Abstract
During angiosperm reproduction, one of the two synergid cells within the female gametophyte undergoes cell death prior to fertilization. The pollen tube enters the female gametophyte by growing into the synergid cell that undergoes cell death and releases its two sperm cells within the degenerating synergid cytoplasm to effect double fertilization. In Arabidopsis (Arabidopsis thaliana) and many other species, synergid cell death is dependent upon pollination. However, the mechanism by which the pollen tube causes synergid cell death is not understood. As a first step toward understanding this mechanism, we defined the temporal relationship between pollen tube arrival at the female gametophyte and synergid cell death in Arabidopsis. Using confocal laser scanning microscopy, light microscopy, transmission electron microscopy, and real-time observation of these two events in vitro, we demonstrate that synergid cell death initiates after the pollen tube arrives at the female gametophyte but before pollen tube discharge. Our results support a model in which a signaling cascade triggered by pollen tube-synergid cell contact induces synergid cell death in Arabidopsis.
During the angiosperm fertilization process, a pollen tube grows into one of the female gametophyte's two synergid cells. The synergid cell that the pollen tube grows into undergoes cell death, either before or upon entry of the pollen tube into this cell. The pollen tube then ceases growth and releases its contents, including the two sperm cells, into the degenerating synergid cytoplasm. Ultimately, the two sperm cells migrate to and fuse with the egg cell and central cell to effect double fertilization (Lord and Russell, 2002; Weterings and Russell, 2004).
Synergid cell death may facilitate several steps of the angiosperm fertilization process. First, synergid cell death may be required for pollen tube entry into the female gametophyte (Russell, 1993; Higashiyama, 2002). Second, the degenerated state may be required for tube growth cessation and release of pollen tube contents (van Went and Willemse, 1984). Third, synergid degeneration is accompanied by a cytoskeletal reorganization that is thought to facilitate migration of the sperm cells to the egg and central cells (Russell, 1993, 1996; Fu et al., 2000). Finally, it is likely that breakdown of the synergid membrane is required to provide the sperm cells direct access to the fertilization targets (Russell, 1993).
Little is known about the molecular processes that regulate and mediate synergid cell death. In many species, synergid cell death is pollination dependent (van Went and Willemse, 1984; Willemse and van Went, 1984; Russell, 1992), indicating that it is not a part of the megagametogenesis developmental program and suggesting that pollen tubes induce a physiological cell death program (i.e. programmed cell death; An and You, 2004). Pollen tubes could induce synergid programmed cell death through either a diffusible or a contact-mediated (i.e. pollen tube-synergid cell contact) signal. Alternatively, pollen tubes may cause cell death through physical disruption of the synergid cell. For example, pollen tube penetration and/or discharge may increase the physical pressure within the synergid cell and result in rupture of this cell (van Went and Willemse, 1984; Willemse and van Went, 1984; Russell, 1992; Higashiyama, 2002).
Several of these models can be distinguished by precisely defining the temporal relationships among synergid cell death, pollen tube arrival at the female gametophyte, and pollen tube discharge. For example, cell death before pollen tube-synergid cell contact would suggest that the pollen tube induces synergid programmed cell death through a long-range, diffusible signal. Alternatively, cell death upon pollen tube-synergid cell contact would suggest that a contact-mediated signal induces programmed cell death in the synergid cell. Finally, cell death after pollen tube discharge would suggest that synergid cell death occurs through physical rupture caused by pollen tube penetration and/or discharge.
The relationship between pollen tube arrival and synergid cell death has been determined in many species and these observations are variable. Synergid cell death occurs before arrival of the pollen tube at the female gametophyte in some species (Jensen and Fisher, 1968; Cass and Jensen, 1970; Maze and Lin, 1975; Mogensen, 1978; Mogensen and Suthar, 1979; Wilms, 1981; Dute et al., 1989; Kuroiwa, 1989; Russell et al., 1990; Yan et al., 1991; Huang et al., 1993; Huang and Russell, 1994) and after arrival in other species (van der Pluijm, 1964; Schulz and Jensen, 1968; van Went, 1970a, 1970b; Newcomb, 1973). Similarly, observations of the cell death process are variable. For example, synergid degeneration appears to occur via programmed cell death in wheat (Triticum aestivum; An and You, 2004) but by physical disruption due to pollen tube discharge in Torenia (Torenia fournieri; Higashiyama et al., 2000). These variable observations might result from differences among species in the induction of synergid cell death. Alternatively, the variation may be due to technical differences; for example, the detection of synergid degeneration has been shown to be fixation dependent (Fisher and Jensen, 1969).
We are using Arabidopsis (Arabidopsis thaliana) as a model system to dissect the molecular processes that regulate and mediate synergid cell death. In Arabidopsis, synergid cell death is pollination dependent (Christensen et al., 1997; Faure et al., 2002). Comparison of the time course of synergid cell death with that of pollen tube discharge in Arabidopsis suggests that degeneration occurs near the time of pollen tube arrival (Faure et al., 2002); however, these experiments did not distinguish between cell death occurrence shortly before or soon after pollen tube arrival. Thus, although these data appear to eliminate cell death induction through a very long-range diffusible signal (e.g. from stigma), they do not distinguish among the other possibilities, including induction by a short-range diffusible signal, induction following pollen tube-synergid cell contact, or physical disruption following pollen tube penetration and/or discharge.
Recently, two Arabidopsis female gametophyte mutants, gfa2 (Christensen et al., 2002) and sirene (srn; Rotman et al., 2003), affected in synergid cell death have been reported. gfa2 and srn synergid cells appear normal and attract wild-type pollen tubes, but fail to undergo cell death following pollination. With gfa2, the precise spatial relationship between the pollen tube and the synergid cell has not been determined. With srn, wild-type pollen tubes enter the female gametophyte and grow between the egg and synergid cells, but fail to enter the synergid cell, cease growth, and release their contents (Rotman et al., 2003). Although these observations indicate that pollen tube-synergid cell contact per se is not sufficient to cause cell death, they do not eliminate the possibility that cell death is caused by physical rupture of the synergid cell following pollen tube discharge. Furthermore, these observations do not eliminate a model in which the pollen tube induces a physiological cell death program since it is possible that the srn mutation affects a signal transduction molecule.
In this article, we defined the temporal relationship between pollen tube arrival at the female gametophyte and synergid cell death in Arabidopsis using light microscopy and transmission electron microscopy (TEM) of fixed material, as well as real-time imaging of these two events in an in vitro pollen tube growth assay. We showed that the synergid cell initiates cell death after the pollen tube arrives at the female gametophyte but before pollen tube discharge. Our observations suggest that the pollen tube triggers cell death by directly interacting with the synergid cell and inducing a physiological cell death program.
RESULTS
Time Course of Pollen Tube Arrival at the Ovule in Arabidopsis
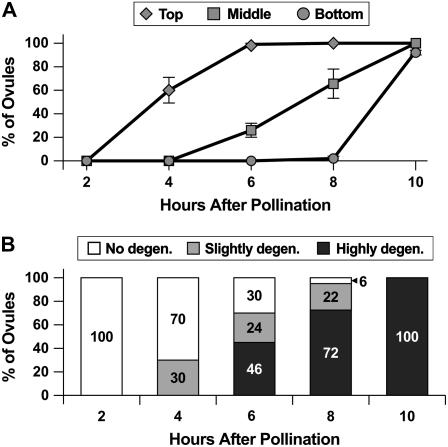
As a first step toward determining the temporal relationship between pollen tube growth and synergid cell death in Arabidopsis, we established the timing of pollen tube arrival at each ovule within the pistil. We performed controlled pollinations (described in “Materials and Methods”), collected pistils at 2 to 10 h after pollination, stained the pollen tubes with congo red, and used confocal laser scanning microscopy (CLSM) to score the number of ovules containing a pollen tube in its micropyle (Palanivelu et al., 2003). To determine whether pollen tubes arrive at the ovules closest to the stigma significantly before those farthest from the stigma, we divided the pistils into three sections, top (the five ovule rows closest to the stigma; rows 1–5), middle (the next five ovule rows; rows 6–10), and bottom (the five ovule rows closest to the pedicle; rows 11–15), and scored the ovules according to position within the pistil. Figure 1A summarizes the analysis of 1,594 ovules and shows that pollen tubes reach the top ovule rows by 4 to 6 h after pollination, the middle ovule rows by 6 to 10 h after pollination, and the bottom ovule rows at over 8 h after pollination. These data indicate that pollen tubes reach ovules in a temporal series, first at the top ovule rows and later at the bottom ovule rows, and that the ovules closest to the stigma receive pollen tubes approximately 6 h before those closest to the pedicle.
Figure 1.
Timing of pollen tube arrival at the ovule micropyle and of synergid cell death. A, Time course of pollen tube arrival at the ovule micropyle. Each data point represents the percentage of ovules with a pollen tube in the micropyle. Top, Ovule rows 1 to 5; middle, ovule rows 6 to 10; bottom, ovule rows 11 to 15. Error bars represent sds. B, Time course of synergid cell death. White bars indicate the percentage of ovules with two intact synergid cells (Fig. 2A), light-gray bars indicate the percentage of ovules with a slightly degenerated synergid cell (Fig. 2B), and dark-gray bars indicate the percentage of ovules that contain a highly degenerated synergid cell (Fig. 2C). Data are for the top ovule rows only.
Time Course of Synergid Cell Death in Arabidopsis
To determine whether synergid cell death occurs significantly before or at about the same time as pollen tube arrival at the ovule in Arabidopsis, we performed controlled pollinations, waited 2 to 10 h, and scored synergid cell death based on its morphology using CLSM (Christensen et al., 1997). Female gametophytes were placed into one of three categories: female gametophytes with two intact synergid cells (Fig. 2A), female gametophytes with a slightly degenerate synergid cell (Fig. 2B), and female gametophytes with a highly degenerate synergid cell (Fig. 2C). Slightly degenerate synergid cells had slightly higher autofluorescence in the cytoplasm, an irregular nucleus, and an intact vacuole (Fig. 2B), and highly degenerate synergid cells were extremely autofluorescent and had neither an observable nucleus nor a vacuole (Fig. 2C).
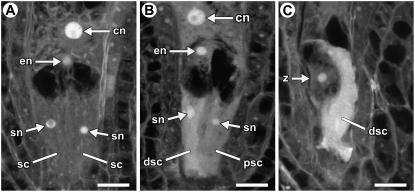
Figure 2.
CLSM analysis of synergid cell death. A, Female gametophyte with two intact synergid cells. The image is a projection of four 1.5-μm optical sections. B, Female gametophyte with a slightly degenerated synergid cell. The cytoplasm has slightly higher autofluorescence and the nucleus is irregular in shape. The image is a projection of three 1.5-μm optical sections. C, Female gametophyte with a highly degenerated synergid cell. The degenerating synergid cell exhibits strong autofluorescence and a nucleus is not observed. The image is a projection of three 1.5-μm optical sections. In all panels, the female gametophytes are oriented with the micropylar pole down and the chalazal pole up. Abbreviations: cn, central cell nucleus (secondary nucleus); dsc, degenerating synergid cell; en, egg cell nucleus; psc, persistent synergid cell; sc, synergid cell; sn, synergid nucleus; z, zygote. Scale bars: 10 μm.
Because pollen tubes reach the ovules along the pistil at different times (Fig. 1A), we limited our analysis to those at the top of the pistil. Figure 1B summarizes the analysis of 629 ovules and shows that synergid cell death was first detected in the top ovule rows at 4 h after pollination. At 4 to 8 h after pollination, the percentage of female gametophytes undergoing synergid cell death (Fig. 1B) was lower than the percentage of ovules with a pollen tube in its micropyle (Fig. 1A). These results suggest that synergid cell death occurs after entry of the pollen tube into the ovule micropyle.
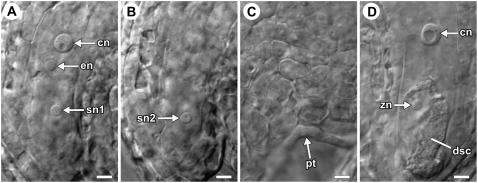
To validate these observations, we scored pollen tube position and synergid cell death within the same ovules. We performed controlled pollinations, collected pistils at 4 to 6 h after pollination, fixed and cleared the tissue, and used differential interference contrast (DIC) microscopy to observe these two events. We scored six pistils and 39 ovules. Of these, 30 ovules had two intact synergid cells (Fig. 3, A and B) and nine ovules had a degenerating synergid cell (Fig. 3D). All of the ovules containing a degenerating synergid cell also had a pollen tube in the micropyle. Most significantly, six of the ovules containing two intact synergid cells had a pollen tube in the micropyle (Fig. 3, A–C). These observations suggest that synergid cell death occurs after the pollen tube enters the ovule micropyle in Arabidopsis.
Figure 3.
DIC analysis of synergid cell death and pollen tube position. A to C, Three optical sections of a female gametophyte showing two persistent synergid cells and a pollen tube within the micropyle. D, An ovule with a degenerating synergid cell. Abbreviations: cn, central cell nucleus (secondary nucleus); dsc, degenerating synergid cell; en, egg cell nucleus; pt, pollen tube; sn, synergid cell nucleus; zn, zygote nucleus. Scale bars: 5 μm.
Pollen Tube Position within the Ovule during Synergid Cell Death in Arabidopsis
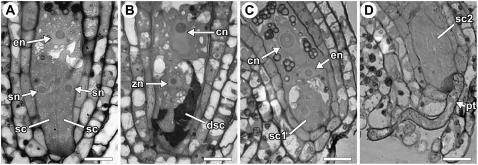
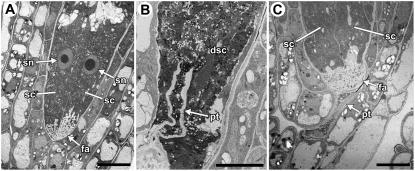
After entering the ovule micropyle, the pollen tube penetrates through the integuments and then enters the female gametophyte. Although the methods used above revealed that synergid cell death occurs after the pollen tube enters the micropyle, they did not allow us to define the position of the pollen tube within an ovule at the time of synergid cell death. To more closely define this spatial relationship, we used two microscopic methods that allowed us to score pollen tube position and synergid cell death within the same ovule. We performed controlled pollinations, waited 4 to 8 h, fixed ovules attached to the placenta (from rows 1–5), embedded the ovules in Spurr's resin, and analyzed plastic sections using light microscopy (Fig. 4) and TEM (Fig. 5). Using these methods, we scored 35 ovules. Of these, 19 ovules had a degenerating synergid cell (Figs. 4B and 5B); all of these also had a pollen tube in the degenerating synergid cell (Fig. 5B). In addition, 16 ovules had two intact synergid cells (Figs. 4A and 5A); in five of these, a pollen tube could be observed within the female gametophyte (Fig. 4, C and D) or contacting the filiform apparatus (Fig. 5C). These results suggest that synergid cell death in Arabidopsis is not initiated until after the pollen tube arrives at the synergid cell.
Figure 4.
Analysis of synergid cell death and pollen tube position using light microscopy of thick plastic sections. A, Female gametophyte with two intact synergid cells. B, Female gametophyte with a degenerating synergid cell. C and D, Two sections of the same ovule showing two intact synergid cells and a pollen tube contacting a synergid cell. Abbreviations: cn, central cell nucleus (secondary nucleus); dsc, degenerating synergid cell; en, egg cell nucleus; pt, pollen tube; sc, synergid cell; sn, synergid cell nucleus; zn, zygote nucleus. Scale bars: 10 μm.
Figure 5.
TEM analysis of synergid cell death and pollen tube position. A, Female gametophyte with two intact synergid cells. B, Female gametophyte with a pollen tube in a degenerating synergid cell. C, Female gametophyte with two intact synergid cells and a pollen tube at the filiform apparatus. Abbreviations: dsc, degenerating synergid cell; fa, filiform apparatus; pt, pollen tube; sc, synergid cell; sn, synergid cell nucleus. Scale bars: A and C, 10 μm; B, 5 μm.
Observations of Pollen Tube Growth and Synergid Cell Death in Vitro
To confirm the temporal relationship between synergid cell death and pollen tube arrival at the female gametophyte, we performed live imaging of pollen tube growth and synergid cell death using an Arabidopsis in vitro assay. We previously showed that this system reflects much of in vivo pollen tube behavior, including pollen tube guidance within an ovule, tube growth arrest at the female gametophyte, and pollen tube discharge (Palanivelu and Preuss, 2006). To observe pollen tube growth, pollen tubes were marked with DsRed driven by the LAT52 promoter (Twell et al., 1989; Francis et al., 2007). To observe synergid degeneration, we labeled the synergid cells with GFP expressed from the MYB98 promoter (Kasahara et al., 2005) and used changes in shape of the synergid cell and/or loss of the synergid GFP signal as indicators of synergid degeneration.
For the in vitro assay, we excised ovules from the pistil, placed them on pollen growth medium, and captured images of pollen tube-ovule interaction by exciting samples every 10 min with light of the appropriate wavelengths and observing the GFP and DsRed signals. To establish the background level of ovule death under the assay conditions, we excised GFP-marked ovules, placed them on pollen growth medium, imaged for 6 h, and scored the number of ovules with loss of GFP in both synergids. In approximately 37% (28/76) of the ovules, complete loss of GFP in both synergid cells was observed, indicating ovule death, and in the remaining ovules (63%, 48/76), no GFP loss was observed, indicating that these ovules survived our assay and image-capture conditions and remained with intact synergids at the end of the experiment. Loss of GFP in just one synergid cell, as with pollen-induced cell death described below, was never observed.
We next monitored synergid degeneration as a consequence of interaction with DsRed-tagged pollen tubes. We followed 59 ovules that had intact synergids over the course of the experiment. Of these, 31 ovules had a pollen tube within 100 μm of the micropyle, and 28 ovules had no pollen tube near the micropyle. Of the 31 ovules with a nearby pollen tube, 23 were penetrated by a pollen tube and eight were not. Of the 23 ovules penetrated by a pollen tube, 18 exhibited synergid degeneration, as evidenced by loss of GFP or change in shape in one of the two synergid cells (Figs. 6 and 7; Supplemental Videos S1–S4). By contrast, in all of the 36 ovules that were not penetrated by a pollen tube, no synergid degeneration was observed. Thus, in our in vitro system, synergid degeneration is a specific event that initiates upon interaction with a pollen tube, which reflects this aspect of in vivo pollen tube-synergid interaction.
Figure 6.
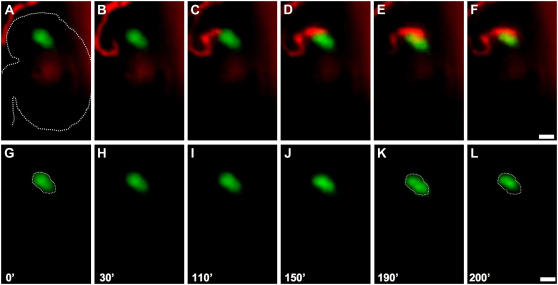
Live imaging of pollen tube growth and synergid cell death using an in vitro assay. All panels are fluorescent images showing DsRed-tagged pollen tubes growing toward and within ovules containing GFP-tagged synergid cells. A to L, A series of time-lapsed images of a pollen tube growing toward an ovule (a representative ovule sample is outlined by a white dashed line in A). Merged images captured in the red and green channels are in A to F. Images captured in the green channel alone are shown in G to L. Initiation of loss of GFP, indicating synergid cell degeneration, is observed in F and L (marked by white spheres), which occurs after the pollen tube reaches the synergid cell in C and I. The entire set of original images is presented in Supplemental Video S1. Time indicated in minutes ('). Scale bars: 25 μm.
Figure 7.
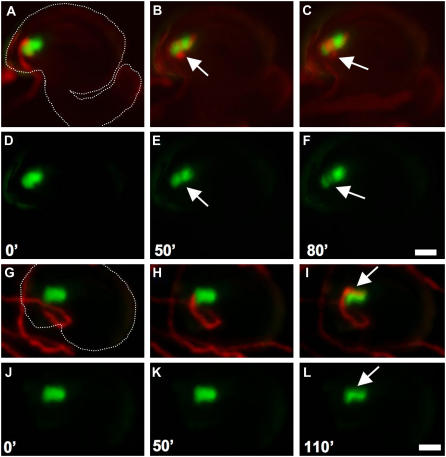
Pollen tube-synergid cell interaction. A to L, A series of time-lapsed images showing two different examples of the interactions between a pollen tube and a synergid cell. A to F, Merged images captured in the red and green channels (A–C) and images captured in the green channel alone (D–F) of a pollen tube extending one time (B and E, arrow) or two times (C and F, arrow) around a synergid cell prior to cell degeneration. A white dashed line in A outlines a representative ovule sample (with funiculus) in this series of images. The entire set of original images (A–L) is presented in Supplemental Video S3. G to L, Merged images captured in the red and green channels (G–I) and images captured in the green channel alone (J–L) of a pollen tube extending parallel to the synergid cell (I and L, white arrow) prior to cell degeneration. A white dashed line in G outlines a representative ovule sample (without funiculus) in this series of images. The entire set of original images (G–I) is presented in Supplemental Video S4. Time indicated in minutes ('). Scale bars: 25 μm.
To determine whether synergid degeneration occurs before or after pollen tube arrival, we scored the temporal relationship between these two events. In all 18 ovules that underwent synergid degeneration, we were able to score both pollen tube arrival and synergid degeneration. With each ovule, we scored (1) the time point at which the DsRed-tagged tube tip first overlapped with the GFP-tagged synergid cell, representing the time point of pollen tube arrival (e.g. Supplemental Video S1, 120-min frame), and (2) the time point at which GFP diminution and/or change in synergid shape were first detected, representing the time point of initiation of synergid degeneration (e.g. Supplemental Video S1, 200-min frame). In all 18 cases, synergid degeneration occurred after pollen tube arrival at the female gametophyte. On average, synergid degeneration was first detected 174 ± 119 (sd) min after the pollen tube reached the female gametophyte. Thus, the observations made in vitro confirm those made in vivo and demonstrate that synergid degeneration does not occur until after the pollen tube arrives at the female gametophyte.
The in vitro assay also allowed us to follow pollen tube behavior after arrival at the female gametophyte and to define the temporal relationship between pollen tube discharge and synergid cell death. In all 18 ovules that underwent synergid degeneration, we were able to clearly observe the entire pollen tube growth pathway until synergid degeneration. In all 18 cases, the pollen tube grew around and subsequently extended over the synergid cell before synergid degeneration; pollen tube growth was perpendicular to the synergids in nine cases (Fig. 7, A–F; 110- and 190-min frames in Supplemental Video S3) and parallel to the synergids in the other nine cases (Fig. 7, G–L; 220-min frame in Supplemental Video S4). These data suggest that synergid cell death does not occur immediately upon arrival of the pollen tube at the female gametophyte and that the pollen tube continues to grow around the synergid cell until discharge occurs.
In 13 of these 18 ovules, we were able to score both pollen tube discharge and synergid degeneration. For these measurements, we used the time point at which the DsRed signal spread out explosively from the pollen tube tip to represent the time point of pollen tube discharge (e.g. Supplemental Video S2, 350-min frame) and synergid degeneration was scored as described above. In all 13 cases, pollen tube discharge occurred after synergid degeneration. On average, pollen tube discharge occurred 102 ± 69 (sd) min after synergid degeneration was first detected. Together, these data suggest that the interaction between a pollen tube and a synergid cell occurs in the following order: (1) pollen tube arrival at the female gametophyte, (2) pollen tube growth around the synergid cell, (3) synergid degeneration, and (4) pollen tube discharge.
DISCUSSION
Synergid Cell Death Occurs after Pollen Tube Arrival But Before Pollen Tube Discharge in Arabidopsis
In Arabidopsis, synergid cell death does not occur in the absence of pollination (Christensen et al., 1997). A dependence of synergid cell death on pollination has also been reported for many other species (van Went and Willemse, 1984; Willemse and van Went, 1984; Russell, 1992). These observations suggest that pollen tube growth within a pistil is responsible for initiation of the synergid cell death process in these species. However, the lack of information about the position of the pollen tube at the time of synergid cell death has limited our understanding of the mechanism by which the pollen tube triggers synergid cell death in Arabidopsis.
In this study, we used a variety of microscopic methods to clarify the temporal relationship between synergid cell death and pollen tube growth in Arabidopsis. Using CLSM (Figs. 1 and 2), light microscopy (Figs. 3 and 4), TEM (Fig. 5), and real-time observation of pollen tubes growing in vitro (Figs. 6 and 7), we show that synergid cell death occurs after arrival of the pollen tube at the female gametophyte. Synergid cell death after arrival of the pollen tube at the female gametophyte has also been reported in other species (Russell, 1992), including Torenia (Higashiyama et al., 2000), Capsella bursa-pastoris (Schulz and Jensen, 1968), sunflower (Helianthus annuus; Newcomb, 1973), and petunia (Petunia hybrida; van Went, 1970a, 1970b).
Using an in vitro assay, we were able to make real-time observations of pollen tube-synergid cell interaction within the same ovule by labeling pollen tubes with DsRed and synergid cells with GFP. In this assay, the pollen tube reaches the synergid cell before synergid degeneration, consistent with our in vivo observations using light microscopy (Fig. 4, C and D) and TEM (Fig. 5C). The pollen tube then continues to grow and extend around the synergid cell for approximately 174 min before synergid disintegration is first detected. Although not commented on previously, prior analysis of pollen tube growth in the ovule has shown continued pollen tube growth near a synergid cell after arriving at the female gametophyte and prior to discharge (e.g. supplemental movie 5, supplemental data, in Rotman et al., 2003). Finally, at approximately 100 min after synergid degeneration is first detected, the pollen tube discharges its contents. In a Torenia in vitro pollen tube guidance assay, it was found that the pollen tube discharges at about the same time as synergid rupture (Higashiyama et al., 2000).
These observations support the proposal that synergid cell death is required for cessation of pollen tube growth and pollen tube discharge (van Went and Willemse, 1984; Higashiyama, 2002). Our in vitro observations that synergid degeneration begins before the pollen tube ceases growth and discharges are consistent with this proposal. Two other observations are consistent with this sequence of events. First, the pollen tube continues to grow in Arabidopsis srn mutants that fail to undergo synergid cell death (Rotman et al., 2003). Second, in cotton (Gossypium hirsutum), pollen tubes that occasionally enter the persistent synergid cell do not cease growth and discharge until they interact with the degenerating synergid cell (Jensen and Fisher, 1968).
Early Steps of Synergid Cell Death in Arabidopsis
Using CLSM, we observed many (>100) ovules that contained one synergid cell exhibiting slightly higher autofluorescence in the cytoplasm, an irregular nucleus, and an intact vacuole (Fig. 2B). The proportion of the synergid cells exhibiting this morphology was highest at early time points and progressively lower at later time points (Fig. 1B), suggesting that these synergid cells are at an early stage of cell death. All other degenerating synergid cells observed resembled those in Figure 2C, suggesting that the cell death process progresses rapidly to a highly degenerated state. These observations suggest that the early steps of synergid cell death include breakdown of the nucleus and a biochemical change in the cytoplasm that results in elevated autofluorescence by CLSM.
We also analyzed degenerating synergid cells at these early time points using TEM. Consistent with the CLSM analysis, the cytoplasm exhibited elevated electron density (Fig. 5B). However, in contrast to the CLSM analysis, nuclei were not observed and the vacuoles were fragmented (data not shown). These differences are likely due to the harsher fixation methods required for TEM analysis. The early steps of synergid cell death have also been described using TEM in other species, including cotton (Jensen and Fisher, 1968), barley (Hordeum vulgare; Cass and Jensen, 1970), Quercus gambelii (Mogensen, 1972), Proboscidea louisianica (Mogensen, 1978), soybean (Glycine max; Dute et al., 1989), tobacco (Nicotiana tabacum; Mogensen and Suthar, 1979; Huang et al., 1993; Huang and Russell, 1994), Brassica campestris (van Went and Cresti, 1988; Sumner, 1992), and sunflower (Yan et al., 1991). Although these observations vary to some extent, the early stages in general are associated with an increase in the electron density of the cytoplasm and breakdown of the nucleus. Later, the plasma membrane and vacuolar membrane break down. Ultimately, during the later stages of cell death, the cytoplasm becomes essentially electron-opaque.
During the cell death process, we observed cytoplasmic material in the narrow space between the chalazal end of the egg cell and the micropylar end of the central cell. This was consistently observed in our CLSM (Fig. 2C), light microscopy (Fig. 4B), and TEM (data not shown) images. Cytoplasmic material between the egg and central cells has been reported previously in several other species (van Went and Cresti, 1988; Huang et al., 1993; Huang and Russell, 1994; Higashiyama et al., 2000) and is thought to be the contents of the pollen tube (Higashiyama, 2002). It is therefore likely that the sperm cells fuse with the fertilization targets in this region (Russell, 1992).
At approximately 10 to 20 min after pollen tube discharge, we consistently observed movement of cytoplasmic contents (i.e. concentration of the GFP signal) to the micropylar end of the degenerating synergid cell in our in vitro assay (e.g. see Supplemental Video S2, between time points 350 and 360 min). It is likely that this movement represents collapse of the synergid cell during the final stages of cell death, as has been observed in TEM analysis of embryo sacs in several other species (Jensen and Fisher, 1968; Cass and Jensen, 1970; Mogensen, 1972; Dute et al., 1989; Huang and Russell, 1992).
Synergid Cell Death Is Not Required for Pollen Tube Guidance in Arabidopsis
Our observations clarify the relationship between pollen tube guidance and synergid cell death. Previous studies using cell ablation in Torenia have found that the synergid cells are the source of a pollen tube attractant (Higashiyama et al., 2001). Our real-time observations of pollen tube growth within the ovule provide supporting data for this model. In this assay, pollen tubes grew only toward a synergid and never to any other cell within the female gametophyte, suggesting very strongly that they are the source of a pollen tube attractant.
Although the Torenia cell ablation studies (Higashiyama et al., 2001) suggest that synergid cell death is not required for guidance of the pollen tube to the ovule micropyle, they left open the possibility that synergid degeneration might be required for the final steps of pollen tube guidance, i.e. pollen tube growth from the micropyle to the female gametophyte (Jensen, 1974; Weterings and Russell, 2004). In this study, we show that synergid degeneration occurs after the pollen tube arrives at the female gametophyte. These observations firmly establish that synergid cell death is not a prerequisite for any aspect of pollen tube guidance in Arabidopsis.
A Model for the Induction of Synergid Cell Death by the Pollen Tube in Arabidopsis
In many other species examined, synergid cell death occurs before arrival of the pollen tube at the female gametophyte (Jensen and Fisher, 1968; Cass and Jensen, 1970; Maze and Lin, 1975; Mogensen, 1978; Mogensen and Suthar, 1979; Wilms, 1981; Dute et al., 1989; Kuroiwa, 1989; Russell et al., 1990; Yan et al., 1991; Huang et al., 1993; Huang and Russell, 1994). These observations suggest that synergid cell death is induced by a long-range diffusible signal in these species. However, our observations that the synergid cell directly interacts with the pollen tube before it degenerates suggest that cell death in Arabidopsis is not induced by a long-range diffusible signal.
Initiation of synergid cell death after arrival of the pollen tube raises several possible means by which degeneration is caused in Arabidopsis. First, the pollen tube may induce a physiological cell death program by a contact-mediated (i.e. pollen tube-synergid cell contact) signal. Alternatively, pollen tube penetration and/or discharge may trigger mechanical breakdown of the synergid cell (van Went and Willemse, 1984; Willemse and van Went, 1984; Russell, 1992).
Mechanical breakdown from pollen tube discharge is thought to be the cause of synergid cell death in T. fournieri (Higashiyama et al., 2000). However, several lines of evidence suggest that synergid degeneration does not result from mechanical breakdown in Arabidopsis. First, we observed many ovules containing two intact synergids and in which the pollen tube is in contact with the synergid cell (Figs. 4, C and D, and 5C). Second, in our in vitro assay, synergid degeneration did not initiate until approximately 174 min after the pollen tube arrived at the female gametophyte, and during this time the pollen tube continued to extend, grow around, and interact with the synergid cell. Third, in our in vitro assay, we did not observe any instance where pollen tube discharge occurred before initiation of synergid degeneration. Instead, synergid degeneration initiated approximately 100 min before pollen tube discharge. Finally, in srn mutants, even though the synergid cells came in contact with wild-type pollen tubes for an extended period of time, synergid degeneration failed to occur (Rotman et al., 2003). Together, these observations suggest very strongly that mechanical impact resulting from pollen tube penetration and/or pollen tube discharge does not cause synergid cell death in Arabidopsis.
Observations in several other species support the conclusion that mechanical breakdown is not the cause of synergid cell death. In both cotton (Jensen and Fisher, 1968) and barley (Cass and Jensen, 1970), instances of the pollen tube entering the persistent synergid have been reported. In Plumbago zeylanica, which does not have synergid cells, the pollen tube contacts the egg cell for several minutes and discharges its contents adjacent to the egg cell, but these events do not cause egg cell death (Russell, 1982, 1983). These observations suggest that pollen tube penetration and pollen tube discharge per se are not sufficient to cause the synergid to undergo cell death.
The data presented here suggest that in Arabidopsis, the pollen tube and the synergid cell interact in the following series of steps: (1) pollen tube arrival at the female gametophyte, (2) pollen tube growth around the synergid cell, (3) synergid degeneration, and (4) pollen tube discharge. In this series, pollen tube penetration and discharge are unlikely to be the cause of synergid breakdown, ruling out the mechanical model for synergid cell death. Our data therefore favor a model in which a signaling cascade triggered by pollen tube-synergid cell contact induces synergid cell death in Arabidopsis. For example, the synergid cell could respond to factors released by the pollen tube, as with cell death in response to fungal pathogens (Ellis et al., 2006). We recently have identified a number of female gametophyte mutants that attract pollen tubes but that fail to undergo synergid cell death (L. Sandaklie-Nikolova, M. F. Portereiko, and G.N. Drews, unpublished data). Analysis of these mutants should lead to molecular dissection of the pathway by which the pollen tube induces synergid cell death.
MATERIALS AND METHODS
Plant Material and Growth Conditions
We used plants of Arabidopsis (Arabidopsis thaliana) Columbia ecotype (Col-0) for all of our experiments. Seeds were sterilized in a solution of 50% bleach (Clorox) and 0.1% Tween 20 and germinated on plates containing 0.5× Murashige and Skoog salts (Sigma M-9274), 0.05% MES, 0.5% Suc, and 0.8% Phytagar (Life Technologies). Ten-day-old seedlings were transferred to Scott's Redi-Earth soil mix and grown under 24 h of illumination. Plants used for in vitro assays were germinated directly on the soil.
Controlled Pollinations
The experiments discussed in this article required pollen tube growth to female gametophytes of the same developmental stage. However, female gametophyte development within a pistil is not perfectly synchronous (Christensen et al., 1997). To synchronize female gametophyte development at the time of pollination, we emasculated flowers at late stage 12 (stage 12c; Christensen et al., 1997), waited 24 h to allow all of the female gametophytes to reach the mature stage (stage FG7), and pollinated with wild-type pollen.
Confocal Microscopy Analysis of Pollen Tube Growth
Congo red staining was performed as described previously (Palanivelu et al., 2003). We emasculated flowers at stage12c (Christensen et al., 1997), waited 24 h, and pollinated with wild-type pollen. Flowers were collected at 2 h after pollination, 4 h after pollination, 6 h after pollination, 8 h after pollination, and 10 h after pollination. The sepals, petals, and stamens were removed from the isolated flowers, and pistils were placed on double-sided tape (Scotch). Cuts were made on both sides of the pistil replum using a 30.5-gauge syringe to expose the ovules to the Congo red stain. The pistils then were transferred to a microscope slide with a drop of 0.4% Congo red solution for staining. The pistil was divided into three sections for ovule scoring. The top section included the first five rows of ovules (rows 1–5), the middle section included the next five rows (rows 6–10), and the bottom section included the last five rows (rows 11–15). We performed three independent experiments per time point, and in each experiment we scored the ovules within 10 pistils (five plants and two pistils per plant). Ovules were analyzed on a Zeiss LSM 510 confocal microscope. The Congo red dye was excited with a HeNe laser at a wavelength of 543 nm. Emission was detected between 585 nm and 650 nm.
Confocal Microscopy Analysis of Synergid Cell Degeneration
Pistils were fixed and mounted for CLSM as previously described (Christensen et al., 1997) with the modification that we used a Zeiss LSM 510 microscope. We emasculated flowers at stage 12c (Christensen et al., 1997), waited 24 to 40 h, pollinated with wild-type pollen, and fixed the pistils at 0 h after pollination, 2 h after pollination, 4 h after pollination, 6 h after pollination, 8 h after pollination, and 10 h after pollination. To prepare the tissue for fixation, we removed the flowers from the plant; removed the sepals, petals, and stamens from the isolated flowers; and placed the pistils on double-sided tape (Scotch) on a microscope slide. We then made cuts on both sides of the pistil replum using a 30.5-gauge syringe to expose the ovules to fixative. The cuts spanned the entire length of the pistil. The pistils were fixed in a solution of 4% glutaraldehyde and 12.5 mm cacodylate buffer, pH 6.9, for 4 h at room temperature, dehydrated in a graded ethanol series (10%, 20%, 40%, 60%, 80%, 95%, for 10 min each), and incubated in 100% ethanol overnight at room temperature and then cleared in a 2:1 mixture of benzyl benzoate:benzyl alcohol for 20 min at room temperature. The cleared pistils were mounted in a drop of immersion oil onto microscope slides. We performed three independent experiments per time point, and in each experiment we scored the ovules within 10 pistils (five plants and two pistils per plant). Observations were made using a Zeiss LSM 510 confocal microscope. Ovules were excited with a HeNe laser at a wavelength of 543 nm. Emission was detected between 543 nm and 719 nm.
DIC Analysis of Ovules
We emasculated flowers at stage 12c (Christensen et al., 1997), waited 24 to 40 h, and pollinated with wild-type pollen. Pistils were harvested 4 and 6 h after pollination. The tissues were processed as described above for the CLSM analysis. The tissue was then mounted on microscope slides in a drop of immersion oil. The ovules were observed with a Zeiss Axioplan microscope using DIC optics. Images were obtained with an Axiocam MRm digital camera (Carl Zeiss) and processed using Adobe Photoshop 7 and Illustrator 10.
Plastic Sections and TEM
We emasculated flowers at stage 12c, waited 24 h, and collected the pistils for fixation. The dissection was the same as described above. The pistils were fixed overnight at 4°C in a solution of 4% glutaraldehyde, 1% paraformaldehyde, and 100 mm cacodylate buffer, pH 7.2. After fixation, the tissue was washed three times with 100 mm cacodylate buffer on ice. The pistils then were postfixed for 4 h in 1% aqueous osmium tetroxide at room temperature, washed three times in distilled water (approximately 20 min each at room temperature), postfixed for 1 h in 1% aqueous uranyl acetate at room temperature, and washed three times in distilled water (approximately 20 min each at room temperature). Pistils were cut into 1-mm pieces containing five to six ovules per piece, and then were dehydrated in a graded ethanol series (20%, 40%, 60%, 80%, 95%, 95%, 100%, 100%, 100% for 20 min each at room temperature) and three times in propylene oxide for 10 min at room temperature. The dehydrated ovules were transferred through intermediate stages of 1:2, 1:1, and 2:1 mixtures of propylene oxide:Spurr's epoxy resin, infiltrated in pure Spurr's epoxy resin, then embedded in pure Spurr's epoxy resin. The ovule blocks initially were sectioned at 1-μm thickness using an LKB Ultratome III microtome. The 1-μm sections were stained with 1% toluidine blue and observed under bright-field optics using an Axioplan Zeiss microscope. For ultrastructural analysis the same blocks were sectioned at 70- to 90-nm thickness using a Reichert-Jung Ultracut E microtome and the sections were transferred to coated slot-grids. Ultrastructural analysis was carried out using a Hitachi H-7100 electron microscope at 75 kV. Images were processed using Adobe Photoshop 7 software.
In Vitro Pollen Tube Targeting Assay and Fluorescence Microscopy
In vitro pollen tube targeting assays were performed essentially as described previously (Palanivelu and Preuss, 2006). The LAT52∷DsRed line (FTL1262) used was described by Francis et al. (2007). The MYB98∷GFP line used was described by Kasahara et al. (2005). Pollen, pistil, and ovules were obtained from stage 14 flowers (Smyth et al., 1990). Pollen tube growth and synergid cell degeneration were observed using an inverted fluorescent microscope fitted with an automated shutter, motorized stage, and CCD camera (Deltavision). GFP and DsRed fluorescence were acquired separately at 10-min intervals. The samples were illuminated with visible light of different wavelengths obtained by passing UV light filtered through appropriate excitation and emission filters. The GFP fluorescence was visualized using a 470/40 excitation filter and a 525/50 emission filter. The DsRed signals were obtained using a 580/20 excitation filter and a 630/60 emission filter. For every time point and in each channel, a stack of optical sections spanning 60 μm was acquired and the images were converted to JPEG format following the manufacturer's instructions. From the optical stack, in-focus images were selected, merged, and assembled into movies using ImageJ image analysis software (http://rsb.info.nih.gov/ij/download.html).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Video S1. Pollen tube interaction with the synergid cell in an in vitro guidance assay, example 1.
Supplemental Video S2. Pollen tube interaction with the synergid cell in an in vitro guidance assay, example 2.
Supplemental Video S3. Pollen tube interaction with the synergid cell in an in vitro guidance assay, example 3.
Supplemental Video S4. Pollen tube interaction with the synergid cell in an in vitro guidance assay, example 4.
Supplementary Material
Acknowledgments
We thank Ramin Yadegari and Mark Johnson for critical review of this manuscript. We thank Carl Boswell of the Molecular Imaging Facility, Department of Molecular and Cellular Biology, University of Arizona, for excellent technical help with time-lapse imaging. We also gratefully acknowledge assistance from J. Jackson, L. Lozano, S. Hewa-Yaddehige, and Y. Huang in performing in vitro assay experiments and image analysis.
This work was supported in part by the U.S. Department of Energy (grant no. DE–FG02–04ER15620 to G.N.D. and grant no. DE–FG02–05ER15651 to G.P.C.) and the College of Agricultural Sciences at the University of Arizona (start-up funds to R.P.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Gary N. Drews (drews@bioscience.utah.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- An LH, You RL (2004) Studies on nuclear degeneration during programmed cell death of synergid and antipodal cells in Triticum aestivum. Sex Plant Reprod 17 195–201 [Google Scholar]
- Cass DD, Jensen WA (1970) Fertilization in barley. Am J Bot 57 62–70 [Google Scholar]
- Christensen CA, Gorsich SW, Brown RH, Jones LG, Brown J, Shaw JM, Drews GN (2002) Mitochondrial GFA2 is required for synergid cell death in Arabidopsis. Plant Cell 14 2215–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen CA, King EJ, Jordan JR, Drews GN (1997) Megagametogenesis in Arabidopsis wild type and the Gf mutant. Sex Plant Reprod 10 49–64 [Google Scholar]
- Dute RR, Peterson CM, Rushing AE (1989) Ultrastructural changes in the egg apparatus associated with fertilization and proembryo development of soybean, Glycine max. Ann Bot (Lond) 64 123–135 [Google Scholar]
- Ellis J, Catanzariti AM, Dodds P (2006) The problem of how fungal and oomycete avirulence proteins enter plant cells. Trends Plant Sci 11 61–63 [DOI] [PubMed] [Google Scholar]
- Faure JE, Rotman N, Fortune P, Dumas C (2002) Fertilization in Arabidopsis thaliana wild type: developmental stages and time course. Plant J 30 481–488 [DOI] [PubMed] [Google Scholar]
- Fisher DB, Jensen WA (1969) Cotton embryogenesis: the identification, as nuclei, of the X-bodies in the degenerated synergid. Planta 84 122–133 [DOI] [PubMed] [Google Scholar]
- Francis KE, Lam SY, Harrison BD, Bey AL, Berchowitz LE, Copenhaver GP (2007) Pollen tetrad-based visual assay for meiotic recombination in Arabidopsis. Proc Natl Acad Sci USA 104 3913–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Yuan M, Huang BQ, Yang HY, Zee SY, O'Brien TP (2000) Changes in actin organization in the living egg apparatus of Torenia fournieri during fertilization. Sex Plant Reprod 12 315–322 [Google Scholar]
- Higashiyama T (2002) The synergid cell: attractor and acceptor of the pollen tube for double fertilization. J Plant Res 115 149–160 [DOI] [PubMed] [Google Scholar]
- Higashiyama T, Kuroiwa H, Kawano S, Kuroiwa T (2000) Explosive discharge of pollen tube contents in Torenia fournieri. Plant Physiol 122 11–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T (2001) Pollen tube attraction by the synergid cell. Science 293 1480–1483 [DOI] [PubMed] [Google Scholar]
- Huang BQ, Russell SD (1992) Synergid degeneration in Nicotiana: a quantitative, fluorochromatic and chlorotetracycline study. Sex Plant Reprod 5 151–155 [Google Scholar]
- Huang BQ, Russell SD (1994) Fertilization in Nicotiana tabacum: cytoskeletal modifications in the embryo sac during synergid degeneration. Planta 194 200–214 [Google Scholar]
- Huang BQ, Strout GW, Russell SD (1993) Fertilization in Nicotiana tabacum: ultrastructural organization of propane-jet-frozen embryo sacs in vivo. Planta 191 256–264 [Google Scholar]
- Jensen WA (1974) Reproduction in flowering plants. In AW Robards, ed, Dynamic Aspects of Plant Ultrastructure. McGraw-Hill, New York, pp 481–503
- Jensen WA, Fisher DB (1968) Cotton embryogenesis: the entrance and discharge of the pollen tube in the embryo sac. Planta 78 158–183 [DOI] [PubMed] [Google Scholar]
- Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN (2005) MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell 17 2981–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa H (1989) Ultrastructural examination of embryogenesis in Crepis capillaris (L.) Wallr.: 1. The synergid cell before and after pollination. Bot Mag Tokyo 102 9–24 [Google Scholar]
- Lord EM, Russell SD (2002) The mechanisms of pollination and fertilization in plants. Annu Rev Cell Dev Biol 18 81–105 [DOI] [PubMed] [Google Scholar]
- Maze J, Lin SC (1975) A study of the mature megagametophyte of Stipa elmeri. Can J Bot 53 2958–2977 [Google Scholar]
- Mogensen HL (1972) Fine structure and composition of the egg apparatus before and after fertilization in Quercus gambelii: the functional ovule. Am J Bot 59 931–941 [Google Scholar]
- Mogensen HL (1978) Pollen tube-synergid interactions in Proboscidea louisianica (Martineaceae). Am J Bot 65 953–964 [Google Scholar]
- Mogensen HL, Suthar HK (1979) Ultrastructure of the egg apparatus of Nicotiana tabacum (Solanaceae) before and after fertilization. Bot Gaz 140 168–179 [Google Scholar]
- Newcomb W (1973) The development of the embryo sac of sunflower Helianthus annuus after fertilization. Can J Bot 51 879–890 [Google Scholar]
- Palanivelu R, Brass L, Edlund AF, Preuss D (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114 47–59 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Preuss D (2006) Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biol 6 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman N, Rozier F, Boavida L, Dumas C, Berger F, Faure JE (2003) Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr Biol 13 432–436 [DOI] [PubMed] [Google Scholar]
- Russell SD (1982) Fertilization in Plumbago zeylanica: entry and discharge of the pollen tube in the embryo sac. Can J Bot 60 2219–2230 [Google Scholar]
- Russell SD (1983) Fertilization in Plumbago zeylanica: gametic fusion and fate of the male cytoplasm. Am J Bot 70 416–434 [Google Scholar]
- Russell SD (1992) Double fertilization. Int Rev Cytol 140 357–388 [Google Scholar]
- Russell SD (1993) The egg cell: development and role in fertilization and early embryogenesis. Plant Cell 5 1349–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SD (1996) Attraction and transport of male gametes for fertilization. Sex Plant Reprod 9 337–342 [Google Scholar]
- Russell SD, Rougier M, Dumas C (1990) Organization of the early post-fertilization megagametophyte of Populus deltoides: ultrastructure and implications for male cytoplasmic transmission. Protoplasma 155 153–165 [Google Scholar]
- Schulz R, Jensen WA (1968) Capsella embryogenesis: The synergids before and after fertilization. Am J Bot 55 541–552 [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner MJ (1992) Embryology of Brassica campestris: the entrance and discharge of the pollen tube in the synergid and the formation of the zygote. Can J Bot 70 1577–1590 [Google Scholar]
- Twell D, Wing R, Yamaguchi J, McCormick S (1989) Isolation and expression of an anther-specific gene from tomato. Mol Gen Genet 217 240–245 [DOI] [PubMed] [Google Scholar]
- van der Pluijm JE (1964) An electron microscopic investigation of the filiform apparatus in the embryo sac of Torenia fournieri. In HF Linskens, ed, Pollen Physiology and Fertilization. North-Holland Publ., Amsterdam, pp 8–16
- van Went J, Cresti M (1988) Pre-fertilization degeneration of both synergids in Brassica campestris ovules. Sex Plant Reprod 1 208–216 [Google Scholar]
- van Went JL (1970. a) The ultrastructure of the fertilized embryo sac of petunia. Acta Bot Neerl 19 468–480 [Google Scholar]
- van Went JL (1970. b) The ultrastructure of the synergids of Petunia. Acta Bot Neerl 19 121–127 [Google Scholar]
- van Went JL, Willemse MTM (1984) Fertilization. In B Johri, ed, Embryology of Angiosperms. Springer-Verlag, Berlin, pp 273–318
- Weterings K, Russell SD (2004) Experimental analysis of the fertilization process. Plant Cell (Suppl) 16 S107–S118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemse MTM, van Went JL (1984) The female gametophyte. In BM Johri, ed, Embryology of Angiosperms. Springer-Verlag, Berlin, pp 159–196
- Wilms HJ (1981) Pollen tube penetration and fertilization in spinach. Acta Bot Neerl 30 101–122 [Google Scholar]
- Yan H, Yang HY, Jensen WA (1991) Ultrastructure of the developing embryo sac of sunflower (Helianthus annuus) before and after fertilization. Can J Bot 69 191–202 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.