Abstract
Objective
We developed assays for measurement of urinary βLH and βFSH under collection and storage conditions typical of non-clinical research settings.
Design and Methods
IEMAs for free βLH and total βFSH were validated by standard methods. Stability of urinary βLH and βFSH was tested across freeze thaws and stored long-term at 4°C or −20°C, or short term at room temperature, and with heating to dissociate the subunits.
Results
The IEMAs exhibited acceptable parallelism, specificity, recovery (averaging 100% for βLH, 97% for βFSH), imprecision (maximum within-run and between run CVs, respectively, 4.8% and 25.7% for βLH, 5.6% and 17.0% for βFSH), and minimum detectable dose (2.5 pmol/L for βLH, 6.8 pmol/L for βFSH). Urine and serum measures were highly correlated (r = 0.95 for LH, 0.86 for FSH). There was no consistent decline with any storage type. Dissociation of subunits by heating was needed for βLH, but not βFSH.
Conclusion
These IEMAs measure free βLH and total βFSH, overcoming inter-individual variability in, and collection and storage effects on, subunit dissociation, without the need for urine preservatives.
Keywords: βLH, βFSH, IEMA, stability
INTRODUCTION
Luteinizing hormone (LH) and follicle stimulating hormone (FSH) are sensitive indicators of hypothalamic-pituitary-ovarian axis function, and are particularly valuable for examining the timing and correlates of puberty (e.g. (1)) and reproductive aging (e.g. (2)). A persistent issue in the use of urinary and serum LH and FSH assays is concern over the effects of collection and storage conditions on dissociation of the alpha and beta (β) subunits of these hormones (3–6). This is particularly relevant for large-scale population-level research where there may be considerable delay between specimen collection from individuals in their homes, or in the field, and time of assay.
Most assays for LH and FSH measure the intact forms of the hormones (7–12), and thus factors influencing dissociation of the subunits are ever present concerns. While limited storage at refrigerated temperatures (up to 6 months) does not appear to affect the stability of intact LH and FSH (7, 9, 13), storage at freezing or room temperature has been found to significantly reduce the amount of intact urinary LH and FSH (7, 9, 10, 13). One study, however, found no effect of up to 10 freeze-thaw cycles on urinary LH and FSH measurements (12). Although refrigerated temperatures do not appear to influence LH and FSH stability, large-scale refrigerator storage is impractical in most settings.
Preservatives, such as glycerol, thymol, bovine serum albumin, boric acid, thimerosal, and sodium azide, or adjustment of specimen pH to neutral, are used to prevent subunit dissociation and ensure accurate measurement of intact LH and FSH (e.g., (7, 9, 10, 13–15)). However, preservatives have some limitations for population level research, including time and expense invested in the advance preparation of specimen collection tubes for home or field collections, potential assay interference, effects on other hormones to be measured in the same specimen, and the fact that dilution volume correction may be necessary for the final urinary result (3, 13). Additionally, preservatives do not correct for any inter-subject variability that might exist in modification of LH and FSH between circulation in the blood and excretion in the urine (3).
Our objective was to develop immunoenzymometric assays to measure LH and FSH in spot urines for population-level research that are robust to non-clinical collection and storage of urine specimens, and do not require specimen preservation or extraction. Following the approach of Qiu et al. (3), we identified antibodies specific to βFSH and βLH and developed assays that would measure the total amount of each β subunit in urine specimens, whether they were in intact or dissociated form.
METHODS
Samples
A total of 799 daily urine and serum specimens were collected over one menstrual cycle from 30 US women in 1997–1998. Thirteen women aged 20–25 years and 17 women 40–45 years old were recruited for a study on reproductive aging. Monetary compensation was provided, participants provided written informed consent, and all procedures were approved by the Institutional Review Board of the University of Washington. All participants were normally cycling, in good health, had a mean body mass index of 22.6 kg/m2 (SD = 2.36, range 18.9 – 27.7), and were not using medications or hormones. Blood specimens were obtained by venipuncture, beginning with the first day of menstrual bleeding and continuing until the first day of menstrual bleeding of the subsequent cycle. Serum specimens were assayed within 1 to 2 months of collection for intact LH and FSH. The LH assay (DELFIA, Wallac Inc., Gaithersburg, MD, USA) cross-reacts less than 1% with FSH, and the inter- and intra-assay CVs were 2.8% and 4.7% respectively. The FSH assay (DELFIA, Wallac Inc., Gaithersburg, MD, USA) cross reacts less than 1% with LH, and the inter- and intra-assay CVs were 2.3% and 4.6% respectively. All cycles were confirmed ovulatory by transvaginal ultrasound. Urine specimens were taken daily in the clinic, usually before noon, at the same time as serum collection and immediately stored at −20° C. Urine specimens remained frozen until thawing two years later for assay and measurement of specific gravity.
Urine Assays
Sandwich immunoenzymometric assays (IEMAs) were developed to measure urinary human βLH and βFSH. For βLH, plates were coated with 100μL of 1μg/mL mouse monoclonal anti-human βLH capture antibody (clone M38259, Fitzgerald Industries International, Inc., Concord, MA, USA) in sodium carbonate coating buffer (pH 9.6). Plates were incubated at 4°C for 18 hours or up to one week. Antibody solution was discarded, and plates were blocked with 200μL per well of 1% w/v bovine serum albumin in phosphate buffered saline (PBS), pH 7.5. After incubation for 2–8 hours at room temperature (RT) or overnight at 4°C, plates were washed, and 100μL of calibrator (βLH, AFP3477A, NIDDK NHPP, A.F. Parlow; calibration curve range 0 to 32.3 pmol/L), controls, and urine specimens, either neat or diluted in assay buffer (PBS with 1% w/v bovine serum albumin), were added. After overnight incubation at 4°C, plates were again washed, and 100μL/well of biotin-conjugated monoclonal antibody directed against the β subunit of human LH (mouse, clone B409, Scantibodies Laboratory, Inc., Santee, CA, USA) (16) and biotinylated in our lab, was diluted to 300ng/mL in PBS containing 1% w/v bovine gamma globulin, and added to the wells. To conjugate the B409 and the biotin, 1 mole of antibody to 12 moles of NHS-LC-biotin ester (Sigma-Aldrich, St. Louis, MO, USA) dissolved in dimethylformamide were combined in pH 7.2 PBS, and allowed to react at room temperature with stirring for 1 hour. The conjugated antibody was separated from free excess biotin by dialysis against PBS, pH 7.2, for 48 hours at 4°C. Biotinylated B409 anti-β subunit LH antibody was stored at −80°C. After incubation with biotinylated antibody overnight at 4°C, plates were washed and 100μL/well of alkaline phosphatase conjugated streptavidin (Zymed, S. San Francisco, CA, USA) diluted in Tris-HCl buffer, pH 7.5, (1:1000 to 1:6000, determined by titration) was added to the wells, and incubated (one hour, RT). After a final wash, 100μL/well of 1mg/mL p-nitrophenyl phosphate in 1M diethanolamine, pH 9.0 was added, and color developed for 2–3 hours in the dark. Absorbance was quantified (405nm test and 570nm reference wavelength) using a Dynatech MR7000 spectrophotometer, and the calibration curve was fitted using a four-parameter logistic regression (Biolinx 2.0 software, Dynex Technologies, Chantilly, VA, USA).
A similar protocol was used to measure βFSH. Plates were coated with 4μg/mL of anti-human βFSH monoclonal antibody (clone FS2.4A10.G10, Scantibodies Laboratory, Inc., Santee, CA, USA) (3). After overnight (or up to one week) incubation, plates were blocked and specimens and calibrators (calibration curve range 0 to 381.0 pmol/L, βFSH, AFP2911A, NIDDK NHPP, A.F. Parlow) were added as described for the βLH assay. A rabbit polyclonal detection antibody directed against human βFSH (NIDDK-anti-hBetaFSH-1, NHPP, A.F. Parlow) was diluted 1:10,000 and added to the wells. After overnight incubation (4°C), plates were washed and incubated for 2–3 hours at RT with 100μL/well of goat-anti-rabbit IgG conjugated to alkaline phosphatase. Plates were washed and substrate solution added as above. After color developed (1–2 hours in the dark), optical density was quantified and a calibration curve was fit as described for βLH.
Urinary hormone values were adjusted by specimen specific gravity (17). Specific gravity measurements were taken with a hand-held urine specific gravity refractometer (Atago Uricon-PN, NSA Precision Cells, Inc).
Dissociation of Subunits
In both the βLH and βFSH assays, the antibodies target the β subunit of the molecule. In the βFSH IEMA, the antibodies bound the β subunit whether free from the alpha subunit or in the molecule’s intact form. In the βLH IEMA, however, there was little cross-reaction with the intact form of LH. For both the βLH and βFSH assays, we tested heating the specimens to boiling to homogenize any inter-sample variation in dissociated hormone levels that might result from differential excretion or specimen treatment before diluting and adding them to the assay (3). We compared results before and after heating using 12 replicates each of 18 in-house urine (male and female, 27–55 years) specimens. Aliquots of neat urine were heated according to the conditions Qiu et al found to be optimal for complete dissociation of FSH into its subunits without degrading the subunits—2 minutes in a heating block at 100°C (3, 18)—and cooled prior to dilution and assay. The specimens were heated and assayed in a single batch for both assays.
Validations
Specificity of each assay was measured as the percentage of cross-reaction with high doses of intact and dissociated subunit forms of hormones of similar structure: LH, human chorionic gonadotropin, and thyroid stimulating hormone.
Recovery was determined as the percentage of added mass recovered from a urine matrix. For each IEMA, zero, low, medium, and high doses of calibrator, diluted in assay buffer, were added as 10% of specimen volume to each of six undiluted urine specimens with low, medium and high endogenous concentrations of βLH and βFSH (19). Six replicate wells for each specimen/dose combination were assayed in 10 separate batches. Recovery was estimated by dividing the observed by the expected values, and expressed as a percentage.
Assay imprecision was estimated using a variance components model (20) to examine intra- and inter-assay variation. Low, medium, and high concentration urine specimens run in duplicate wells on 10 plates were used to estimate imprecision for each IEMA.
Minimum detectable dose was estimated as the lowest dose giving results significantly different from zero (p <0.05) (based on duplicate wells, n = 20 microtiter plates) (21).
To assess assay parallelism, we used linear mixed effects models to test whether the slopes of serially diluted specimens’ concentration plotted against urine volume per well was significantly different from zero (19). Urine specimens (n = 8 for βLH; n = 11 for βFSH) were diluted in assay buffer, and assayed in six replicate wells of each dilution and specimen combination.
Urinary βLH and βFSH IEMA performance was also evaluated by comparing results with established serum intact LH and FSH measures. Pearson correlations of paired urinary and serum LH and FSH measures were calculated using averaged data from the 30 menstrual cycles, aligned by day of serum LH surge (n = 799 specimens, 34 cycle days).
Stability
Large volume, pooled, in-house specimens (n = 8) were used to examine (1) the effects of long-term storage at 4°C and at −20°C, and (2) stability during short-term RT storage and repeated freeze-thaw cycles (FTC), on βLH and βFSH measured in urine specimens stored without preservatives. Specimens were assayed on the day of collection to establish a baseline, then divided into 1mL aliquots. To test the effects of long-term storage on both βLH and βFSH, specimens were assayed after 1, 2, 4, 8, 16, 32, and 64 weeks stored at 4°C and at −20°C. One additional storage condition was added for βLH, in which specimens were first heated, and then stored refrigerated. To examine the stability of βLH and βFSH in urine specimens subjected to short-term storage at RT and through repeated freeze-thaw cycles, specimens were kept for 0, 1, 2, 4, and 8 days at RT, and then underwent 0, 1, 2, 4, or 8 FTC to determine if there was any interaction between specimen treatment regimes. For the RT-FTC experiment, the specimens were assayed in the same batch after all treatment scenarios had been completed. Thus, the specimens were stored frozen, or refrigerated in the case of specimens with 0 FTCs, for lengths of time varying from 7 to 28 days. For adequate statistical power to detect changes in concentration, four aliquots for each specimen were subjected to each of the storage time and temperature conditions. Each of those aliquots was assayed in quadruplicate, with two wells on one plate and two identical wells on another plate, to minimize the potential for bias resulting from within-plate effects. This design balances within and between plate effects in the experiment to make treatment effects apparent. The same specimens and baseline values were used as the basis for comparison with results from both sets of treatment experiments. Results were analyzed using linear mixed effects models.
RESULTS
Heating specimens prior to assay to dissociate the alpha and β subunits significantly increased βLH, but not βFSH, concentration (Table 1). βLH values were 220% higher on average after heating, and the proportion of assay wells without detectable βLH values decreased from 33.3% before heating to 1.4% after heating. βFSH values were not significantly different in specimens before and after heating, and the proportion of wells with βFSH concentrations below the detectable range changed only slightly (0% before heating to 2.3% after). We thus retained the specimen heating step for the βLH assay, but not for the βFSH assay. All urine specimens used in experiments to evaluate the performance of the β LH assay were heated before use, as were βLH urine controls.
Table 1.
βLH and βFSH before and after heating.
| β LH | β FSH | |||
|---|---|---|---|---|
| not heated | heated | not heated | heated | |
| N | 216 | 213 | 216 | 216 |
| # undetectable | 72 | 3 | 0 | 5 |
| # detectable | 144 | 210 | 216 | 211 |
| Mean (SD) pmol/L | 14.7 (21.5) | 36.4 (36.2) | 85.4 (80.8) | 71.5 (65.7) |
| Mean (SD) pmol/L differences, heated - not heated | 21.7* (23.9) | −13.9† (28.8) | ||
p = 0.0013
p = 0.0571
Cross-reactivity with hormones of similar structure, shown in Table 2, was very low for both assays. The βLH assay showed slight (7.6%) cross-reaction with intact LH. The βLH IEMA thus preferentially measures free β subunit of LH after specimens have been heated to dissociate the heterodimer into its alpha and β subunits. The βFSH assay binds to β in both its intact and free forms, but showed higher affinity (263% cross-reaction at 50% response) for intact FSH than for the free β subunit of FSH.
Table 2.
Specificity for βLH and βFSH IEMA.
| β LH | β FSH | ||
|---|---|---|---|
| analyte | maximum dose tested (pmol/L)§ | % cross reaction at maximum dose tested | % cross reaction at maximum dose tested |
| hLH alpha | 384,615 | 0.00 | 0.05 |
| hCG (CR127) | 87,720 | 0.00 | 0.03 |
| Intact TSH | 176,680 | 0.00 | 0.02 |
| β TSH | 326,795 | 0.00 | 0.05 |
| Intact LH | 175,440 | 7.60* | 0.02 |
| β LH | 322,580 | 100.00 | 0.69 |
| Intact FSH | 147,060 | 0.00 | 263.00† |
| β FSH | 238,095 | 0.00 | 100.00 |
Maximum dose is equivalent to 5 μg/mL for each analyte.
Cross reaction at 50% response given, rather than at maximum dose; 101.8 pmol/L intact LH yielded 50% response relative to a βLH curve.
Cross reaction at 50% response given, rather than at maximum dose; 23.5 pmol/L intact FSH yielded 50% response relative to a βFSH calibration curve.
Percentages of added mass at low, medium, and high doses recovered from a urine matrix are shown in Table 3. Table 4 shows measures of intra- and inter-assay imprecision; within-assay CVs for both IEMAs were below 6%, but between-assay CVs were considerably higher, particularly for the βLH assay. Detection limits, estimated as the lowest dose for which results were significantly different from zero (p <0.05) (21), were 2.5 pmol/L for βLH and 6.8 pmol/L for βFSH (based on n = 20 microtiter plates). Both assays exhibited parallelism (Figure 1); linear mixed effects models found that the slopes of serially diluted specimens (n = 8 for βLH; n = 11 for βFSH) concentration plotted against urine volume per well were not significantly different from zero (βLH: average slope = −0.014, SD 0.013, p = 0.3; βFSH: average slope = −0.06, SD 0.07, p = 0.4).
Table 3.
Recovery of added metabolites in urine.
Table 4.
Imprecision (CV), n = 10 plates
| Assay | Mean concentration (pmol/L) | CV (%) |
|---|---|---|
| LH | ||
| Within-run | 4.7 | 4.8 |
| 9.3 | 4.7 | |
| 15.2 | 2.7 | |
| Between-run | 4.7 | 25.7 |
| 9.3 | 20.3 | |
| 15.2 | 23.2 | |
| FSH | ||
| Within-run | 40.0 | 3.9 |
| 75.8 | 4.6 | |
| 123.3 | 5.6 | |
| Between-run | 40.0 | 14.5 |
| 75.8 | 14.5 | |
| 123.3 | 17.0 | |
Figure 1.
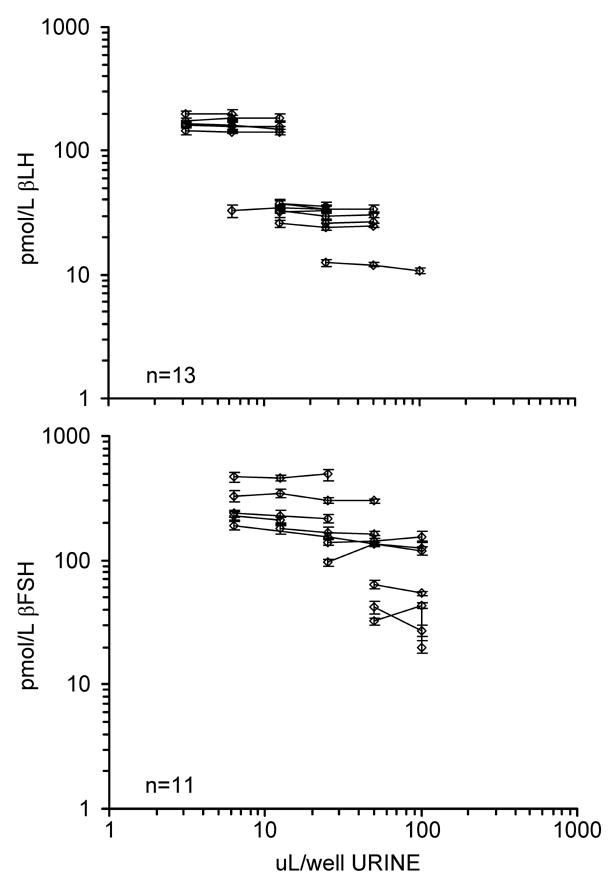
βLH and βFSH assay parallelism. Log concentration of urinary βLH (top panel) and urinary βFSH (bottom panel) plotted against log urine volume. Error bars are ± 2 SE.
Urinary βLH and βFSH values paralleled the serum intact LH and FSH results across the menstrual cycle (Figure 2). Pearson correlations between the urine and serum values for 30 averaged cycles were 0.86 for FSH and 0.95 for LH (n = 34 cycle days). Incorporating lag effects between serum and urine results did not improve correlations. Correlations with a lag of urine one day after serum were 0.68 for FSH and 0.50 for LH.
Figure 2.
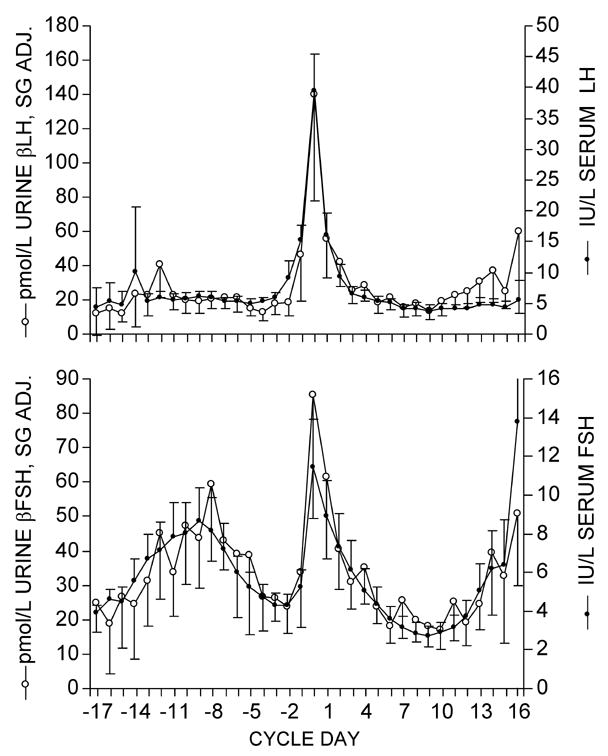
Mean (± 2 SE) gonadotropin profiles of 30 cycles from daily paired urine and serum specimens. Top panel: serum intact LH and urinary βLH; Bottom panel: serum intact FSH and urinary βFSH. Cycles are aligned by day of serum LH peak (day 0). Number of observations varies by cycle day, with a minimum of 4 observations on cycle days -17 and 16 and a maximum of 28 to 30 observations for cycle days -10 through 11. pmol/L SG Adj. = urinary hormone concentration adjusted for specific gravity.
There was no evidence of a consistent trend in measurements of βLH and βFSH at intervals varying from zero to 64 weeks of storage at 4°C and at −20°C. Individual-level data are shown in Figure 3 and average concentrations across all specimens for each time point are shown in Table 5. Estimates from the mixed effects models showed that freezer storage of βLH for 64 weeks resulted in a 3% decline from baseline. Larger decreases were seen for βLH with storage for 64 weeks in the refrigerator before (17%) and after (8%) applying heat to dissociate the subunits. However, βLH showed no consistent trend across time for any storage type (p = .2) (Figure 3, panel A). There were differences from baseline in βFSH results after storage refrigerated or frozen for up to 64 weeks, but with no consistent trend in direction (Figure 3, panel B). βFSH increased by less than 1% (p = .053) up to 32 weeks of refrigerator storage. There was no significant trend in βFSH up to 32 weeks for freezer storage (p = .7). There was a 40% decline from week 0 in βFSH by 64 weeks (p<.0091), which was consistent across storage type (p = .6).
Figure 3.
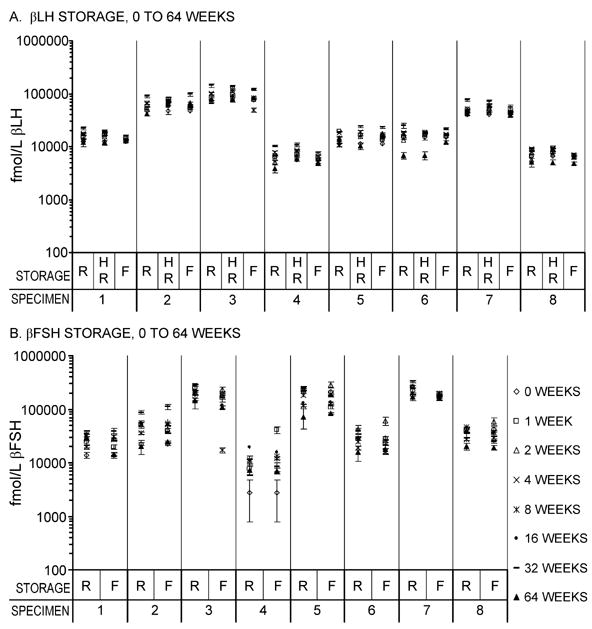
Long-term stability of βLH and βFSH in urine specimens. Log urinary βLH (Panel A) and urinary βFSH (Panel B) measured after 0, 1, 2, 4, 8, 16 (βFSH only), 32, and 64 weeks of storage refrigerated (R) or frozen at −20°C (F). Panel A, βLH, includes an additional treatment where specimens were heated to dissociate subunits before storage in the refrigerator (HR). Values at 16 weeks of storage are missing for βLH because laboratory errors led to assay failure. Error bars are ± 2 SE.
Table 5.
Mean (SD) βLH and βFSH concentrations across specimens (n = 8) over 64 weeks of storage.
| β LH (pmol/L) | β FSH (pmol/L) | ||||
|---|---|---|---|---|---|
| week | refrigerator | heat-refrigerator | freezer | refrigerator | freezer |
| 0 | Baseline: 26.8 (25.2) | Baseline: 87.2 (91.0) | |||
| 1 | 30.1 (26.7) | 33.0 (31.2) | 28.5 (25.7) | 111.2 (112.6) | 76.3 (64.3) |
| 2 | 27.4 (24.9) | 32.4 (30.3) | 29.7 (27.3) | 123.6 (104.4) | 115.8 (106.5) |
| 4 | 35.9 (33.7) | 37.8 (36.8) | 30.0 (27.9) | 102.4 (98.8) | 89.7 (79.4) |
| 8 | 34.7 (34.3) | 43.8 (42.1) | 25.2 (19.8) | 72.7 (72.9) | 60.5 (68.4) |
| 16 | - | - | - | 87.0 (75.9) | 73.1 (63.7) |
| 32 | 53.7 (50.9) | 45.2 (44.6) | 43.7 (43.5) | 134.2 (131.4) | 88.2 (72.3) |
| 64 | 25.6 (26.5) | 30.2 (31.1) | 30.1 (30.0) | 59.5 (63.0) | 54.2 (55.9) |
The association between the number of FTCs and the number of days of storage at RT for βFSH and βLH are shown in Figure 4. βLH increased slightly across FTC (1% increase/FTC, p = .2) and days RT (3% increase/day RT, p = .0083) compared to baseline. βFSH varied across FTC (maximum of 14% increase from baseline at FTC 1, p = .029), and across days RT (maximum 7% increase from baseline at 2 days RT, p=.044), but showed no consistent decline over FTC, (p = .2) or days RT (p = .9) with a <1% change in βFSH per unit for either variable.
Figure 4.
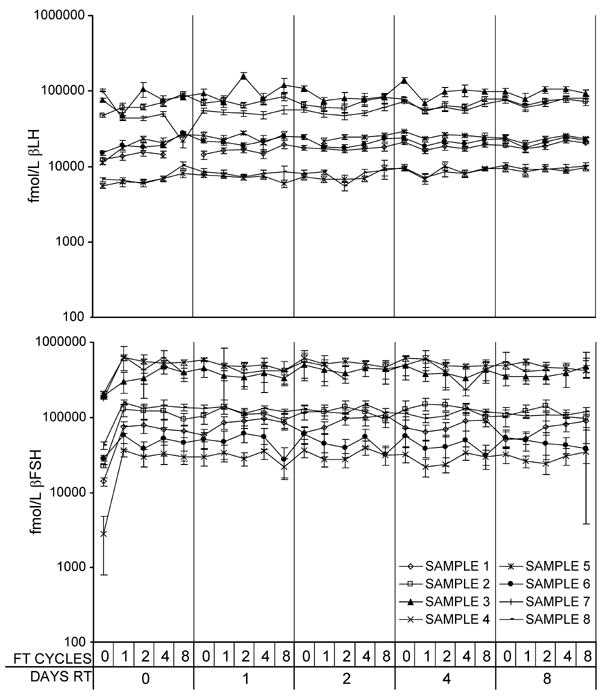
Effects of short-term room temperature storage and repeated freeze-thaw cycles. Log urinary βLH (Panel A) and urinary βFSH (Panel B) measured after 0, 1, 2, 4, or 8 days at room temperature (RT), and 0, 1, 2, 4 or 8 freeze thaw (FT) cycles. Error bars are ± 2 SE.
DISCUSSION
The assays for βLH and βFSH described here were developed for large-scale, population-level research, where collecting blood specimens is not logistically feasible, and commercially available kit assays are prohibitively expensive. Validation and stability experiments demonstrate their suitability for non-clinical research projects involving specimen collection in study participants’ homes, where storage of the specimens is out of the investigators’ control, and conditions may be less than ideal. These assays for urinary free βLH and total βFSH are inexpensive compared to commercial kits, the reagents are readily available, and they are robust to the types of collection and storage conditions characteristic of non-clinical, population-level research. Hormone patterns obtained from the urinary assays closely parallel those derived from serum measures, but the urine specimens are less cumbersome and invasive to collect and process.
The βLH and βFSH IEMAs showed acceptable performance in evaluations of recovery, parallelism, minimum detectable dose, and specificity. For both assays, within-assay imprecision was low. Between-assay imprecision was higher, suggesting a need for careful quality control to detect differences between assay batches. For βLH, all specimens used in the validation experiments were heated as suggested for routine use of the assay, and all results suggest accurate and reliable performance with heated specimens. Results of the cross-reactivity experiments were congruent with those of the pre- and post-heating experiments for both assays. The βLH assay had very little cross-reactivity with intact LH, but the βFSH assay detects the β subunit whether in free or heterodimer form. Parallelism in serially-diluted specimens (Figure 1) and the parallel dose-response curves of βFSH and intact FSH (data not shown) used to calculate cross-reactivity demonstrate that although the assay preferentially binds intact FSH, calibration by βFSH provides valid results. However, these results suggest that it would be possible to use intact FSH reference preparations to calibrate this assay in place of the free β subunit reference preparation used here.
Both assays showed very little within-assay noise, and acceptable between-assay variability with imprecision estimated from ten microtiter plates using a standard method described by Rodbard (20). However, βFSH assay between-batch variability in the long-term storage experiments appeared to be higher than would be expected given our estimates of assay imprecision. Given the timed nature of the storage experiment, and that the experiment was designed to look for change in concentration across time and treatment, some results that may have been rejected under strict quality control criteria were included in the analyses. This was necessary to avoid biasing results in the direction of finding no effect of storage time. Four aliquots were made for each specimen/treatment combination, and each of those four aliquots was assayed in quadruplicate, with the aim of increasing statistical power for detecting differences between storage time and treatment. These two elements of the experiments design may, however, have contributed to the appearance of higher βFSH assay imprecision in the long-term storage experiments, as even outliers among the 16 replicate results for each specimen/treatment combination were retained to avoid introducing bias.
Linear mixed effects models did not find any evidence of a consistent trend in either direction over storage time for either assay. With respect to long term storage, we found that βLH was stable at 4ºC (17% decline) and −20ºC (3% decline) for up to 64 weeks. βLH measures for week 16 of the long-term storage experiment are missing because an error was made while carrying out the assay. However, because there was little change between weeks 8 and 32 of the experiment, a change in βLH at week 16 is unlikely. Storing specimens frozen after heating was not tested for the βLH assay; this storage scenario is unlikely to be efficient for processing and assaying large numbers of specimens.
There was no net decline in βFSH by 32 weeks at either 4ºC or −20ºC storage, but there was a significant decline (40%) between weeks 32 and 64 for both storage conditions, as well as higher variability across storage time as compared to βLH. For βFSH, results of the storage experiment are difficult to interpret. Specimens were not heated to ensure complete dissociation of the subunits for the βFSH assay. It may be that the relative proportions of βFSH in the free and intact forms changed with long term storage, and this result may reflect the assay’s preference for measuring βFSH still in the intact form over free βFSH, demonstrated in the cross-reactivity experiment. If this is the case, long term storage beyond 32 weeks may present a problem unless there is uniformity in storage time and conditions for all specimens across a single study. However, batch and time effects are confounded in the long-term storage experiment, but the lack of a consistent trend in βFSH values over time is suggestive that storage time may be less important than batch. Perhaps the most informative long term storage experiment we conducted was unintentional: urine specimens frozen for two years before assay yielded gonadotropin patterns highly correlated with serum specimens assayed soon after collection (Figure 2); the overall patterns were clearly retained.
Our findings for βLH and βFSH stored long-term at 4ºC are in agreement with other studies reporting that urinary intact LH and FSH are stable without preservatives for several months at 4ºC (7, 9, 13). While we found no significant loss in βLH or βFSH for up to 32 weeks at −20C, other investigators report significant loss of the intact forms of these hormones when stored frozen without preservatives for 1 to 24 weeks (7, 9, 10, 13). We conclude that long term storage at refrigerated or frozen temperatures is feasible when using assays targeting the dissociated and intact forms of LH and FSH.
Overall, number of days at room temperature and number of freeze-thaw cycles had no significant effect on the stability of βLH and βFSH as measured with our IEMAs. There was a significant and unusual increase from baseline to the first FTC and first day at RT for βFSH (Figure 4). The apparent increase in FSH between the baseline and all other treatments in the RT and FTC treatment experiments was not seen in the long-term storage experiment. There was some duplication of conditions between the two experiments, and they used the same baseline values (from assay on the day of collection). Because the increase was not consistent between the two experiments, we hypothesize that the increase may be an assay batch effect. Further experiments are needed to determine if the increase reflects equilibration of the subunits or other changes in composition of the specimens that occurs immediately after collection. If this pattern is reproduced in future experiments, variability resulting from this increase could be minimized or avoided by allowing all specimens to equilibrate for at least one day before they are assayed.
Previous work found significant declines in urinary intact LH and FSH with storage at room temperature (9, 13) at 7 days and 7 weeks. Similarly, while previous work found effects of three to ten freeze-thaw cycles on intact LH and FSH (9, 12), we did not find any effects of up to 8 freeze-thaw cycles on βLH and βFSH (less than 1% change per cycle for each). As with the long term storage, we attribute the differences in these findings to the ability of our IEMAs to target dissociated forms of LH and FSH.
Our βLH and βFSH IEMAs were developed using the approach taken by Qiu et al. (3) and Clough et al. (22) targeting the subunits of the LH and FSH molecules for immunoassay. The Clough et al. (22) assay targets intact LH and the alpha subunit of LH; a limitation of their assay is cross-reactivity with the alpha subunits of thyroid stimulating hormone, human chorionic gonadotropin, and FSH. Our βLH IEMA is highly specific for the dissociated β subunit of LH and is thus useful for research where high specificity for LH is required. The main limitation for our βLH IEMA is the added step of heating urine specimens prior to assay. Our FSH IEMA offers few advantages over the Qiu et al. (3) βFSH IEMA, except that heating specimens prior to assay is not needed and both of the antibodies are widely available for our assay.
Patterns of β-subunit LH and FSH may not be identical in all cases to those of the intact hormones in serum, but are nonetheless likely to be biologically meaningful, and proportional to pituitary production of intact LH and FSH. The β-subunits of LH and FSH are regulated, produced and secreted separately from each other and from the common alpha subunit. Free excess alpha may be found in the blood, whereas the free β subunits of each, which confer specific biological actions, are rarely found in the plasma in healthy individuals (23, 24). Regulation of bioactive LH and FSH is most likely mediated by production of the β-subunits; for example, changes in the frequency and amplitude of gonadotropin releasing hormone pulses differentially stimulate synthesis of the β-subunits of each (25, 26). Production of the β-subunit is likely the limiting step in production of the bioactive heterodimer forms of both LH and FSH (23), and would thus be expected to be proportional to the amount of bioactive hormone in circulation.
Close monitoring of quality control data or running all specimens from each participant together in the same batch are important for the βFSH and βLH assays. These assays are designed for population-level, rather than for individual-level, diagnostic use. The results of our storage time and temperature experiments suggest these assays provide reasonable estimates of βLH and βFSH, even under the worst-case scenarios tested here.
These IEMAs for βLH and βFSH are effective and efficient for use in population-level studies where practicality and cost prohibit the use of serum assays. Additionally, preservatives and extraction are not needed: because they target the β subunit in urine specimens, these IEMAs are robust to collection and storage conditions encountered in non-clinical research settings.
Acknowledgments
We thank the National Hormone & Peptide Program (NHPP), NIDDK, and Dr. A.F. Parlow for providing antibodies and purified hormones.
Footnotes
SOURCES OF FINANCIAL SUPPORT: NIA RO1AG015141; NIA RO1AG14579 NICHD 2P30HD028263; NICHD R24 HD042828
Population Research Institute, Pennsylvania State University; Center for Studies in Demography and Ecology, University of Washington
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57 (Suppl 2):2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- 2.Prior JC. Perimenopause: The complex endocrinology of the menopausal transition. Endocrine Reviews. 1998;19:397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- 3.Qiu Q, Kuo A, Todd H, Dias JA, Gould JE, Overstreet JW, Lasley BL. Enzyme immunoassay method for total urinary follicle-stimulating hormone (FSH) beta subunit and its application for measurement of total urinary FSH. Fertil Steril. 1998;69:278–85. doi: 10.1016/s0015-0282(97)00475-5. [DOI] [PubMed] [Google Scholar]
- 4.Reyna R, Traynor KD, Hines G, Boots LR, Azziz R. Repeated freezing and thawing does not generally alter assay results for several commonly studied reproductive hormones. Fertility and Sterility. 2001;76:823–5. doi: 10.1016/s0015-0282(01)01986-0. [DOI] [PubMed] [Google Scholar]
- 5.Taieb J, Benettar C, Birr AS, Lindenbaum A, Frydman R, Olivennes F. Delayed assessment of serum and whole blood estradiol, progesterone, follicle-stimulating hormone, and luteinizing hormone kept at room temperature or refrigerated. Fertility and Sterility. 2000;74:1053–4. doi: 10.1016/s0015-0282(00)01546-6. [DOI] [PubMed] [Google Scholar]
- 6.Kwekkeboom DJ, de Jong FH, Lamberts SW. Confounding factors in the interpretation of gonadotropin and gonadotropin-subunit release from cultured human pituitary adenomas. J Steroid Biochem. 1989;33:777–82. doi: 10.1016/0022-4731(89)90491-3. [DOI] [PubMed] [Google Scholar]
- 7.Kesner JS, Knecht EA, Krieg EF. Stability of urinary female reproductive hormones stored under various conditions. Reproductive Toxicology. 1995;9:239–44. doi: 10.1016/0890-6238(95)00005-u. [DOI] [PubMed] [Google Scholar]
- 8.Taylor A, Khoury RH, Crowley JWF. A comparison of 13 different immunometric assay kits fo rgonadotropins: Implications for clinical investigation. Journal of Clinical Endocrinology and Metabolism. 1994;79:240–7. doi: 10.1210/jcem.79.1.8027235. [DOI] [PubMed] [Google Scholar]
- 9.Demir A, Alfthan H, Stenman U-H, Voutilainen R. A clinically useful method for detecting gonadotropins in children: assessment of luteinizing hormone and follicle-stimulating hormone from urine as an alternative to serum by ultrasensitive time-resolved immunofluorometric assays. Pediatric Research. 1994;36:221–6. doi: 10.1203/00006450-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Saketos M, Sharma N, Adel T, Raghuwanshi M, Santoro N. Time-resolved immunofluorometric assay and specimen storage conditions for measuring urinary gonadotropins. Clinical Chemistry. 1994;40:749–53. [PubMed] [Google Scholar]
- 11.Kulin H, Demers L, Chinchilli V, Martel J, Stevens L. Usefulness of sequential urinary follicle-stimulating hormone and luteinizing hormone measurements in the diagnosis of adolescent hypogonadotropism in males. J Clin Endocrinol Metab. 1994;78:1208–11. doi: 10.1210/jcem.78.5.8175980. [DOI] [PubMed] [Google Scholar]
- 12.Landy H, Schneyer A, Whitcomb RW, Crowley JWF. Validation of Highly Specific and Sensitive Radioimmunoassays for Lutropin, Follitropin, and Free Alpha Subunit in Unextracted Urine . Clin Chem. 1990;36:340–4. [PubMed] [Google Scholar]
- 13.Livesey JH, Roud HR, Metcalf MG, Donald RA. Glycerol prevents loss of immunoreactive follicle-stimulating hormone and leteinizing hormone from frozen urine. Journal of Endocrinology. 1983;98:381–4. doi: 10.1677/joe.0.0980381. [DOI] [PubMed] [Google Scholar]
- 14.Miro F, Parker SW, Aspinall LJ, Coley J, Perry PW, Ellis JE. Relationship between follicle-stimulating hormone levels at the beginning of the human menstrual cycle, length of the follicular phase and excreted estrogens: The FREEDOM study. Journal of Clinical Endocrinology and Metabolism. 2004;89:3270–5. doi: 10.1210/jc.2003-031732. [DOI] [PubMed] [Google Scholar]
- 15.Santoro N, Isaac B, Neal-Perry G, Adel T, Weingart L, Nussbaum A, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. The Journal of Clinical Endocrinology and Metabolism. 2003;88:5502–9. doi: 10.1210/jc.2002-021839. [DOI] [PubMed] [Google Scholar]
- 16.Krichevsky A, Birken S, O’Connor J, Bikel K, Schlatterer JP, Canfield RE. The development of a panel of monoclonal antibodies to human luteinizing hormone and its application to immunological mapping and two-site assays. Endocrine. 1994;2:511–20. [Google Scholar]
- 17.Miller RC, Brindle E, Holman DJ, Shofer JB, Klein NA, Soules MR, O’Connor KA. Comparison of specific gravity and creatinine methods for normalizing urinary reproductive hormone concentrations. Clin Chem. 2004;50:924–32. doi: 10.1373/clinchem.2004.032292. [DOI] [PubMed] [Google Scholar]
- 18.Liu JH, Kao L, Rebar RW, Muse K. Urinary β-FSH subunit concentrations in perimenopausal and postmenopausal women: a biomarker for ovarian reserve. Menopause: The Journal of the North American Menopause Society. 2003;10:526–33. doi: 10.1097/01.GME.0000070524.74726.18. [DOI] [PubMed] [Google Scholar]
- 19.O’Fegan P. Validation. In: Gosling JP, editor. Immunoassays: A Practical Approach. Oxford: Oxford University Press; 2000. pp. 211–38. [Google Scholar]
- 20.Rodbard D. Statistical quality control and routine data processing for radioimmunoassays and immunoradiometric assays. Clin Chem. 1974;20:1255–70. [PubMed] [Google Scholar]
- 21.Rodbard D. Statistical estimation of the minimal detectable concentration (“sensitivity”) for radioligand assays. Anal Biochem. 1978;90:1–12. doi: 10.1016/0003-2697(78)90002-7. [DOI] [PubMed] [Google Scholar]
- 22.Clough KM, Cole FX, Seaver SS, Vesprini A, Kuo AY, Lasley BL. Enzyme immunoassay method for total alpha gonadotropin in human urine samples. Fertil Steril. 1992;57:1241–6. [PubMed] [Google Scholar]
- 23.Katznelson L, Alexander JM, Bikkal HA, Jameson JL, Hsu DW, Klibanski A. Imbalanced follicle-stimulating hormone beta-subunit hormone biosynthesis in human pituitary adenomas. J Clin Endocrinol Metab. 1992;74:1343–51. doi: 10.1210/jcem.74.6.1375599. [DOI] [PubMed] [Google Scholar]
- 24.McCann SM, Ojeda SR. The Anterior Pituitary and Hypothalamus. In: Griffen JE, Ojeda SR, editors. Textbook of Endocrine Physiology. 3. New York: Oxford University Press; 1996. pp. 101–33. [Google Scholar]
- 25.Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK. Regulation of the rat follicle-stimulating hormone beta-subunit promoter by activin. Mol Endocrinol. 2003;17:318–32. doi: 10.1210/me.2002-0081. [DOI] [PubMed] [Google Scholar]
- 26.Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of Gonadotropin Gene Expression. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Vol. 1. New York: Raven Press; 1994. pp. 1793–813. [Google Scholar]


