Abstract
Two new iodinated fluoro- and hydroxy-pegylated aza-diphenylacetylene derivatives, 1 and 2, targeting β-amyloid (Aβ) plaques have been successfully prepared. In vitro binding carried out in tissue homogenates prepared from postmortem AD brains with [125I]IMPY (6-iodo-2-(4′-dimethylamino-)phenyl-imidazo[1,2-a]pyridine) as the radioligand indicated good binding affinities (Ki = 9.2 and 16.8 nM for 1 and 2, respectively). Brain penetrations of the corresponding radioiodinated ligands, evaluated in the normal mice, showed good initial brain penetrations (3.55 and 5.67% ID/g for [125I]1 and [125I]2 at 2 min post-injection). The washout from normal mice brain was relatively fast (0.33 and 0.91% ID/g at 2 hr post-injection). The specific binding of these radioiodinated ligands to β-amyloid plaques was clearly demonstrated using film autoradiography of AD brain sections. Taken together, these preliminary results strongly suggest that these novel iodinated aza-diphenylacetylenes may be potentially useful for imaging Aβ plaques in the living human brain.
Keywords: Alzheimer’s disease, Radioiodination, Biodistribution, Autoradiography and Sonogashira coupling reaction
Formation and accumulation of aggregated protein deposits is a common feature of a number of neurodegenerative diseases1, 2. Major neuropathology observations of postmortem Alzheimer’s disease (AD) brains depict the presence of senile plaques (containing β-amyloid (Aβ) aggregates) and neurofibrillary tangles (highly phosphorylated tau proteins)3, 4. The exact mechanisms leading to the development of AD are not fully understood; however, the formation of Aβ plaques, consisting of β-sheets of Aβ protein aggregates, in the brain is a pivotal event in the pathogenesis of AD5–8. Thus, developing Aβ plaque-specific probes for in vivo imaging may be important for the diagnosis and treatment monitoring of AD4, 9, 10.
Positron emission tomography (PET) imaging with [11C]PIB (2-(4′-(methylaminophenyl)-6-hydroxybenzothiazole) has demonstrated the feasibility of visualizing Aβ plaques in patients suffering from AD11, 12. However, the short half-life (20 min) of C-11 limits the usefulness of the agent for a widespread clinical application. To achieve a widespread availability, one major focus of our effort is in the development of I-123 (T1/2 = 13 hr) labeled Aβ plaque-specific imaging agents that can be used for single photon emission tomography (SPECT) imaging. The development of [123I]IMPY (6-iodo-2-(4′-dimethylamino-)phenyl-imidazo[1,2-a]pyridine), a unique thioflavin derivative with an [1,2,a]imidazopyridine ring, showed the feasibility of developing SPECT imaging agents for targeting Aβ plaques13, 14. However, certain undesirable characteristics of [123I]IMPY, which including high lipophilicity, less in vivo stability and insufficient signal to noise ratio, prompted us to pursue the development of a second generation of I-123 labeled SPECT imaging agents. In addition to the benzothiazole series, we have also explored radioiodinated derivatives of other backbone structures, including stilbenes15 and fluorenes16. So far these attempts have met with a limited success. Recently, we reported a novel series of iodinated styrylpyridine derivatives showing promising results17. Based upon the successful results obtained from the fluorinated diphenylacetylene ligands for PET18, we decided to further extend our search of SPECT ligands using a similar aza-diphenylacetylene structure. Several iodinated aza-diphenylacetylene derivatives, in which one of the phenyl rings was replaced with a pyridine ring, were prepared and tested for their plaque binding affinities. Reported herein are the synthesis and the initial biological evaluations of these iodinated aza-diphenylacetylene ligands for targeting amyloid plaques.
Synthesis of the aza-diphenylacetylene derivatives is illustrated in Scheme 1. The key step in the synthesis is using a palladium catalyzed Sonogashira coupling reaction. All bromo- substituted compounds (5a–d, 10) were readily assembled at room temperature by reacting alkynes (3a–b, 8) with iodo- substituted pyridine compounds (4a–b). The phenol compound 11 was obtained from 10 through pyridinium p-toluenesulfonate catalyzed deprotection of tetrahydropyran (THP) protecting group. Next, the desired organotin compounds (6a–c) were successfully prepared by using the palladium catalyzed trans-stannylation from their bromide precursors (5a–b, 5d). The subsequent iododestannylation reaction afforded iodinated targets (1, 2, 7) 17,20.
Scheme 1.
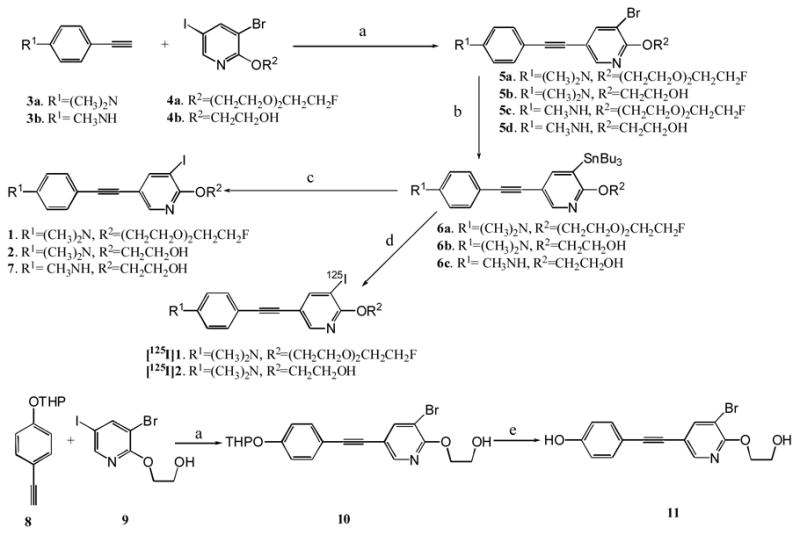
Reagents and conditions: (a) Pd(PPh3)4, CuI, Et3N, CH3CN, 0 ºC to rt; (b) (Bu3Sn)2, Pd(PPh3)4, toluene, 110 ºC; (c) I2, THF, 0 ºC to rt; (d). H2O2, Na125I, HCl, EtOH; (e). PPTS, EtOH, 55 ºC, 3 h.
The binding affinities of the non-radioactive ligands (1–2, 5a–d, 7, 11) were screened via the binding competition with [125I]IMPY using postmortem AD brain homogenates19. All the brominated and iodinated aza-diphenylacetylene derivatives examined displayed excellent to good binding affinities in comparison to [125I]IMPY binding. It was evident that all of the brominated derivatives and the corresponding iodinated ligands displayed similar excellent binding affinities (Ki values shown in Table 1 between series 5b and 5d vs 2 and 7).
Table 1.
Potencies (Ki) of compounds on competition of [125I]IMPY binding to amyloid plaques in AD brain homogenates
| Compound | Ki (nM ± SEM) |
|---|---|
| 1 | 16.8 ± 1.8 |
| 2 | 9.2± 1.7 |
| 5a | 11.2 ± 0.8 |
| 5b | 6.7 ± 1.3 |
| 5c | 13.1 ± 1.9 |
| 5d | 1.6 ± 0.5 |
| 7 | 12.5 ± 2.5 |
| 11 | 6.2 ± 1.2 |
| IMPY | 5.0 ± 0.4 19 |
Each value was determined three times with duplicate for each measurement.
The hydroxyl pegylated derivatives, i.e. 5b, 5d, 11, 2 and 7 competed effectively with [125I]IMPY binding with Ki values of 6.7 ± 1.3, 1.6 ± 0.5, 6.2 ± 1.2, 9.2 ± 1.7 and 12.5 ± 2.5 nM. The addition of a fluoropegylated chain to the 2′-position of the pyridine group displayed similar binding affinities to β-amyloid plaques. Compounds 5a, 5c and 1 showed Ki values of 11.2 ± 0.8,13.1 ± 1.9 and 16.8 ± 1.8 nM (Table 1). Similarly, there is no significant difference in the binding affinities between N,N-dimethylamino derivatives, 5b, 2, and N-monomethylamino derivatives, 5d, 7. In addition, after replacing the substituted amino group with a hydroxy group attached to the 4-position of one end of the phenyl ring, the binding affinity remained the same (Ki = 6.2 ± 1.2 and 6.7 ± 1.3 nM for 11 and 5b, respectively).
On the basis of the encouraging binding data observed for these series of ligands, we chose two representatives, 1 and 2, to carry out further biological evaluations with the I-125 labeled probes. Radioiodination was successfully carried out with the corresponding tributyltin precursors, following the standard iododestannylation reaction, using hydrogen peroxide as the oxidant (Scheme 1)13. The final HPLC-purified ligands, [125I]1 and [125I]2, showed greater than 98% radiochemical purities with high overall yields (>60%) and high specific activities (~2000 Ci/mmol). The two radioiodinated probes measured under the experimental conditions showed moderate lipophilicity17 (logP = 2.6 and 2.8), a desirable property for Aβ-targeting imaging agents. When evaluated for whole animal biodistribution after an iv injection in normal mice, [125I]1 and 2, displayed good initial penetrations of the intact blood-brain barrier with excellent initial brain uptakes (3.55 and 5.67 % ID/g at 2 min after tracer injection). The high brain uptakes of these iodinated ligands were subsequently followed by relatively fast washouts with 0.33 and 0.91% ID/g remaining in the brain at two hours after the tracer injection (Table 2). The kinetics of fast brain uptake and washout from normal brain (containing no Aβ plaques) is highly desirable for an Aβ plaque-targeting imaging agent 10.
Table 2.
Biodistribution in normal mice after an iv injection of [125I]ligand (%ID/g, mean ± SD, n = 3 mice per group)
| [125I]1 (LogP = 2.60) | ||||
|---|---|---|---|---|
| Organ | 2 min | 30 min | 1 hr | 2 hr |
|
| ||||
| Blood | 4.23 ± 0.67 | 2.84 ± 0.32 | 3.19 ± 0.41 | 2.70 ± 0.09 |
| Heart | 15.8 ± 3.83 | 2.59 ± 0.68 | 1.53 ± 0.24 | 1.07 ± 0.03 |
| Muscle | 0.95 ± 0.27 | 1.43 ± 0.31 | 0.99 ± 0.20 | 0.67 ± 0.08 |
| Lung | 12.9 ± 3.20 | 4.00 ± 1.39 | 2.84 ± 0.43 | 2.07 ± 0.13 |
| Kidney | 16.3 ± 2.96 | 3.56 ± 0.82 | 2.78 ± 0.68 | 1.94 ± 0.10 |
| Spleen | 4.99 ± 0.21 | 1.76 ± 0.36 | 1.73 ± 0.34 | 1.46 ± 0.15 |
| Liver | 24.1 ± 4.06 | 11.2 ± 0.31 | 3.07 ± 0.62 | 1.76 ± 0.27 |
| Skin | 0.89 ± 0.07 | 2.12 ± 0.40 | 2.85 ± 0.23 | 2.75 ± 0.24 |
| Brain | 3.55 ± 0.91 | 3.10 ± 0.38 | 1.36 ± 0.10 | 0.33 ± 0.01 |
| [125I]2 (LogP = 2.80) | ||||
| Organ | 2 min | 30 min | 1 hr | 2 hr |
|
| ||||
| Blood | 3.45 ± 0.29 | 3.38 ± 0.51 | 2.71 ± 0.43 | 3.29 ± 0.61 |
| Heart | 13.0 ± 2.47 | 2.31 ± 0.37 | 1.41 ± 0.33 | 1.33 ± 0.26 |
| Muscle | 0.98 ± 0.30 | 1.23 ± 0.20 | 0.71 ± 0.09 | 0.78 ± 0.11 |
| Lung | 13.0 ± 2.32 | 4.03 ± 0.41 | 2.60 ± 0.56 | 2.77 ± 0.86 |
| Kidney | 16.6 ± 2.48 | 4.10 ± 0.54 | 2.85 ± 0.50 | 2.40 ± 0.47 |
| Spleen | 6.33 ± 1.03 | 1.98 ± 0.34 | 1.42 ± 0.40 | 1.73 ± 0.45 |
| Liver | 17.2 ± 2.86 | 11.2 ± 1.59 | 5.21 ± 2.05 | 2.24 ± 0.45 |
| Skin | 0.87 ± 0.14 | 2.22 ± 0.64 | 2.46 ± 0.27 | 3.18 ± 0.17 |
| Brain | 5.67 ± 1.49 | 4.51 ± 0.56 | 2.14 ± 0.21 | 0.91 ± 0.17 |
To confirm the specific labeling of radioiodinated aza-diphenylacetylenes for Aβ plaques, we performed the in vitro film autoradiography. A human brain macroarray containing both AD and control cases allowed us to efficiently compare the plaque labeling with various radiolabeled probes. As shown in Figure 2, [125I]1 distinctively labeled plaques on AD brain sections with low background labeling, but not for the control section (indicated by an arrow), which is consistent with the immunohistochemistry staining with Aβ antibody 4G8.
Figure 2.
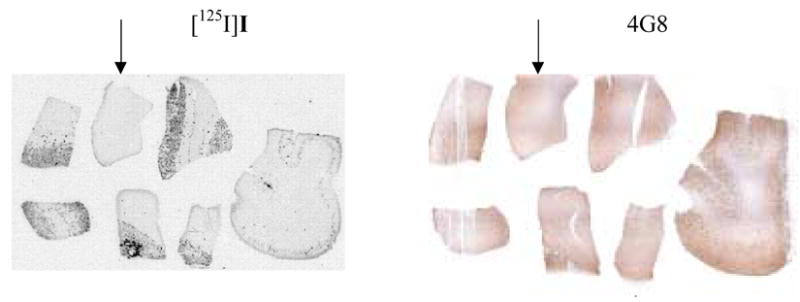
In vitro autoradiography to detect amyloid plaques with [125I]1. The human macroarray brain sections were constructed from six postmortem AD cases plus one control (marked by arrowhead). The plaques were confirmed with 4G8 antibody immunohistochemistry staining.
In conclusion, we have demonstrated that iodinated aza-diphenylacetylenes can be successfully prepared. They showed high binding affinities to β-amyloid plaques by in vitro binding assay. The radioiodinated derivatives, [125I]1 and [125I]2, displayed desirable in vivo properties, with excellent brain penetrations and relatively fast rates of washout in normal mice (resulting in low background signal). Specific Aβ plaque labeling was clearly demonstrated by in vitro autoradiography of postmortem AD brain section. This series of SPECT probes warrants further investigation to confirm the usefulness for imaging amyloid plaques in AD.
Figure 1.
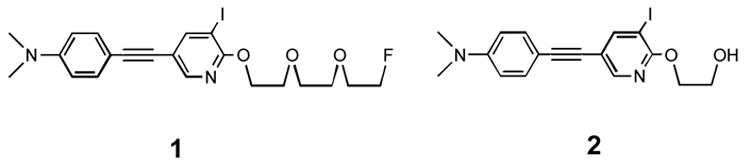
Structures of two iodinated aza-diphenylacetylene derivatives
Acknowledgments
This work was supported by grants from the National Institutes of Health (AG-021868 to M.P.K). The author thanks Pathology Core Laboratories at The Childrens Hospital of Philadelphia for assembling the human macro-array brain sections. The authors also thank Drs. Daniel Skovronsky and Rajesh Manchanda for their helpful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference and notes
- 1.Kelly JW. Curr Opin Struct Biol. 1996;6:11. doi: 10.1016/s0959-440x(96)80089-3. [DOI] [PubMed] [Google Scholar]
- 2.Shastry BS. Neurochem Int. 2003;43:1. doi: 10.1016/s0197-0186(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg SD, Schmidt ML, Crino PB, Eberwine JH, Lee VM-Y, Trojanowski JQ. Molecular pathology of Alzheimer’s disease and related disorders. In: Peters A, Morrison JH, editors. Cerebral cortex: neurodegenerative and age-related changes in structure and function of cerebral cortex. Kluwer Academic/Plenum; New York: 1999. pp. 603–654. [Google Scholar]
- 4.Selkoe DJ. JAMA. 2000;283:1615. doi: 10.1001/jama.283.12.1615. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J, Selkoe DJ. Science. 2002;297:353. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Hardy J. Curr Alzheimer Res. 2006;3:71. doi: 10.2174/156720506775697098. [DOI] [PubMed] [Google Scholar]
- 7.Golde TE. Brain Pathol. 2005;15:84. doi: 10.1111/j.1750-3639.2005.tb00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchesi VT. Proc Natl Acad Sci U S A. 2005;102:9093. doi: 10.1073/pnas.0503181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blennow K, Zetterberg H. Nat Med. 2006;12:753. doi: 10.1038/nm0706-753. [DOI] [PubMed] [Google Scholar]
- 10.Mathis CA, Wang Y, Klunk WE. Curr Pharm Des. 2004;10:1469. doi: 10.2174/1381612043384772. [DOI] [PubMed] [Google Scholar]
- 11.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang G-f, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Ann Neurol. 2004;55:306. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 12.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. Neurology. 2006;67:446. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 13.Zhuang ZP, Kung MP, Hou C, Skovronsky D, Gur TL, Trojanowski JQ, Lee VMY, Kung HF. J Med Chem. 2001;44:1905. doi: 10.1021/jm010045q. [DOI] [PubMed] [Google Scholar]
- 14.Kung MP, Hou C, Zhuang ZP, Zhang B, Skovronsky DM, Gur T, Lee VMY, Trojanowski JQ, Kung HF. Brain Res. 2002;956:202. doi: 10.1016/s0006-8993(02)03436-4. [DOI] [PubMed] [Google Scholar]
- 15.Kung HF, Lee CW, Zhuang ZP, Kung MP, Hou C, Plossl K. J Am Chem Soc. 2001;123:12740. doi: 10.1021/ja0167147. [DOI] [PubMed] [Google Scholar]
- 16.Lee CW, Kung MP, Hou C, Kung HF. Nucl Med Biol. 2003;30:573. doi: 10.1016/s0969-8051(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 17.Qu W, Kung MP, Hou C, Benedum TE, Kung HF. J Med Chem. 2007 doi: 10.1021/jm070025+. ASAP. [DOI] [PubMed] [Google Scholar]
- 18.Chandra R, Oya S, Kung MP, Hou C, Jin LW, Kung HF. J Med Chem. 2007 doi: 10.1021/jm070090j. In Press. [DOI] [PubMed] [Google Scholar]
- 19.Kung MP, Hou C, Zhuang ZP, Skovronsky D, Kung HF. Brain Res. 2004;1025:89. doi: 10.1016/j.brainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Typical procedure for Sonogashira coupling reaction: 4-((5-bromo-6-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)ethynyl)-N,N-dimethylbenzenamine (5a). A mixture of 4-ethynyl-N,N-dimethylaniline (0.080 g, 0.55 mmol), 4a (0.217 g, 0.5 mmol), Pd(Ph3)4 (0.029 g, 0.025 mmol) in 3 mL Et3N and 3 mL CH3CN was deoxygenated by purging with nitrogen for 15 min. The reaction mixture was cooled with an ice bath and CuI (0.009 g, 0.05 mmol) was added into reaction flask. After 5 min at 0 ºC, the reaction mixture was stirred at r.t. for 1 h. It was then concentrated and purified by flash chromatography (EtOAc/Hexanes, 25/75) to yield 5a as a light yellow solid (0.180 g, 79%). 1H NMR (200 MHz, CDCl3) δ 8.20 (d, 1H, J = 2.0 Hz), 7.92 (d, 1H, J = 2.0 Hz), 7.39 (dt, 2H, J1 = 8.9 Hz, J1 = 2.3 Hz), 6.66 (dt, 2H, J1 = 8.9 Hz, J1 = 2.3 Hz), 4.69 (t, 1H, J = 4.2 Hz), 4.55 (t, 2H, J = 4.9 Hz), 4.45 (t, 1H, J = 4.2 Hz), 3.94–3.68 (m, 8H), 3.01 (s, 6H). 13C NMR (50 MHz, CDCl3) δ 158.3, 150.4, 143.5, 138.0, 129.6, 129.5, 127.7, 125.2, 118.8, 112.5, 107.5, 85.0, 81.6, 71.2, 71.0, 70.8, 70.4, 69.6, 66.7, 40.5. HRMS calcd for C21H24BrFN2O3 (M+), 450.0954; found, 450.0944.


