea (Thomma et al., 1998; Veronese et al., 2004) and mutants with induced or constitutively activated systemic acquired resistance (SAR) and SAR gene expression generally were more susceptible to B. cinerea (Kachroo et al., 2001; Govrin and Levine, 2002). However, Govrin and Levine (2002) also reported that removal of basal SA accumulation by expression of the NahG gene or by infiltration of 2-aminoindan-2-phosphonic acid increased B. cinerea disease symptoms. In addition, SA accumulation was shown to play a role in local resistance to B. cinerea infection in Arabidopsis mutants that constitutively express SAR, but do not have a cell death phenotype (Ferrari et al., 2003). Finally, it was shown before that the role of SA signaling in defense against necrotrophic pathogens might differ according to the host plant (Achuo et al., 2004).
Necrotrophic fungi, such as B. cinerea, feed upon dead plant tissue, implying that the pathogen is able to kill host cells during infection. B. cinerea induces ROS formation in plants, resulting in hypersensitive cell death that facilitates fungal colonization (Elad, 1992; von Tiedemann, 1997; Govrin and Levine, 2000; Schouten et al., 2002; Govrin et al., 2006). We propose that timing, localization, and function of the increase in ROS are crucial in its role on B. cinerea development. Under our experimental conditions, H2O2 in wild-type tomato started to accumulate after 24 h in mesophyll tissue colonized by B. cinerea and was associated with spreading cell death. In sitiens, however, H2O2 accumulation was observed from 4 hpi specifically in the epidermis, where it caused cell wall modification, which is known to be an essential part of the plant's defense response and to pose physical barriers to invading pathogens. From 12 hpi, intracellular H2O2 was present in HR-like epidermal cells in sitiens. Considering that these HR cells in sitiens might result from overaccumulation of defense compounds and that, given the low number of cells involved, total nutrient release will be limited, HR-like cells can be expected to have only a small effect on total fungal development. It seems unlikely that the H2O2 accumulation in sitiens has a direct fungitoxic effect on B. cinerea because this pathogen possesses an array of enzymes to protect itself against damage by ROS and has the ability to grow in relatively high concentrations of H2O2 (Schouten et al., 2002; Lyon et al., 2004). Moreover, neither in sitiens nor in wild type did we observe signs of hyphal death of the invading fungus.
The importance of H2O2 accumulation in resistance to Botrytis was shown by increased susceptibility in sitiens after application of different antioxidants. In wild-type plants, catalase treatment had no significant effect on disease development, but in sitiens it partially eliminated H2O2 accumulation and increased the number of spreading B. cinerea lesions, although wild-type control levels were not reached. Catalase has a specific, well-characterized mode of action and eliminates H2O2 generated in the cell wall and the plasma membrane (Mellersh et al., 2002). The fact that catalase did not remove all extracellular H2O2 nor fully restore susceptibility in sitiens might be explained by inadequate uptake or activity of the enzyme. Profuse B. cinerea symptom development was observed on both genotypes after ascorbic acid treatment. This strong antioxidant not only removes pathogen-induced H2O2 accumulation in sitiens, but appears to disrupt all defenses, including the ones that are normally capable of retarding pathogen development in wild-type tomato. It cannot be excluded that ascorbate treatment has provoked unwanted changes and disrupted normal plant metabolism because it is known that ascorbate serves as an important redox compound controlling redox homeostasis and can have effects on plant growth, photosynthesis, cell cycle, and hormone production (Noctor, 2006). In addition to antioxidant treatments, inhibition of ROS generation with DPI increased susceptibility in sitiens to wild-type levels. Together, these findings point to the existence of additional H2O2-dependent defense mechanisms in the sitiens mutant that are not adequately expressed in wild-type tomato. Our observations are consistent with other studies that have also demonstrated that accumulation of ROS can be essential in successful defense against B. cinerea. Previously, resistance of tomato plants against B. cinerea infection has been demonstrated to result from early stimulation of H2O2 and superoxide radical generation by NADH peroxidase and superoxide dismutase in the apoplastic space (Patykowski and Urbanek, 2003). Moreover, whereas an aggressive isolate of B. cinerea induces expanding pale-brown necrosis, a nonaggressive isolate is arrested by biphasic oxidative burst and HR-like necrosis on bean leaf tissue (Unger et al., 2005).
Two major mechanisms of ROS production in plants upon pathogen attack have been described: membrane-bound NADPH oxidases, inhibited by DPI, and cell wall peroxidases, inhibited by azide (Lamb and Dixon, 1997; Torres et al., 2006). Whereas the effect of azide on pathogen-induced ROS accumulation could not be established due to its antimicrobial effect (data not shown), application of DPI to sitiens leaf discs inhibited H2O2 production upon B. cinerea infection and increased susceptibility, pointing to the involvement of NADPH oxidases as a source of ROS in sitiens. However, because the specificity of DPI to exclusively block NADPH oxidases has been questioned (Bolwell et al., 1998), we cannot make conclusive statements regarding the source of sitiens ROS generation.
Early H2O2 accumulation at the site of plant-fungal contact has been shown to be decisive for the outcome of several tomato-pathogen interactions. H2O2 is critical to determine resistance of tomato to Cladosporium fulvum (Borden and Higgins, 2002). The same was found for the interaction of tomato with the anthracnose fungus (Colletotrichum coccodes; Mellersh et al., 2002) and with the powdery mildew fungus (O. neolycopersici; Mlíčková et al., 2004). In these interactions, penetration failure and resistance result from early accumulation of H2O2 in the anticlinal epidermal cell walls in close contact with the fungal structures. The sitiens response to B. cinerea shows striking analogy in timing and localization of H2O2 accumulation with these tomato defense reactions to biotrophic and hemibiotrophic pathogens, but are even more forcefully triggered and therefore effective in arresting development of the necrotroph B. cinerea.
Fortification of the cell wall is a multistep process that involves H2O2-mediated cross-linking of preformed molecules and induction of transcription-dependent defenses (Bradley et al., 1992; Ribeiro et al., 2006). In our system, expression of defense-related cell wall genes was observed early after infection and coincided with microscopically visible cell wall modification (8 hpi). Genes encoding Hyp- and Gly-rich proteins, structural plant cell wall proteins that can be cross-linked (Showalter, 1993), are expressed more strongly in sitiens than in wild type. Also, genes encoding enzymes involved in the phenylpropanoid biosynthesis pathway or in the peroxidative incorporation of these phenolic compounds into the cell wall, such as the suberization-associated peroxidase and the cinnamic acid 4-hydroxylase, are transcriptionally activated. Furthermore, microarray data also revealed a role for enzymes that function in pectin and cellulose modification (e.g. xyloglucan endotransglycosilases and pectinesterases). In view of the infection process of B. cinerea, the anticlinal cell wall of the epidermis is a promising site for reinforcement (Fig. 9). After breaching the plant's cuticle, B. cinerea hyphae grow within the epidermal outer periclinal cell wall before invading the underlying tissue. This penetration to the mesophyll layer occurs intercellularly between epidermal cells and implies the collapse of anticlinal cell walls and dissolution of the middle lamella (Clark and Lorbeer, 1976). Hence, obstruction at the anticlinal cell walls blocks passage for B. cinerea to the underlying tissue. Besides forming a mechanical barrier to physical fungal penetration, cell wall reinforcements are known to decrease susceptibility to cell wall-degrading enzymes, to impede nutrient diffusion to the pathogen, and to possibly restrict diffusion of toxins (Brisson et al., 1994; Bestwick et al., 1998; van Kan, 2006). Again, the anticlinal wall is a supreme target to hinder cell wall degradation during early invasion of the epidermal layer because anticlinal walls are very rich in pectin and pectin decomposition and consumption are essential in the development of B. cinerea on all hosts (van Kan, 2006). Moreover, Kars et al. (2005) showed that action of B. cinerea pectinases, needed for the growth in the anticlinal walls, is required for normal primary lesion formation. Although a number of studies indicate the importance of cell wall modification in the defense against Botrytis on several hosts (Mansfield and Hutson, 1980; Mitchell et al., 1994; McLusky et al., 1999; van Baarlen et al., 2004), the requirement of localized ROS accumulation in this response to Botrytis was, to our knowledge, never presented before.
Figure 9.
Schematic model of the location and sequence of fungal growth, H2O2 accumulation, and cell wall modification in wild-type and sitiens leaf tissue infected with B. cinerea. Wild-type tissue displays only very few zones with H2O2 accumulation or cell wall modification until 24 hpi. Between 24 and 48 hpi, strong H2O2 accumulation appears mainly in the mesophyll cell layer and is associated with cell death and fungal spreading. In sitiens, H2O2 accumulation and modifications coincide in a first phase (around 8 hpi) mainly in the anticlinal walls of epidermal cells. At later time points (24 and 48 hpi), fungal growth inside sitiens is blocked by these modifications and is thereby limited to the epidermal outer periclinal wall. Intracellular H2O2 is mainly restricted to HR-like epidermal cells. co, Conidium; cu, cuticula; ep, epidermal cell layer; me, mesophyll cell layer; pe, point of penetration.
The relative contribution to B. cinerea resistance of cell wall modifications and concomitant production of secondary antimicrobial metabolites has often been questioned (for review, see van Baarlen et al., 2004). In our interaction, we cannot exclude a role for secondary defense compounds in sitiens because there are several reports dealing with phytoalexin production during B. cinerea infection and ROS can serve as a signal for phytoalexin production (Thoma et al., 2003; van Baarlen et al., 2004). However, due to the rapid and extensive nature of cell wall modifications in sitiens, we propose that wall strengthening will have an important effect in delaying fungal colonization and progress, with antimicrobial secondary metabolites acting during subsequent stages of plant defense.
As opposed to the generally accepted theorem that plant defense-related ROS formation aids necrotrophs in their pathogenicity, we state that timely production of H2O2 and fast induction of defense responses, including cell wall modifications, are efficient in protecting the sitiens tomato mutant against B. cinerea. We propose that resistance due to low ABA content originates from higher basal defense-related transcript accumulation and subsequent fast and strong defense activation upon pathogen challenge.
MATERIALS AND METHODS
Plant Material, Exogenous ABA Application, and Antioxidant Treatments
Tomato (Solanum lycopersicum, previously Lycopersicon esculentum) sitiens mutants (Taylor et al., 1988, 2000), provided by Prof. M. Koornneef (Wageningen University) and the corresponding wild-type ‘Moneymaker’ were grown in potting compost soil (Substrat 4; Klasmann-Deilmann) at 22°C. Plants were raised in a growth chamber with 75% relative humidity in a 16-h light/8-h dark regime. ABA treatment was performed as described by Achuo et al. (2006). Plants were sprayed until runoff with 100 μm ABA twice a week during their development. After 4 to 5 weeks, when seedlings were at the fifth leaf stage, leaf discs were punched out of the tertiary leaves with a 1-cm-diameter cork bore and placed floating with the adaxial side up in 24-well plates (VWR). Each well was filled with 1.5 mL of distilled water or, if indicated, with 1.5 mL of the solution of the chemical treatments. The treatments used consisted of 5 mm ascorbic acid (Sigma-Aldrich), 1,100 units/mL catalase (Sigma-Aldrich), 50 μm DPI (Sigma-Aldrich), and distilled water as control. The discs of the ABA-treated plants were placed in distilled sterile water as in the control treatment. For transcription profiling, whole tertiary leaves of 5-week-old plants were used for infection.
Fungal Material and Infection Method
Conidia of Botrytis cinerea strain R16 (Faretra and Pollastro, 1991) were obtained as described by Audenaert et al. (2002). The conidial suspension was centrifuged for 10 min at 10,000g. After removal of the supernatant and resuspension of the conidia in distilled water, an inoculation suspension was prepared containing 6.25 × 104 conidia/mL in 16.7 mm KH2PO4 and 25 mm Glc. Conidia were pregerminated for 2 h in the inoculation suspension at 22°C. For leaf disc assays, two 5-μL droplets were used to inoculate each tomato leaf disc. The plates containing the leaf discs were incubated at 22°C under dark conditions. Symptoms were evaluated after 4 d. Each inoculation droplet was classified as a spreading or nonspreading lesion and the data were analyzed with a binary logistic regression. At least 72 inoculation drops were evaluated for each treatment. For RNA extraction, a spray inoculation was used to get a uniform infection covering the total leaf area. Leaves were sprayed with an inoculation solution containing 5 × 104 spores/mL, 0.01 m KH2PO4 (pH 5), and 6.67 mm Glc, and placed in plastic trays with high humidity according to Audenaert et al. (2002) and incubated at 22°C under dark conditions. Mock inoculation consisted of spraying plant leaves with a solution of 0.01 m KH2PO4 (pH 5) and 6.67 mm Glc.
Visualization of Defense Responses
To compare the defense responses of sitiens and wild-type leaf tissue, different staining techniques were used and evaluation was done macroscopically and microscopically. Leaf discs were inoculated with B. cinerea as described above and sampled at 4, 8, 12, 16, 20, 24, 48, and 72 hpi by clearing and fixing in 100% ethanol. For each time point, at least three discs originating from different plants of sitiens and wild type were used. For H2O2 accumulation, staining was according to the protocol of Thordal-Christensen et al. (1997). Three hours before each sampling time point, tomato leaf discs infected with B. cinerea were placed under light conditions and floated in a solution of 1 mg/mL DAB-HCl (pH 4). Polymerization of the DAB molecule at the site of H2O2 and peroxidase accumulation results in a brown, reddish color that is macroscopically visible and because of the high spatial and temporal distribution of the oxidized DAB molecule, it can be visualized using bright-field microscopy. A subset of the ethanol-fixed DAB-stained samples was embedded in Technovit 7100 histo-embedding medium (Heraeus Kulzer) according to the manufacturer's instructions and semithin cross sections (4 μm) were cut with a Leica RM2265 motorized rotary microtome (Leica Microsystems). To detect superoxide, leaf discs were floated in 0.05% NBT for 30 min before fixation in 100% ethanol, according to the protocol of Doke (1983). For protein cross-linking, staining was performed as described by Mellersh et al. (2002). Ethanol-fixed samples were placed in 1% SDS at 80°C for 24 h and stained with 0.1% Coomassie Blue in 40% ethanol/10% acetic acid for 15 min and subsequently washed in 40% ethanol/10% acetic acid. To visualize cell wall modifications, safranin-O staining was according to Lucena et al. (2003). Leaf discs were incubated in 0.01% safranin-O in 50% ethanol for 3 min. Accumulation of phenolics was detected by staining with 0.05% toluidine blue in citrate/citric acid buffer (50 mm, pH 3.5; Mellersh et al., 2002). Fungal structures were stained with 0.02% trypan blue in lactophenol. After staining, leaf discs were mounted in 50% glycerol. Fluorescence and bright-field microscopy were performed with an Olympus BX-51 microscope and images were captured with a ColorView III camera and edited with the software package CELL-F (Olympus Soft Imaging Solutions).
Extracellular peroxidase activity was measured with the TMB assay based on Ros Barceló (1998). Leaf discs, inoculated with two drops of a B. cinerea conidial suspension or with the appropriate mock solution, were fixed in ethanol. After subsequent washing in distilled water, the discs were incubated in 1.5 mL of 50 mm Tris-acetate buffer (pH 5.0) containing 0.1 mg/mL TMB and 0.03% H2O2 for 20 min. Peroxidase activity of the discs was determined by measuring the absorbance of the incubation solution at 654 nm.
Sampling and RNA Preparation
Leaf samples were taken at 0 and 8 hpi. Two leaflets per leaf were excised and immediately frozen in liquid nitrogen. Three days after inoculation, infection levels on the three remaining leaflets were scored. For each genotype/treatment combination (i.e. wild-type mock, wild-type infected, sitiens mock, and sitiens infected), four leaflets were sampled and pooled. Total RNA was prepared from the sample pools with the RNeasy plant mini kit (Qiagen), according to the manufacturer's instructions. Total RNA quality was checked on an agarose gel and concentrations were determined with a ND-1000 spectrophotometer (Nanodrop Technologies).
Microarrays
The tomato TOM1 microarray used was obtained from the Center for Gene Expression Profiling of the Boyce Thompson Institute and consisted of 12,899 EST clones representing 8,600 independent tomato genes. The functional annotation of the genes related to the spotted cDNAs can be viewed at http://bti.cornell.edu/CGEP/CGEP.html.
Target Labeling and Hybridizations
Target labeling and hybridizations were carried out at the Flanders Institute of Biotechnology MicroArray Facility (http://www.microarrays.be). Total RNA (5 μg) of each sample was reverse transcribed to double-stranded cDNA and further amplified according to a modified version of the protocol described in Puskás et al. (2002; http://www.microarrays.be/service.htm). Subsequent Cy5 and Cy3 (GE-Healthcare) labeling, hybridization, posthybridization washing, and scanning were performed according to protocols accessible through the Web site (http://www.microarrays.be/service.htm).
Experimental Design and Statistical Analysis of Microarray Data
We constructed a loop design (Supplemental Fig. S3) consisting of 14 two-dye TOM1 microarray experiments in which two independent pools of four leaflets harvested from 5-week-old mock-sprayed and spray-infected plants at 0 and 8 hpi were compared. Expression data were analyzed in two steps: (1) a within-slide analysis aimed at removing variation associated with differential dye responses to binding and scanning, as noise; and (2) a between-slide analysis aimed at estimating the mean differences between treatments (e.g. genotypes and infection treatments) and their se. To correct for dye intensity differences, we used the robust scatter plot smoother LOWESS (Yang et al., 2002) as implemented in Genstat (Payne and Lane, 2005), where the response variable is the log2 ratio of the artifact-removed total foreground Cy3 and Cy5 fluorescence intensities measured at the 12,899 spots. The fraction of the data used for estimating the local LOWESS fit was set at 20%.
For between-slide analysis, a two-step mixed-model ANOVA (Wolfinger et al., 2001) was used and variance components were estimated by residual maximum likelihood (REML), as implemented in Genstat (Payne and Lane, 2005) and described previously (Vuylsteke et al., 2005; Nettleton, 2006). Following Wolfinger et al. (2001), the mixed-model analysis on the LOWESS fit to the spot measurements consisted of two steps. First, array and channel effects were removed from the expression responses by a normalization ANOVA model of the form:
 |
(1) |
where the response variable represents the corrected log2-transformed Cy3 and Cy5 fluorescence intensity measurements of the 12,899 ESTs. Array models the hybridization effects of each of the 14 microarrays, dye models the effects of each of the two dyes, and dye by array models the 28-channel effects. Array, dye, and dye by array were added as random terms.
In a second step, the residuals from model 1 were analyzed for each of the 12,899 ESTs separately by a mixed model of the following form:
 |
(2) |
partitioning gene-specific variation into gene-specific fixed dye effects, fixed replicate effects, fixed genotype (wild type and sitiens) effects, fixed treatment (mock and infected), fixed genotype-specific treatment effects, and fixed genotype-specific treatment effects across time. The random array term models the effects for each spot and equals the (EST × array) interaction effect. These models were fitted by REML and Wald statistics were calculated to assess significance of the fixed effects in the gene model. No further adjustments for multiple testing were done. From REML analysis, we saved the vector of estimated genotype, treatment, and interaction effects with the corresponding estimated variance-covariance matrix for each gene. Test statistics for contrasts were constructed from the parameter estimates divided by their ses. These ratios were supposed to follow approximately a t distribution, with the degrees of freedom equal to those for the error term in the gene-specific model. On the basis of the t approximation to the test statistics for the contrasts, P values were calculated.
Quantitative RT-PCR Analysis
Poly(dT) cDNA was synthesized from 2 μg of total RNA with SuperScript II reverse transcriptase (Invitrogen) and quantified on a LightCycler480 RT-PCR detection system (Roche Diagnostics) with the SYBR Green I RT-PCR core kit (Roche Diagnostics). PCR was carried out in triplicate. Gene-specific primer pairs were designed with Beacon Designer 4.0 (Premier Biosoft International): SGN-U143838 (FWD, AACCTAGCTGCCGCTTTCCC; REV, TTACGCCACACCACCTGAGTATAG); SGN-U143866 (FWD, CATCAAAGGGTAAGTGCCCAAAGG; REV, AAGGCAAACAGCAGCATCAAGATC); SGN-U134316 (FWD, TCGCCACCAACATTCACATAACAG; REV, AGAATGTGATGGCAAGTTGTTCCC); SGN-U144656 (FWD, GGACGATGGTCTAGCAGCCTATG; REV, CAGCACCAGCAGCGTTTAGC); SGN-U144488 (FWD, TTGTGGTGGAGTCCTGGATTGC; REV, TGGCTGTGCATTGAATTGGATGAC); SGN-U147913 (FWD, CGGCGGTCGTGGTAGAGG; REV, AACAACCATTGCGGCAGTACC).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Superoxide accumulation detected with NBT in wild-type and sitiens tomato leaf discs inoculated with B. cinerea.
Supplemental Figure S2. Symptom development of wild-type and sitiens tomato leaves 3 d after spray inoculation with 5 × 104 B. cinerea conidia/mL.
Supplemental Figure S3. Experimental design consisting of 14 TOM1 cDNA arrays to examine transcript levels in RNA samples collected from wild-type and sitiens plants inoculated either with an infection solution or a mock solution at 0 and 8 hpi.
Supplemental Figure S4. Gene expression kinetics of osmotin-like protein (SGN-U144488), endo-1,3-β-glucanase (SGN-U143416), and PR1 protein (SGN-U143838; Table I), PR1A1 protein (SGN-U144656), Pro-rich protein EIG-I30 (SGN-U143866; Table II), and cell wall protein (SGN-U147913; Supplemental Table S2) in wild-type and sitiens leaves spray-inoculated with 5 × 104 B. cinerea conidia/mL.
Supplemental Table S1. Genes significantly differentially expressed in wild-type and sitiens plants after infection with B. cinerea (P < 0.01) with FC of at least 1.5.
Supplemental Table S2. Nonredundant list of cell wall-related genes significantly differentially expressed upon B. cinerea infection at 8 hpi (P < 0.05) with FC of at least 1.5 in sitiens plants.
Supplementary Material
Acknowledgments
We thank Ilse Delaere, Greet De Backer, Carlos Puig, Michael Vandorpe, Stijn Morsa, and Korneel Vandenbroucke for technical assistance, Sepp van Rompaey for help with preparing figures, Paul Van Hummelen for the Flanders Institute of Biotechnology Microarray Facility, and Martine De Cock for help in preparing the manuscript.
This work was supported by the Research Fund of Ghent University (Geconcerteerde Onderzoeksacties grant no. 12051403), the “Fonds voor Wetenschappelijk Onderzoek-Vlaanderen” (grant nos. G.0350.04 and G.0061.03), and the “Instituut voor de Aanmoediging van Innovatie door wetenschap en Technologie in Vlaanderen” (predoctoral fellowship to K.C.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Monica Höfte (monica.hofte@ugent.be).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- AbuQamar S, Chen X, Dhawan R, Bluhm B, Salmeron J, Lam S, Dietrich RA, Mengiste T (2006) Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. Plant J 48 28–44 [DOI] [PubMed] [Google Scholar]
- Achuo EA, Audenaert K, Meziane H, Höfte M (2004) The salicylic acid-dependent defence pathway is effective against different pathogens in tomato and tobacco. Plant Pathol 53 65–72 [PubMed] [Google Scholar]
- Achuo EA, Prinsen E, Höfte M (2006) Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici. Plant Pathol 55 178–186 [Google Scholar]
- Alba R, Fei Z, Payton P, Liu Y, Moore SL, Debbie P, Cohn J, D'Ascenzo M, Gordon JS, Rose JKC, et al (2004) ESTs, cDNA microarrays, and gene expression profiling: tools for dissecting plant physiology and development. Plant J 39 697–714 [DOI] [PubMed] [Google Scholar]
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16 3460–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert K, De Meyer GB, Höfte MM (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol 128 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito EP, ten Have A, van't Klooster JW, van Kan JAL (1998) Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea. Eur J Plant Pathol 104 207–220 [Google Scholar]
- Bestwick CS, Brown IR, Mansfield JW (1998) Localized changes in peroxidase activity accompany hydrogen peroxide generation during the development of a nonhost hypersensitive reaction in lettuce. Plant Physiol 118 1067–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM (1998) Comparative histochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol 116 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden S, Higgins VJ (2002) Hydrogen peroxide plays a critical role in the defence response of tomato to Cladosporium fulvum. Physiol Mol Plant Pathol 61 227–236 [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ (1992) Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70 21–30 [DOI] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb C (1994) Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 6 1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CA, Lorbeer JW (1976) Comparative histopathology of Botrytis squamosa and B. cinerea on onion leaves. Phytopathology 66 1279–1289 [Google Scholar]
- Conrath U, Pieterse CMJ, Mauch-Mani B (2002) Priming in plant-pathogen interactions. Trends Plant Sci 7 210–216 [DOI] [PubMed] [Google Scholar]
- Doke N (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol 23 345–357 [Google Scholar]
- Elad Y (1992) The use of antioxidants (free radical scavengers) to control grey mould (Botrytis cinerea) and white mould (Sclerotinia sclerotiorum) in various crops. Plant Pathol 41 417–426 [Google Scholar]
- Faretra F, Pollastro S (1991) Genetic basis of resistance to benzimidazole and dicarboximide fungicides in Botryotinia fuckeliana (Botrytis cinerea). Mycol Res 95 943–951 [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM (2003) Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J 35 193–205 [DOI] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9 436–442 [DOI] [PubMed] [Google Scholar]
- Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43 205–227 [DOI] [PubMed] [Google Scholar]
- Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10 751–757 [DOI] [PubMed] [Google Scholar]
- Govrin EM, Levine A (2002) Infection of Arabidopsis with a necrotrophic pathogen, Botrytis cinerea, elicits various defense responses but does not induce systemic acquired resistance (SAR). Plant Mol Biol 48 267–276 [DOI] [PubMed] [Google Scholar]
- Govrin EM, Rachmilevitch S, Tiwari BS, Solomon M, Levine A (2006) An elicitor from Botrytis cinerea induces the hypersensitive response in Arabidopsis thaliana and other plants and promotes the gray mold disease. Phytopathology 96 299–307 [DOI] [PubMed] [Google Scholar]
- Gu YQ, Wildermuth MC, Chakravarthy S, Loh YT, Yang C, He X, Han Y, Martin GB (2002) Tomato transcription factors Pti4, Pti5, and Pti6 activate defense responses when expressed in Arabidopsis. Plant Cell 14 817–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Warren RF, Zhao T, Shan L, Zhu L, Tang X, Zhou JM (2001) Overexpression of Pti5 in tomato potentiates pathogen-induced defense gene expression and enhances disease resistance to Pseudomonas syringae pv tomato. Mol Plant Microbe Interact 14 1453–1457 [DOI] [PubMed] [Google Scholar]
- Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44 321–334 [DOI] [PubMed] [Google Scholar]
- Henfling J, Bostock R, Kuc J (1980) Effect of abscisic acid on rishitin and lubimin accumulation and resistance to Phytophthora infestans and Cladosporium cucumerinum in potato tuber tissue slices. Phytopathology 70 1074–1078 [Google Scholar]
- Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF (2001) A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci USA 98 9448–9453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kars I, Krooshof GH, Wagemakers L, Joosten R, Benen JAE, van Kan JAL (2005) Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. Plant J 43 213–225 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Nguyen V, Schroeder JI (2006) The role of reactive oxygen species in hormonal responses. Plant Physiol 141 323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48 251–275 [DOI] [PubMed] [Google Scholar]
- Lucena MA, Romero-Aranda R, Mercado JA, Cuartero J, Valpuesta V, Quesada MA (2003) Structural and physiological changes in the roots of tomato plants over-expressing a basic peroxidase. Physiol Plant 118 422–429 [Google Scholar]
- Lyon GD, Goodman BA, Williamson B (2004) Botrytis cinerea perturbs redox processes as an attack strategy in plants. In Y Elad, B Williamson, P Tudzynski, N Delen, eds, Botrytis: Biology, Pathology and Control. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 119–141
- Malolepsza U, Urbanek H (2002) o-Hydroxyethylorutin-mediated enhancement of tomato resistance to Botrytis cinerea depends on a burst of reactive oxygen species. J Phytopathol 150 616–624 [Google Scholar]
- Mansfield JW, Hutson RA (1980) Microscopical studies on fungal development and host responses in broad bean and tulip leaves inoculated with five species of Botrytis. Physiol Plant Pathol 17 131–143 [Google Scholar]
- Mauch-Mani B, Mauch F (2005) The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol 8 409–414 [DOI] [PubMed] [Google Scholar]
- McLusky SR, Bennett MH, Beale MH, Lewis MJ, Gaskin P, Mansfield JW (1999) Cell wall alterations and localized accumulation of feryloyl-3′-methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis allii are associated with actin polarisation, peroxidase activity and suppression of flavonoid biosynthesis. Plant J 17 523–534 [Google Scholar]
- Mehdy MC (1994) Active oxygen species in plant defense against pathogens. Plant Physiol 105 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellersh DG, Foulds IV, Higgins VJ, Heath MC (2002) H2O2 plays different roles in determining penetration failure in three diverse plant-fungal interactions. Plant J 29 257–268 [DOI] [PubMed] [Google Scholar]
- Mengiste T, Chen X, Salmeron J, Dietrich R (2003) The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 15 2551–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell HJ, Hall JL, Barber MS (1994) Elicitor-induced cinnamyl alcohol dehydrogenase activity in lignifying wheat (Triticum aestivum L.) leaves. Plant Physiol 104 551–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlíčková K, Luhová L, Lebeda A, Mieslerová B, Peč P (2004) Reactive oxygen species generation and peroxidase activity during Oidium neolycopersici infection on Lycopersicon species. Plant Physiol Biochem 42 753–761 [DOI] [PubMed] [Google Scholar]
- Mohr PG, Cahill DM (2001) Relative roles of glyceollin, lignin and the hypersensitive response and the influence of ABA in compatible and incompatible interactions of soybeans with Phytophthora sojae. Physiol Mol Plant Pathol 48 31–41 [Google Scholar]
- Mohr PG, Cahill DM (2007) Suppression by ABA of salicylic acid and lignin accumulation and the expression of multiple genes, in Arabidopsis infected with Pseudomonas syringae pv tomato. Funct Integr Genomics 7 181–191 [DOI] [PubMed] [Google Scholar]
- Nagel OW, Konings H, Lambers H (1994) Growth rate, plant development and water relations of the ABA-deficient tomato mutant sitiens. Physiol Plant 92 102–108 [Google Scholar]
- Nettleton D (2006) A discussion of statistical methods for design and analysis of microarray experiments for plant scientists. Plant Cell 18 2112–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G (2006) Metabolic signaling in defence and stress: the central roles of soluble redox couples. Plant Cell Environ 29 409–425 [DOI] [PubMed] [Google Scholar]
- Patykowski J, Urbanek H (2003) Activity of enzymes related to H2O2 generation and metabolism in leaf apoplastic fraction of tomato leaves infected with Botrytis cinerea. J Phytopathol 151 153–161 [Google Scholar]
- Payne RW, Lane PW (2005) GenStat® Release Reference Manual, Part 3: Procedure Library PL16. VSN International, Oxford
- Puskás LG, Zvara Á, Hackler L Jr, Van Hummelen P (2002) RNA amplification results in reproducible microarray data with slight ratio bias. Biotechniques 32 1330–1340 [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Pereira CS, Soares NC, Vieira AM, Feijó JA, Jackson PA (2006) The contribution of extensin network formation to rapid, hydrogen peroxide-mediated increases in grapevine callus wall resistance to fungal lytic enzymes. J Exp Bot 57 2025–2035 [DOI] [PubMed] [Google Scholar]
- Ros Barceló A (1998) Hydrogen peroxide production is a general property of the lignifying xylem from vascular plants. Ann Bot (Lond) 82 97–103 [Google Scholar]
- Schopfer P, Plachy C, Frahry G (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol 125 1591–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten A, Tenberge KB, Vermeer J, Stewart J, Wagemakers L, Williamson B, van Kan JAL (2002) Functional analysis of an extracellular catalase of Botrytis cinerea. Mol Plant Pathol 3 227–238 [DOI] [PubMed] [Google Scholar]
- Showalter AM (1993) Structure and function of plant cell wall proteins. Plant Cell 5 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor IB, Burbidge A, Thompson AJ (2000) Control of abscisic acid synthesis. J Exp Bot 51 1563–1574 [DOI] [PubMed] [Google Scholar]
- Taylor IB, Linforth RST, Al-Naieb RJ, Bowman WR, Marples BA (1988) The wilty tomato mutants flacca and sitiens are impaired in the oxidation of ABA-aldehyde to ABA. Plant Cell Environ 11 739–745 [Google Scholar]
- Thaler JS, Bostock RM (2004) Interactions between abscisic-acid-mediated responses and plant resistance to pathogens and insects. Ecology 85 48–58 [Google Scholar]
- Thara VK, Tang X, Gu YQ, Martin GB, Zhou JM (1999) Pseudomonas syringae pv tomato induces the expression of tomato EREBP-like genes Pti4 and Pti5 independent of ethylene, salicylate and jasmonate. Plant J 20 475–483 [DOI] [PubMed] [Google Scholar]
- Thoma I, Loeffler C, Sinha AK, Gupta M, Krischke M, Steffan B, Roitsch T, Mueller MJ (2003) Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J 34 363–375 [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11 1187–1194 [Google Scholar]
- Torres MA, Jones JDG, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger C, Kleta S, Jandl G, von Tiedemann A (2005) Suppression of the defence-related oxidative burst in bean leaf tissue and bean suspension cells by the necrotrophic pathogen Botrytis cinerea. J Phytopathol 153 15–26 [Google Scholar]
- Urbanczyk-Wochniak E, Usadel B, Thimm O, Nunes-Nesi A, Carrari F, Davy M, Bläsing O, Kowalczyk M, Weicht D, Polinceusz A, et al (2006) Conversion of MapMan to allow the analysis of transcript data from Solanaceous species: effects of genetic and environmental alterations in energy metabolism in the leaf. Plant Mol Biol 60 773–792 [DOI] [PubMed] [Google Scholar]
- van Baarlen P, Staats M, van Kan JAL (2004) Induction of programmed cell death in lily by the fungal pathogen Botrytis elliptica. Mol Plant Pathol 5 559–574 [DOI] [PubMed] [Google Scholar]
- van Baarlen P, Woltering EJ, Staats M, van Kan JAL (2007) Histochemical and genetic analysis of host and non-host interactions of Arabidopsis with three Botrytis species: an important role for cell death control. Mol Plant Pathol 8 41–54 [DOI] [PubMed] [Google Scholar]
- van Kan JAL (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci 11 247–253 [DOI] [PubMed] [Google Scholar]
- van Loon LC, van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55 85–97 [Google Scholar]
- Veronese P, Chen X, Bluhm B, Salmeron J, Dietrich R, Mengiste T (2004) The BOS loci of Arabidopsis are required for resistance to Botrytis cinerea infection. Plant J 40 558–574 [DOI] [PubMed] [Google Scholar]
- von Tiedemann A (1997) Evidence for a primary role of active oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea. Physiol Mol Plant Pathol 50 151–166 [Google Scholar]
- Vuylsteke M, van Eeuwijk F, Van Hummelen P, Kuiper M, Zabeau M (2005) Genetic analysis of variation in gene expression in Arabidopsis thaliana. Genetics 171 1267–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfinger RD, Gibson G, Wolfinger ED, Bennett L, Hamadeh H, Bushel P, Afshari C, Paules RS (2001) Assessing gene significance from cDNA microarray expression data via mixed models. J Comput Biol 8 625–637 [DOI] [PubMed] [Google Scholar]
- Xiong L, Yang Y (2003) Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell 15 745–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP (2002) Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 30 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.