Abstract
Fifteen new betulinic acid analogues were designed, synthesized, and tested for anti-inflammatory activity. Many of these analogues effectively suppress nitric oxide (NO) production in RAW cells stimulated with interferon-γ. Analogue 10 is highly and orally active in vivo for induction of the anti-inflammatory and cytoprotective enzyme, heme oxygenase-1.
Keywords: triterpene, betulinic acid, inhibitors of nitric oxide production, RAW 264.7 cells, inducer of heme oxygenase-1
Betulinic acid (BA, 1) is a pentacyclic lupane–type triterpene isolated from various plants.1,2 Betulinic acid selectively inhibits the growth of human melanoma cells in vitro and in vivo,3 suppresses proliferation of neuroblastoma4 and ovarian carcinoma cells,5 and enhances differentiation and apoptosis of primary leukemia cells.6 Despite a lack of toxicity in animal studies even at high concentrations,3 the relatively low potency of betulinic acid itself lessens its clinical utility as an anti-cancer drug.
Our ongoing efforts to improve the anti-inflammatory and anti-proliferative activity of oleanolic acid (2), a naturally occurring oleanane–type triterpene, led us to discover 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) and related compounds (e.g., CDDO-Me, CDDO-Im, and TP-222, see Figure 1).7–9 CDDO and CDDO-Me are currently being evaluated in phase I clinical trials for the treatment of leukemia and solid tumors. In these investigations, we discovered two important structure–activity relationships (SARs). The combination of enone functionalities with cyano and carboxyl groups in ring A and an enone functionality in ring C is an essential structure feature for high potency in various bioassays related to inflammation and cancer,8 and modifications of the C-17 carboxyl group affect both potency and pharmacokinetics.9 Thus, we envisioned incorporating the same structures into betulinic acid for increasing its anti-inflammatory activity.
Figure 1.
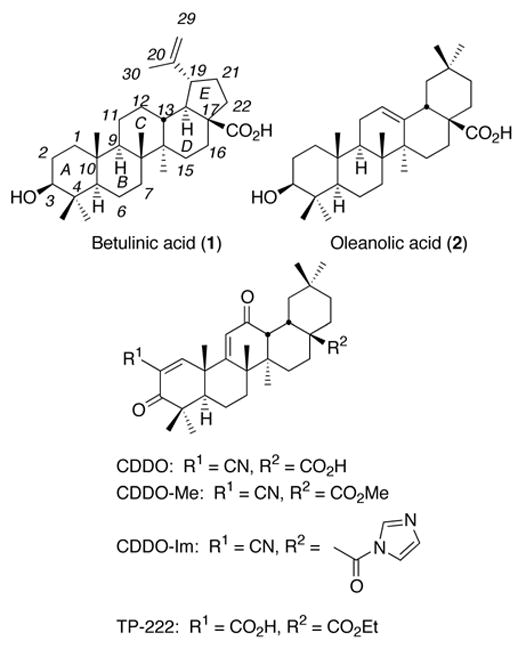
Structures of betulinic acid, oleanolic acid, CDDO, and CDDO analogues.
We initially designed and synthesized methyl ester 310 and fifteen new betulinic acid analogues 4–1811 without the enone functionality in ring C (Table 1) because betulinic acid differs from oleanolic acid and is devoid of functionality in ring C. We evaluated the potency of the new analogues for inhibition against nitric oxide (NO) production in RAW 264.7 cells (in vitro) and induction of the anti-inflammatory, cytoprotective enzyme, heme oxygenase-1 (in vivo).12 We report here that several new semi-synthetic betulinic acid analogues display highly potent anti-inflammatory activity in vitro, and we show that 10 is also highly and orally active in vivo.
Table 1.
Inhibition of NO production in RAW cells stimulated with interferon-γ by methyl ester 3 and new betulinic acid analogues 4–18.
| Compds | R1 | R2 | R3 | Yield (%) | Inhibition of NO IC50, μMc |
|---|---|---|---|---|---|
| 3 | CN | CO2Me | H | Ref. 10 | 0.03 |
| 4 | CN | CO2H | H | a | 0.2 |
| 5 | CN | CO2Et | H | a | 0.02 |
| 6 | CN | CONH2 | H | 74b | 0.03 |
| 7 | CN | CONHMe | H | 70b | 0.05 |
| 8 | CN | CONHEt | H | 79b | 0.07 |
| 9 | CN | CONMe2 | H | 77b | 0.03 |
| 10 | CN |
|
H | 91b | 0.03 |
| 11 | CN |
|
H | 62b | 0.05 |
| 12 | CN |
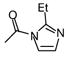
|
H | 60b | 0.03 |
| 13 | CO2Me | CO2Me | H | a | 0.6 |
| 14 | CO2Me | CO2Me | OH | a | 1 |
| 15 | CO2Me | CO2Me | F | a | 0.3 |
| 16 | CO2H | CO2Me | H | a | 0.3 |
| 17 | CO2H | CO2Me | OH | a | 0.9 |
| 18 | CO2H | CO2Me | F | a | 0.3 |
| BA (1) | inactive | ||||
| CDDO | 0.02 |
Yield is shown in the text.
Yield from 19.
RAW 264.7 cells were treated with various concentrations of compounds and interferon-γ (10 ng/mL) for 24 h. Supernatants were analyzed for NO by the Griess reaction.
Compounds 4–12 with a cyano enone functionality in ring A were synthesized by the sequence shown in Scheme 1. Acid 4 was obtained in 61% yield by cleavage of methoxycarbonyl group of 3 with AlBr3 in Me2S.13 Ethyl ester 5 was prepared in 78% yield from 4 using EtI and DBU in toluene.14 Reaction of 4 with oxalyl chloride gave acyl chloride 19 in quantitative yield. Amides 6–9 and imidazolides 10–12 were synthesized in moderate yields by condensation reactions between 19 and the corresponding amines and imidazoles (Scheme 1 and Table 1).
Scheme 1.
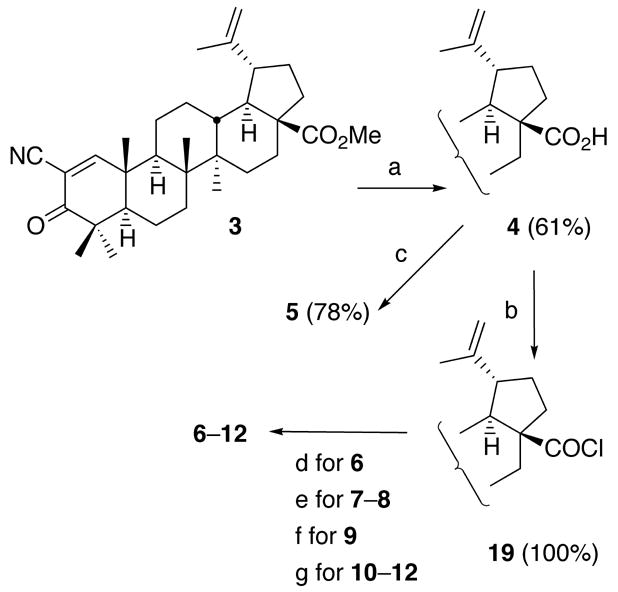
(a) AlBr3, Me2S (room temp.); (b) (COCl)2, CH2Cl2 (room temp.); (c) EtI, DBU, toluene (reflux); (d) NH3, PhH (room temp.); (e) amine hydrochloride, NaHCO3, PhH, water (reflux); (f) Me2NH, PhH (reflux); (g) imidazole, PhH (room temp.).
Compounds 13–18 with a carboxyl or methoxycarbonyl enone functionality in ring A were synthesized by the sequence shown in Scheme 2. Methyl ester 21 was prepared in 85% yield from methyl betulonate (20), which was easily synthesized in 95% yield in 2 steps (methylation with CH2N2 and Jones oxidation) from 1, by Stiles’ reagent (methoxymagnesium methyl carbonate) in DMF,15 followed by methylation with CH2N2. The 1H NMR spectrum showed that 21 is the single tautomer in CDCl3 as depicted in Scheme 2. Initially, we expected that addition of phenylselenyl chloride (PhSeCl, 2 equivalents) to 21 and subsequent oxidation/elimination of the selenated intermediate with H2O2 would give only 13. However, these conditions16 afforded 14 (28% yield) and 22 (14%) in addition to 13 (22%). We believe that PhSeCl produces the rearranged allylic selenide 23 from 13 and/or 21 (Scheme 3).17 Oxidation of 23 with H2O2 forms allylic selenoxide 24, which is converted to allylic selenenic ester 25 by a [2,3]sigmatropic rearrangement, followed by hydrolysis of 25 to give allylic alcohol 14 and/or 22.18 Allylic fluoride 15 was obtained in 65% yield from 14 with DAST in CH2Cl2.19 Acids 16–18 were prepared from the corresponding methyl esters 13–15 by alkaline hydrolysis (86%, 100%, and 86% yield, respectively).
Scheme 2.
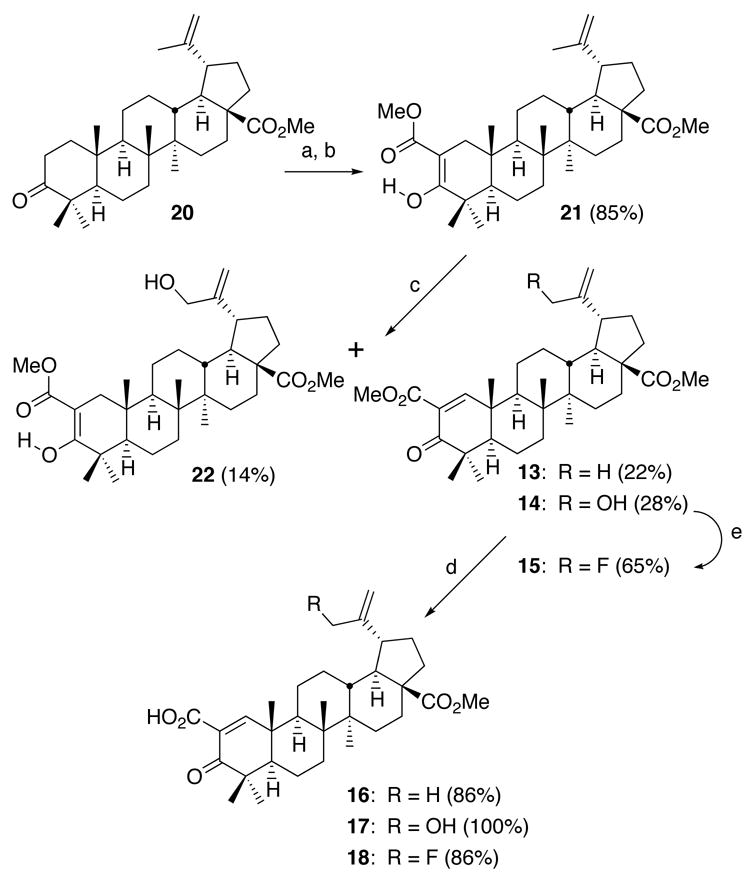
(a) Stiles’ reagent, DMF (at 110 °C); (b) CH2N2, Et2O, THF (room temp.); (c) PhSeCl, pyr., CH2Cl2 (at 4 °C); 30% H2O2, CH2Cl2 (at 4 °C); (d) aqueous KOH, MeOH (reflux); (e) DAST, CH2Cl2 (−78 °C).
Scheme 3.
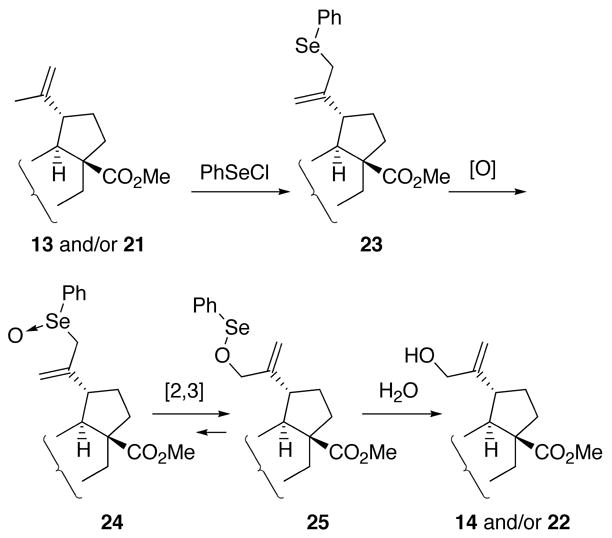
Conversion of 13/21 to 14/22 using PhSeCl–H2O2
We have evaluated the potency of methyl ester 3 and new analogues 4–18 for inhibition of NO production in RAW 264.7 cells stimulated with interferon-γ, and induction of the anti-inflammatory, cytoprotective enzyme, heme oxygenase-1 in the liver (in vivo). In the RAW cell assay (Table 1), compounds 3–12 with a cyano enone functionality in ring A are highly active, with a potency that is similar to that of CDDO, whereas betulinic acid is inactive. The series of compounds 13–18 with a carboxyl or methoxycarbonyl enone functionality in ring A are less active, with a potency more than 10 fold less than that of CDDO. Most importantly, we have found that one of the new analogues, 10, is significantly more potent in vivo than both betulinic acid and the oleanolic acid analogue, CDDO. Thus, as shown in Figure 2, oral dosing of 2 μmoles of compound 10 caused significant induction of the anti-inflammatory, cytoprotective enzyme, heme oxygenase-1, in the liver, while betulinic acid and CDDO were both ineffective at this low dose. There is major interest in stimulating heme oxygenase-1 as a protective enzyme in many chronic disease conditions in which inflammation and oxidative stress play a key role.12
Figure 2. Analog 10 induces heme oxygenase-1 (HO-1) in vivo in liver.
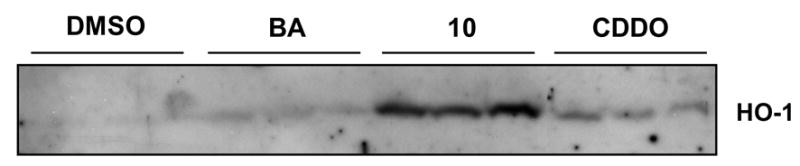
Male CD-1 mice (3 mice per group) were gavaged with triterpenoids (2 μmole) in DMSO. After 6 h, livers were collected and analyzed by Western blot for heme oxygenase-1.20
In summary, in a series of new betulinic acid analogues, we have found the following interesting SARs: (1) A cyano enone functionality in ring A is necessary for exhibiting potent inhibitory activity against NO production in RAW cells. (2) Noteworthy is that an enone functionality in ring C is not necessary for the potency. The SARs are entirely different from those of oleanolic acid analogues.7,8 (3) The methoxycarbonyl and carboxyl enone functionalities in ring A are not important for the potency. (4) C-17 modifications do not affect the potency in the RAW cell assay. These results are also different from those of CDDO analogues.9 However, interestingly, only the acyl imidazole increases the potency in vivo. (5) Isopropenyl side chains do not affect the potency. Overall, it is important to note that our present results are different from the oleanolic acid analogues that we have studied previously. Further syntheses and biological evaluation of new betulinic acid analogues are in progress.
Acknowledgments
This investigation was supported by funds from NIH Grant 1 R01 CA78814, the Norris Cotton Cancer Center, the Dartmouth College Class of 1934, and the National Foundation for Cancer Research. M. B. S. is Oscar M. Cohn Professor.
Footnotes
The new betulinic acid analogue 10, at oral dosing of 2 μmoles, causes significant induction of the anti-inflammatory, cytoprotective enzyme, heme oxygenase-1, in the liver.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Cole BJ, Bentley MD. Holzforshung. 1991;45:265. [Google Scholar]
- 2.Maurya SK, Devi S, Pandey VB. Fitoterapia. 1989;5:468. [Google Scholar]
- 3.Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown DM, Wani MC, Wall ME, Heiken TJ, Das Gupta TK, Pezzuto JM. Nature Med. 1995;1:1046. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt ML, Kuzmanoff KL, Ling-Indeck L, Pezzuto JM. Eur J Cancer. 1997;33:2007. doi: 10.1016/s0959-8049(97)00294-3. [DOI] [PubMed] [Google Scholar]
- 5.Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti-Passerini C, Formelli F. Cancer Lett. 2002;175:17. doi: 10.1016/s0304-3835(01)00718-2. [DOI] [PubMed] [Google Scholar]
- 6.Ehrhardt H, Fulda S, Fuhrer M, Debatin KM, Jeremias I. Leukemia. 2004;18:1406. doi: 10.1038/sj.leu.2403406. [DOI] [PubMed] [Google Scholar]
- 7.Honda T, Gribble GW, Suh N, Finlay HJ, Rounds BV, Bore L, Favaloro FG, Jr, Wang Y, Sporn MB. J Med Chem. 2000;43:1866. doi: 10.1021/jm000008j. [DOI] [PubMed] [Google Scholar]
- 8.Honda T, Rounds BV, Bore L, Finlay HJ, Favaloro FG, Jr, Suh N, Wang Y, Sporn MB, Gribble GW. J Med Chem. 2000;43:4233. doi: 10.1021/jm0002230. [DOI] [PubMed] [Google Scholar]
- 9.Honda T, Honda Y, Favaloro FG, Jr, Gribble GW, Suh N, Place AE, Rendi MH, Sporn MB. Bioorg Med Chem Lett. 2002;12:1027. doi: 10.1016/s0960-894x(02)00105-1. [DOI] [PubMed] [Google Scholar]
- 10.Shortly after we started this project, a Korean group reported methyl ester 3; You YJ, Kim Y, Nam NH, Ahn BZ. Bioorg Med Chem Lett. 2003;13:3137. doi: 10.1016/s0960-894x(03)00724-8.This compound was synthesized from betulinic acid (1) by the same method as they reported
- 11.All new compounds 4–18 provided acceptable HRMS data (+/− 5 ppm) and 1H NMR spectra that exhibit no discernible impurities. Compound 10: amorphous solid. [α]25D +0.7° (c 0.57, CHCl3). CD (c 0.0016, EtOH) Δε265 +1.71 and Δε346 −2.32. 1H NMR (CDCl3) δ 8.30 (1H, brs), 7.82 (1H, s), 7.55 (1H, brs), 7.07 (1H, brs), 4.79 (1H, d, J = 1.46 Hz), 4.68 (1H, t, J = 1.46 Hz), 2.98 (1H, ddd, J = 4.76, 10.98, 10.98 Hz), 2.77 (1H, ddd, J = 3.66, 12.27, 12.27 Hz), 2.48 (1H, ddd, J = 3.29, 3.29, 13.91 Hz), 2.34 (1H, q, J = 6.59 Hz), 1.72, 1.18, 1.13, 1.12, 1.021, 1.016 (each 3H, s); 13C NMR (CDCl3) δ 198.4, 173.1, 170.8, 149.5, 137.5, 130.0, 117.6, 115.2, 114.2, 110.7, 57.7, 52.8, 51.4, 45.3, 45.2, 44.2, 42.7, 42.1, 41.0, 37.2, 34.2, 33.6, 33.2, 30.7, 29.8, 27.9, 25.4, 21.6, 19.6, 19.0, 18.6, 16.7, 14.7. MS (ESI+) m/z 528 [M+H]+; HRMS (ESI+) calcd for C35H45N3O2 + H 528.3590, found 528.3580.
- 12.Ryter SW, Alam J, Choi AM. Physiol Rev. 2006;86:583. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 13.Node M, Nishide K, Sai M, Fuji K, Fujita E. J Org Chem. 1981;46:1991. [Google Scholar]
- 14.Ono N, Yamada T, Saito T, Tanaka K, Kaji A. Bull Chem Soc Jpn. 1978;51:2401. [Google Scholar]
- 15.Finkbeiner HL, Stiles M. J Am Chem Soc. 1963;85:616. [Google Scholar]
- 16.Liotta D, Barnum C, Puleo R, Zima G, Bayer C, Kezar HS., III J Org Chem. 1981;46:2920. [Google Scholar]
- 17.Hori T, Sharpless KB. J Org Chem. 1979;44:4208. [Google Scholar]
- 18.Clive DLJ. Tetrahedron. 1978;34:1049. [Google Scholar]
- 19.Rozen S, Faust Y, Ben-Yakov H. Tetrahedron Lett. 1979:1823. [Google Scholar]
- 20.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. Cancer Res. 2005;65:4789. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]


