Abstract
CD8+ cytotoxic T lymphocytes are key effectors of adaptive immunity for the control of virus infections. Epitope-specific responses are hierarchical and the rules for dominance are not well defined. Here we show that the H2-Kd-restricted RSV M282–90 (KdM282–90) epitope dominates the H2-Db-restricted RSV M187–195 (DbM187–195) epitope and influences epitope-specific effector function in the acute and memory phase of the immune response to primary RSV infection in H-2d/b hybrid mice. The hybrid mouse model provides a system to define rules of epitope hierarchy and better understand how antigen presentation and epitope competition affect the phenotype of effector and memory T cells.
Keywords: RSV, CD8+ CTL, epitope, mice, immunodominance
INTRODUCTION
Respiratory syncytial virus (RSV) is a pneumovirus that carries a considerable public health cost. Development of a vaccine for RSV is therefore considered a top priority in developed countries (Collins et al., 2001). In the majority of virus infections, including RSV, the CD8+ T cell is responsible for the elimination of virus-infected cells (Cannon et al., 1988; Doherty and Christensen, 2000; Graham et al., 1991; Harty et al., 2000; Wong and Pamer, 2003). CD8+ T cells recognize processed virus antigens in the context of class I MHC expressed by APCs, resulting in a highly specific response (Zinkernagel and Doherty, 1974). Immunogenic peptide epitopes vary in the magnitude of the response that they induce resulting in a hierarchy of epitope dominance (Kedl et al., 2003). Factors that determine whether a specific epitope is immunodominant or subdominant include antigen abundance, the efficiency of antigen processing, binding affinity to the class I MHC groove, competition for class I MHC molecules, recognition and binding affinity of the MHC:peptide complex for the TCR, and signaling pathways triggered at the time of TCR interaction (Kedl et al., 2003; Yewdell and Bennink, 1999). Moreover, subdominant epitopes can ascend to dominance if the immunodominant epitope is eliminated (Chen et al., 2000; Johnstone et al., 2004), during secondary responses (Belz et al., 2000 and 2001; Selin et al., 1999), or depending on the density of antigen expression (Moskophidis et al., 1993; Probst et al., 2003; Zajac et al., 1998).
We have recently identified an H2-Db-restricted CD8+ T cell epitope from the RSV M protein (DbM187–195, NAITNAKII) in C57Bl/6 mice (Rutigliano et al., 2005). This allowed us to begin investigating epitope hierarchy in H-2d/b hybrid CB6F1/J mice, which are the F1 generation of a BALB/c x C57Bl/6 mating. In this report, we compare the CD8+ T cell responses to KdM282–90, SYIGSINNI, and DbM187–195 in RSV-infected CB6F1/J mice to the responses in the parent strains.
RESULTS
The illness pattern and viral replication in CB6F1/J mice mimics the parental strains
We first examined illness as defined by weight loss in RSV-infected CB6F1/J mice. RSV-induced illness in CB6F1/J mice was intermediate between that of BALB/c and C57Bl/6 mice (Fig. 1A), but the hybrid mice showed no physical signs of illness, such as ruffled fur, inactivity, rapid breathing, or gaunt posture (data not shown), commonly seen in BALB/c mice. Viral titers in the lungs of C57Bl/6 mice were 1.5 to 2 logs lower than those of CB6F1/J and BALB/c mice on day 4 post-infection, which is the peak of virus replication in the lungs of mice. All three strains of mice had similar kinetics of viral clearance, with a reduction in titer on day 6, and little to no detectable virus by day 8 post-infection (Fig. 1B).
Figure 1. A) Illness in RSV-infected mice.
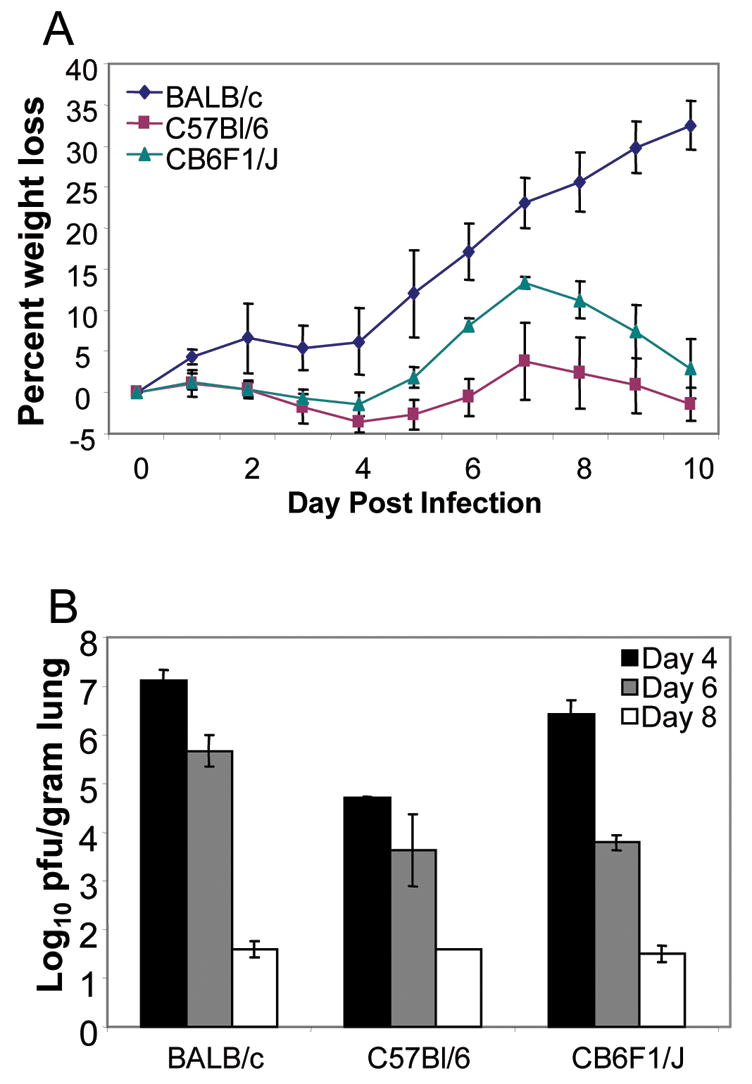
RSV-A2 infected parent and hybrid mice were weighed daily to monitor virus-induced illness. Averages and standard deviations were calculated from results with 5 mice per group. The data represent 3 independent experiments. B) Clearance of virus-infected cells from the lungs of RSV-infected mice. Lung viral titers were determined by a standard plaque assay as described (11) in all three strains of RSV-A2 infected mice. The limit of detection is 1.8 log10 pfu/gram of lung tissue. Geometric means and standard deviations were calculated from results with 5 mice per group. The data represent 3 independent experiments. The day 4 virus titer in C57Bl/6 mice is significantly lower than the titer in BALB/c or CB6F1/J mice (P<0.05, Student’s T-test).
Enumeration of epitope-specific CD8+ T cells in CB6F1/J mice reveals a hierarchy of epitope specifity
We have previously determined that the CD8+ T cell response to the novel DbM187–195 epitope in C57Bl/6 mice is similar to the response induced by the KdM282–90 epitope in BALB/c mice (Rutigliano et al., 2005). To characterize the CD8+ T cell response in the hybrid mouse we first measured the total T cell response in the lungs of both parent strains and the hybrid mice after RSV infection. The percentage and absolute numbers of CD8+ T cells in the lung rose through day 8 and waned by day 14, nearly returning to baseline levels (Fig. 2). CD8+ T cell numbers were similar in all three strains of mice at each time point. Next we defined the time course of the epitope–specific CD8+ T cell response in each strain with tetramers that recognize CD8+ T cells specific for each epitope (KdM282–90 tetramer and DbM187–195 tetramer) as described (Rutigliano et al., 2005). The percentage of KdM282–90 tetramer positive CD8+ T cells approached 50% in BALB/c mice at day 10 (Fig 3A), and DbM187–195 tetramer positive CD8+ T cells approached 40% in C57Bl/6mice (Fig 3B). In CB6F1/J mice, the number of CD8+ T cells specific for KdM282–90 was more than 3–fold higher than DbM187–195 -specific CD8+ T cells in 4 independent experiments (Figs. 3C and D), demonstrating a clear epitope hierarchy.
Figure 2. Quantitation of CD8+ T cells in the lungs of RSV infected mice.
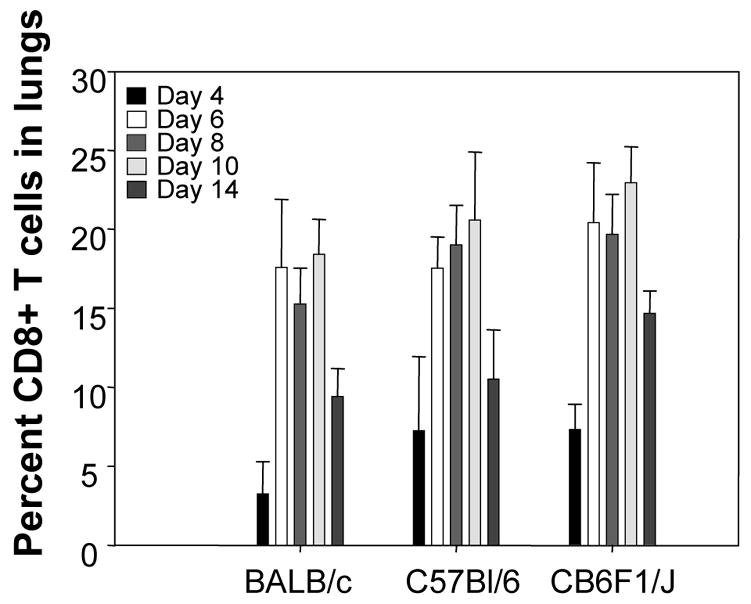
Percentages of CD3+CD8+ T cells were determined by flow cytometry of lung lymphocytes from mice sacrificed at 4, 6, 8, 10, and 14 days post RSV-infection. The data represent 3 independent experiments.
Figure 3. Epitope-specific CD8+ T cell responses in the lungs of RSV-infected parent strains and hybrid mice.
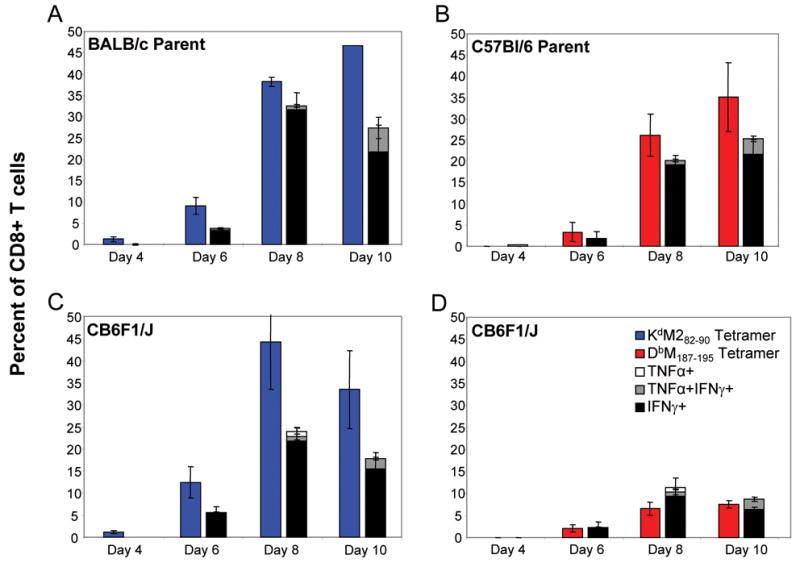
KdM282–90- and DbM187–195-specific CD8+ T cells were quantitated by tetramer staining lung lymphocytes at days 4, 6, 8, and 10 post-infection. KdM282–90 tetramer staining was done for BALB/c and CB6F1/J mice, and DbM187–195-tetramer staining was done for C57Bl/6 and CB6F1/J mice. Antiviral cytokine production by CD3+CD8+ T cells was determined separately by ICS after in vitro peptide stimulation with M282–90 or M187–195 peptides. Panels A (KdM282–90 responses) and B (DbM187–195 responses) indicate responses for the BALB/c and C57Bl/6 parent strains, respectively. Panels C (KdM282–90) and D (DbM187–195) represent quantitated epitope-specific responses in the CB6F1/J hybrid. The data are representative of 3 independent experiments, with background (DbNP366–374 tetramer stained, or flu peptide, ASNENMETM, -stimulated) subtracted.
The DbM187–195 epitope elicits a lower magnitude of T cell responses that more consistently produce cytokines compared to responses elicited by the KdM282–90 epitope in both primary and memory responses
Functional differences in the epitope-specific responses between parent and hybrid strains were assessed by intracellular cytokine staining (ICS) for antiviral cytokine production as described (Rutigliano et al., 2005), in parallel with tetramer staining by stimulating additional lung lymphocyte samples with either M282–90 or M187–195 peptide in combination with anti-CD49d and anti-CD28 antibodies for co-stimulation. We found that between 40 and 80% of the epitope-specific cells (as quantitated by surface tetramer staining) in BALB/c and C57Bl/6 parent strains were able to respond to peptide by the production of IFN-γ, TNF-α, or both cytokines simultaneously (Figs 3A and B). As previously indicated by tetramer staining, the magnitude of the M282–90 response was higher than the M187–195 response in hybrid mice (Figs. 3C and D). Interestingly, despite lower frequencies of DbM187–195 -specific cells in CB6F1/J mice, and the subdominant status of this epitope, a higher proportion of the DbM187–195 epitope-specific cells were capable of cytokine production following in vitro peptide stimulation (Fig. 3D).
The predominant cytokine response to stimulation with either the M82–90 or the M187–195 peptides was the production of IFN-γ. Increased production of TNF-α was seen on day 10 post-infection. However most of these cells produced IFN-γ. Very few antigen-specific T cells produced only TNF-α, suggesting that cells producing TNF-α are a subset of cells producing IFN-γ during RSV infection. This phenomenon has also been demonstrated in influenza infection (Barber et al., 2005; La Gruta et al., 2004).
We next asked if KdM282–90 –specific CD8+ T cells remained dominant in the memory phase after infection of CB6F1/J. We sacrificed RSV-infected CB6F1/J mice 42 days post-infection and analyzed the memory CD8+ T cell responses in the spleen. We observed a higher frequency of KdM282–90 tetramer positive cells than DbM187–195 tetramer positive cells in the spleens of hybrid mice, indicating that the dominance in the magnitude of response to this epitope was maintained (Fig. 4A). Only a fraction of the RSV-specific CD8+ T cells to either epitope produced cytokines upon in vitro peptide stimulation. Again, TNF-α-producing cells were a subset of IFN-γ-producing CD8+ T cells. These results indicate that the KdM282–90 epitope maintains dominance in the memory population. As an additional measure of functionality, we measured the geometric mean fluorescence intensity (MFI) of IFN–γ production by CD8+ T cells following stimulation with M282–90 and M187–195 peptides (Fig. 4B). During primary infection, when a higher proportion of DbM187–195-specific cells produce IFN-γ following stimulation, more IFN-γ per cell is also produced as measured by MFI. However, during the memory phase, the KdM282–90-specific cells dominate quantitatively, as measured by tetramer staining, and also produce more IFN-γ per cell than DbM187–195-specific cells (Fig. 4A and B).
Figure 4. Memory CD8+ T cell responses to RSV infection in CB6F1/J mice.
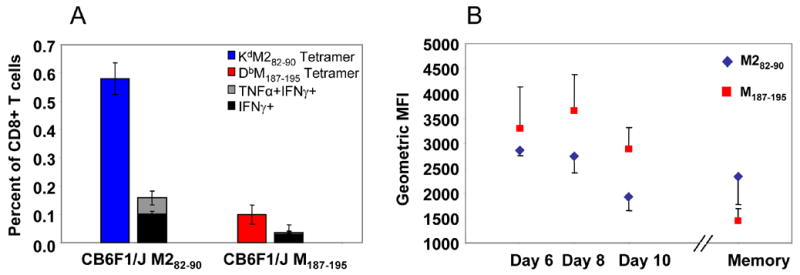
A) KdM282–90-and DbM187–195-specific CD8+ T cells were measured by tetramer staining of CB6F1/J spleen lymphocytes 42 days after RSV infection. Antiviral cytokine production by memory CD3+CD8+ T cells was determined separately by ICS after stimulation with epitope-specific peptide and co-stimulation with anti-CD49d and anti-CD28. Averages and standard deviations were calculated from results for 5 mice per group, and the data are representative of 3 independent experiments. P<0.05 between KdM82–90 and DbM187–195 epitopes for both tetramer and cytokine responses, Student’s T-test. B) Geometric mean fluorescence intensity (MFI) of IFN-γ production by CD8+ T cells following M282–90 and M187–195 peptide stimulation of lung (days 6–10) and spleen (memory) lymphocytes.
DISCUSSION
We have described the CD8+ T cell response to two dominant epitopes, KdM282–90 and DbM187–195 (Kulkarni et al., 1993; Rutigliano et al., 2005), in RSV-infected H-2d/b hybrid CB6F1/J mice where the consequences of epitope competition on the T cell response can be independently studied. These mice have been used previously to study the hybrid resistance effect in LCMV infection (Doherty and Allan, 1986a and 1986b). We suggest that the hybrid mouse model provides a system to define the rules of epitope hierarchy and to better understand the determinants of CD8+ T cell effector functions. The system allows for adoptive transfer of cells from either parent strain into the hybrid mouse to define the role of antigen presentation or CD4+ T cell help on determining the functional characteristics of epitope-specific CD8+ T cell responses. It provides a system to address whether patterns of epitope hierarchy can be modified by vaccination or the conditions under which antigen presentation occurs. In addition, sorting and clonotyping epitope-specific T cells following vaccination or RSV infection will augment our understanding of how clonal selection and TCR clonotype patterns influence CD8+ T cell phenotypes. These questions are under active investigation in our laboratory.
Despite exhibiting dominance in parent C57Bl/6 mice, the DbM187–195 epitope became subdominant to the KdM282–90 epitope in H-2d/b mice. It has previously been shown that there is no haplotype preference in H-2d/b mice (Thomsen and Marker, 1989). However, another study that looked at CB6F1/J chimeras infected with Listeria monocytogenes strain EJL243, which co-expresses the secreted LCMV NP protein, also showed that the H2-Kd–restricted response was dominant compared to the H2-Db-restricted response (Lenz et al., 2000). Evaluation of relative MHC class I expression in F1 hybrid mice suggested that H-2b and H-2d MHC class I molecules are similarly expressed on the cell surface in H-2b/d mice, although the mixing of alleles in other F1 hybrids may result in unequal expression (Tourdot and Gould, 2002), and epitope-specific effects may occur. Studies from Harty et. al. have shown that CD8+ T cell contraction may be preprogrammed and controlled by early inflammation (Badovinac et al., 2002 and 2004). Similarly, it has recently been proposed by Doherty’s group that clonal expansion of CD8+ T cells in response to certain influenza epitopes may ensue from a preset pattern (Thomas et al., 2006). Our studies, combined with the recent description of minor Db-restricted epitopes (Lukens et al., 2006), may provide a system to help define the principles underlying CD8+ T cell response patterns after RSV infection or immunization.
Interestingly, we found that although the DbM187–195 response was quantitatively subdominant in CB6F1/J hybrid mice, almost all M187–195 -specific cells produce IFN-γ after peptide stimulation. In contrast, only a fraction of KdM282–90-specific cells produce IFN-γ after stimulation. During primary infection, DbM187–195-specific cells also produce more IFN-γ per cell than KdM282–90-specific as measured by MFI. These differences are not maintained in the memory phase, where the M2 response is both quantitatively and functionally dominant. These data suggest that the more functionally active DbM187–195-specific cells may have the capacity for effector function during primary infection, but less often survive into the memory phase. Using the hybrid mouse model to further define ancillary responses and host factors involved in establishing epitope hierarchy, and the function of epitope-specific cells, will help establish the contribution of subdominant T cell epitopes in viral pathogenesis and potentially inform T cell-based vaccine design.
Materials and Methods
Mice
Pathogen-free BALB/c, C57Bl/6, and CB6F1/J female mice between the ages of 6 and 10 weeks were purchased from Charles River Laboratories (Raleigh, NC) or Jackson Laboratories (Bar Harbor, ME). CB6F1/J mice are the F1 generation of a C57Bl/6 x BALB/c mating, and therefore express both H-2b and H-2d class I MHC proteins. All animal work was approved by the NIH Animal Care and Use Committee. The mice were cared for in accordance with the Guide for the Care and Use of Laboratory Animals, as described previously (Graham et al., 1988). Experiments were performed with age-matched groups.
Cell Lines and Antibodies
HEp-2 cells were used to titer RSV from lungs. Cells were maintained in Eagle’s minimal essential media containing 10% fetal bovine serum (10% EMEM), and were supplemented with 2mM glutamine, 10 U of penicillin G per ml, and 10 μg of streptomycin sulfate per ml. Cells were determined to be free of mycoplasma contamination by analysis with the polymerase chain reaction (ATCC, Manassas, VA).
Virus Infection
The RSV challenge stock was derived from the A2 strain of RSV by sonication of HEp-2 monolayers as previously described (Graham et al., 1988). Mice were anesthetized intramuscularly with ketamine (40 μg/g body weight) and xylazine (6 μg/g body weight) prior to intranasal inoculation with 107 PFU live RSV in 100 μl of 10% EMEM. Mice were weighed daily after infection, and percent weight lost was used to assess the severity of illness.
Plaque Assays
Mice were sacrificed and lung tissue was removed and quick-frozen in 10% EMEM. Thawed tissues were kept chilled while individually ground. Dilutions of clarified supernatant were inoculated on 80% confluent HEp-2 cell monolayers in triplicate and overlaid with 0.75% methyl cellulose in 10% EMEM. After incubation for 4 days at 37° C, the monolayers were fixed with 10% buffered formalin and stained with hematoxylin and eosin. Plaques were counted and expressed as log10 PFU/gram of tissue. The limit of detection is 1.8 log10 PFU/gram of tissue in this assay.
Synthetic peptides
RSV M2 82–90 (SYIGSINNI) and RSV M187–195 (NAITNAKII) were derived from the M2 and M proteins, respectively, of the RSV A2 strain (Kulkarni et al., 1993; Rutigliano et al., 2005). M2 82–90 is H-2Kd-restricted and M187–195 is H-2Db-restricted. Negative control peptides were derived from the influenza virus A/Puerto Rico/8/34 nucleoprotein (NP) 147–155 (TYQRTRALV), which is H-2Kd-restricted. Influenza virus NP 366–374 (ASNENMETM) is derived from influenza virus A/Puerto Rico/8/34 and is H-2Db-restricted. Peptides were synthesized by Anaspec, Inc. (San Jose, CA), and confirmed to be >95% pure by analytical high-performance liquid chromatography at the NIAID peptide core facility (Bethesda, MD).
Tetramers
Class I MHC tetramers were synthesized by Beckman Coulter (San Diego, CA). Virus-specific T cells were enumerated with PE-labeled tetrameric complexes of H-2Kd plus the RSV M2 82–90 peptide or APC-labeled tetrameric complexes of H-2Db plus the RSV M187–195 peptide. For negative controls, PE-labeled tetrameric complexes of H-2Db plus the influenza NP 366–374 peptide, H-2Kd plus the influenza NP 147–155 peptide, or H-2Kb plus the ovalbumin 257–264 (SIINFEKL) epitope were used.
Tetramer and intracellular cytokine staining (ICS)
Mice were sacrificed and lungs harvested at various times between days 2–14 post-infection. Spleens were also harvested between 4–8 weeks post-infection to analyze memory responses. Lymphocytes were isolated manually by grinding organ tissue between the frosted ends of two sterile glass microscope slides in RPMI containing 10% FBS (10% RPMI). Lymphocytes were isolated by centrifugation on a cushion of Ficoll/Hypaque at room temperature, washed, and resuspended in 10% RPMI. Lymphocytes were incubated at 37°C for 5–6 hours with 1 μg of the appropriate peptide and 1 μg/ml of the co-stimulatory antibodies against CD28 and CD49d. After 1 hr of incubation, 1 μg/ml of monensin was added to retain newly synthesized proteins within the cell. As a positive control, cells were stimulated with 10 ng/ml PMA and 1 μM ionomycin. After the incubation, cells were fixed and permeabilized according to the manufacturer’s instructions (BD Pharmingen, San Diego, CA) and stained with fluorochrome-conjugated antibodies against CD3, CD8, IFN-γ, and TNF-α (BD Pharmingen) for 20 min at 4°C. In some experiments, cells were stained with a fluorochrome-conjugated antibody against CD49b to identify natural killer (NK) cells. For tetramer analysis, cells were stained with M2tet and M187tet in addition to antibodies against CD3 and CD8. Tetramers that recognized the influenza NP 366–374 or the SIINFEKL ovalbumin epitope were used as negative controls. After staining, cells were washed and analyzed by flow cytometry. Cells were analyzed on a FACSCaliber (Becton Dickinson, San Jose, CA) argon-ion laser at 15mW and 488nm. Data were analyzed by using FlowJo version 6.3 (Tree Star, San Carlos, CA).
Statistical analysis
Two-tailed Student’s t test was used for comparison of means, and values of p<0.05 were considered statistically significant.
Acknowledgments
Reagents for initial experiments were provided by the NIH Tetramer Core Facility, Emory University, Atlanta, GA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8+ T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2005;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Belz GT, Xie W, Altman JD, Doherty PC. A previously unrecognized H–2Db-restricted peptide prominent in the primary influenza A virus-specific CD8+ T cell response is much less apparent following secondary challenge. J Virol. 2000;74:3486–3493. doi: 10.1128/jvi.74.8.3486-3493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Xie W, Doherty PC. Diversity of epitope and cytokine profiles for primary and secondary influenza A virus-specific CD8+ T cell responses. J Immunol. 2001;166:4627–4633. doi: 10.4049/jimmunol.166.7.4627. [DOI] [PubMed] [Google Scholar]
- Cannon MJ, Openshaw PJ, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Anton LC, Bennink JR, Yewdell JW. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity. 2000;12:83–93. doi: 10.1016/s1074-7613(00)80161-2. [DOI] [PubMed] [Google Scholar]
- Collins PL, Chanock RM, Murphy BR. Respiratory syncytial virus. In: Knipe DN, Howley PW, Griffin D, editors. Fields Virology. Lippincott-Raven Publishers; Philadelphia, PA: 2001. pp. 1443–1486. [Google Scholar]
- Doherty PC, Allan JE. Differential effect of hybrid resistance on the localization of virus–immune effector T cells to spleen and brain. Immunogenetics. 1986a;24:409–415. doi: 10.1007/BF00377960. [DOI] [PubMed] [Google Scholar]
- Doherty PC, Allan JE. Hybrid resistance modulates the inflammatory process induced by lymphocytic choriomeningitis virus-immune T cells. Immunology. 1986b;57:515–519. [PMC free article] [PubMed] [Google Scholar]
- Doherty PC, Christensen JP. Accessing complexity: the dynamics of virus-specific T cells responses. Ann Rev Immunol. 2000;18:561–592. doi: 10.1146/annurev.immunol.18.1.561. [DOI] [PubMed] [Google Scholar]
- Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Ann Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- Johnstone C, de Leon P, Medina F, Melero JA, Garcia-Barreno B, Del Val M. Shifting immunodominance pattern of two cytotoxic T-lymphocyte epitopes in the F glycoprotein of the Long strain of respiratory syncytial virus. J Gen Virol. 2004;85:3229–3238. doi: 10.1099/vir.0.80219-0. [DOI] [PubMed] [Google Scholar]
- Kedl RM, Kappler JW, Marrack P. Epitope dominance, competition, and T cell affinity maturation. Curr Opin Immunol. 2003;15:120–127. doi: 10.1016/s0952-7915(02)00009-2. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Morse HC, III, Bennink JR, Yewdell JW, Murphy BR. Immunization of mice with vaccinia virus-M2 recombinant induces epitope- specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J Virol. 1993;67:4086–4092. doi: 10.1128/jvi.67.7.4086-4092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gruta NL, Turner SJ, Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J Immunol. 2004;172:5553–5560. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- Lenz LL, Butz EA, Bevan MJ. Requirements for bone marrow-derived antigen-presenting cells in priming cytotoxic T cell responses to intracellular pathogens. J Ex Med. 2000;192:1135–1142. doi: 10.1084/jem.192.8.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens MV, Claassen EAW, de Graaff PMA, van Dijk MEA, Hoogerhout P, Toebes M, Schumacher TN, van der Most RG, Kimpen JLL, van Bleek GM. Characterization of the CD8+ T cell responses directed against respiratory syncytial virus during primary and secondary infection in C57BL/6 mice. Virology. 2006;352:157–168. doi: 10.1016/j.virol.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Moskophidis D, Lechner F, Pircher H, Zinkernagel R. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Probst HC, Tschannen K, Gallimore A, Martinic M, Basler M, Dumrese T, Jones E, van den Broek MF. Immunodominance of an antiviral cytotoxic T cell response is shaped by the kinetics of viral protein expression. J Immunol. 2003;171:5415–5422. doi: 10.4049/jimmunol.171.10.5415. [DOI] [PubMed] [Google Scholar]
- Rutigliano JA, Rock MT, Johnson AK, Crowe JE, Jr, Graham BS. Identification of an H-2Db-restricted CD8+ cytotoxic T lymphocyte epitope in the matrix protein of respiratory syncytial virus. Virology. 2005;337:335–343. doi: 10.1016/j.virol.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Selin LK, Lin MY, Kraemer KA, Pardoll DM, Schneck JP, Varga SM, Santolucito PA, Pinto AK, Welsh RM. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity. 1999;11:733–742. doi: 10.1016/s1074-7613(00)80147-8. [DOI] [PubMed] [Google Scholar]
- Thomas PG, Brown SA, Yue W, So J, Webby RJ, Doherty PC. An unexpected antibody response to an engineered influenza virus modifies CD8+ T cell responses. PNAS. 2006;103:2764–2769. doi: 10.1073/pnas.0511185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen AR, Marker O. Class I gene regulation of haplotype preference may influence antiviral immunity in vivo. Cell Immunol. 1989;122:365–376. doi: 10.1016/0008-8749(89)90084-1. [DOI] [PubMed] [Google Scholar]
- Tourdot S, Gould KG. Competition between MHC class I alleles for cell surface expression alters CTL responses to influenza A virus. J Immunol. 2002;169:5615–5621. doi: 10.4049/jimmunol.169.10.5615. [DOI] [PubMed] [Google Scholar]
- Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu Rev Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Ann Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- Zajac A, Blattman J, Murali-Krishna K, Sourdive D, Suresh M, Altman J, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Ex Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]


