Abstract
Merkel cell carcinoma is a skin cancer with 30% mortality and an incidence that has tripled in the past 15 years. There is agreement that surgical excision with negative margins is an appropriate therapeutic first step and that sentinel lymph node biopsy is a powerful prognostic indicator. Following excision of detectable cancer, optimal adjuvant therapy is not well established. A role for adjuvant radiotherapy is increasingly supported by retrospective data suggesting a nearly four-fold decrease in local recurrences if radiation is added to surgery. In contrast, a role for adjuvant chemotherapy is not well supported. The rationale for chemotherapy in this disease is based on small-cell lung cancer, a more common neuroendocrine tumor for which chemotherapy is the primary treatment modality. Several issues call into question the routine use of adjuvant chemotherapy in Merkel cell carcinoma: lack of evidence for improved survival; the associated morbidity and mortality; important differences between small-cell lung cancer and Merkel cell carcinoma; and rapid development of resistance to chemotherapy. Importantly, chemotherapy suppresses immune function that plays an unusually large role in defending the host from the development and progression of Merkel cell carcinoma. Taken together, these arguments suggest that adjuvant chemotherapy for Merkel cell carcinoma patients should largely be restricted to clinical trials.
Merkel cell carcinoma is a neuroendocrine cancer that typically presents as a rapidly growing non-specific nodule on sun exposed skin in people over 65 years of age. The recent increase in incidence to over 1000 cases a year in the United States has led Merkel cell carcinoma to become the second most common cause of non-melanoma skin cancer death.1,2 Optimal management for Merkel cell carcinoma beyond surgical excision is not agreed on, and no randomized trials have been carried out. Sentinel lymph node biopsy has been shown to be powerful in predicting subsequent recurrences as well as in determining if further nodal treatment is indicated.3,4
Data supports adjuvant radiotherapy for Merkel cell carcinoma
Adjuvant radiotherapy is associated with a marked decrease in local recurrences and a trend to improved survival in multiple retrospective studies. Although no prospective trials of radiation therapy have been performed, many retrospective studies find that adjuvant radiotherapy is associated with better outcomes in MCC. A meta-analysis was carried out on 1254 Merkel cell carcinoma patients previously reported in the literature who met the following criteria: a single primary tumor arising on skin that was excised with negative surgical margins on whom follow-up data was included regarding recurrence and survival.5 In this study, patients who received adjuvant radiation therapy had improved outcomes compared to those who received surgical excision only. Specifically, local recurrences at 5 years were three times less likely (12% vs 39%, p < .001) if adjuvant radiation was given, and a similar association was found for regional recurrences (23% vs 56%, p < .001) (see Figure 1). Patients who received adjuvant radiation also had an improved overall and cause specific survival, although this was not statistically significant. In a subgroup analysis excluding single-patient case reports and non-comparative studies there was a significant cause-specific survival advantage associated with adjuvant radiation to the local site (hazard ratio for death = 0.62, p = 0.04). Although one large single institution study did not find a statistically significant improvement in outcomes if radiation therapy was given, only 13% of patients in this study received adjuvant radiation and those that received surgery mono-therapy had exceptional results with only 8% experiencing local recurrences.4 Although retrospective and not randomized, in aggregate these studies strongly suggest improved outcomes in Merkel cell carcinoma with the addition of adjuvant radiation therapy.
Figure 1. Adjuvant radiation therapy and local recurrence of Merkel cell carcinoma.
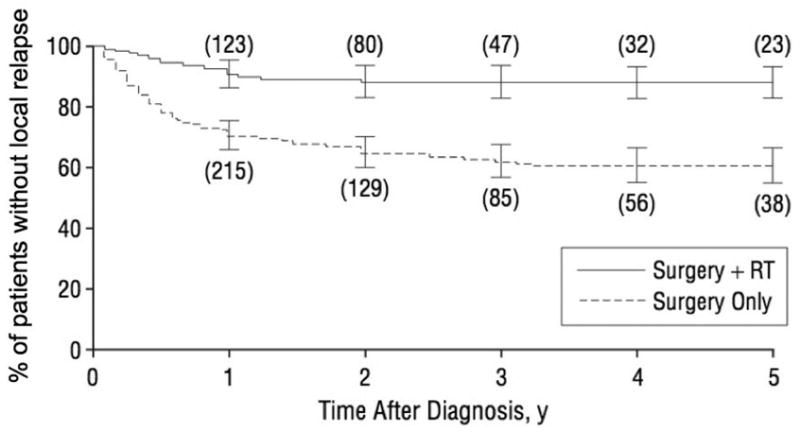
The fraction of patients who are free of local relapse is plotted for five years after diagnosis. Numbers in parentheses indicate the number of patients in each group that were at risk at that time point. All patients received surgery and those who also received radiation (solid line) are plotted separately from those who did not (dashed line; p < 0.001 for difference). Bars represent 95% confidence limits. (From Lewis, K. G. et al. Arch Dermatol 2006; 142:693 Copyright 2006 American Medical Association; permission to reprint has been granted.)
Adjuvant chemotherapy for Merkel cell carcinoma is not currently supported by data
Many Merkel cell carcinoma patients receive adjuvant chemotherapy in analogy to a more common neuroendocrine tumor, small-cell carcinoma of the lung. However, there are several important differences between small-cell lung cancer and Merkel cell carcinoma that suggest that therapy established for one disease should not automatically be extended to the other. Below are six issues that raise concern about the routine use of adjuvant chemotherapy in Merkel cell carcinoma.
1. Adjuvant chemotherapy for Merkel cell carcinoma has not been shown to improve survival
Several recent studies have failed to demonstrate that adjuvant chemotherapy provides a survival benefit for Merkel cell carcinoma. A 2003 Australian study appeared to show favorable outcomes for adjuvant chemotherapy in Merkel cell carcinoma.6 However, a 2006 reanalysis of the data by the same authors using multivariate analysis to account for stage at presentation found no significant improvement in disease-specific survival.7 Indeed, another recent study of 76 patients with stage II disease suggests that chemotherapy may actually reduce survival (Figure 2).4 Specifically, node-positive patients that received adjuvant chemotherapy (n=23) had a 4 year survival of 42% as compared to patients that did not receive adjuvant chemotherapy (n=53) who had a 4 year survival of 60%. Although these node-positive patients were not randomized and this difference was not statistically significant, the 18% lower survival in the group that received adjuvant chemotherapy does not imply a survival benefit for this treatment.
Figure 2. Adjuvant chemotherapy and survival in patients with Merkel cell carcinoma spread to lymph nodes.
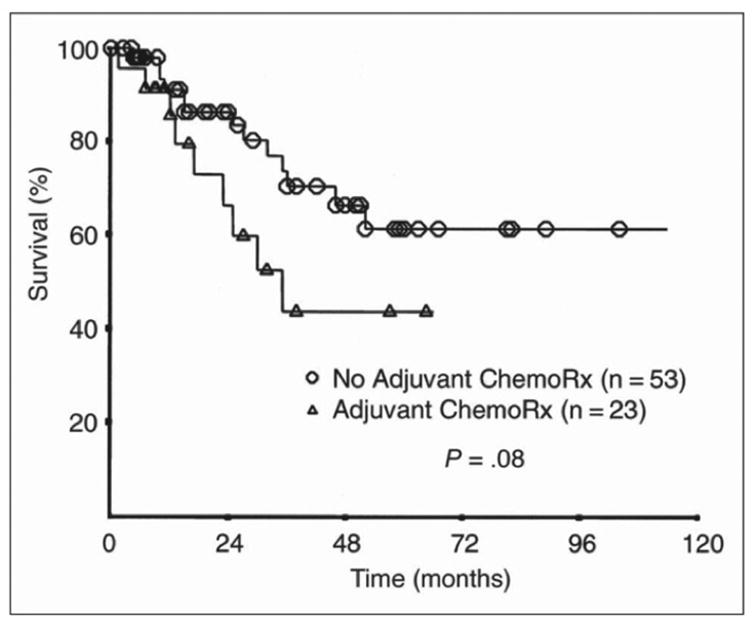
The group that received adjuvant chemotherapy had lower survival, although this was not statistically significant. (From Allen, P. J. et al. J Clin Oncol; 23:2300, reprinted with permission from the American Society of Clinical Oncology).
2. Chemotherapy for Merkel cell carcinoma is associated with significant treatment-related mortality
The largest single report of 204 Merkel cell carcinoma patients treated with chemotherapy observed a toxic death rate of 3.4%.8 A separate analysis of patients older than 65 found that 16% of older Merkel cell carcinoma patients died of complications of chemotherapy.9 Given that the median age of Merkel cell carcinoma presentation is over 70, and that the most commonly used regimen (cis-platinum and etoposide) is identical for both adjuvant and therapeutic regimens, this death rate is significant. Although this mortality risk is similar to other cancers, it is more troubling in Merkel cell carcinoma given a lack of evidence for improved outcomes.
3. Adjuvant chemotherapy adds significant morbidity
In the largest clinical trial of Merkel cell carcinoma patients who were given adjuvant chemotherapy, 63% experienced serious skin toxicities with moist desquamation in areas that were also irradiated.10 More importantly, 40% of patients were hospitalized with neutropenia.10 Although tolerable if necessary, these morbidities negatively impact quality of life, increase infection risk, and are especially difficult for elderly Merkel cell carcinoma patients to tolerate.
4. The rationale for adjuvant chemotherapy is based on a tumor that is biologically different than Merkel cell carcinoma
The more common neuroendocrine tumor, small-cell lung cancer (SCLC), is routinely treated with chemotherapy as the primary modality. Although MCC and SCLC share striking histologic similarity, these tumors have distinct presentations, prognoses and biological behavior. Accessibility of the primary lesion is one fundamental difference: small-cell lung cancer is visceral with over 75% of patients presenting with advanced disease that is not amenable to cure with surgery and radiation. In contrast, Merkel cell carcinoma originates in the skin often allowing earlier detection when surgery and radiation can be curative.3,11,12 Given the high cure rate for node-negative MCC using loco-regional therapy only, exposing low risk patients to the known toxicities of adjuvant chemotherapy is not indicated. Even among high risk (node-positive) MCC patients there is approximately a 50% chance of cure by surgery and radiation and no evidence for improved survival by adjuvant chemotherapy as noted above. In contrast, chemotherapy in SCLC is better justified due to a proven survival benefit and the low chance of cure by surgery and radiation.12 Furthermore, the biology of these cancers may be distinct. Specifically, the Ras/Raf/MEK/Map kinase pathway has been shown to be active in fewer than 5% of Merkel cell carcinomas and active in ~50% of small-cell lung cancers.13,14 As discussed below, MCC is highly sensitive to immune function regarding incidence and survival. In contrast, SCLC appears to be less related to immune function. Although small-cell lung cancer incidence is 2.1 fold increased in HIV patients as compared to the general population, this is far less than the 13.4-fold increase in Merkel cell carcinomas in HIV and the lung cancer finding has been partly attributed to risk factors such as smoking.15 Given these diverse differences, a role for chemotherapy in Merkel cell carcinoma patients should be based on data from that disease rather than extrapolated from SCLC.
5. Resistance to chemotherapy develops frequently
Initially 40–70% of Merkel cell carcinomas are sensitive to chemotherapy with partial or complete responses.9 In contrast, there are no good salvage mechanisms for recurrent disease initially treated with chemotherapy. As with many other malignancies, Merkel cell carcinoma seems to gain broad resistance to chemotherapeutic agents following an initial course of chemotherapy. Since there are no data to suggest adjuvant chemotherapy is beneficial in Merkel cell carcinoma it may make sense to withhold chemotherapy for palliation when surgery and radiation are no longer an option.
6. The immune system is unusually important in controlling Merkel cell carcinoma
Merkel cell carcinoma is distinct from many cancers because its incidence and prognosis are strongly related to immune function. This cancer occurs more frequently and behaves more aggressively in diverse immune compromised populations. There is about a 10 fold increase in incidence in solid organ transplant recipients and a 13.4-fold increase among HIV patients.16,17 Immune compromised patients present with Merkel cell carcinoma at a more advanced stage (68% have nodal disease vs. 39% of immune competent MCC patients) and at a younger age (53 years vs. 74 years).3,11,16 Finally, Merkel cell carcinoma is more deadly in immune compromised patients (56% disease specific mortality vs. 25–35%).16 These data suggest that a healthy immune system is critical in both preventing the development and halting the progression of Merkel cell carcinoma. We speculate that the negative impact of chemotherapy on immune function may counteract benefits that come from reducing tumor cell burden in this unusually immune-sensitive disease.
In summary, there is a growing consensus that surgical excision, sentinel lymph-node biopsy and radiation therapy are important components in managing Merkel cell carcinoma. When the biological underpinnings of this cancer are better understood, it is hoped this will lead to more effective molecularly targeted therapies. However, given the known morbidity, mortality, and immune suppression coupled with a lack of evidence of survival benefit, present data do not support the routine use of standard adjuvant chemotherapy in Merkel cell carcinoma.
Acknowledgments
Funding sources: Harvard/NCI Skin Cancer SPORE, Merkel cell carcinoma patient fund, NIH K02-AR050993
Footnotes
Conflict of Interest Disclosure: We declare no conflicts of interest.
This manuscript has not been presented elsewhere
Reprint requests: Paul Nghiem
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1–4. doi: 10.1002/jso.20167. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Wang L, Nghiem P. Merkel cell carcinoma: information for patients and their physicians, 6/06 update. http://www.merkelcell.org.
- 3.Gupta SG, Wang LC, Penas PF, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685–690. doi: 10.1001/archderm.142.6.685. [DOI] [PubMed] [Google Scholar]
- 4.Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300–2309. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 5.Lewis KG, Weinstock MA, Weaver AL, et al. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142:693–700. doi: 10.1001/archderm.142.6.693. [DOI] [PubMed] [Google Scholar]
- 6.Poulsen MG, Rischin D, Walpole E, et al. High risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: A Trans-Tasman Radiation Oncology Group Study TROG 96:07. J Clin Oncol. 2003;21:4371–4376. doi: 10.1200/JCO.2003.03.154. [DOI] [PubMed] [Google Scholar]
- 7.Poulsen MG, Rischin D, Porter I, et al. Does chemotherapy improve survival in high-risk stage I and II Merkel cell carcinoma of the skin? Int J Radiat Oncol Biol Phys. 2006;64:114–119. doi: 10.1016/j.ijrobp.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 8.Tai PT, Yu E, Winquist E, et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol. 2000;18:2493–2499. doi: 10.1200/JCO.2000.18.12.2493. [DOI] [PubMed] [Google Scholar]
- 9.Voog E, Biron P, Martin JP, et al. Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer. 1999;85:2589–2595. doi: 10.1002/(sici)1097-0142(19990615)85:12<2589::aid-cncr15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Poulsen M, Rischin D, Walpole E, et al. Analysis of toxicity of Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group study. Int J Radiat Oncol Biol Phys. 2001;51:156–163. doi: 10.1016/s0360-3016(01)01577-2. [DOI] [PubMed] [Google Scholar]
- 11.Agelli M, Clegg LX. Epidemiology of Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–841. doi: 10.1016/s0190-9622(03)02108-x. [DOI] [PubMed] [Google Scholar]
- 12.Stupp R, Monnerat C, Turrisi AT, 3rd, et al. Small cell lung cancer: state of the art and future perspectives. Lung Cancer. 2004;45:105–117. doi: 10.1016/j.lungcan.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Houben R, Michel B, Vetter-Kauczok CS, et al. Absence of classical MAP kinase pathway signalling in Merkel cell carcinoma. J Invest Dermatol. 2006;126:1135–1142. doi: 10.1038/sj.jid.5700170. [DOI] [PubMed] [Google Scholar]
- 14.Blackhall FH, Pintilie M, Michael M, et al. Expression and prognostic significance of kit, protein kinase B, and mitogen-activated protein kinase in patients with small cell lung cancer. Clin Cancer Res. 2003;9:2241–2247. [PubMed] [Google Scholar]
- 15.Chaturvedi A, Pfeiffer R, Chang L, et al. Elevated risk of lung cancer among people with AIDS. AIDS. 2007;21:207–213. doi: 10.1097/QAD.0b013e3280118fca. [DOI] [PubMed] [Google Scholar]
- 16.Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717–1721. doi: 10.1097/00007890-199912150-00015. [DOI] [PubMed] [Google Scholar]
- 17.Engels EA, Frisch M, Goedert JJ, et al. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–498. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]


