Abstract
The boronic acid functional group plays very important roles in sugar recognition, catalysis, organic synthesis, and supramolecular assembly. Therefore, understanding the unique properties of this functional group is very important. 8-Quinolineboronic acid (8-QBA) is found to be capable of self-assembling in solid state through a unique intermolecular B-N bond mechanism reinforced by intermolecular boronic anhydride formation, π-π stacking, and hydrogen bond formation. NMR NOE and diffusion studies indicate that intermolecular B-N interaction also exists in solution with 8-QBA. In contrast, a positional isomer of 8-QBA, 5-quinolineboronic acid (5-QBA) showed very different behaviors in crystal packing and in solution and therefore different supramolecular network. Understanding the structural features of this unique 8-QBA assembly could be very helpful for the future design of new sugar sensors, molecular catalysts, and supramolecular assemblies.
Keywords: Quino lineboronic acid, Self-assembly, Crystal structure, NMR
1. Introduction
Boron compounds are very useful in a wide variety of ways in organic, bioorganic, and medicinal chemistry. For example, because of its open shell, boron-based compounds have been widely used as Lewis acids for chelation and catalysis applications.1-3 Boronic acids are known to bind the diol moiety and thus have been widely used in the field of sensing and sugar recognition.4-14 In addition, there is a strong interest in the synthesis of new boronic acids as potential boron neutron capture therapy (BNCT) agents,15-17 antiviral agents,18,19 and enzyme inhibitors.20-24,19,25-29 Recently, boronic acids have been used as promising building blocks in crystal engineering, in which various types of novel supramolecular assemblies have been generated.30-35 For example, Strongin and co-workers found that a tetraarylboronic acid resorcinarene could form an infinite two-dimensional array through extensive hydrogen bond interactions.30 Wuest and Galoppini recently reported a new molecular tectonics with 3-D supramolecular channel networks using the –B(OH)2 moiety of tetraboronic acid.31 The Hopfl and Pedireddi labs generated different types of hydrogen-bonding supramolecular assemblies systems utilizing phenylboronic acid and its derivatives.32,33 Lavigne and colleagues reported self-repairing polymers36,37 based on boronic acid-diol interactions.8,38,27 Jäkle and co-workers also developed boron-containing polyolefins as Lewis acid catalysts and precursor to luminescent materials, sensors and other materials.39-41
We envision that the boronic acid functional group can also be used to build supramolecular architectures by taking advantage of its unique and strong Lewis acidity. Therefore, a boronic acid compound with an appropriately positioned Lewis base should allow for tight self-assembly of the boronic acid compound.35,42 Herein we report one such example in which 8-quinolineboronic acid (8-QBA) is shown to self-assemble into a dimer in solid state as determined by X-ray crystallography. NMR studies also indicate the same tendency to self-assembly in solution.
2. Results and Discussion
Again, we are interested in taking advantage of the unique Lewis acidity of the boron due to its open shell for constructing unique boronic acid-based self-assembly systems. In doing so, one can envision an approach that uses B-N bond formation as a way to achieve self-recognition. B-N bond formation involving a boronic acid in an intramolecular fashion has been reported, especially in crystalline states and in aprotic solvents.43-53,35 For example, Wulff and colleagues reported reversible formation of a B-N bond when an amine is in a 1,5-relationship with a boronic acid.43 A boronic acid protease inhibitor was found to have intramolecular B-N bond formation when the boron atom and an amine group is in a 1-6 relationship.46 Livant reported B-N bond formation involving trapped boric acid which is positioned closely to an amino group.44 In all these examples, strong intramolecular B-N interactions only occur under favorable entropic conditions. Therefore, for B-N mediated self-assembly to work there needs to have a secondary reinforcement for the interaction to be strong enough. Another relevant system worth mentioning is an anthracene-based fluorescent boronic acid reported by the Shinkai lab, which changes fluorescent properties upon sugar binding.54,55 Initially, it was thought that strong B-N bond formation was the reason for the increased fluorescent intensity upon sugar binding. Our lab has recently shown that solvolysis is responsible for the observed fluorescent intensity changes.52,56 Such a mechanism is further substantiated by crystal structural studies of the Anslyn lab.42
Upon a close examination of the structure of 8-QBA (Figure 1), it appears that the relative orientation and positions of the quinoline nitrogen and the boronic acid moiety are perfect for bidentate self-assembly. Hence, we were very interested in studying the supramolecular properties of 8-QBA to see whether self-association happens or not. As a comparison, we were also interested in studying 5-QBA.
Figure 1.

The structures of 8-QBA and 5-QBA
The crystals of 8-QBA and 5-QBA were obtained from methanol solution and their structures were determined by X-ray diffraction. Indeed, 8-QBA self-assembles through the formation of two complementary B-N bonds leading to the formation of a dimer (Figure 2). The B-N interactions are reinforced by the formation a boronic anhydride group resulting from the loss of one water molecule. 57 To the best of our knowledge, this is the first example where a boronic acid supramolecular structure is based on this kind of double B-N interactions. The shape of the dimer resembles that of a chair with an angle of 104.7 °, which is consistent with the sp3 hybridization state of the boron atoms at the hinge position. This dimeric structure is in direct contrast to that of 5-QBA (Figure 3), which exists in a monomeric form.58 It seems that the ability for 8-QBA to form two B-N bonds is the main reason that differentiates these two and allows for dimer formation in 8-QBA. The further ordering of the dimeric units of 8-QBA is dependent on π-stacking of the quinoline rings and hydrogen bond formation between the boronic anhydride units (Figures 4 and 5). There are two hydrogen bonds between two neighboring boronic anhydride units, which are reinforced by the presence of four additional hydrogen bonds involving two water molecules (Figure 4). The hydrogen bond distances range from 1.67 to 1.91 Å, indicating strong interactions. There also exist strong π-π stacking in the supramolecular structure. In each dimeric unit (Figure 5), one quinoline ring shows π-π overlap with two adjacent aromatic rings and the other shows stacking with only one. In a face to face stacking, the distance between two aromatic rings is 3.6 Å (Figure 4), which approaches the van der Waals minimally allowable radius, again indicating strong interactions.
Figure 2.
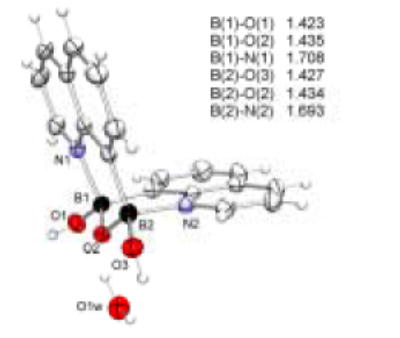
Crystal structure of 8-QBA dimer with thermal ellipsoids shown at 50% probability level
Figure 3.
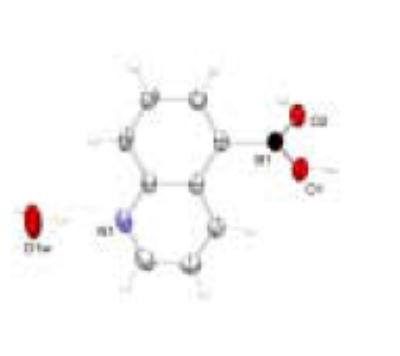
Crystal structure of 5-QBA with thermal ellipsoids shown at 50% probability level
Figure 4.
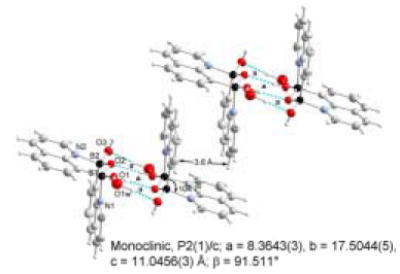
Perspective view of the molecular recognition pattern of 8-QBA: a hydrogen bondeddimer of H2(B2O3)(C9NH6)˙H2O.
Figure 5.
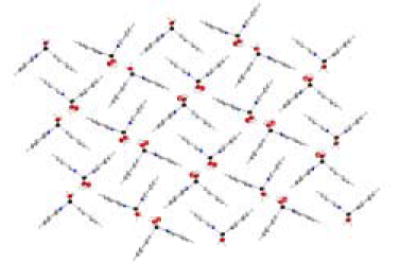
3-D supramolecular network of 8-QBA through π‐π interactions and hydrogen bonds
As a comparison, the crystal structure of 5-QBA (Figure 3) has also been examined. The pattern of recognition and self-assembly between two 5-QBA units (Figure 6) is understandably different from that of 8-QBA. First, B-N bond formation does not play any role in the intermolecular interactions in 5-QBA. Instead, the assembly is controlled by head to head hydrogen bond formation between boronic acid units. There is one water molecule bridging between the quinoline nitrogen and a boronic acid hydroxyl group on a neighboring molecule. As consequence, each water molecule is engaged in three hydrogen bonds, one through its oxygen and two through its two hydrogen atoms. Similarly, each hydroxyl group of the boronic acid moiety is engaged in two hydrogen bond interactions, one through its hydrogen and one through its oxygen. This intricate hydrogen bond network seems to be the dominant force in crystal packing. Second, the boron atom is in the sp2 hybridized form, giving it a planary shape. However, the boron atom is twisted out of the plane of the aromatic system allowing hydrogen bond formation in a “vertical” fashion and π–π-stacking between the aromatic systems.
Figure 6.
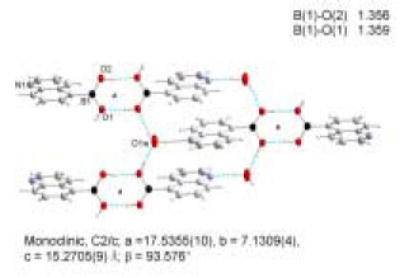
A cyclic hydrogen bond network is the dominant force in crystal packing for 5-QBA.
| (Equation 3) |
With B-N bond formation in 8-QBA, the boron atom exists in the tetrahedral form. The tetrahedral character THCDA[%] of the two boron atoms in 8-QBA structure have been calculated from a formula introduced by Höpfl, that includes all six bond angles around the boron atom(Equation 3). For the B1 atom, the d (N1→B1) bond length is 1.708 (3) Å and the tetrahedral character THCDA[%] is 77.53. For B2 atom, the d(N2→B2) bond length is 1.693 (3) Å and the tetrahedral character THCDA[%] is 80.58. The results agree with those listed by Höpfl and Norrild for related compounds.32,49,45 5-QBA on the other hand has no N-B interaction and exists in the trigonal form.
Although the crystal structure clearly shows intermolecular B-N bond formation in 8-QBA, it was not clear whether in solution such interactions would be strong enough to promote dimer formation. In order to probe this issue, we have used NMR to examine the NOE effect. Therefore, 2-D NOESY experiments were conducted (Figure 7 and 8). If 8-QBA exists in a dimer form in solution, we would expect to see NOE effect between HA and HD (Figure 7). Indeed, the NOESY spectrum at 115 mM in deuterated methanol shows an intense cross peak (HA/HD) corresponding to the interaction between HA proton of one 8-QBA and HD proton of the other 8-QBA unit (Figure 8). This could only arise from dimer formation since the intermolecular distance between HA and HD in a non-associated form would be too long to allow for the observed NOE.
Figure 7.
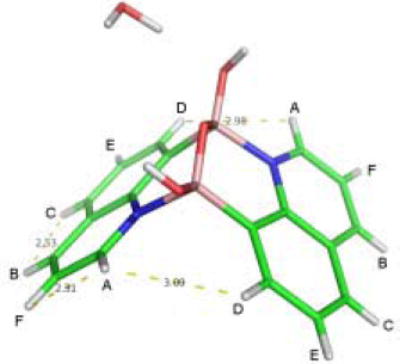
The structure of 8-QBA dimer
Figure 8.
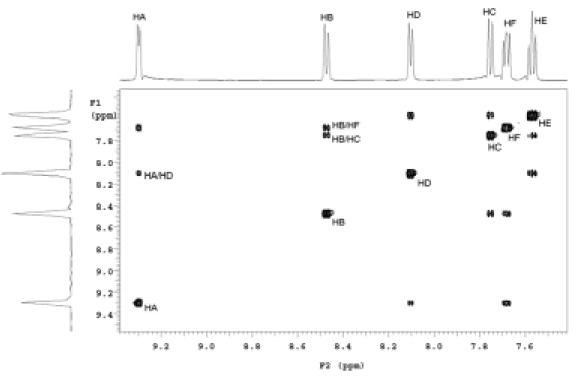
2D-NOESY spectrum of 8-QBA measured in CD3OD
Furthermore, there was no NOE observed between H E and H F , which has the same relationship as H A and H D if there was no dimer formation. Using the X-ray HA-HF distance of 2.30 Å as reference, the calculated H A -H D distance determined by NOESY in solution is 2.71 Å, which is close to that obtained from X-ray crystallographic studies (2.98 Å). The results suggest that 8-QBA exist as a dimer in methanol solution under the conditions of the experiments. In contrast, 5-QBA showed no such intermolecular NOE effect.
In addition to the NOE experiments, we were also interested in studying the molecular radius of 8-QBA at different concentrations. We envisioned that if self-association happens, one would expect to see increased apparent molecular radius. The pulsed field gradient (PFG) NMR techniques have long been used for the direct measurement of diffusion coefficients,59 which can be converted to molecular radii. 60 Therefore, 8-QBA was dissolved in methanol-d6 at 1.0, 25, 50, 115 mM concentrations with dioxane spiked in as an internal reference (about 100 mM).61 The diffusion constants were obtained via fitting the integrated area of the resonance of each arrayed spectrum into the Stejskal-Tanner equation.62 The same experiments were conducted with 5-QBA as a comparison.
Table 1 summarizes the results of the molecular diffusion experiments. At 115 mM, the molecular radius of 8-QBA (5.43 Å) is 20% greater than that of 5-QBA (4.50 Å) at the same concentration. The molecular radius of 8-QBA also increases by 20% when its concentration changes from 1 mM to 115 mM. It needs to be noted that since 8-QBA is not a spherical molecule, one would not expect the dimer to have a molecular radius twice that of the monomer. Furthermore, self association in solution is not an “all or none” situation. It is a concentration dependent event with the fraction of those in the dimer form related to the association constant and 8-QBA concentration. Thus the molecular radii determined are the average results of the monomer and dimer forms and are depending on the fraction of 8-QBA in the dimer form and the true molecular radius of the dimer. The combined results of the NOE and diffusion studies indicate that in solution 8-QBA exists in the dimer form in sufficient quantity at 115 mM to give rise to a strong NOE effect between HA and HD and to show an increased apparent molecular size. All indications are that self-association of 8-QBA does occur in solution (methanol) as well.
Table 1.
The results of molecular diffusion experiments for 8-QBA and 5-QBA.
| Concentration | 5-QBA Studies | 8-QBA Studies | ||||||
| 5-QBA | Internal dioxane reference | 8-QBA | Internal dioxane reference | |||||
| Diffusion Constant(×10-6 cm2∕s) | Molecular Radius (Å) | Diffusion Constant(×10-6 cm2∕s) | Molecular Radius (Å) | Diffusion Constant(×10-6 cm2∕s) | Molecular Radius (Å) | Diffusion Constant(×10-6 cm2∕s) | Molecular Radius (Å) | |
| 1 mM | 10.6 | 3.96 | 18.5 | 2.27 | 9.32 | 4.50 | 19.4 | 2.16 |
| 25 mM | 10.6 ±0.2 | 3.95±0.08 | 16.7 ±0.06 | 2.53±0.01 | 8.08 ±0.13 | 5.20±0.08 | 18.9 ±2.6 | 2.25±0.29 |
| 50 mM | 11.3 ±0.2 | 3.72±0.07 | 16.8 ±0.15 | 2.49±0.02 | 7.87 ±0.04 | 5.33±0.03 | 17.0 ±0.2 | 2.46±0.02 |
| 115 mM | 9.32 ±0.06 | 4.50±0.03 | 15.9 ±0.6 | 2.65±0.10 | 7.74 ±0.34 | 5.43±0.24 | 16.6 ±0.1 | 2.53±0.02 |
3. Conclusion
In conclusion, we have demonstrated that 8-quinoline boronic acid (8-QBA) forms a dimer through the formation of two B-N bonds reinforced by intermolecular anhydride formation, hydrogen bonds, and π–π-stacking in crystal form. NMR studies indicate that the same dimer form exists in solution as well. Such results may help the future design of boronic acid-based new molecules, supramolecular assemblies, and materials for organic, bioorganic, medicinal and crystal engineering applications.
4. Experimental
Chemicals and solvents were obtained from Frontier Scientific, Aldrich, and Acros and used without purification. Crystals were grown from a mixture of methanol and methylene chloride.
4.1 X-ray crystallographic studies
Suitable crystals each of 5-QBA and 8-QBA were coated with Paratone-N oil, suspended in small fiber loops and placed in a cooled nitrogen gas stream at 173 K on a Bruker D8 SMART 1000 CCD sealed tube diffractometer using CuKα radiation. Data were measured using a series of combinations of phi and omega scans with 10 s frame exposures and 0.3° frame widths. Data collection, indexing and initial cell refinements were all carried out using SMART 63 software. Frame integration and final cell refinements were done using SAINT software.64 The SADABS65 program was used to carry out absorption corrections.
The structures were solved using Direct methods and difference Fourier techniques (SHELXTL, V6.12).66 All the hydrogen atoms were located in a difference Fourier map and were included in the final cycles of least squares with isotropic Uij‘s or as riding atoms; all non-hydrogen atoms were refined anisotropically. Scattering factors and anomalous dispersion corrections are taken from the International Tables for X-ray Crystallography.67 Structure solution, refinement, graphics and generation of publication materials were performed by using SHELXTL, V6.12 software. Additional details of data collection and structure refinement are given in Table 1 of the Supplemental Materials section.
4.2 NMR studies
The NOE studies were conducted in deuterated methanol at 115 mM. Pulsed field gradient (PFG) NMR techniques were used for the direct measurement of diffusion coefficients.59 8-QBA and 5-quinolineboronic acid (5-QBA) were dissolved in methanol-d4 at 1.0, 25, 50, 115 mM respectively. The spectra were collected using a modified PG-SLED pulse sequence with 16 or 8 K complex data points for each FID with dioxane spiked in as an internal reference (about 100 mM) at 25 °C.61 The diffusion constants were obtained via fitting the integrated area of the resonance of each arrayed spectrum into the Stejskal-Tanner equation ( Equation 1 ).62
| (Equation 1) |
Where γ is the gyromagnetic ratio of proton (26752), δ is the PFG duration time (2 ms), and Δ is the time between PFG pulses (200 ms). The gradient strength (G) was arrayed from 0.195 G/cm to 28.32 G/cm using 25 steps. A is the integrated area of desired resonances at each array spectrum after subtraction of baselines. A0 is the integrated area of the desired resonances when the PFG strength is minimal.68 The data are treated by plotting the log of the signal intensity against (γδG)2 (Δ - δ/3), the slope of which gives the diffusion coefficient.
The diffusion coefficient (D) is related to the size of the diffusing object according to the Einstein-Stokes equation ( Equation 2 ). 60
| (Equation 2) |
where K is Boltzmann constant (1.38×10−23 J/K), η is viscosity of methanol-d4 at 25 °C (5.2×10−4 Pa∙s), and T is absolute temperature (298 K).
Supplementary Material
Some crystallographic lattice parameters are depicted in supplementary. CCDC 635580 and 635581 also contain the supplementary crystallographic data for this paper, which can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Acknowledgments
Financial support from the National Institutes of Health (CA123329, CA88343, CA113917, NO1-CO-27184), the Georgia Cancer Coalition through a Distinguished Cancer Scientist Award, and the Georgia Research Alliance through an Eminent Scholar endowment and Eminent Scholar Challenge grants is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roy CD. Aust J Chem. 2006;59:657–659. [Google Scholar]
- 2.Cho BT. In: In Boronic Acids. Hall DG, editor. Wiley-VCH; Weinheim : 2005. pp. 411–439. [Google Scholar]
- 3.Ishihara K. In: In Boronic Acids. Hall DG, editor. Wiley-VCH; Weinheim : 2005. pp. 101–121. [Google Scholar]
- 4.Wang W, Gao X, Wang B. Curr Org Chem. 2002;6:1285–1317. [Google Scholar]
- 5.Cao HS, Heagy MD. J Fluoresc. 2004;14:569–584. doi: 10.1023/b:jofl.0000039344.34642.4c. [DOI] [PubMed] [Google Scholar]
- 6.Hall DG, editor. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine. Wiley-VCH; 2005. [Google Scholar]
- 7.Lorand JP, Edwards JO. J Org Chem. 1959;24:769–774. [Google Scholar]
- 8.Springsteen G, Wang B. Tetrahedron. 2002;58:5291–5300. [Google Scholar]
- 9.Wiskur SL, Lavigne JJ, Metzger A, Tobey SL, Lynch V, Anslyn EV. Chem-A Eur J. 2004;10:3792–3804. doi: 10.1002/chem.200305737. [DOI] [PubMed] [Google Scholar]
- 10.James TD, Linnane P, Shinkai S. Chem Commun. 1996:281–288. [Google Scholar]
- 11.Ward CJ, Patel P, James TD. Org Lett. 2002;4:477–479. doi: 10.1021/ol016923w. [DOI] [PubMed] [Google Scholar]
- 12.Alexeev VL, Sharma AC, Goponenko AV, Das S, Lednev IK, Wilcox CS, Finegold DN, Asher SA. Anal Chem. 2004;75:2316–2323. doi: 10.1021/ac030021m. [DOI] [PubMed] [Google Scholar]
- 13.Shinkai S, Takeuchi M. Trend Anal Chem. 1996;15:188–193. [Google Scholar]
- 14.Jiang S, Rusin O, Escobedo JO, Kim KK, Yang Y, Fakode S, Warner IM, Strongin RM. J Am Chem Soc. 2006;128:12221–12228. doi: 10.1021/ja063651p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soloway AH, Tjarks W, Barnum BA, Rong FG, Barth RF, Codogni IM, Wilson JG. Chem Rev. 1998;98:1515–1562. doi: 10.1021/cr980493e. [DOI] [PubMed] [Google Scholar]
- 16.Hawthorne MF, editor. Angew Chem Int. Vol. 32. 1993. pp. 950–984. [Google Scholar]
- 17.Kabalka GW. Expert Opin Ther Pat. 1998;8:545–551. [Google Scholar]
- 18.Chen X, Bastow K, Goz B, Kucera L, Morris-Natschke SL, Ishaq KS. Antivir Chem Chemother. 1996;7:108–114. [Google Scholar]
- 19.Bacha U, Barrila J, Velazquez-Campoy A, Leavitt SA, Freire E. Biochemistry. 2004;43:4906–4912. doi: 10.1021/bi0361766. [DOI] [PubMed] [Google Scholar]
- 20.Suenaga H, Yamamoto H, Shinkai S. Pure Appl Chem. 1996;68:2179–2186. [Google Scholar]
- 21.Adams J, Behnke M, Chen SW, Cruickshank AA, Dick LR, Grenier L, Klunder JM, Ma YT, Plamondon L, Stein RL. Bioorg Med Chem Lett. 1998;8:333–338. doi: 10.1016/s0960-894x(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 22.Lebarbier C, Carreaux F, Carboni B, Boucher JL. Bioorg Med Chem Lett. 1998;8:2573–2576. doi: 10.1016/s0960-894x(98)00455-7. [DOI] [PubMed] [Google Scholar]
- 23.Teicher BA, Ara G, Herbst R, Palombella VJ, Adams J. Clin Cancer Res. 1999;5:2638–2645. [PubMed] [Google Scholar]
- 24.Adams J, Kauffman M. Can Invest. 2004;22:304–311. doi: 10.1081/cnv-120030218. [DOI] [PubMed] [Google Scholar]
- 25.Johnson LL, Houston TA. Tetrahedron Lett. 2002;43:8905–8908. [Google Scholar]
- 26.Myung J, Kim KB, Crews CM. Med Res Rev. 2001;21:245–273. doi: 10.1002/med.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan J, Fang H, Wang B. Med Res Rev. 2005;25:490–520. doi: 10.1002/med.20038. [DOI] [PubMed] [Google Scholar]
- 28.Yang W, Gao S, Wang B. In: In Organoboronic Acids. Hall D, editor. John Wiley and Sons; New York: 2005. pp. 481–512. [Google Scholar]
- 29.Yang W, Gao X, Wang B. Med Res Rev. 2003;23:346–368. doi: 10.1002/med.10043. [DOI] [PubMed] [Google Scholar]
- 30.Lewis PT, Davis CJ, Fronczek FR, Strongin RM. Org Lett. 2001;3:2443–2445. doi: 10.1021/ol0160194. [DOI] [PubMed] [Google Scholar]
- 31.Fournier JH, Maris T, Wuest JD, Guo WZ, Galoppini E. J Am Chem Soc. 2003;125:1002–1006. doi: 10.1021/ja0276772. [DOI] [PubMed] [Google Scholar]
- 32.Höpfl H. J Organomet Chem. 1999;581:129–149. [Google Scholar]
- 33.Pedireddi VR, Lekshmi NS. Terrahedron Lett. 2004;45:1903–1906. [Google Scholar]
- 34.Rodriguez-Cuamatzi P, Arillo-Flores OI, Bernal-Uruchurtu MI, Höpfl H. Cryst Growth Des. 2005;5:167–175. [Google Scholar]
- 35.Barba V, Hápfl H, Farfán N, Santillan R, Beltran HI, Zamudio-Rivera LS. Chem Commun. 2004:2834 – 2835. doi: 10.1039/b410148k. [DOI] [PubMed] [Google Scholar]
- 36.Niu WJ, Rambo B, Smith MD, Lavigne JJ. Chem Commun. 2005;41:5166–5168. doi: 10.1039/b507475d. [DOI] [PubMed] [Google Scholar]
- 37.Niu WJ, O’Sullivan C, Rambo BM, Smith MD, Lavigne JJ. Chem Commun. 2005;34:4342–4344. doi: 10.1039/b504634c. [DOI] [PubMed] [Google Scholar]
- 38.Yan J, Springsteen G, Deeter S, Wang B. Tetrahedron. 2004;60:11205–11209. [Google Scholar]
- 39.Qin Y, Cheng G, Sundararaman A, Jäkle F. J Am Chem Soc. 2002;124:12672–3. doi: 10.1021/ja020773i. [DOI] [PubMed] [Google Scholar]
- 40.Jäkle F. J Inorg Organomet Polym Mater. 2005;15:293–307. [Google Scholar]
- 41.Qin Y, Pagba C, Piotrowiak P, Jäkle F. J Am Chem Soc. 2004;126:7015–8. doi: 10.1021/ja039133l. [DOI] [PubMed] [Google Scholar]
- 42.Zhu L, Shabbir SH, Gray M, Lynch VM, Sorey S, Anslyn EV. J Am Chem Soc. 2006;128:1222–1223. doi: 10.1021/ja055817c. [DOI] [PubMed] [Google Scholar]
- 43.Burgemeister T, Grobe-Einsler R, Grotstollen R, Mannschreck A, Wulff G. Chem Ber. 1981;114:3403–3411. [Google Scholar]
- 44.Livant PD, Northcott DJD, Shen YP, Webb TR. J Org Chem. 2004;69:6564–6571. doi: 10.1021/jo040199o. [DOI] [PubMed] [Google Scholar]
- 45.Norrild JC, Sotofte I. J Chem Soc Perkin Trans. 2001;2:727–732. [Google Scholar]
- 46.Snow RJ, Bachovchin WW, Barton RW, Campbell SJ, Coutts SJ, Freeman DM, Gutheil WG, Kelly TA, Kennedy CA, Krolikowski DA, Leonard SF, Pargellis CA, Tong I, Adams J. J Am Chem Soc. 1994;116:10860–10869. [Google Scholar]
- 47.Sudmeier JL, Gunther UL, Gutheil WG, Coutts SJ, Snow RJ, Barton RW, Bachovchin WW. Biochemistry. 1994;33:12427–12438. doi: 10.1021/bi00207a009. [DOI] [PubMed] [Google Scholar]
- 48.Toyota S, Futawaka T, Asakura M, Ikeda H, Oki M. Organometallics. 1998;17:4155–4163. [Google Scholar]
- 49.Norrild JC. J Chem Soc Perkin Trans. 2001;2:719–726. [Google Scholar]
- 50.Fjeldberg T, Gundersen G, Jonvik T, Seip HM, Saeboe S. Acta Chem Scand Series A: Phys Inorg Chem. 1980;34:547–565. [Google Scholar]
- 51.Ostby KA, Gundersen G, Haaland A, Noth H. Dalton Transaction. 2005;13:2284–2291. doi: 10.1039/b500659g. [DOI] [PubMed] [Google Scholar]
- 52.Franzen S, Ni W, Wang B. J Phys Chem B. 2003;107:12942–12948. [Google Scholar]
- 53.Ni W, Kaur G, Springsteen G, Wang B, Franzen S. Bioorg Chem. 2004;32:571–81. doi: 10.1016/j.bioorg.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Sandanayake KRAS, Shinkai S. J Chem Soc Chem Commun. 1994:1083–1084. [Google Scholar]
- 55.James TD, Sandanayake KRAS, Shinkai S. Chem Commun. 1994:477–478. [Google Scholar]
- 56.Ni W, Kaur G, Springsteen G, Wang B, Franzen S. Bioorg Chem. 2004;32:571–81. doi: 10.1016/j.bioorg.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Crystal data for 8-QBA: , (C18H16B2N2O4), M = 345.95, Monoclinic, P2(1)/c; 0.33 × 0.22 × 0.17 mm, a = 8.3643(3), b = 17.5044(5), c = 11.0456(3) Å,α = 90, β= 91.511(2), γ = 90°, V= 1616.65(9) Å3, Z= 4, D = 1.421 g/cm3, μ = 0.810 mm-1,8341 total reflections, R1 = 0.0508 and wR2 = 0.1448. CDDC 635581.
- 58.Crystal data for 5-QBA: , (C9H10BNO3), M = 190.99, Monoclinic, C2/c; 0.35 × 0.33 × 0.31mm, a =17.5355(10), b = 7.1309(4), c = 15.2705(9) Å, α=90, β= 93.576(3), γ= 90°, V=1905.76(19) Å3, Z= 8, D = 1.331 g/cm3, μ = 0.815 mm-1, 44121905.76(19) total reflections, R1 = 0.0467 and wR2 = 0.1259. CDDC 635580.
- 59.Altieri AS, Hinton DP, Byrd RA. J Am Chem Soc. 1995;117:7566–7567. [Google Scholar]
- 60.Kholodenko AL, Douglas JF. Phys Rev E. 1995;51:1081–1090. doi: 10.1103/physreve.51.1081. [DOI] [PubMed] [Google Scholar]
- 61.Martichonok K, Jones JB. Bioorg Med Chem. 1997;5:679–684. doi: 10.1016/s0968-0896(97)00008-4. [DOI] [PubMed] [Google Scholar]
- 62.Stejskal EO, Tanner JE. J Chem Phys. 1965;42:288–292. [Google Scholar]
- 63.SMART Version 5.625. Bruker AXS, Inc., Analytical X-ray Systems: 5465 East Cheryl Parkway, Madison WI 53711-5373, 2002
- 64.SAINT Version 6.36A. Bruker AXS, Inc., Analytical X-ray Systems 5465 East Cheryl Parkway, Madison WI 53711-5373, 2002
- 65.Sheldrick G, SADABS V2.10. University of Göttingen, 2003
- 66.SHELXTL V6.12. Bruker AXS, Inc., Analytical X-ray Systems 5465 East Cheryl Parkway, Madison WI 53711-5373, 2002
- 67.Wilson AJC. Ed International Tables for X-ray Crystallography. C Academic Publishers; Dordrecht: 1992. [Google Scholar]
- 68.Lee HW, Yang W, Ye Y, Liu ZR, Glushka J, Yang JJ. Biochim Biophys Acta. 2002;1598:80–87. doi: 10.1016/s0167-4838(02)00338-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Some crystallographic lattice parameters are depicted in supplementary. CCDC 635580 and 635581 also contain the supplementary crystallographic data for this paper, which can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.


