Abstract
The evolution of communicative signals involves a major hurdle; signals need to effectively stimulate the sensory systems of their targets. Therefore, sensory specializations of target animals are important sources of selection on signal structure. Here we report the discovery of an animal signal that uses a previously unknown communicative modality, infrared radiation or “radiant heat,” which capitalizes on the infrared sensory capabilities of the signal's target. California ground squirrels (Spermophilus beecheyi) add an infrared component to their snake-directed tail-flagging signals when confronting infrared-sensitive rattlesnakes (Crotalus oreganus), but tail flag without augmenting infrared emission when confronting infrared-insensitive gopher snakes (Pituophis melanoleucus). Experimental playbacks with a biorobotic squirrel model reveal this signal's communicative function. When the infrared component was added to the tail flagging display of the robotic models, rattlesnakes exhibited a greater shift from predatory to defensive behavior than during control trials in which tail flagging included no infrared component. These findings provide exceptionally strong support for the hypothesis that the sensory systems of signal targets should, in general, channel the evolution of signal structure. Furthermore, the discovery of previously undescribed signaling modalities such as infrared radiation should encourage us to overcome our own human-centered sensory biases and more fully examine the form and diversity of signals in the repertoires of many animal species.
Keywords: animal communication, signal evolution, multimodal communication
How do we account for the astonishing diversity in the forms of animal signals? Answers to this long-standing question in biology have historically focused on both the variety of messages that animals must encode in signals and the multiplicity of media through which signals must travel (1, 2). However, animal signals must also work through the sensory systems of signal targets and, as a consequence, sensory specializations of target animals are additional important sources of selection on signal structure (2–6). Here we report evidence that the infrared sensory system of northern Pacific rattlesnakes has shaped the evolution of the tail-flagging display used by California ground squirrels while harassing these predators. These squirrels differentiated infrared-sensitive rattlesnakes from infrared-insensitive gopher snakes, adding an infrared component to tail flagging only during encounters with rattlesnakes. Biorobotic playbacks of tail flagging revealed that adding an infrared component to this antipredator display enhanced its efficacy as a deterrent of rattlesnake predatory efforts. This discovery provides exceptionally strong support for the hypothesis that sensory systems guide the evolution of signal structure and is particularly remarkable given that the infrared component of tail flagging is almost certainly not detectable by the squirrels' own perceptual systems.
For adult California ground squirrels, defending pups against predation by rattlesnakes and gopher snakes has been an important part of successful reproduction for millions of years (7). These squirrels have evolved an arsenal of behavioral and physiological defenses against snakes, including a capacity to neutralize rattlesnake venom (8), skill in confronting snakes, and a snake-specific signal called tail flagging, consisting of side-to-side motions of the elevated, piloerected tail (3, 9). These capacities develop more fully as squirrels mature, making adults potent defenders of their pups against snake predation.
Snakes appear to be the primary targets of tail flagging (3, 10). Ground squirrels typically vocalize during encounters with avian and mammalian predators but shift to the visual signal of tail flagging when dealing with snakes (9). Airborne acoustic signals produced by squirrels are undetectable to snakes (11), whereas visual signals complement their sensory capabilities (12). Tail flagging places snakes on the defensive when combined with other harassment activities (13) and therefore could deter snakes from remaining in the vicinity of burrows with squirrel pups.
In addition to their visual abilities, rattlesnakes have evolved a specialized sensory innovation, infrared-sensitive pit organs, that has significantly enhanced their effectiveness as predators on small mammals (12, 14, 15). In turn, such a predatory innovation could have set the stage for an antipredator counterinnovation; protective mothers who have used olfactory and auditory cues to confirm that their adversary is a rattlesnake (13, 16) might well benefit from adding an infrared component to tail flagging, thereby enhancing the conspicuousness and efficacy of this signal. Such an infrared component however, would provide no additional benefits while dealing with gopher snakes, who lack a specialized infrared sensory system. Squirrels could add an infrared component to tail flagging through a combination of tail piloerection and increased blood flow from the warm body core to the tail, abilities commonly used in thermoregulation by small rodents (17). We tested the hypotheses (i) that tail flagging by California ground squirrels includes an infrared component but only when confronting infrared sensitive rattlesnakes and (ii) that the inclusion of this infrared component in tail flagging increases its effectiveness in shifting rattlesnakes from predatory to defensive behavior.
Results
Experiment 1: Ground Squirrel Infrared Emission.
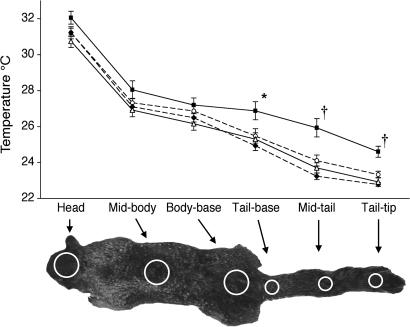
During laboratory interactions, ground squirrels engaged both rattlesnakes and gopher snakes, but not the control (conspecific) stimuli, with behavioral repertories similar to those seen in natural encounters (3, 10). Snakes elicited cautious approaches, elongate investigatory postures, tail flagging, and even some attempted substrate throwing (despite the absence of loose substrate). Although anti-snake behavior was qualitatively similar for the two snake species, video records of the encounters taken with an infrared imaging camera told a different story. Infrared emission from these squirrels varied with stimulus condition, and these differences were confined to the tail regions. Of the six regions measured on each squirrel (three from the head and body and three from the tail), only the three tail regions differed significantly in infrared emission among the stimulus conditions: the Tail-base (F3,33 = 6.388, P = 0.002), Mid-tail (F3,33 = 15.799, P < 0.001), and Tail-tip (F3,33 = 16.021, P < 0.001) (Fig. 1). Paired comparisons between the stimulus conditions for each of these tail regions revealed that the squirrels were increasing infrared emission from their tails during encounters with infrared-sensitive rattlesnakes compared with each of the other three conditions (gopher snake: Tail-base t11 = 3.61, P = 0.004, d = 1.06; Mid-tail t11 = 4.708, P = 0.001, d = 1.57; Tail-tip t11 = 6.364, P < 0.001, d = 1.74; baseline (no stimulus present): Tail-base t11 = 2.174, P = 0.05, d = 0.89; Mid-tail t11 = 3.29, P = 0.007, d = 1.25; Tail-tip t11 = 3.398, P = 0.006, d = 1.44; control (conspecific): Tail-base t11 = 3.91, P = 0.002, d = 1.39; Mid-tail t11 = 5.681, P < 0.001, d = 1.42; Tail-tip t11 = 6.223, P < 0.001, d = 1.89). In contrast, tail infrared emission was not significantly elevated over baseline or control conditions while interacting with gopher snakes (Fig. 1). The higher level of tail infrared emission with rattlesnakes than gopher snakes is clearly visible in video clips and stills from these trials [Fig. 2 and supporting information (SI) Movies 1 and 2]. These results demonstrate that California ground squirrels differentiate between rattlesnakes and gopher snakes, only increasing their tail infrared emission during encounters with rattlesnakes, the species specializing in thermoreceptivity.
Fig. 1.
Mean surface temperatures in each of the testing conditions across the six measured regions of the subjects' head, body and tail. Testing conditions are coded as, ■, rattlesnake; ▵, gopher snake; ♦, conspecific; ○, baseline. Squirrels increased emission of tail-infrared radiation over baseline and control levels during rattlesnake trials but not during gopher snake trials. *, P < 0.01 compared with conspecific and gopher snake; †, P < 0.01 compared with baseline, conspecific, and gopher snake. All points represent means ± SEMs, and planned comparisons were performed by using paired samples t tests. Points are connected for ease of reading and do not imply continuous measurement.
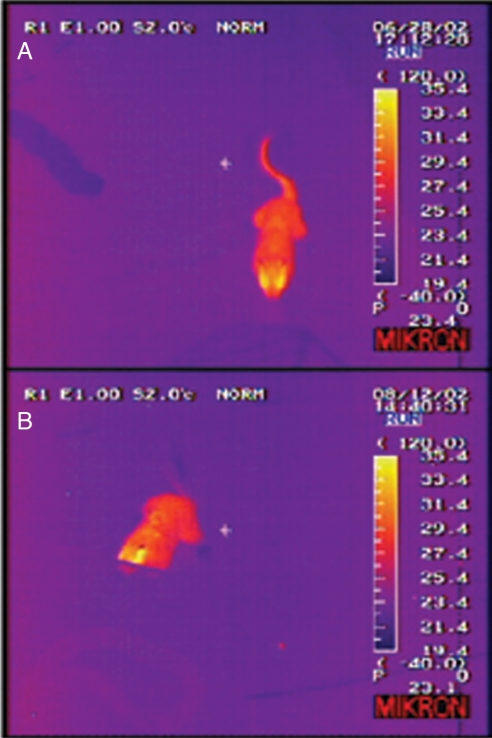
Fig. 2.
Infrared video frames of a squirrel interacting with a rattlesnake (A) and a gopher snake (B) during experimental trials. Pixel color corresponds to object surface temperature. Note that the tail regions of the squirrel are considerably warmer than the background during the rattlesnake trial but not the gopher snake trial. Stimulus cage partially obstructs the squirrel's head in B.
Comparisons of arousal and motor activity provide insight into the proximate processes mediating tail temperature changes during rattlesnake encounters. These data indicate that tail temperature changes involve more than generalized effects of increases in sympathetic nervous system arousal or squirrel activity. Tail fur piloerection has been shown to be a highly reliable measure of sympathetic nervous system arousal in small mammals (18–20). We found no significant difference in this index of arousal, as measured by tail fur piloerection at the mid tail [paired samples t tests of first third of the rattlesnake and gopher snake trials (t11 = 0.442, P = 0.667); middle third (t11 = 0.865, P = 0.405); and last third (t11 = 0.326, P = 0.751)]. Previous laboratory studies of encounters between California ground squirrels and these snake species have also found no difference in arousal (20, 21). Overall activity levels, which might affect the generation of metabolic heat, did not differ between stimulus conditions (F3,33 = 0.508, P = 0.68, repeated measures ANOVA). Neither the number of tail-flagging bouts (t11 = 0.813, P = 0.434; paired samples t test) nor the frequency of these bouts (t11 = 0.775, P = 0.455; paired samples t test) was significantly higher for rattlesnake than gopher snake trials. And finally, significant temperature differences were found only in the squirrels' three tail regions and not in their head or two body regions.
These data indicate that the addition of an infrared component to tail flagging has undergone evolutionary refinement, serving to increase the efficacy of tail flagging in persuading rattlesnakes to seek a meal elsewhere. These infrared-sensitive predators could be expected to respond to infrared-augmented tail flagging in this way for several reasons: (i) Tail flagging is associated with aggressive and dangerous harassment by squirrels. The frequent pairing of these noxious stimuli and tail flagging may produce a conditioned association (22, 23) that would enable squirrels to use tail flagging alone to induce rattlesnakes to shift from predatory to defensive behavior. The addition of an infrared component to tail flagging should make this signal even more salient to rattlesnakes and, therefore, more effective in producing a memorable conditioning effect (1, 24). (ii) Increased emission from the tail during rattlesnake encounters may serve to increase the apparent size of the squirrel and, thereby, more effectively dissuade the snake by exploiting its unconditioned avoidance response to cues that an adversary is large (23). (iii) Tail flagging likely reduces the chances of a successful ambush by elevating the level of snake-related vigilance in nearby squirrels (10).
Experiment 2: Biorobotic Playback.
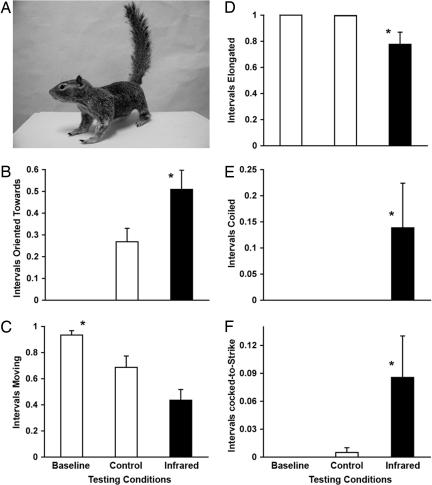
The biorobotic squirrel model (Fig. 3A) was placed adjacent to a snake food source, simulating a squirrel mother protecting her young. Encounters between the squirrel model and snake subjects were similar to those seen in natural encounters (3, 10, 25). Snakes initially entered the testing arena exhibiting predatory search behaviors, as observed in our baseline condition trials (no squirrel model present). But compared with baseline trials, the robotic tail-flagging squirrel in both the control condition (tail flagging with no tail infrared emission) and the infrared condition (tail flagging with augmented tail infrared emission) elicited more cautious behavior by the rattlesnakes. This was evidenced by reductions in both the proportion of intervals spent moving in the testing chamber and the elongated posturing associated with seeking food, as well as increases in the more defensive postures of coiling and cocking-to-strike (Fig. 3 C–F). (See SI Text for further discussion of the defensive behavior of rattlesnakes.) Thus, tail flagging induced hunting rattlesnakes to become more cautious.
Fig. 3.
Rattlesnake responses to the robotic squirrel models. (A) One of the robotic squirrels used for playbacks of tail flagging with (infrared) and without (control) an infrared component. Proportion of time intervals spent by the rattlesnake subjects oriented toward the robotic model (B), moving in the testing chamber (C), in an elongated posture (D), in a coiled posture (E), and in a cocked-to-strike posture (F). Bars represent means ± SEMs. *, P < 0.05 by a Wilcoxon signed-ranks test compared with all other conditions.
This shift from predatory to defensive behavior was much more pronounced when tail flagging included the infrared component than when it did not, during the control condition (Fig. 3). Compared with control tail-flagging trials, rattlesnakes in the infrared trials spent a greater proportion of time oriented toward the robotic model (control mean ± SEM = 0.268 ± 0.061; infrared mean ± SEM = 0.509 ± 0.088; Z = 2.355, n = 14, P = 0.019, Wilcoxon signed-ranks test; Fig. 3B) and a marginally smaller proportion of time moving (control mean ± SEM = 0.686 ± 0.087; infrared mean ± SEM = 0.435 ± 0.081; Z = 1.852, n = 14, P = 0.064, Wilcoxon signed-ranks test; Fig. 3C). Rattlesnakes in our playback study took longer to enter the squirrel burrow in the infrared condition (mean ± SEM = 35.1 ± 19.3 min) than during baseline trials (mean ± SEM = 4.1 ± 1.6 min; Z = 2.48, n = 14, P = 0.013, Wilcoxon signed-ranks test) and tended to enter with longer latencies than during control trials (mean ± SEM = 7.9 ± 2.4 min; Z = 1.287, n = 14, P = 0.198, Wilcoxon signed-ranks test). Snake posture also differed significantly among the treatment conditions in the proportion of time intervals spent in an elongated posture (χ22 = 12.08, P = 0.002, Friedman's ANOVA; Fig. 3D) and a cocked-to-strike posture (χ22 = 8.86, P = 0.012, Friedman's ANOVA; Fig. 3F). Furthermore, the snake's defensive reaction to the infrared-emitting model involved additional behavioral features not present when the model's tail emitted no infrared, including the coiled defensive posture (χ22 = 10.0, P = 0.007, Friedman's ANOVA; Fig. 3E) and rattling (χ22 = 10.0, P = 0.007; Friedman's ANOVA). Both are key indicators of arousal and defensiveness in rattlesnakes (26).
Discussion
Inclusion of the infrared component in tail flagging by the biorobotic squirrel demonstrated its communicative function; infrared-augmented tail flagging produced a much stronger shift from predatory to defensive behavior in rattlesnakes than the visual effects of tail flagging alone. Such induction of a defensive state may dissuade snakes from remaining in the vicinity of a tail flagger's vulnerable pups; snakes in this experiment tended to take longer to enter the simulated squirrel burrow when the infrared signal was present in the robotic squirrel model's tail. Such rattlesnake-dissuading effects of infrared emission would likely be augmented during playbacks by inclusion of the additional harassment activities that often accompany tail flagging, such as substrate throwing and looming. Similar confrontation and tail flagging by chipmunks (Tamias striatus) and gray squirrels (Sciurus carolinensis) induces foraging timber rattlesnakes (Crotalus horridus) to abandon their predatory efforts (ref. 27; but no measures of infrared were available for these observations).
The discovery of infrared signaling explains a finding that has long seemed paradoxical: California ground squirrels tail flag at higher rates to rattlesnakes in a dark room, when the visual component of this signal would not be effective, than in a normally lighted room (28). But the infrared component of tail flagging would be especially salient during such low-light conditions, which are characteristic of the periods when rattlesnakes are often most active (twilight in the spring and nighttime in the summer) (26). Thus, even though lower light levels reduce the visual detectability of tail flagging (29), the cool background of these conditions should highlight the infrared component (30). The mean and peak tail temperatures measured here during rattlesnake encounters are high enough to reliably exceed average ambient spring and summer temperatures in central California at twilight and nighttime (http://www4.ncdc.noaa.gov/cgi-win/wwcgi.dll?wwDI∼StnSrch∼StnID∼20002686) (SI Fig. 4). Differences of this magnitude are sufficient to produce an effective thermal signal to rattlesnakes (31), especially within the distances that ground squirrels often approach snakes. The multimodal nature of this signal thus allows tail flagging to maintain its efficacy in a wide variety of background lighting conditions.
To human and ground squirrel observers, tail flagging to rattlesnakes and gopher snakes appears identical; however, infrared video technology reveals a far more sophisticated form of communication, in which squirrels discriminate between species of snake predators and produce an infrared signal only to the snakes capable of detecting it. The pit organs of rattlesnakes constitute the most sensitive known infrared sensory system, an attribute that makes these snakes very effective rodent predators. But California ground squirrels have countered this adaptation with a defensive signal that exploits this infrared sensitivity and places rattlesnakes on the defensive. This case of an infrared signaling system may not be an isolated example. A variety of taxa are now known to possess specialized infrared sensory systems (32) and a closer examination of these species may reveal that coevolutionary processes have forged additional infrared signaling systems. Findings such as those reported here highlight the need to consider the full range of sensory and perceptual capabilities of all of the participants in communicative systems to develop a more complete understanding of the evolution and function of animal signals.
Materials and Methods
Infrared Video Analysis of Ground Squirrels.
We used an infrared imaging video camera (Mikron 7102) to film 12 adult female California ground squirrels during laboratory encounters with snakes and control stimuli. The resulting video records allowed for remote measurements of emitted thermal radiation (back calculated by the imager to read surface temperature) from six regions of the head, body, and tail (Fig. 1). Squirrels were live trapped in March 2002 from Winters, CA, an area containing a large population of northern Pacific rattlesnakes. Each subject received six experimental trials, one every three days, in a 1.52 m × 1.83 m × 1.07 m indoor testing chamber (SI Fig. 5A) lined with aluminum sheeting painted flat black to minimize reflection to the infrared camera. This series began with a baseline trial (no stimulus present in the chamber), followed by a trial with a conspecific (control) stimulus (to control for increases in infrared emission generated by interactions with a live stimulus). Subjects then received one trial with a northern Pacific rattlesnake and one trial with a Pacific gopher snake, balanced for order of presentation. After the snake trials, each subject then received a second conspecific and then baseline trial to test for any differences in infrared emission after exposure to the snake stimuli.
Trials were initiated by placing a squirrel's home cage adjacent to an opening in one wall of the testing chamber, allowing free access. Ten-minute experimental trials commenced after a squirrel first engaged the stimulus animal in the chamber with an investigative approach, tail-flag, or overt orientation. Stimulus animals were presented in a split-oval shaped wire mesh cage allowing visual, olfactory, auditory, and thermal exchange while preventing direct contact. Three exemplars of each stimulus type were used equally often and randomly assigned to individual subjects. Consecutive trials for individual subjects were separated by 2 days to limit carry-over effects.
The average temperature of each region measured from the squirrels was derived from instantaneous samples every 20 sec of the infrared video by using Image J software (version 1.29: National Institute of Health). We checked the reliability of the scored temperature measurements by having nine randomly selected trials rescored by independent observers blind to the stimulus condition for each trial. A comparison of the original and rescored data yielded high interobserver ratings (Interclass R = 0.993, coefficient α = 0.996). The six regions were individually tested for differences among the stimulus conditions by using repeated measures ANOVAs, Bonferroni corrected for an experiment-wide α of 0.05. No significant differences were found between either the two baseline or the two conspecific trials, pre- and post-snake exposure; therefore, composite scores for these conditions were used in the ANOVAs. Planned comparisons between treatment conditions were conducted with paired-sample t tests for each region that differed significantly among testing conditions. SPSS was used for all comparisons (version 11.0.4, 2005; SPSS).
Robotic Playbacks.
Fourteen adult northern Pacific rattlesnakes (190–765 g) were captured from March to August 2004 from several locations on the western edge of the central valley of California known to contain large populations of California ground squirrels. Simulated squirrel encounters occurred in a two-chamber apparatus (SI Fig. 5B) consisting of a 0.51 m × 0.63 m × 0.79 m starting chamber connected by an enclosed runway to a 1.17 m × 1.22 m × 0.79 m testing chamber containing a simulated squirrel burrow. The testing room was maintained at ≈70 Lux illumination and 22°C, the average temperature in Winters, CA, at dusk when rattlesnakes typically hunt squirrel pups in the spring and summer (27).
All snakes were conditioned to feed in the testing chamber once every 2 weeks for a total of seven trials. During conditioning a snake was placed in the starting chamber and allowed to move into the testing chamber, where a frozen/rewarmed rat pup was available inside the simulated squirrel burrow. Rat pups were used because they are similar in size to squirrel pups and squirrels are extremely difficult to breed in captivity. We video recorded the seventh trial as a baseline for the experimental trials.
Experimental trials involved the use of biorobotic California ground squirrel models engineered to independently produce surface temperatures of 31°C in the body (mean body temperature from experiment 1) and 28°C in the tail (peak tail temperature of a rattlesnake-engaged squirrel from experiment 1). The models were configured to tail flag at random intervals with an average rate of 5 bouts/min. The number of cycles per bout (the number of times the tail completes 360° of side-to-side motion) was contingent on the distance of the rattlesnake subject from the model, varying from one when the snake was farthest to three when closest [values derived from field presentations of tethered snakes (3)]. The squirrel model was placed adjacent to the squirrel burrow in the testing chamber 40 cm from and oriented toward the runway entrance (SI Fig. 5B). One of two identical robotic models was randomly assigned to each subject. These highly realistic models were constructed from taxidermic mounts of California ground squirrels trapped from Winters, CA, and stored in used squirrel bedding to impregnate them with squirrel odor.
Each snake received two playback trials with the robotic squirrel, a control trial (only body heated and tail flagging) and an infrared trial (body and tail heated and tail flagging). Each of the trials was separated by 2 weeks to minimize carry-over effects and counterbalanced for order of presentation. Trials commenced when the snake crossed the threshold from the runway to the testing chamber and continued either until the snake entered the squirrel burrow or 2 h had elapsed [observed interactions in the wild can last for several hours (10)]. Video records of all trials were scored for the following measures: (i) latency to enter the squirrel burrow; (ii) instantaneous samples every 10 sec of snake (a) distances to the robot and squirrel burrow, (b) orientation with reference to the robot (toward or away), (c) posture (elongated, coiled, or cocked-to-strike), and (d) movement (yes or no); and (iii) all occurrences of rattling. Because these data were not normally distributed, we used Friedman ANOVAs followed by Wilcoxon signed-ranks tests for planned comparisons of measures that were statistically significant (using SPSS version 11.0.4, 2005).
Supplementary Material
Acknowledgments
We thank C. Sullivan for assistance in programming the robotic squirrel models; J. Schank for the use of his infrared imaging camera; and R. Chancellor, J. Davis, G. Patricelli, J. Watters, A. Krakauer, and R. Mehta for comments on earlier versions of this manuscript. This work was supported by grants from the American Society of Mammalogists, Sigma Xi, the Animal Behavior Society, the American Museum of Natural History, and the Chicago Herpetological Society (to A.S.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 14177.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702599104/DC1.
References
- 1.Dawkins MS, Guilford T. In: Perspectives in Ethology. Owings DH, Beecher MD, Thompson NS, editors. Vol 12. New York: Plenum; 1997. pp. 55–75. [Google Scholar]
- 2.Owings DH, Morton ES. Animal Vocal Communication: A New Approach. New York: Cambridge Univ Press; 1998. [Google Scholar]
- 3.Hennessy DF, Owings DH, Rowe MP, Coss RG, Leger DW. Behavior. 1981;78:188–226. [Google Scholar]
- 4.Guilford T, Dawkins MS. Anim Behav. 1986;42:1–14. [Google Scholar]
- 5.Ryan MJ, Rand AS. Evolution (Lawrence, Kans) 1990;44:305–314. doi: 10.1111/j.1558-5646.1990.tb05200.x. [DOI] [PubMed] [Google Scholar]
- 6.Endler JA. Am Nat. 1992;139:S125–S153. [Google Scholar]
- 7.Coss RG. In: Geographic Variation in Behavior: Perspectives on Evolutionary Mechanisms. Foster SA, Endler JA, editors. Oxford: Oxford Univ Press; 1999. pp. 180–208. [Google Scholar]
- 8.Poran NS, Coss RG, Benjamini E. Toxicon. 1987;25:767–777. doi: 10.1016/0041-0101(87)90127-9. [DOI] [PubMed] [Google Scholar]
- 9.Owings DH, Coss RG. Behavior. 1977;62:50–69. [Google Scholar]
- 10.Hersek MJ, Owings DH. Anim Behav. 1993;46:129–138. [Google Scholar]
- 11.Young BA. J Penn Acad Sci. 1997;71:39–46. [Google Scholar]
- 12.Kardong KV, Berkhoudt H. Brain Behav Evol. 1999;53:20–28. doi: 10.1159/000006579. [DOI] [PubMed] [Google Scholar]
- 13.Rowe MP, Owings DH. Behavior. 1978;66:252–267. [Google Scholar]
- 14.Bullock TH, Diecke FPJ. J Physiol. 1956;134:47–87. doi: 10.1113/jphysiol.1956.sp005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haverly JE, Kardong KV. Copeia. 1996;1996:419–428. [Google Scholar]
- 16.Hennessy DF, Owings DH. Behaviour. 1978;65:115–124. [Google Scholar]
- 17.Gordon CJ. Temperature Regulation in Laboratory Rodents. Cambridge, UK: Cambridge Univ Press; 1993. [Google Scholar]
- 18.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Chem Senses. 2004;29:35–40. doi: 10.1093/chemse/bjh004. [DOI] [PubMed] [Google Scholar]
- 19.Dettling A, Pryce CR, Martin RD, Dobeli M. Dev Psychobiol. 1998;33:21–31. doi: 10.1002/(sici)1098-2302(199807)33:1<21::aid-dev3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Ennis M, Coss RG. Behav Neurosci. 2006;120:1299–1307. doi: 10.1037/0735-7044.120.6.1299. [DOI] [PubMed] [Google Scholar]
- 21.Coss RG, Biardi JE. J Mammal. 1997;73:294–310. [Google Scholar]
- 22.Steger RW, Caldwell RL. Science. 1983;221:558–560. doi: 10.1126/science.221.4610.558. [DOI] [PubMed] [Google Scholar]
- 23.Owren MJ, Rendall D. In: Perspectives in Ethology. Owings DH, Beecher MD, Thompson NS, editors. Vol 12. New York: Plenum; 1997. pp. 299–346. [Google Scholar]
- 24.Garcia J, McGowan BK, Hager JL. In: Biological Boundaries of Learning. Seligman MEP, Prokasy WF, editors. New York: Appleton–Century–Crofts; 1972. pp. 21–43. [Google Scholar]
- 25.Swaisgood RR, Owings DH, Rowe MP. Anim Behav. 1999;57:1033–1044. doi: 10.1006/anbe.1998.1069. [DOI] [PubMed] [Google Scholar]
- 26.Klauber LM. Rattlesnakes: Their Habits, Life Histories, and Influence on Mankind. Berkeley, CA: Univ of Calif Press; 1982. [Google Scholar]
- 27.Clark RW. Behav Ecol Sociobiol. 2005;59:258–261. [Google Scholar]
- 28.Coss RG, Owings DH. Z Tierpsychol. 1978;48:421–435. [Google Scholar]
- 29.Barrett R, Maderson PFA, Meszler RM. In: Biology of the Reptilia. Gans C, editor. London: Academic; 1970. pp. 277–314. [Google Scholar]
- 30.Theodoratus DH, Chiszar D, Smith HM. Psychol Rec. 1997;47:461–472. [Google Scholar]
- 31.Molenaar GJ. In: Biology of the Reptilia. Gans C, editor. London: Academic; 1992. pp. 367–453. [Google Scholar]
- 32.Campbell AL, Naik RR, Sowards L, Stone MO. Micron. 2002;33:211–225. doi: 10.1016/s0968-4328(01)00010-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.