Summary
The expression of the VAV proto-oncogene in 57 patients with chronic myeloproliferative disease (CMD), B-cell acute lymphoblastic leukaemia (BALL) and B-cell non-Hodgkin Lymphoma (B-NHL), and 61 with B-cell chronic lymphocytic leukaemia (B-CLL) was analysed. VAV overexpression was observed in 19.5% of cases and 81% of VAV-positive tumours also displayed VAV phosphorylation. Overexpression was not observed in B-ALL or CMD, but 13% of B-NHL and 34.4% of B-CLL patients (P = 0.002) overexpressed VAV. The overexpression and phosphorylation of VAV was detected more frequently in 13q- chronic lymphocytic leukaemias (71.4%) versus other B-CLLs (23.4%, P = 0.001). Overexpression of VAV protein is a frequent event in patients with B-CLL displaying loss of 13q sequences.
Keywords: VAV proto-oncogene, haematological malignancies, chronic lymphocytic leukaemia, 13q
The VAV family shows a wide phylogenetic distribution, with members present in all animal metazoans (Bustelo, 2000). All these proteins work as a group of signal transduction proteins with oncogenic potential that catalyse GDP/GTP exchange on GTPases of the Rho/Rac family. The deregulation of VAV family genes leads to cell transformation. Despite the genetic and in vitro evidence, there is still little information regarding the implication of VAV proteins in human tumours. Recently, it has been shown that immortalised neuroblastoma tumour cell lines show ectopic expression of the VAV proto-oncogene. In addition, pancreatic tumours seem to show upregulation of VAV expression, probably by altering the methylation pattern of the promoter of this gene (Fernández-Zapico et al, 2005). However, the implication of the VAV proto-oncogene on other malignancies remains unknown.
Given that the activation of the endogenous Rho/Rac proteins seems to represent an important step for the biology of cancer cells (Etienne-Manneville & Hall, 2002), the status of the VAV proto-oncogene product in bone marrow (BM) cells of different types of haematological malignancies was studied.
Patients
118 unselected patients with different types of haematolog-ical malignancies were included. Thirty-two had a diagnosis of chronic myeloproliferative disease (CMD), 10 had B-cell acute lymphoblastic leukaemia (B-ALL) and 15 had non-Hodgkin lymphoma (NHL). The remaining 61 cases were identified as having B-cell chronic lymphocytic leukaemia (B-CLL). All samples were obtained with informed consent.
Methods
Antibodies, protein extraction and immunoblot analysis
To evaluate the status of the VAV pathway, total cellular lysates from BM cells were obtained. These lysates were then subjected to immunoblot analysis using two different antibodies. Total VAV protein expression was monitored by incubating the electrophoretically-fractionated samples with a polyclonal antibody that recognises the VAV zinc finger region. To assess VAV activation, the phosphorylation level of the key regulatory residue of Vav (Y174) was studied (Lopez-Lago et al, 2000). When dephosphorylated, Y174 establishes intramolecular interactions with the catalytic region of VAV, leading to a closed conformation of VAV that is not compatible with the binding of the GTPase substrates. In contrast, the phosphorylation of this residue breaks such intramolecular interactions, leading to an open conformation of VAV that can bind to Rho/Rac GTPases (Llorca et al, 2005). To track the phosphorylation of this residue in the experimental samples, a phosphospecific anti-VAV antibody that recognises phosphorylated Y174 but not other phosphorylated residues of VAV or other signalling proteins was used (Lopez-Lago et al, 2000). Equal loading of samples was estimated by staining the filters with Ponceau-S solution and by analysing in parallel the levels of actin by immunoblot.
Fluorescence in situ hybridisation (FISH) analysis
In B-CLL patients, FISH studies with specific probes to 13q14, chromosome 12, 11q22.3 and 17p13 regions were performed, as previously described (Hernández et al, 2000).
Statistical methods
The statistical correlation of VAV phosphorylation and protein levels with specific types of tumours or genetic abnormalities was performed using a chi-squared test. Values were considered significant when P < 0.05.
Results
Table I summarises the results obtained. Notably, neither VAV protein nor its phosphorylated form could be detected in any of the 42 samples obtained from CMD and B-ALL patients. In contrast, increased levels of VAV protein and tyrosine-phosphorylated VAV were detected in 34.4% and 27.9% of the protein extracts obtained from BM samples of patients with B-CLL, respectively (P = 0.002). Two patients with a NHL (13%) also showed VAV overexpression.
Table I.
Overexpression of theVAV protooncogene product according to the type of haematological tumour analysed.
| Positive†,‡ | |||
|---|---|---|---|
| Disease type | n* | VAV overexpression | VAV phosphorylation |
| CMD | |||
| Polycythaemia vera | 11 | 0† | 0‡ |
| Essential thrombocythaemia | 12 | 0 | 0 |
| Agnogenic myeloid metaplasia | 3 | 0 | 0 |
| Chronic myeloid leukaemia | 3 | 0 | 0 |
| Non-classificable MDS/CMD | 3 | 0 | 0 |
| B-ALL | 10 | 0 | 0 |
| B-NHL | 15 | 2 | 2 |
| B-CLL | |||
| Normal FISH karyotype | 33 | 7† | 6‡ |
| 13q deletion | 14 | 10 | 8 |
| Chromosome 12 trisomy | 6 | 1 | 0 |
| 17p deletion | 6 | 2 | 2 |
| 11q deletion | 2 | 1 | 1 |
Number of patients in the indicated disease group.
Number of positives for VAV overexpression
Number of positives for VAV phosphorylation on the regulatory Y 174 residue, as determined by immunoblot analysis.
MDS/CMD, Myelodysplastic syndrome/chronic myeloproliferative disorder; CMD, Chronic myeloproliferative disease; B-ALL, B-cell acute lymphoblastic leukaemia; B-NHL, B-cell leukae-mic non-Hodgkin lymphoma; B-CLL, B-cell chronic lymphocytic leukaemia; FISH, Fluorescence in situ hybridization analysis.
B-cell chronic lymphocytic leukaemia samples were subdivided according to the presence of specific cytogenetic alterations. A high percentage of B-CLL with a 13q deletion showed increased levels of both VAV protein (71.4%) and tyrosine phosphorylated VAV (57.14%) (Table I, Fig 1A). By contrast, B-CLL without FISH abnormalities displayed signi-ficantly lower levels of VAV overexpression (21.2%) or tyrosine phosphorylation (18.2%). Moreover, in B-CLL patients with either +12 or 17p- the frequency of overexpres-sion of VAV protein was also lower (16.6% and 33.3% of cases, respectively) than in B-CLL with 13q- (Fig 1B).
Fig 1.
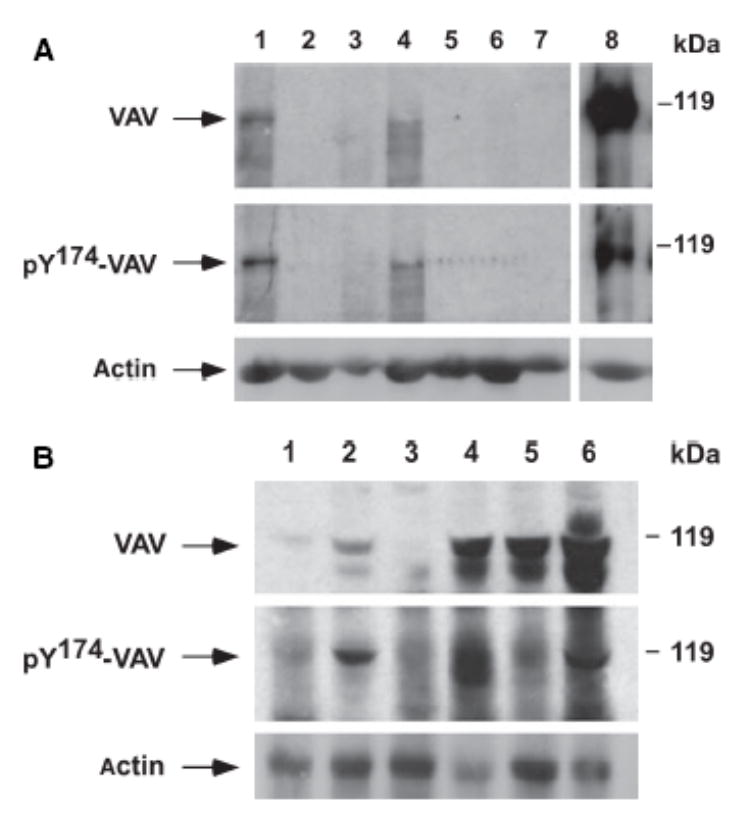
(A) Analysis of VAV protein levels in bone marrow (BM) cells from B-cell chronic lymphocytic leukaemia (B-CLL) patients. Total cellular lysates obtained from BM cells from either B-CLL patients (lanes 1–4) or healthy individuals (lanes 5–7) were subjected to immunoblot analysis with anti-VAV antibodies (upper panel). After stripping, the same filter was subjected to immunoblot analysis with anti-phosphoVAV Y174 antibodies (lower panel). As a positive control, a total cellular extract from quiescent COS-1 cells ectopically expressing an epitope-tagged version of VAV that were stimulated with epidermal growth factor for 10 min at 37°C was included (lane 8). (B) Analysis of VAV protein expression levels in BM cells from B-CLL patients. Total cellular lysates, derived from BM cells from six independent B-CLL patients (lanes 1 to 6, top), were analysed as indicated in (A). The electrophoretic mobility of VAV and phosphorylated VAV proteins is indicated by arrows (left side of panels). The position of molecular weight markers is indicated on the right.
Discussion
The present study has shown that VAV is another signal transduction molecule involved B-CLL, mainly in patients with 13q deletions. To our knowledge, this is the first time that a deregulated expression of the VAV proto-oncogene has been reported in a haematological disorder. Whether this increase is linked to direct genetic alterations induced by the 13q- or because of the biological needs of VAV positive chronic lymphocytic leukaemia (CLL) cells, remains to be determined. It is likely that the hyperphosphorylation of VAV protein is an indirect consequence of its overexpression, as it has been shown before in transient transfections of T-cell and B-cell lines with the VAV proto-oncogene product (Wu et al, 1995). Although the reason for this is unknown, it has been argued that VAV overexpression probably overcomes the basal activity of the tyrosine phosphatase that regulates the dephosphory-lation the endogenous VAV under normal physiological conditions.
The deregulation of VAV protein in B-CLL is not a surprising finding given the key role of VAV family genes in B-lymphocyte signal transduction. These proteins promote proper B-cell proliferation and are required for the engagement of many downstream elements such as the Ras/extracel-lular signal-regulated kinase route, the phosphoinositide 3 kinase/protein kinase B pathway, and protein kinase C-dependent routes (Caloca et al, 2003). Based on these results, it is possible that VAV overexpression could help in the activation of other signalling pathways that promote cell survival, proliferation or migration of B-CLL cells. Further work in this area will shed light on the specific molecular roles of VAV in CLL.
Acknowledgments
This work was supported by the US National Cancer Institute (5RO1-CA73735-08 to XRB), the Biomedicine Program of the Spanish Ministry of Education and Science (SAF2003-00028 to XRB), the Fondo de Investigaciones Sanitarias of the Spanish Health Ministry FIS-FEDER (PI020103 and PI02/1358 to XRB and JMH, respectively), and the Council from Castilla y León (‘Proyectos de Biomedicina del SACYL’). RMP-S is a student of the Molecular and Cellular Cancer Biology graduate program of the CIC and the University of Salamanca who is supported by an FPU fellowship (AP2000-3829) of the Spanish Ministry of Education and Science. JLG is supported by a Spanish FIS grant (01/1353). The Centro de Investigación del Cáncer is supported by endowments from the CSIC, University of Salamanca, Castilla-Leó n Autonomous Government, the Spanish Cooperative Network of Cancer Centres (C03/10, Spanish Ministry of Health), and the Foundation for Cancer Research of Salamanca (FICUS).
References
- Bustelo XR. Regulatory and signalling properties of the Vav family. Molecular and Cellular Biology. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caloca MJ, Zugaza JL, Matallanas D, Crespo P, Bustelo XR. Vav mediates Ras stimulation by direct activation of the GDP/GTP exchange factor Ras GRP1. EMBO Journal. 2003;22:3326–3336. doi: 10.1093/emboj/cdg316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Fernández-Zapico ME, González-Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R, Smyrk TC, Chari ST, Urrutia R, Billadeau DD. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell. 2005;7:39–49. doi: 10.1016/j.ccr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Hernández JM, González MB, Granada I, Gutiérrez N, Chillón C, Ramos F, Ribera JM, González M, Feliu E, San Miguel JF. Detection of inv(16) and t(16;16) by fluorescence in situ hybridization in acute myeloid leukaemia M4Eo. Haematologica. 2000;85:481–485. [PubMed] [Google Scholar]
- Lopez-Lago M, Lee H, Cruz C, Movilla N, Bustelo XR. Tyrosine phosphorylation mediates both activation and down-modulation of the biological activity of Vav. Molecular and Cellular Biology. 2000;20:1678–1691. doi: 10.1128/mcb.20.5.1678-1691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca O, Arias-Palomo E, Zugaza JL, Bustelo XR. Global conformational rearrangements during the activation of the GDP/GTP exchange factor Vav3. EMBO Journal. 2005;24:1330–1340. doi: 10.1038/sj.emboj.7600617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Katzav S, Weiss A. A functional T-cell receptor signalling pathway is required for p95vav activity. Molecular and Cellular Biology. 1995;15:4337–4346. doi: 10.1128/mcb.15.8.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]


