Summary
Non-visual responses to light, such as photic entrainment of the circadian clock, involve intrinsically light sensitive melanopsin-expressing ganglion cells as well as rod and cone photoreceptors. However, previous studies have been unable to demonstrate a specific contribution of cones in the photic control of circadian responses to light. Using a mouse model that specifically lacks mid-wavelength (MW) cones we show that these photoreceptors play a significant role in light entrainment and in phase shifting of the circadian oscillator. The contribution of MW cones is mainly observed for light exposures of short duration and towards the longer wavelength region of the spectrum, consistent with the known properties of this opsin. Modelling the contributions of the various photoreceptors stresses the importance of considering the particular spectral, temporal and irradiance response domains of the photopigments when assessing their role and contribution in circadian responses to light.
Keywords: Animals; Circadian Rhythm; physiology; radiation effects; Cones (Retina); abnormalities; physiology; Gene Expression; physiology; Light; Male; Mice; Mice, Knockout; Models, Biological; Motor Activity; Opsin; genetics; physiology; Photoperiod; Retinal Ganglion Cells; physiology; Thyroid Hormone Receptors beta; genetics
Introduction
Entrainment of the circadian clock and other non-visual responses to light involves intrinsically light sensitive ganglion cells (ipRGCs) that express the rhabdomeric photosensory pigment melanopsin (Berson et al., 2002; Arendt et al., 2004) and that receive modulatory inputs from both rods and cones (Berson et al., 2002; Belenky et al., 2003). Although the functional absence of rods, cones and melanopsin abolishes all non-visual responses to light (Hattar et al., 2003), defining the relative roles and contributions of the different photopigments in non-visual responses has proven to be challenging. The main strategies employed have involved investigation of these non-visual responses in mutant/transgenic mice lacking selected photopigments resulting from retinal degeneration of rods and/or cones (rd, rds, cl, rd/cl, rdta/cl), transgenic knockout mice with invalidation of rod/cone phototransduction pathways (gnat1−/−, cnga3−/−) or of the photopigment melanopsin gene (Opn4−/−).
Depending on the retinal alteration and light stimulus, the functional absence of one or more of these photopigments generally leads to anomalies in light induced phase shifts, entrainment, pupillary light reflex (PLR), or masking. For example, melanopsin knockout mice (Opn4−/−) exhibit attenuated phase shifts of circadian locomotor activity in response to a light pulse, coupled with diminished entrainment to low light levels and an attenuated PLR in bright light (Panda et al., 2002; Panda et al., 2003; Lucas et al., 2003; Hattar et al., 2003). Some individual Opn4−/− mice may become active during the light phase and masking of activity by light is also altered (Mrosovsky and Hattar, 2005).
Mice with intact ipRGCs but lacking both rods and cones (rd/rd cl) show a significant loss in sensitivity in their PLR (Lucas et al., 2001; 2003) but not in their ability to phase shift, at least if bright white light pulses are used(Semo et al., 2003). Rodless-coneless gnat1−/−, cnga3−/− mice are reported to show the same pupil response as the rd/rd cl mouse (Hattar et al., 2003). In contrast, mice that that predominantly lack rods are reported to show normal phase shifts (rd/rd, Foster et al., 1991; rds, Argamanso et al., 1995), but fail to entrain at low (<1 lux) light levels (Ebihara and Tsuji, 1980; Mrosovsky, 2003) and have altered masking responses (Mrosovsky, 2003). In addition, rd/rd mice show diminished sensitivity for PLR (Panda et al., 2003; Van Gelder et al., 2003).
Only a single study has examined the phase shifting response in a mouse lacking cones (Freedman et al., 1999). In the transgenic cl/cl mouse model almost all mid-wavelength (MW) cones and the majority of short-wavelength (SW) cones are eliminated. Despite this deletion, phase shifts for a range of irradiances using mid-wavelength light were reported to be essentially similar in the cl/cl and wild-type mice suggesting that cones do not play a significant role in the response.
Interpretation of these findings obviously poses a number of problems related to the anatomical alteration of the retina, the response investigated and the quantitative and qualitative properties of the light stimulus employed. First, the nature of the retinal deletion (mutation, transgene, altered transduction pathway) can lead to different consequences related to retinal plasticity during development including effects of transcriptional regulation on conserved photopigments (Sakamoto et al., 2004). Second, the relative contribution of rods, cones and melanopsin ipRGCs differs according to the response assayed and the associated structures involved. The suprachiasmatic nucleus (SCN) is almost exclusively innervated by ipRGCs, whereas the olivary pretectal nucleus (involved in the PLR) receives significant additional input from non-ipRGCs (Hattar et al., 2006). Masking of activity by light relies on a more complex and distributed network of non-visual and visual structures (Mrosovsky and Hattar, 2005). Finally, with the exception of the PLR (Lucas et al., 2001; Hattar et al., 2003; Lucas et al., 2003), the majority of studies in mice with altered retinas have not characterised circadian responses in relation to irradiance, duration and wavelength of the light stimulus. Most reports have used broadband white light or a single wavelength (480 – 500 nm) limiting the scope of analysis of the spectral, irradiance and temporal response domains of the underlying photopigments. For example, rhodopsin in mice has a maximum of absorbance at 498 nm and dominates retinal function in low light (scoptopic) levels, whereas MW and SW cones respond at higher irradiances but each in different spectral domains (λmax = 508 nm and 359 nm respectively (Jacobs et al., 1991). Melanopsin (λmax = 479–484 nm) also requires high irradiances but differs in it’s temporal domain with more sluggish responses to changes in light intensity and resistance to bleaching by light (Berson et al., 2002; Wong et al., 2005).
To dissect out the roles of different photopigments in the photic regulation of the circadian clock, we used TRβ−/− knockout mouse for both isoforms β1 and β2 of the thyroid hormone receptor (Gauthier et al., 1999) which is essential for the development of MW cones in vitro and in vivo (Ng et al., 2001; Forrest et al., 2002). The TRβ2 deletion in mice induces a complete and selective loss of MW-cone opsin without significant changes in total cone numbers. All cones instead express SW-opsin (Ng et al., 2001). We examined entrainment to shifts of the light/dark cycle coupled with a decrease of the light level and phase shifts to brief pulses of monochromatic light at different irradiances and duration, specifically designed to preferentially influence either SW, MW or melanopsin photopigments based on their peak spectral sensitivity. Our results show that MW cones significantly contribute to photic entrainment of circadian responses in a manner coherent with the irradiance, temporal and spectral response properties of the MW-opsin photopigment. Furthermore, modelling the comparative profiles of the responses of the two genotypes provides insight into the relative contributions of both melanopsin and MW cone opsin in the mid-wavelength region of the spectrum.
Results
MW-coneless mice overexpress SW opsin and melanopsin
The lack of MW cones in the TRβ2−/− mice has previously been reported (Ng et al., 2001). The double knockout TRβ−/− mice used in this study present a similar pattern of cone differentiation to the TRβ2−/− mice although the opsin content has not previously been characterized (Gauthier et al., 1999). In order to confirm the absence of MW-opsin and to validate this model, we first examined the expression of MW- and SW-opsins in wild-type and TRβ−/− mice using immunohistochemistry (Figures 1a–b) and real time PCR (Figure 1c). Immunohistochemical labelling using specific antibodies against SW- and MW-opsins demonstrates the absence of MW-expressing cones in the TRβ−/− mice compared to wild-type animals (Figure 1a). All cones instead express SW-opsin as has been described for TRβ2−/− mice (Ng et al., 2001). Figure 1c shows the relative levels of the different opsins (rhodopsin, SW-, MW-opsins and melanopsin) using real time PCR. Transcript of SW-opsin is overexpressed (1.55 fold; p<0.001) in the TRβ−/− mice whereas MW-opsin mRNA is completely absent, confirming the immunohistochemical staining pattern. The rhodopsin mRNA content is equivalent in the wild-type and the knockout mice. Melanopsin transcripts are slightly over-expressed (1.35 fold; p<0.05) in TRβ−/− mice compared to normal mice. However, immunohistochemical labelling and confocal analysis did not reveal any apparent differences between the genotypes in the dendritic morphology or distribution of melanopsin-RGCs (Figure 1b).
Figure 1.
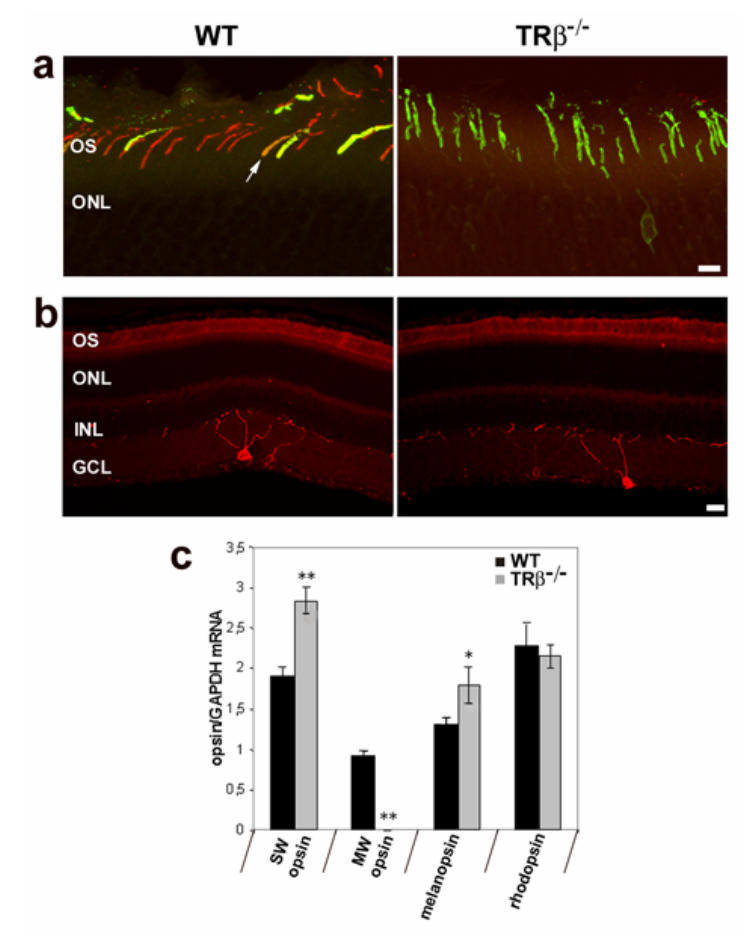
Cone opsins and melanopsin expression in the retina of wild-type (WT) and MW-coneless (TRβ−/−) mice, a) Confocal photomicrographs showing immunohistochemical labelling of MW (red fluorescence) and SW (green fluorescence) opsins in retinal sections. In wild-type mice, both MW and SW opsins are present with co-expression of both opsins in the outer segments of some cones (white arrow, yellow fluorescence). In TRβ−/− mice, MW-immunoreactive cones are not detected and all cones express SW opsin. Scale = 10μm b) Confocal photomicrographs of melanopsin-immunopositive ganglion cells (red fluorescence) in retinal sections of WT and MW-coneless mice showing that the relative number and distribution of melanopsin-containing ganglion cells are similar for both genotypes. Scale = 20 μm; OS: outer segment; ONL: outer nuclear layer; INL: inner nuclear layer; GCL: ganglion cell layer, c) Relative Opsins (SW, MW and rhodopsin) and melanopsin mRNA levels in the retina of WT (black bars) and MW-coneless (grey bars) mice using real time PCR. Results are expressed as mean ± SEM (n = 4 for each genotype). The TRβ−/− knockout mouse is characterized by a total absence of MW opsin and over-expression of SW opsin. The relative level of melanopsin is also up-regulated whereas rhodopsin levels are equivalent in both genotypes. Asterisks indicate a statistically significant difference (Mann-Whitney U test *:p<0.05; **: p<0.01).
Deficits of photic entrainment in MW-coneless mouse
Daily locomotor activity of MW-coneless (TRβ−/−) and wild-type littermate mice were initially synchronized for 3 weeks to a 12L/12D cycle at a light level of 100 lux. Under these high light conditions, both wild-type and MW-coneless mice entrained with normal phase angles and consolidated their locomotor activity to the dark period of the light/dark cycle (Figures 2a–b). We then examined the ability of mice to entrain to a new light/dark cycle that was phase delayed by 6 hrs and coupled with a 1 log unit decrease of irradiance to 10 lux. Although both genotypes adjusted their daily locomotor activity to the new light cycle, a significant difference was observed in the rate and phase angle of re-entrainment. In control mice, stable re-entrainment was achieved after 14.7 ± 2 days whereas locomotor activity of MW-coneless required 21.3 ± 1.1 days (p < 0.001, Figures 2b and 3). In addition, the MW-coneless mice exhibit an abnormal phase angle of entrainment, with a significantly delayed onset of activity with respect to light offset (1.91 ± 0.04 hr, p<0.001). When the same experiment was repeated again using a 6 hr delay of the light/dark cycle with a further decrease in light level (1 lux), wild-type animals entrained but required more time than at 10 lux (23.2 ± 1.1 days, p<0.05) to adjust the onset of locomotor activity whereas knockout mice do not display stable entrainment at least during the 43 days of exposure (Figures 2b and 3).
Figure 2.
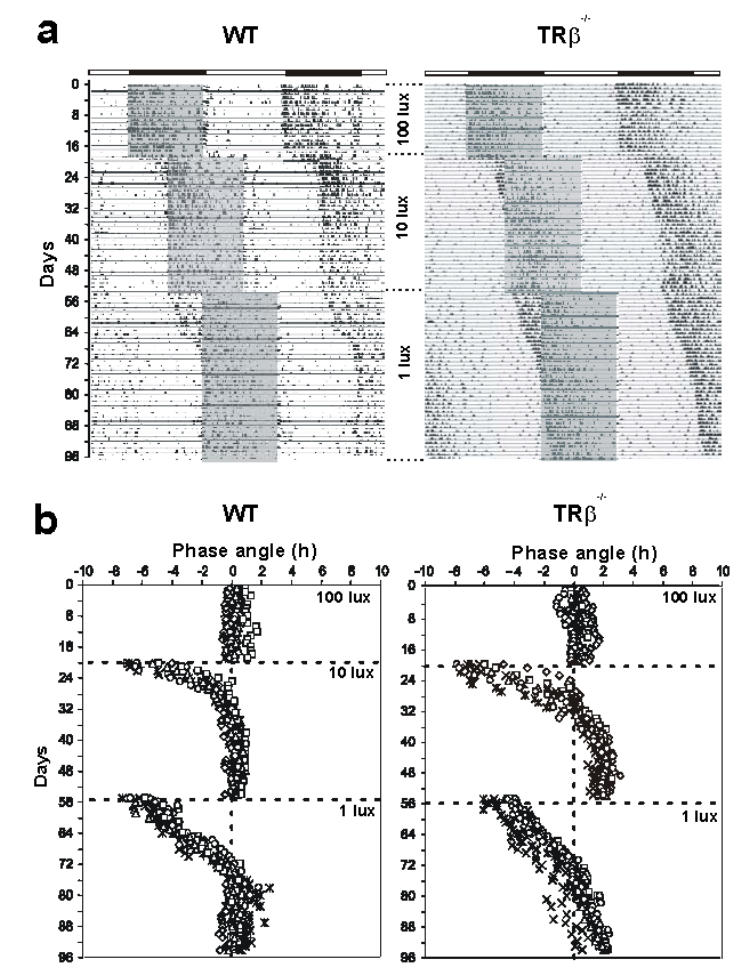
Impaired photic entrainment in MW-coneless mice, a) Two representative actograms of locomotor activity for WT and MW-coneless (TRβ−/−) mice under a 12L/12D cycle, double-plotted on a 24 h timescale. The numbered lines represent successive days and the bar above the actograms indicates the original light/dark cycle. The grey rectangles indicate the dark phase of the subsequent shifted 12L/12D cycles. After 3 weeks of entrainment under a 12L/12D cycle, mice were exposed to successive 6h-delayed light/dark cycle, associated with a decrease of light intensity (from 100 to 10 lux, and from 10 to 1 lux). In the WT mice, locomotor activity had a rhythm of 24 h and was phase-locked to the onset of darkness, showing photic entrainment from 100 to 1 lux. In contrast, MW-coneless mice entrained normally at a high light level (100 lux), entrained with an abnormal phase angle at 10 lux and at 1 lux do not show stable entrainment at least after 43 days, b) Phase angles of activity onsets showing the differences in entrainment for all individual WT and MW-coneless mice (n=8 for both genotypes).
Figure 3.
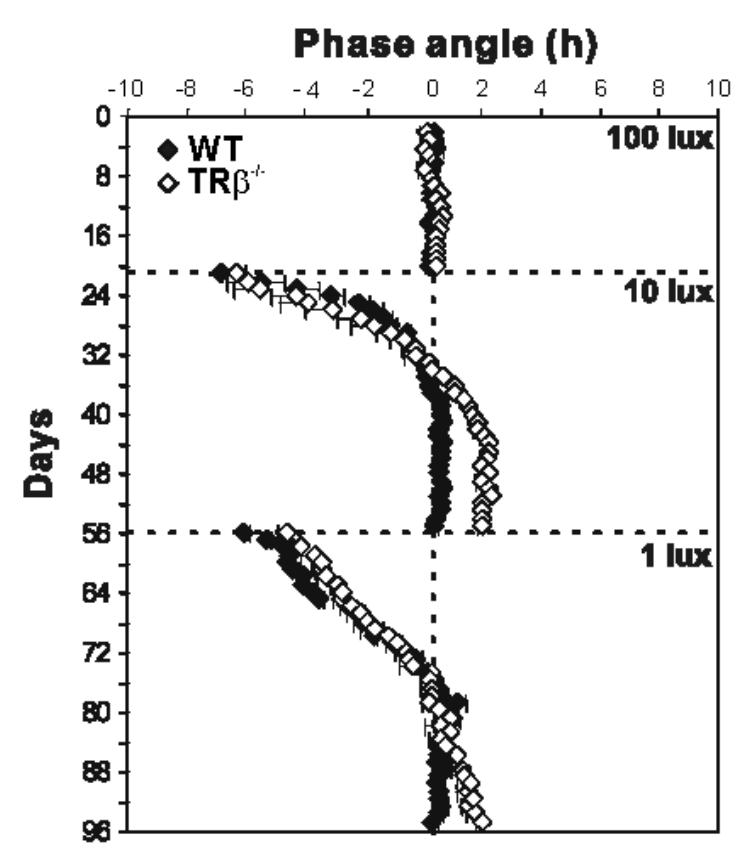
Mean (± SEM) phase angles of activity onsets in wild-type and MW-coneless mice (see figure 2b for individual data). In both genotypes (n=8), the number of days to achieve a stable phase angle of entrainment increases as the light intensity during the light phase decreases Wild-type mice achieve stable re-entrainment (phase angle = 0 hr) after the shift from 100 to 10 lux and from 10 to 1 lux (respectively 14.7 ± 2 and 23.2 ± 1.1 days). In contrast, MW-coneless (TRβ−/−) mice re-entrain with an abnormal phase angle (1.91 ± 0.04 hr, ANOVA, p<0.001) after 21.3 ± 1.1 days for the decrease from 100-10 lux (Mann-Whitney U test; p<0.05) and when the light level is decreased to 1 lux still do not display a stable phase angle of entrainment at least after 43 days.
The spectral and temporal responses for light-induced phase shifts are altered in MW-coneless mouse
The magnitude of light-induced phase shifts of circadian locomotor activity rhythms depends on irradiance and wavelength of light exposure. To assess these effects, we examined the amplitude of phase shifts induced by a 15 min pulse of monochromatic light at 3 different wavelengths (370, 480 and 530 nm) and 4 irradiance levels (Figure 4). For 360 and 480 nm light stimulations, no significant difference in the magnitude of the phase shift of locomotor activity is observed between knockout and wild-type mouse at all irradiance levels used (Figure 4b). For the two wavelengths, and in both genotypes the lowest irradiance stimulus delivered (2.8 × 1010 photons/cm2/s) does not induce a phase shift significantly different compared to dark control animals (0.22 ± 0.03 hrs, data not shown). At higher irradiance levels the magnitude of the phase shift increases proportionately with increased photon flux (from 2.8 × 1011 to 2.8 × 1014 photons/cm2/s). In contrast, the magnitude of the response at 530 nm is significantly attenuated in MW-coneless mice compared to wild-type animals in particular for the higher irradiances (Figure 4b).
Figure 4.
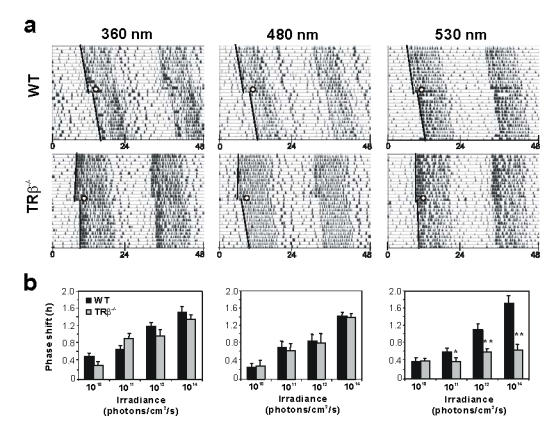
Attenuated phase shifting response to light in MW-coneless mice, a) Representative actograms of locomotor activity of WT and MW-coneless (TRβ−/−) mice exposed to 15-min pulses of monochromatic light (370, 480 and 530 nm) at 4 different irradiances and at CT16 (white circle), b) Mean ± SEM (n=8 for each genotype) phase shifts for WT and TRβ−/− mice at the 3 wavelengths tested. A statistically significant difference was observed between the genotypes only for monochromatic stimulation at 530 nm. Control animals handled in the same way but that did not receive a light pulse show no significant difference between genotypes. Asterisks indicates a statistically significant difference between the two genotypes (ANOVA: p<0.05; post-hoc Student Newman-Keuls tests comparing genotypes at each irradiance: *:p<0.05; **:p<0.01).
The similarity in responsiveness of the circadian system for the two genotypes at 480 nm is unexpected considering the reduction in the response at 530 nm in the MW-coneless mice. To further understand this discrepancy, we repeated a series of 480 nm light exposures at a constant irradiance of 2.8 × 1014 photons/cm2/s but with shorter durations to assess whether there were temporal differences in responsiveness. With the shorter light exposures of 1 and 5 min-duration, knockout mice show significantly attenuated phase delays (p<0.05) compared to wild-type mice (Figure 5) whereas the response is similar between genotypes after 15 min of light stimulation.
Figure 5.
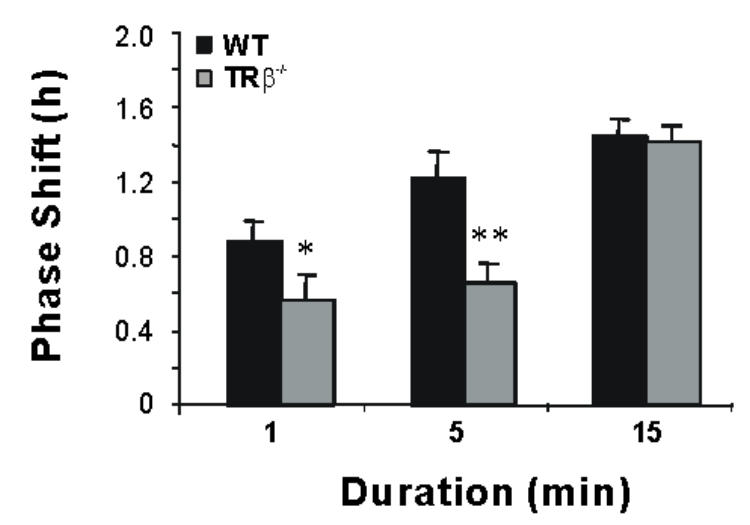
Attenuated phase shifting response to short duration light pulses at 480 nm in MW-coneless mice. A significant difference between genotypes is only observed for light pulses of short duration (Means ± SEM; n=8 for each genotype). Asterisks indicate a statistically significant difference between the two genotypes (ANOVA: p<0.05; post-hoc Student Newman-Keuls tests comparing genotypes at each duration:*:p<0.05; **: p<0.01).
Modelling the relative contributions of MW-opsin and melanopsin
Although this latter result reveals a difference between the 2 genotypes in their temporal responsiveness to light, the similarity of the responses for 15 min exposures at 480 nm coupled with a decrease in the response at 530 nm for the MW-coneless mice is enigmatic. To understand the properties underlying this difference, we modelled the behavioural response based on the hypothesis that the response function in the wild-type mouse is derived from the combined sensitivities of melanopsin and MW-opsin whereas the responsivity of the MW-coneless mice only involves melanopsin. The relative response sensitivities were based on the opsin absorption curves calculated using the Lamb nomogram (Lamb, 1995). Deriving this type of response function is similar to classical procedures applied for example, to determine the underlying additive contributions of MW and long wavelength (LW) cones in the human photopic sensitivity function (Kaiser and Boynton, 1996). The rod response was not taken into consideration since although rods remain intact in both genotypes, previous studies on phase shifts in rd mice suggest little or no contribution of rods at these irradiance levels and the irradiances used in our study are above the response range of rods (Nathan et al., 2006). Likewise, SW opsin was not taken into account since the relative sensitivity of this photopigment is reduced by more than 3 log units in the 480–530 nm region. For the model to be valid, we consider that this hypothesis should fulfil two conditions. The first condition is that the response function calculated for the combined melanopsin and MW-opsin nomograms should correspond to the action spectrum for phase shifting and the PLR in wild-type mice (Foster et al., 1991; Lucas et al., 2001). The second condition requires that the difference between the combined melanopsin and MW-opsin response function (wild-type mice) and the melanopsin curve alone (MW-coneless mice) correspond to the experimentally observed differences in the behavioural responses at 480 and at 530 nm for the two genotypes. If this assumption is correct, then the model should predict a single combination of underlying relative sensitivities for each of the opsins. The equations used and the results are graphically illustrated in Figure 6.
Figure 6.
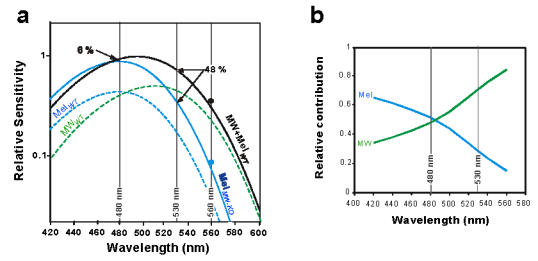
Model of the relative contributions of MW opsin and melanopsin responsiveness derived from the phase shifting response in wild-type and MW-coneless mice, a) The difference in the response functions are based on the mean differences in light-induced phase shifts in MW-coneless mice compared to wild-type for each irradiance at 480 and 530 nm, for values significantly different from the dark controls, respectively 6 % and 48 %. The model (a) is based on additivity of the sensitivities of the two photopigments constrained by the Lamb nomogram and multiplied by the coefficients of sensitivity in the following equations:
where: a = coeff. for sensitivity of melanopsin in the wild-type mouse
b = coeff. for sensitivity of MW-opsin in the wild-type mouse
c = coeff. for sensitivity of melanopsin in the knockout mouse
σ corresponds to the relative sensitivity of the photopigment (Lamb, 1995)
The response function in the wild-type mouse (MW+MelWT, solid black line) corresponds to the combined contribution of melanopsin (MelWT, λmax = 480 nm, dashed blue line) and MW-opsin (MWWT, λmax = 508 nm, dashed green line). The response function in the MW-coneless mouse only involves melanopsin (MelMW-KO, solid blue line). The model predicts that the behavioral responsiveness in the wild-type mouse is derived from relative contributions of 1.0 melanopsin: 1.12 of MW-opsin, with a predicted maximum of sensitivity at 492 nm, which is close to the spectral sensitivity of the circadian system previously reported in the wild-type mouse (Foster et al., 1991; Yoshimura and Ebihara, 1996; Lucas et al., 2001). In a post-hoc experiment to validate the model, we measured light induced phase shifts in response to a longer wavelength (560 nm, 15 min, 2.8 × 1014 photons/cm2/s) in the two genotypes. The amplitudes of the phase shifts were entirely consistent with the predicted values for both the wild-type mouse (0.92 ± 0.16 hrs, black circle) and the MW-coneless mouse (0.21 ± 0.13 hrs, blue circle, see text for further details), (b) Using the lamb nomogram and the derived coefficients for the wild-type mouse, the model allows prediction of the relative contributions of melanopsin and MW-opsin across the spectrum. This shows that in the wild-type mouse, the relative contribution of MW cones is greater for wavelengths above 490 nm while, inversely, at wavelengths shorter than 490 nm melanopsin dominantly contributes to the response.
The model generates a unique set of coefficients that resolve these two equations. Figure 6a illustrates the relative contributions in the wild-type mouse of melanopsin (a = 1.0, dashed blue line) and of MW-opsin (b = 1.12, dashed green line) and the resulting combined response function (black line). The response function of the MW-coneless mouse corresponds to the sensitivity curve for melanopsin alone (c = 1.82, solid blue line). This implies that the response in wild-type mice is derived from relative contributions of 1:1.12 of melanopsin and MW-opsin respectively. In the MW-coneless mice, the relative increase responsiveness of 1.82 may be related to the up-regulation of melanopsin mRNA (Figure 1b). As illustrated, the model also predicts (Figure 6b) that in the wild-type mouse the relative contributions of the MW-opsin and melanopsin systems vary according to wavelength, expressed as a progressively increasing relative contribution of MW-opsin for wavelengths greater than 490 nm and inversely of melanopsin at wavelengths less than 490 nm.
Discussion
Our findings in a mouse model in which a single cone opsin is invalidated demonstrate that MW cones play an essential role in phase shifting responses and in light entrainment. Use of a model based on the underlying photoreceptor response sensitivities, constrained by the opsin nomograms and additivity, provides insight into (and allows prediction of) the relative contributions of melanopsin and MW cone opsin in the mid-wavelength region of the spectrum. Our results also underline the need to consider the particular wavelength, irradiance and temporal response domains of the photopigments when assessing their role in different circadian responses to light.
Deletion of thyroid hormone receptor β induces loss of MW cone opsin in the mouse
Nuclear thyroid hormone receptors are known to play a specific role in retinal development (Forrest et al., 2002) by inducing cone differentiation in cultures of mammalian retinal progenitor cells (Kelley et al., 1995), regulating the development of UV cones in the trout smolt (Browman and Hawryshyn, 1992) and of several retinal cell types in the rat (Sevilla-Romero et al., 2002). In humans, recessive resistance to thyroid hormone causes alterations in the photopic electroretinogram to mid-wavelength light, suggesting a related role for TRβ2 in human retina (Newell and Diddie, 1977). Treatment with T3 hormones of rodent retinal cells in vitro increases the numbers of MW cones and reduces the number of SW cones (Kelley et al., 1995). TRβ2 is prominently expressed in the outer nuclear layer of the embryonic retina and is considered to be necessary for activation of MW-opsin gene promoter function (Yanagi et al., 2002; Roberts, 2005). Deletion of this receptor induces a selective loss of MW-opsin without significant morphological changes in the outer retinal layers or in total cone numbers (Ng et al., 2001), in contrast with rd/rd cl and rdta/cl transgenic mouse models (Freedman et al., 1999; Lucas et al., 1999). In the TRβ−/− mouse, all cones express SW-opsin whereas the coneless cl mouse displays a nearly complete anatomical lack of MW cones (<1%) coupled with a substantial reduction of SW cones (>95% loss; Freedman et al., 1999; Lucas et al., 1999). Our results also provide evidence that the selective absence of MW-cones results in a transcriptional up-regulation of melanopsin mRNA. This result differs from a previous study that suggested a down regulation of melanopsin by rods and cones, obtained, however, in a different model of retinally degenerate RCS rats (Sakamoto et al., 2004).
MW-coneless mice show impaired photic entrainment
Photic entrainment is the primary process by which animals synchronize their circadian rhythms with the environment. The maintenance of an appropriate phase relationship between internal and environmental time has been reported to be dependent on the period of the internal clock as well as the strength of environmental cues (Pittendrigh and Daan, 1976). Our data show that entrainment to a light/dark cycle with decreased light levels is impaired in MW-coneless mice suggesting that in the absence of this opsin the photic signal relayed to the circadian clock is altered. At 100 lux, photic entrainment is identical in both genotypes and the daily rhythms of locomotor activity reflect an appropriate state of entrainment rather than simply masking by light, since activity onsets in darkness can be extrapolated back to the time of activity onsets under the 12L/12D cycle in each individual animal. At a reduced intensity of 10 lux, the photic stimulus is sufficient to entrain the rhythm in MW-coneless mice, but insufficient to maintain a normal phase angle of entrainment. At the lowest light intensity (1 lux) MW-coneless mice do not display a stable phase angle of entrainment during the 43 days in our test conditions, although, it cannot be excluded that after an additional number of days in these conditions a stable phase angle of entrainment could be achieved. In comparison, photoreceptor deficient mice, (rd/rd, rds/rds, rdta, rd/rd cl, rdta/cl) were originally reported to display normal entrainment to a light/dark cycle, prompting the conclusion that rods and cones do not play a role in photic entrainment (Foster et al., 1991; Argamaso et al., 1995; Freedman et al., 1999). However, all these studies used relatively high irradiance levels, whereas when low light levels are employed (≤ 1 lux) rd/rd mice fail to entrain (Ebihara and Tsuji, 1980; Mrosovsky, 2003).
Mrosovsky et al. (2003) have suggested that thresholds for entrainment may be a more sensitive assay of the deficit compared to phase shifts using a single light pulse. However, we would argue that detection of the photic deficit also depends on the relative luminous, spectral and temporal response domains of the photoreceptors. In the case of rods, for example, a deficit in the response is more likely to be detected within scotopic levels, whereas in the case of cones (as in the present study), as is the case of Opn4−/− mice (Panda et al., 2002; Ruby et al., 2002) the alteration is more likely to be detected at photopic levels. Furthermore, the use of saturating light levels may conceal the detection of possible response deficits, as shown by the normal entrainment to light/dark cycles of high irradiance in MW-coneless mice (present study), Opn4−/− mice (Ruby et al., 2002) and other strains (Mrosovsky and Hattar, 2005).
MW-coneless mice show attenuated phase shifts
Since MW-coneless mice also lack nuclear thyroid hormone receptors expression in the SCN compared to the wild-type mouse (data not shown), it may be postulated that the reduced entrainment to a white light LD cycle may result from an alteration of signal transduction in the SCN. However, the results obtained for phase shifting responses to different monochromatic lights support the idea that observed alterations in photic sensitivity are due to a wavelength specific retinal mechanism rather than a general downstream effect. The similar response amplitudes observed at 480 nm in wild-type and MW-coneless mice are consistent with the values predicted from the proposed model of circadian photoreceptor contributions since at wavelengths ≤ 480 nm the response functions for melanopsin in the MW-coneless mouse and the combined curve for MW-opsin and melanopsin in the wild-type mouse are virtually identical. Thus it would be expected that at wavelengths in the vicinity of 480 nm, responses in the 2 genotypes would also be similar. In contrast, at wavelengths greater than 480 nm the two curves increasingly diverge, and as predicted by the model, the MW-coneless mice thereby show a deficit at 530 nm that is consistent with this difference. Despite the similar magnitudes of the phase shifts at 480 nm following a 15 min duration light pulse, shorter exposures result in significantly attenuated phase shifts in the MW–coneless mice. This difference in temporal responsiveness is consistent with a shorter latency contribution of the MW-cones response compared to the melanopsin RGC response (Berson et al., 2002; Dacey et al., 2005; Tu et al., 2005) and with the idea that MW-cones provide the initial sensitivity of the light signals for non-image-forming functions, whereas the intrinsic response of the melanopsin RGCs provides a sustained signal throughout the light stimulus and in long temporal integration (Dacey et al., 2005).
Modelling photoreceptor contributions of non-visual responses
Although our findings differ from a previous study that described no attenuation of phase shifts in cl coneless mice (Freedman et al., 1999) their result is not surprising given the use of a single wavelength (509 nm light). Our photopigment additivity model predicts that in this spectral region, the expected difference in responsiveness between wild-type and MW-coneless mouse would be relatively small.
On the other hand, a significant contribution of MW cones in phase shifting is a predictable feature considering the known action spectra for phase shifts and for the PLR in wild-type mice (Takahashi et al., 1984; Foster et al., 1991; Provencio and Foster, 1995; Yoshimura and Ebihara, 1996; Lucas et al., 2001). In all these studies the peak region of spectral sensitivity is located around 500 nm. In rd/rd and rd/rd cl mice that lack rods or both rods and cones but conserve melanopsin ipRGCs intact, the peak sensitivity is shifted to shorter wavelengths around 480 nm (Yoshimura and Ebihara, 1996; Lucas et al., 2001; Lucas et al., 2003) in agreement with the known spectral properties of ipRGCs (Berson et al., 2002; Dacey et al., 2005; Tu et al., 2005). These ipRGCs receive inputs from rods and cones via synaptic contacts with bipolar and amacrine cells (Belenky et al., 2003) and Berson et al. (2002) have shown that these inputs provide excitatory modulation of ipRGCs. This suggests that rod and cone opsin response sensitivity combines additively with melanopsin sensitivity as proposed for the murine circadian photoreception (Bullough et al., 2005). Accordingly, the shift the peak of the response in the wild-type mouse towards 500 nm must necessarily involve a longer wavelength contribution from rods (λmax=498 nm) and/or MW-cones (λmax=508 nm). Conversely, their absence would lead to both a shift in the spectral peak to shorter wavelengths and a loss in luminous sensitivity. Indeed, for the PLR, rodless rd/rd mice show a 1 log unit loss and rodless coneless rd/rd cl mice a 2.5 log unit loss of sensitivity compared to wild-type mice suggesting a significant contribution from cones (Lucas et al., 2001). However, it appears unlikely that rods provide a dominant component to light induced phase shifts, since in addition to the fact that the shift of the peak sensitivity to 500 nm in wild-type mice would require an unproportionately large rod contribution, the functional (scotopic) irradiance domain of rods differs from that of cones. At photopic levels (roughly >1 lux in the mouse) the rod contribution is normally limited due to the slow kinetics of rhodopsin regeneration and the uncoupling of the AII amacrine cells that convey rod signals to cone bipolar and ganglion cells (Bloomfield and Dacheux, 2001). A recent study (Nathan et al., 2006) using GNAT1−/− mice that lack the α subunit of rod transducin and GNAT2−/− that carry a missense mutation of the in the cone-transducin gene shows that the operating range for rod sensitivity extends from 1×105 to 5×109 photons/cm2/s whereas cones responses require at least 5×l08 photons/cm2/s. In our experimental conditions (as in most phase shifting studies) the minimal irradiance values required to elicit a phase shift exceed the functional range of rod responses. Although an eventual rod contribution in non-visual responses in elevated light levels is presently difficult to exclude, in vitro recordings of ipRGC’s in photopic conditions by Dacey et al. (2005) would argue that the neuronal responses are mediated almost exclusively by contributions from cones and melanopsin.
Cryptochromes, which function as circadian photopigments in Arabidopsis and Drosophila are also expressed in the mammalian eye and have been suggested to contribute to non visual responses (Van Gelder et al., 2003). However, the pupillary reflex and the masking response to light of double knockout Cry1−/− Cry2−/− mice is normal (Mrosovsky, 2001). Coupled to the finding that triple knockout mice (Opn4−/−, Gnat1−/−, Cnga3−/−) that express normal levels of Cry1 and Cry2 completely loose all sensitivity to light argues against a contribution of cryptochromes at least in phase shifting (Hattar et al., 2003).
In order to validate the predictions of the model, we conducted two complementary sets of experiments. First we explored phase shifting responses of wild-type compared with MW coneless mice to an additional wavelength of 560 nm (15 min pulse, 2.8 × 1014 photons/cm2/sec). The model predicts that under these conditions the response of MW coneless mice would be indistinguishable from that of dark controls and that wildtype mice would show a phase shift of 0.8 hrs. The observed phase shifts (mean ± SD) are identical to the predicted values (see Figure 6). The MW-coneless mice show a phase shift of 0.21 ± 0.13 hrs, compared to 0.22 ± 0.06 hrs in the dark controls (p=0.84). The wild-type mice show a phase shift of 0.92 ± 0.16 hrs, which is not significantly different from the predicted value of 0.8 hrs (p=0.58).
In a second test we assayed the amplitude of a phase shift in the Opn4−/− mouse (provided by S Hattar) that lacks melanopsin but retains functional MW cones with the combined prediction (i) of a reduction in the phase shift amplitude and (ii) that this reduction should be complementary to that observed in the MW-coneless mouse compared to the wild-type mouse. Following a 15 min pulse at 530 nm (2.8 × 1012 photons/cm2/sec), the observed phase shift in the Opn4−/− mouse (0.52±0.28 hrs) is not statistically different from the predicted value (0.78 hrs, p=0.10). In further accordance with the model, addition of the phase shift amplitudes for the melanopsin knockout mouse and for the MW-coneless mouse (respectively 0.52 ± 0.28 hrs and 0.60 ± 0.23 hrs) at 530 nm is equivalent to the phase shift observed in the wild-type mouse (1.12 ± 0.39 hrs) in which both photopigment systems are functional. This result is consistent with the model’s assumption of additive photopigment contributions.
Additional evidence in favor of the model is provided by the only other phase shift study using monochromatic light in the Opn4−/− mouse by Panda et al. (2002). Using a 480 nm stimulation, Panda et al; show a 40–50% reduction in amplitude for light induced phase shifts in melanopsin knockout mice compared to wild-type that is in agreement with the 47% decrease predicted by the model at this wavelength.
In summary, both MW cones and melanopsin contribute to resetting the circadian clock by light, but in varying proportions depending on the wavelength, level of irradiance and duration of exposure. While the role of rods still remains to be elucidated, knowledge of the specific response domains is crucial to understanding the relative contributions of retinal photopigments in non-visual responses. This is particularly important in the functional context of dynamic changes in irradiance and spectral composition of light in the natural environment for which, depending on lighting conditions throughout the day, the different photoreceptor systems may be brought into play.
Experimental procedures
Animals
In TRβ−/− null mice the gene is inactivated downstream of exon 3 such that both TRβ1 and TRβ2 mRNAs are not transcribed, precluding expression of MW expressing cones (Gauthier et al., 1999). Using RT-PCR (primer sequences for TRβ are sens, CTCTTCTCACGGTTCTCCTC and reverse, AACCAGTGCCAGGAATGT) we find that nuclear thyroid hormone receptors are present in the SCN of the wildtype mouse but absent in the TRβ−/− null mouse. Homozygous animals display hyperthyroxinemia and hearing defects, but are nevertheless fertile and show no other neurological or behavioral alterations (Forrest et al., 1996; Gauthier et al., 1999). Heterozygous mice were derived in an inbred 129SV background. Control homozygous animals were from the same genetic background and were obtained by inter-crossing heterozygous animals. All experiments were done with male mice between 6 to 8 weeks of age at the start of the experiment. Animals were housed in plexiglass cages under a 12L/12D light-dark cycle, with food and water ad libitum. All treatment of animals was in strict accordance with current international regulations on animal care, housing, breeding and experimentation.
Real time quantitative PCR
Total RNA was extracted using GenElute™ Mammalian Total RNA Miniprep Kit (Sigma) according to the manufacturer’s instructions and subsequently subjected to DNase digestion. The two retinas from each animal were pooled (n=4 animals for both wild-type and TRβ−/− mouse). Total RNAs was reverse transcribed using random primers and MMLV Reverse Transcriptase (Invitrogen). Real-time PCR was then performed on a LightCycler™ system (Roche Diagnostics) using the light Cycler-DNA Master SYBR Green 1 mix. The efficiency and the specificity of the amplification were controlled by generating standard curves and carrying out melting curves and agarose gels of the amplicons respectively. Relative transcript levels of each gene were calculated using the second derivative maximum values from the linear regression of cycle number versus log concentration of the amplified gene. Amplification of the control gene GAPDH was used for normalization. Primer sequences were the following: SW opsin sens, CAGCCTTCATGGGATTTG and reverse, GTGCATGCTTGGAGTTGA; MW opsin sens, GCTGCATCTTCCCACTCAG and reverse, GACCATCACCACCACCAT; Rhodopsin sens, GCCACCACTCAGAAGGCAG and reverse, GATGGAAGAGCTCTTAGCAAAG; Melanopsin sens, TGCGAGTTCTATGCCTTCTG and reverse, GGCACGTAGGCACTCCAAC; GAPDH sens, GACCTCAACTACATGGTCTACA and reverse, ACTCCACGACATACTCAGCAC. Each reaction was performed in duplicate.
Immunohistochemistry
Animals (n=3 for each genotype) were rapidly anesthetized with halothane followed by an intraperitoneal injection of nembutal (100 mg/kg i.p.) then perfused intracardially with warm heparinized saline followed by Zamboni’s fixative at 4°C. The eyes were removed and post-fixed overnight in the same fixative at 4°C, and subsequently stored in 0.1 M phosphate buffer (PB, pH 7.4) with 0.1% sodium azide. Eyes were cryoprotected in 30% sucrose in PB for 24 hours and serial sections of the retina were made at 15 μm on a freezing microtome. To identify SW and MW/LW cone opsins in mouse retinal sections, we used respectively the affinity-purified rabbit antisera JH455 (1/5000) directed against the human SW cone opsin (gift of Dr J. Nathans (Wang et al., 1992) and the mouse monoclonal antibody COS-1 (1/100) generated to chick opsins (Szel et al., 1986). The secondary antisera were goat anti-rabbit Alexa 488 IgG (H+L) conjugate (100μg/ml, Molecular Probes USA) and goat anti-mouse Alexa 568 IgG (H+L) conjugate (100μg/ml, Molecular Probes USA) for 2 h. An antiserum against melanopsin (gift from I. Provencio; 1/1200; Provencio et al., 2002) and a secondary goat anti-rabbit Alexa 568 IgG (H+L) conjugate (100μg/ml, Molecular Probes USA)was used to identify melanopsin expressing cells. Micrographs were obtained using confocal microscopy (Leica TCS SP). Laser lines and emission filters were optimized with the Leica PowerScan software.
Assessment of Behavioral Rhythms
For monitoring locomotor activity, mice were housed individually in cages equipped with passive infrared motion captors placed over the cages and a computerized data acquisition system (Circadian Activity Monitoring System, INSERM, France). Activity records were analyzed with the Clocklab software package (Actimetrics, Evanston, IL).
Light-entrainment assay
Adult (7 week-old) male mice (n=8 of each genotype) were initially maintained for 2 weeks under a 12L/12D cycle with broad-band white light. Mouse subsequently underwent a 6 hr phase delay of the light/dark cycle, associated with successive decreases of light intensity (from 100 to 10 lux and subsequently from 10 to 1 lux). To analyse entrainment, the phase angle defined as the time difference between the onset of the activity rhythm and lights off of the light/dark cycle to which the animal is entrained, was determined for each animal.
Phase shifting assay
Singly housed male mice (n=16 for each genotype) were first entrained in a 12L/12D cycle for 20 days. Subsequently animals were maintained in constant darkness (DD) to examine free running period calculated by periodogram analysis using ClockLab software (Actimetrics). The endogenous free running period in the knockout mice is slightly shorter than the wild-type animals (23.94 ± 0.04 vs 24.07 ± 0.03 hr; p<0.05). Phase shifts were studied using a single 15-min monochromatic light pulse using three different wavelengths (370, 480 and 530 nm, half-bandwidth, 10 nm) at 4 different irradiance levels (2.8 × 1010 to 2.8 × 1014 photons/cm2/s) applied at CT16 (4 h after activity onset). Temporal responses to 480 nm light exposures were studied using an equivalent irradiance (2.8 × 1014 photons/cm2/s) of different durations (1,5 and 15 min).
To avoid effects due to the age of the animals, to previous light pulses and to the length of exposure to darkness (Shimomura and Menaker, 1994; Refinetti, 2003) the order of presentation of irradiance, duration and wavelength (and dark control) were randomized. Each animal was exposed to two irradiances of all three wavelengths, and at 480 nm to at least 2 durations plus a dark control. The age of the mice was 7 weeks at the beginning of the initial entrainment to the 12L/12D cycle and 32–38 weeks at completion of the series of light pulses.
The stimulator (light source and chamber) has been described previously (Dkhissi-Benyahya et al., 2000). After the light pulse, animals were returned to their home cages and activity was monitored in DD for an additional 15–20 days before the next light pulse. The magnitude of a light-induced phase-shift was determined from the difference between the regression lines of the activity onsets before and after the light stimulation, extrapolated to the day following the light pulse. The transient responses on the 3–4 days immediately after the pulse were discounted (Daan and Pittendrigh, 1976).
Statistical analyses
Mann-Whitney U test was used to compare results obtained in the two genotypes for 1) the relative levels of opsin mRNAs and 2) the number of days necessary to achieve stable entrainment. Two way ANOVA, and post-hoc Student Newman-Keuls when necessary, were used to evaluate the differences in behavioral measures (phase angles and phase shift). Statistical significance was considered for p<0.05. Values are shown as mean ± SEM.
Acknowledgments
This work was supported by grants from FP6-EUCLOCK, INSERM ACT, Emergence-Rhône-Alpes. French Ministery of Research (ACI Neurosciences;ACI Biologie Cellulaire, Moléculaire et Structurale), Ligue contre le Cancer, FP5-CASCADE (CT-2004-506319). The authors thank J. Samarut who provided the TRβ−/− and S. Hattar for the Opn4−/− mouse models
References
- Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–871. doi: 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]
- Argamanso SM, Froehlich AC, McCall MA, Nevo E, Provencio I, Foster RG. Photopigments and circadian systems of vertebrates. Biophys Chem. 1995;56:3–11. doi: 10.1016/0301-4622(95)00009-m. [DOI] [PubMed] [Google Scholar]
- Argamaso SM, Froehlich AC, McCall MA, Nevo E, Provencio I, Foster RG. Photopigments and circadian systems of vertebrates. Biophys Chem. 1995;56:3–11. doi: 10.1016/0301-4622(95)00009-m. [DOI] [PubMed] [Google Scholar]
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–384. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Browman HI, Hawryshyn CW. Thyroxine induces a precocial loss of ultraviolet photosensitivity in rainbow trout (Oncorhynchus mykiss, Teleostei) Vision Res. 1992;32:2303–2312. doi: 10.1016/0042-6989(92)90094-y. [DOI] [PubMed] [Google Scholar]
- Bullough JD, Figueiro MG, Possidente BP, Parsons RH, Rea MS. Additivity in murine circadian phototransduction. Zoolog Sci. 2005;22:223–227. doi: 10.2108/zsj.22.223. [DOI] [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curvesHeavy water and constant light: homeostasis of frequency. J Comp Neurol. 1976;106:253–266. [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Sicard B, Cooper HM. Effects of irradiance and stimulus duration on early gene expression (Fos) in the suprachiasmatic nucleus: temporal summation and reciprocity [In Process Citation] J Neurosci. 2000;20:7790–7797. doi: 10.1523/JNEUROSCI.20-20-07790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara S, Tsuji K. Entrainment of the circadian activity rhythm to the light cycle: effective light intensity for a Zeitgeber in the retinal degenerate C3H mouse and the normal C57BL mouse. Physiol Behav. 1980;24:523–527. doi: 10.1016/0031-9384(80)90246-2. [DOI] [PubMed] [Google Scholar]
- Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, Wehner JM, Curran T. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: evidence for tissue-specific modulation of receptor function. Embo J. 1996;15:3006–3015. [PMC free article] [PubMed] [Google Scholar]
- Forrest D, Reh TA, Rusch A. Neurodevelopmental control by thyroid hormone receptors. Curr Opin Neurobiol. 2002;12:49–56. doi: 10.1016/s0959-4388(02)00289-1. [DOI] [PubMed] [Google Scholar]
- Foster R, Provencio I, Hudson D, Fiske S, DeGrip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd) J Comp Physiol [A] 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Gauthier K, Chassande O, Plateroti M, Roux JP, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J. Different functions for the thyroid hormone receptors TRalpha and TRbeta in the control of thyroid hormone production and post-natal development. Embo J. 1999;18:623–631. doi: 10.1093/emboj/18.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GH, Neitz J, Deegan JF. Retinal photoreceptors in rodents maximally sensitive to ultraviolet light. Nature. 1991;353:655–656. doi: 10.1038/353655a0. [DOI] [PubMed] [Google Scholar]
- Kaiser PK, Boynton RM. Human Color Vision. 2. Optical Society of America; 1996. [Google Scholar]
- Kelley MW, Turner JK, Reh TA. Ligands of steroid/thyroid receptors induce cone photoreceptors in vertebrate retina. Development. 1995;121:3777–3785. doi: 10.1242/dev.121.11.3777. [DOI] [PubMed] [Google Scholar]
- Lamb T. Photoreceptor spectral sensitivities: common shape in the long-wavelength region. Vis Res. 1995;35:3083–3091. doi: 10.1016/0042-6989(95)00114-f. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin- knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Further characterization of the phenotype of mCry1/mCry2-deficient mice. Chronobiol Int. 2001;18:613–625. doi: 10.1081/cbi-100106076. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Contribution of classic photoreceptors to entrainment. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2003;189:69–73. doi: 10.1007/s00359-002-0378-7. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Hattar S. Diurnal mice (Mus musculus) and other examples of temporal niche switching. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:1011–1024. doi: 10.1007/s00359-005-0017-1. [DOI] [PubMed] [Google Scholar]
- Nathan J, Reh R, Ankoudinova I, Ankoudinova G, Chang B, Heckenlively J, Hurley JB. Scotopic and Photopic Visual Thresholds and Spatial and Temporal Discrimination Evaluated by Behavior of Mice in a Water Maze. Photochem Photobiol. 2006 doi: 10.1562/2006-02-27-RA-818. [DOI] [PubMed] [Google Scholar]
- Newell FW, Diddie KR. [Typical monochromacy, congenital deafness, and resistance to intracellular action of thyroid hormone (author’s transl)] Klin Monatsbl Augenheilkd. 1977;171:731–734. [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J Comp Physiol [A] 1976:291–332. [Google Scholar]
- Provencio I, Foster RG. Circadian rhythms in mice can be regulated by photoreceptors with cone- like characteristics. Brain Res. 1995;694:183–190. doi: 10.1016/0006-8993(95)00694-l. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- Refinetti R. Effects of prolonged exposure to darkness on circadian photic responsiveness in the mouse. Chronobiol Int. 2003;20:417–440. doi: 10.1081/cbi-120021443. [DOI] [PubMed] [Google Scholar]
- Roberts SG. Transcriptional regulation by WT1 in development. Curr Opin Genet Dev. 2005;15:542–547. doi: 10.1016/j.gde.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Tosini G. Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. J Neurosci. 2004;24:9693–9697. doi: 10.1523/JNEUROSCI.2556-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semo M, Peirson S, Lupi D, Lucas RJ, Jeffery G, Foster RG. Melanopsin retinal ganglion cells and the maintenance of circadian and pupillary responses to light in aged rodless/coneless (rd/rd cl) mice. Eur J Neurosci. 2003;17:1793–1801. doi: 10.1046/j.1460-9568.2003.02616.x. [DOI] [PubMed] [Google Scholar]
- Sevilla-Romero E, Munoz A, Pinazo-Duran MD. Low thyroid hormone levels impair the perinatal development of the rat retina. Ophthalmic Res. 2002;34:181–191. doi: 10.1159/000063885. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Menaker M. Light-induced phase shifts in tau mutant hamsters. J Biol Rhythms. 1994;9:97–110. doi: 10.1177/074873049400900201. [DOI] [PubMed] [Google Scholar]
- Szel A, Takacs L, Monostori E, Diamantstein T, Vigh-Teichmann I, Rohlich P. Monoclonal antibody-recognizing cone visual pigment. Exp Eye Res. 1986;43:871–883. doi: 10.1016/0014-4835(86)90066-7. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Van Gelder RN, Wee R, Lee JA, Tu DC. Reduced pupillary light responses in mice lacking cryptochromes. Science. 2003;299:222. doi: 10.1126/science.1079536. [DOI] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Merbs SL, Zack DJ, Klaunberg B, Bennett J, Gearhart J, Nathans J. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992;9:429–440. doi: 10.1016/0896-6273(92)90181-c. [DOI] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Yanagi Y, Takezawa S, Kato S. Distinct functions of photoreceptor cell-specific nuclear receptor, thyroid hormone receptor beta2 and CRX in one photoreceptor development. Invest Ophthalmol Vis Sci. 2002;43:3489–3494. [PubMed] [Google Scholar]
- Yoshimura T, Ebihara S. Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+) mice. J Comp Physiol [A] 1996;178:797–802. doi: 10.1007/BF00225828. [DOI] [PubMed] [Google Scholar]


