Abstract
The retinoid acid receptors (RAR) and peroxisome proliferator-activated receptors (PPAR) have been implicated in the regulation of inflammatory reactions. Both receptor families contain ligand-activated transcription factors which form heterodimers with retinoid X receptors (RXR). We review data that imply RAR/RXR and PPAR/RXR pathways in physiological reactions after spinal cord injury. Experiments show how RAR signaling may improve axonal regeneration and modulate reactions of glia cells. While anti-inflammatory properties of PPAR are well documented in the periphery, their possible roles in the central nervous system have only recently become evident. Due to its anti-inflammatory function this transcription factor family promises to be a useful target after spinal cord or brain lesions.
1. INTRODUCTION
During the last decade much progress has been made disentangling the physiological responses after central nervous system (CNS) injury. Yet, there is still no effective treatment available to prevent the devastating effects that result from major CNS lesions. Although neurite sprouting and axonal growth is observed after spinal cord injury (SCI), successful regeneration beyond the lesion-induced scar does not occur. In contrast, axonal regeneration with functional recovery is possible in the peripheral nervous system (PNS). The different outcomes of CNS and PNS lesions are largely due to differences in the cellular and molecular signals that neurons encounter in these environments. Three important problems after CNS injury are (1) development of a growth inhibitory glial scar, (2) secondary neuronal and glial degeneration as a delayed consequence of the lesion, and (3) the failure of axonal regeneration in white matter tracts. Responding to these problems, most attention has been directed either to overcome the inhibitory barrier of the glial scar or to promote the growth of axon collaterals and thus compensate for permanently severed connections [1–3]. A subsidiary approach is to modify inflammatory reactions in order to limit the secondary degeneration. Synthetic corticosteroids, e.g., methylprednisolone, are the only pharmacological tools currently in clinical use [4]. In this review, we will discuss two nuclear receptor families that have recently been discovered as possible therapeutic targets for the treatment of SCI and brain damage. Retinoic acid receptor (RAR) signaling may improve axonal regeneration, influence glial differentiation, and modulate inflammatory reactions [5]. Peroxisome proliferator-activated receptors (PPAR) have a major function in anti-inflammatory processes [6]. Both receptor classes, RAR and PPAR, heterodimerize with retinoid X receptors (RXR) to form active transcription factors.
2. ENDOGENOUS RAR/RXR AND PPAR/RXR ACTIVITY IN THE INJURED NERVOUS SYSTEM
2.1. RAR/RXR signal transduction
The RAR/RXR transcription factor complex is activated by ligand binding, its natural ligand being all-trans retinoic acid (all-trans RA). Retinoids are obtained from the diet in the form of vitamin A (retinol and retinal), retinyl esters, or β-carotene. Following cellular uptake of all-trans retinol from the plasma, the intracellular synthesis of retinoic acid occurs in two steps. (1) Retinol is oxidized to retinal, predominantly involving the alcohol dehydrogenases ADH-1, -3, and -4. (2) The critical step is the subsequent oxidation of retinal to retinoic acid by retinaldehyde dehydrogenases (RALDH) [7]. Retinoic acid is released in a paracrine or autocrine fashion. The fact that RA activates nuclear transcription factors was discovered in 1987 [8, 9]. Retinoid receptors belong to the same superfamily as PPAR, thyroid hormone receptors (TR), and steroid receptors. They can be grouped into two families, the RARs and the RXRs, each consisting of three isoforms encoded by separate genes: RAR α, RARβ, RAR γ (also: NR1B1-3) and RXRα, RXRβ, RXRγ (NR2B1-3). All-trans RA and 9-cis RA bind to the RAR family, whereas only 9-cis RA is a high-affinity ligand for the RXR [7, 10]. The active transcription factor complex consists of an RAR/RXR heterodimer, ligand, and coactivators. It interacts with retinoic acid response elements (RARE) in the promoters of target genes. About 500 genes have been suggested to be regulated by RAR/RXR signaling, however a much lower number was experimentally shown to be activated via the classical RARE driven pathway. Proven target genes include enzymes, transcription factors, cytokines, and cytokine receptors [5, 11]. In addition, many cases of gene suppression and nongenomic modes of action of RA and its receptors have been described [7].
2.2. RAR/RXR signaling after spinal cord injury
The discovery that enzyme activity of a retinaldehyde dehydrogenase increased after spinal cord contusion injury was the first direct evidence that retinoids play a role in the physiological responses to SCI. Contusion injury caused a significant increase in RALDH2 enzyme activity, which peaked 8–14 days following the lesion [12]. While in the noninjured rat spinal cord RALDH2 is only present in the meninges, oligodendrocytes and in pericytes, around the lesion site, its immunoreactivity also appeared in a population of NG2-positive glia cells [13] (Figure 1(a)). NG2 is a chondroitin sulfate proteoglycan, expressed in cells that have been described as oligodendrocyte precursor cells, synantocytes or polydendrocytes. These cells respond to injury with increased production of NG2 and a subpopulation of them, close to the lesion site, appears to be involved in the local production of RA. Alternatively or additionally, RA-synthesizing cells migrate from the adjacent arachnoid membrane and from blood vessels toward the site of injury [13]. While only minor changes in the quantitative expression of retinoid receptors were observed after SCI, their cellular distribution changed remarkably (Figures 1(b), 1(c)). In the noninjured tissue, retinoid receptors were found in the cytosol of motorneurons and glia, but close to the injury site macrophages and surviving neurons displayed a nuclear localization of RARα, RXRα, and RXRβ [14]. In the context of locally rising RA synthesis, the observation that retinoid receptors translocate into the cell nuclei indicates that neurons, glia, and macrophages are targets of RA signaling after SCI. This interpretation is consistent with data on spinal cord development, where RA has been shown to be a regulator of cell differentiation [15–18].
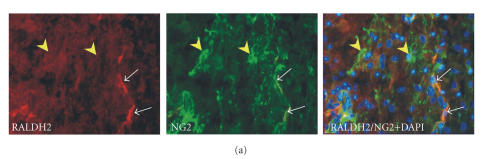
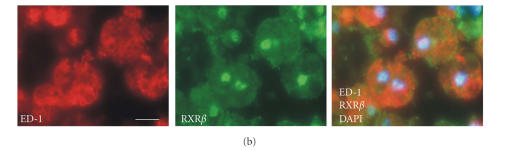
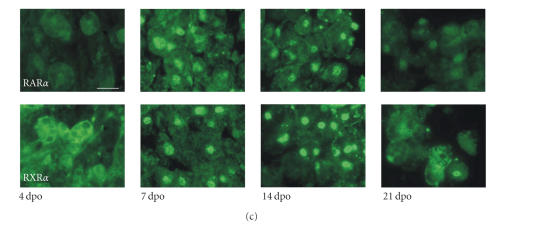
Figure 1.
Retinoic acid synthesis and nuclear translocation of RAR/RXR after spinal cord injury. (a) Double immunostaining of rat spinal cord sections shows RALDH-2 immunoreactivity (red) in a subpopulation of NG2-positive glia (green) 7 days after contusion injury. RALDH2/NG2 expressing cells were only detected in the vicinity of the lesion site, many NG2 cells do not contain the RA-synthesizing enzyme (yellow arrow heads). White arrows point to DAPI-stained cell nuclei of RALDH2/NG2 cells in superimposed photographs. (b) At 7 dpo, ED1-positive macrophages at the lesion site express RXRβ in their cell nuclei. (c) SCI-induced transient translocation of RARβ and RXRα from the cytosol into the nuclei of activated macrophages/microglia. All scale bars are 20μ m (sources: [12, 14]).
2.3. RAR/RXR signaling after peripheral nerve injury
RA signaling is implicated in the differentiation of neurons whose axons grow in the PNS, [19–22] and again in neurite regeneration [23, 24]. Several mediators of the RA signaling pathway have been shown to be induced after peripheral nerve injury in the rat. Experimental lesion of the sciatic nerve induced expression of the cellular retinol binding protein-I (CRBP-I) [25], which is implicated in retinoid metabolism [26]. Similarly, transcript and protein concentrations of the cellular retinoic acid binding protein-II (CRABP-II) increased strongly [25]. CRABP-II is probably involved in the intracellular transport of RA to the cell nucleus [27]. As after SCI, the RA-synthesizing enzyme RALDH2 was present in the injured nerve. After sciatic nerve injury in transgenic reporter mice, local activation of RARE was detectable in the regenerating nerve, indicating that RA-dependent gene expression is induced during peripheral nerve regeneration [25]. The expression of retinoid receptors also changed significantly. Following sciatic nerve crush, mRNA of all RARs and of RXRα was increased, and at 4, 7, and 14 days after the injury protein levels of RARα, RARβ, and RXRα were augmented [28]. To study Wallerian degeneration in the absence of axonal regeneration, the distal nerve segment can be cut off and sutured to the peroneus muscle. Under these conditions RARα and RARβ were upregulated in the degenerating nerve.
Cell culture experiments indicated that RARβ is required for RA-induced axonal regeneration [24]. Consequently, experiments with RARβ-deficient mice corroborated this interpretation because there were significantly less GAP43-positive, regenerating axons after sciatic nerve crush in the knockout animals [29]. Since RALDH2 [23] and RARβ [30] are induced by NGF, RA appears to act downstream of this neurotrophin. RA/RARβ signaling seemed to be necessary for the neurotrophic activity of NGF [23]. In addition, neurotrophin-independent RA/RARβ activity contributes to axon outgrowth [29]. Double labeling with cell type specific markers in the sciatic nerve demonstrated expression of retinoid receptors mostly in macrophages and Schwann cells. In cultured primary Schwann cells, RA downregulated CNTF [31] and raised expression of ErbB3, a neuregulin receptor that is necessary for normal myelination. Finally, RXRα labeling was shown to colocalize with some regenerating axons, indicative of a direct role for RXRα in nerve regeneration [28].
2.4. PPAR/RXR signal transduction
Peroxisome proliferator-activated receptors belong to the same superfamily as the RAR. The first PPAR was discovered, cloned, and sequenced in 1990, and it was named after its property to be activated by drugs that cause proliferation of peroxisomes in hepatocytes [32]. Until now, three different isoforms of PPAR, encoded by separate genes, have been identified: PPARα (NR1C1), PPARβ/δ (NUC1, NR1C2), and PPARγ (NR1C3). The different isoforms have similar structural features, however each isoform exhibits its own specific tissue expression pattern and distinct physiological functions depending on ligand activation [33, 34]. PPAR also heterodimerize with RXR, then bind as a complex to their response elements (PPRE). These consist of two repeats of the consensus sequence AGGTCA separated by one or two nucleotides (direct repeats DR-1 and DR-2) [35]. Natural ligands of PPAR are fatty acids, prostaglandins, and oxidized fatty acid derivatives. They are also activated by synthetic ligands like the lipid-lowering fibrates and the anti-diabetic glitazones [36]. PPAR, which have been implicated in lipid metabolism, cellular proliferation and inflammatory responses, are widely expressed, for example, in colon, spleen, retina, the cardiovascular system, liver, skeletal muscles, and in adipose tissue. Their expression by monocytes, dendritic cells, endothelial cells, megakaryocytes, and lymphocytes may be related to immune functions [34, 36]. PPARγ can also influence gene expression independently of PPRE. The activity of a number of transcription factors, for example, NFκB, AP-1, and STAT-1, are inhibited by PPARγ via direct interaction or by competition for limiting supplies of coactivators [6].
2.5. PPAR/RXR signaling after spinal cord injury
PPAR are expressed in the developing and adult CNS. PPARα and PPARβ/δ, but not PPARγ were found in cervical, thoracic, and lumbar segments of the adult spinal cord, in the thalamus and cerebral cortex [37] (Figure 2). Immunohistochemical staining showed that PPARβ/δ is the main isoform present in neuronal cell bodies of the spinal cord gray matter. Both receptors, PPARα and PPARβ/δ, were concentrated in cell nuclei. In the white matter PPARα appeared particularly strong in PPARβ/δ-negative astrocytes, whereas oligodendrocytes expressed only PPARβ/δ [38]. PPARβ/δ is a factor in neuronal differentiation, and functions of this receptor in various aspects of neural physiology have been suggested [38, 39]. While in two studies PPARγ could not be detected in brain and spinal cord [37, 38], this receptor is expressed in microglial primary cultures [40] and may be upregulated after injury. In a different report, all PPAR and RXR were found immunohistochemically throughout the adult rat CNS [41].
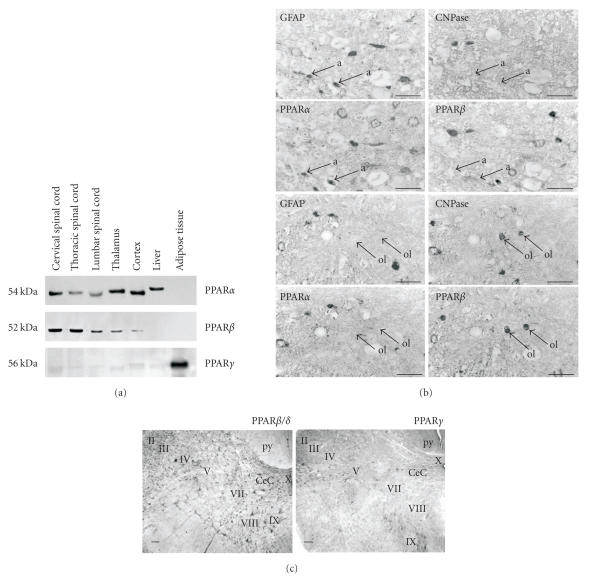
Figure 2.
Localization of PPARα and PPARβ/δ in astrocytes and oligodendrocytes in the spinal cord. (a) Western blotting shows PPARα and PPARβ but not PPARγ in spinal cord, telencephalon, and diencephalon. Identical expression patterns were detected with RT-PCR. (b) Detection of PPAR immunoreactive cells in the white matter of rat spinal cord. GFAP-positive/CNPase-negative astrocytes are immunoreactive for PPARα (marked a, upper four panels) while GFAP-negative/CNPase-positive oligodendrocytes express PPARβ (marked ol, lower panels) (scale bars: 25μ m, source: [38]). (c) Distribution of PPARβ/δ and PPARγ immunoreactive cells in the spinal cord of the adult rat (cervical level, coronal sections, scale bar: 100μ m, source: [41]).
Endogenous PPAR ligands may play a role in modulating the inflammatory response after SCI, possibly preventing the expansion of the initial damage. Genovese and coworkers [42] examined the effects of endogenous PPARα ligands in an experimental model of spinal cord trauma. SCI was induced in wild-type and in PPARα-deficient (−/−) mice. The injury resulted in severe trauma characterized by edema, loss of myelin, neutrophil infiltration, apoptosis, and increased production of TNFα. Compared to wild-type animals, all these parameters were augmented in PPARα−/− mice. The absence of PPARα also interfered with recovery of limb function [42]. Studies using animal models of chronic pain indicate that PPARα is involved in the neural processing of pain [43, 44].
3. REGULATION OF INFLAMMATORY PROCESSES BY RAR/RXR SIGNALING IN THE SPINAL CORD
Although the inflammatory response limits the effects of a pathogenic insult, it is also responsible for most of the secondary damage incurred after spinal cord injury. The regulation of these events is therefore of primary therapeutic concern. Both pathways, RAR/RXR and PPAR/RXR signaling are implicated.
3.1. Inflammatory reactions in the spinal cord
The physiological events following spinal cord injury can be differentiated into an acute phase (the first 24hrs), followed by a subacute phase (24–72hrs), and a late phase (3–90 days). Events after this time are considered chronic. CNS injury directly leads to release of inflammatory signals resulting in the production of vasoactive mediators and chemotactic factors. Primary damage consists mainly of severed axons, neuronal and oligodendrocyte cell death. Expansion of the initial damage is then caused by a disruption of the blood supply and an extended inflammatory reaction. Depending on the type of injury, the blood-brain barrier is disrupted. This event determines the extent to which blood-derived cells participate in the inflammatory process [45, 46]. When the blood-brain barrier breaks down, the release of cytokines and chemokines leads to a massive recruitment of inflammatory cells from the periphery, including hematogenous neutrophils and perivascular/meningeal macrophages. These cells contribute to tissue damage by the production of proteolytic enzymes and reactive oxygen species. Microglia and macrophages phagocytose dead or injured neurons and glia, and are responsible for the clearance of cellular debris [45].
Microglia, perivascular macrophages, and activated astrocytes are the most important local sources of cytokines. Astrocytes are further involved in the formation of a glial scar, thereby insulating the healthy tissue from uncontrollable processes in the damaged area. Acute inflammation can develop into a chronic process when feedback mechanisms fail to inhibit amplification of the inflammatory response. Chronic inflammation leads to a continuous influx of neutrophils, macrophages, lymphocytes, and eosinophils from the circulation, causing more tissue destruction and scarring.
3.2. RAR/RXR signaling is involved in inflammation
Before the molecular mechanisms of the retinoid signaling pathway were understood, anti-inflammatory properties of RA had already been described. In 1983, a study showed that oral administration of retinoids affected dermal inflammatory cells and reduced elevated skin temperature [47]. Retinoic acid has beneficial effects in diseases with an inflammatory-based pathology including asthma, arthritis, and atherosclerosis [48, 49]. A number of cell culture studies support the hypothesis that RA reduces inflammatory activation of monocytes, macrophages, myeloma cells, and polymorphonuclear cells (neutrophil granulocytes). Signals that were found to be downregulated by retinoids include IL-1α, IL-1β, IL-6, TNFα, IL-8, prostaglandin E2, production of reactive oxygen species, and release of lysosomal enzymes. A table with these effects and references is presented in [5]. In contrast to a majority of findings that indicate an anti-inflammatory role of RAR/RXR signaling, it was also reported that 9-cis RA induced secretion of MCP-1 and thereby stimulated monocyte migration [50]. In combination with T-cell stimulating agents, all-trans RA, 9-cis RA, and an RAR agonist, TTNPB, indirectly increased proliferation of human T lymphocytes and IL-2 secretion [51].
One of the major transcription factors responsible for the regulation of inflammatory cytokines is NFκB. It was shown that RXR inhibits NFκB-dependent gene expression (IL-12) [52]. In the experiments, interference of RXR with DNA binding of NFκB required the presence of the RXR ligand 9-cis RA. The negative RXR/NFκB interaction appears to be mutual because increasing levels of transfection with NFκB subunits also abrogated expression of a retinoid reporter construct [52].
3.3. The role of RAR/RXR in inflammatory reactions after CNS injury
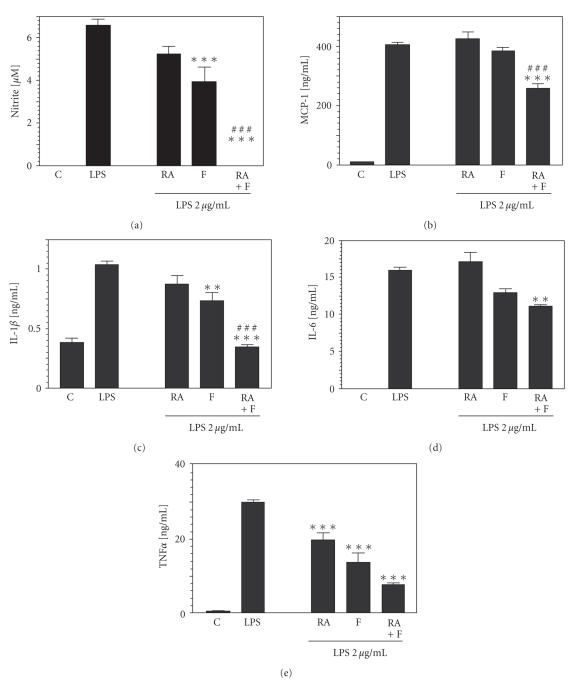
Although retinoic acid has so far not been used to modify inflammatory reactions in the CNS in vivo, cell culture experiments demonstrate anti-inflammatory effects on microglia and astrocytes [5]. Primary cultures of microglia and astrocytes are most frequently prepared from cerebral cortex of perinatal rats and mice. One can expose these cultures to LPS to simulate a bacterial infection, or treat them with β-amyloid peptide (Aβ) to mimic the immunogenic stimulus of Alzheimer's disease. In this kind of experiment, RA treatment suppressed the induction of TNFα and iNOS. Effects correlated with enhanced expression of RARβ, TGFβ, and inhibition of NFκB nuclear translocation [53]. Xu and Drew demonstrated that 9-cis RA suppressed LPS-induced production of NO as well as of proinflammatory cytokines TNFα, IL-1β, and IL-12p40 in primary mouse microglia, while IL-6 secretion and MCP-1 production were not significantly affected (Figure 3) [54]. In astrocytes from rat brain and in C6 astroglioma cells, 9-cis RA and all-trans RA inhibited IFNγ-induced inflammatory responses [55].
Figure 3.
Anti-inflammatory effects of PPAR and RAR agonists in astrocytes. Astrocyte primary cultures from cortices of newborn mice were treated with 9-cis RA (RA) and/or the PPARα agonist fenofibrate (F) for 1hour and then stimulated with 2μg/mL LPS for 24 hours. (a) Nitrite production as an indicator of NOS activity was reduced by 1μ M RA or 100μ M F, and completely suppressed with RA plus F. Pretreatment of RA and/or F also decreased the release of (b) the chemokine MCP-1 (RA: 2μ M, F: 200μ M), and cytokines (c) IL-1β (RA: 1μ M, F: 100μ M), (d) IL-6 (RA: 2μ M, F: 50μ M), and (e) TNFα (RA: 1μ M, F: 100μ M) in response to LPS. Cytokine production was measured with ELISA. Experiments with microglia cultures revealed similar effects, except for MCP-1, whose production was stimulated by 9-cis RA in microglia. Error bars indicate SEM, asterisks indicate significant differences compared to the LPS condition (sources: [54, 78]).
Matrix metalloproteinases (MMP) are involved in the breakdown of the extracellular matrix and other proteins. These proteases are products of leukocytes and endothelial cells and are released in response to various cytokines and growth factors. Increased proteolytic activity of MMP can lead to disruption of the blood-brain barrier and escalate the inflammatory response [56, 57]. Retinoic acid has negative effects on secretion and expression of these enzymes. It reduced mRNA, protein synthesis, and secretion of MMP-9 (gelatinase B) in lymphocytes from patients with chronic B-lymphocytic leukemia and in human monocytes [58]. Other metalloproteinases including MMP-1 and MMP-13 appeared to be influenced by RA as well [59].
4. REGULATION OF INFLAMMATORY PROCESSES BY PPAR/RXR SIGNALING IN THE SPINAL CORD
4.1. PPAR/RXR signaling counteracts inflammatory processes
For the PPAR family, anti-inflammatory effects are well documented. One mechanism involves direct interaction of PPAR with proinflammatory transcription factors, most importantly NFκB and AP-1, and the subsequent reduction of gene transcription. This has been observed in vitro in human vascular endothelial cells [60], human aorta smooth muscle cells [61], in C2C12 skeletal muscle cells (in a model for insulin resistance in type 2 diabetes) [62], and in fibroblasts from rheumatoid arthritis patients [63]. PPAR knockout studies supplement and confirm these results. One of the first reports indicating that PPARα is involved in attenuating inflammation demonstrated that the eicosanoid LTB4 binds and activates PPARα. Subsequently, PPARα-deficient mice were shown to have a prolonged inflammatory response when challenged with LTB4 or arachidonic acid [64].
An interaction of PPARα with NFκB is indicated by studies of inflammatory cytokine production in aging. In aged mice, NFκB becomes constitutively active in many tissues due to oxidative stress, which eventually leads to the production of cytokines. Administration of PPARα activators was found to restore the cellular redox balance, to suppress the constitutive activation of NFκB and to eliminate the spontaneous production of IL-6 and IL-12 [65, 66]. Other examples where PPARα−/− mice suffer from more severe inflammatory reactions are carrageenan-induced hypersensitivity [67], airway inflammation [68], and experimental colitis [69]. On the molecular level, these investigations demonstrated Fas-ligand, IL-1β, TNFα, keratinocyte-derived chemokine, MIP-2, MCP-1, ICAM-1, and enzyme activities of MMP-9, myeloperoxidase, and iNOS to be influenced by PPARα signaling [67, 69, 70].
PPARγ-deficient mice die in utero, but heterozygotic PPARγ+/− mice with 50% expression of the receptor survive and can be studied. In an investigation of PPARγ functions after intestinal and gastric ischemia/reperfusion injury, PPARγ+/− mice displayed more severe lesions of the gastric intestinal mucosa. Treatment with the PPARγ ligand BRL-49653 limited the damage of intestinal injury and resulted in downregulation of cell adhesion molecules and proinflammatory cytokines in the intestine and stomach [71, 72]. As mentioned before, NFκB and inflammatory mediators are raised during the aging process. While this is accompanied by reduced PPARγ levels, the PPARγ ligand 2,4-TZD was shown to reduce age-related oxidative stress, the translocation of NFκB p65 subunit to the nucleus and NFκB-regulated transcription of iNOS, COX-2, IL-1β, IL-6, and the cell-adhesion molecule VCAM-1 [73]. Another PPARγ agonist, rosiglitazone, relieved renal injury in a nephrotoxicity model, also acting via inhibiton of NFκB [74]. It seems therefore that both receptors PPARα and PPARγ play a general role in modulating the inflammatory response in a wide variety of tissues.
4.2. Effect of PPAR activation on macrophages, microglia, and astrocytes
Given that PPAR agonists influence peripheral macrophages [75], it was to be expected that they act on brain macrophages after disruption of the blood-brain barrier. PPARα is already present in undifferentiated monocytes, while PPARγ expression is induced during differentiation [76, 77] and upregulated in the course of macrophage activation [78–82]. A number of cell culture studies with macrophages and microglia demonstrate that agonists of PPARα and PPARγ elicit the same anti-inflammatory effects that we have discussed above. When cells were activated with bacterial antigens or Aβ, the PPAR ligands typically prevented or reduced the inflammatory response. This effect was observed on the following levels: (1) activity of transcription factors NFκB, AP-1, and STAT-1, (2) secretion of proinflammatory cytokines and chemokines, (3) enzyme activities of COX-2, iNOS, and MMP-9, and (4) the formation of reactive oxigen radicals. A synopsis of these results is presented in Table 1.
Table 1.
Anti-inflammatory effects of PPARα and PPARγ agonists on the molecular level.
| Experimental system | Nuclear receptor | Regulated signals | Reference |
|
| |||
| Cytokine- and LPS-stimulated microglia | PPARα, RXR | NO synthesis, IL-1β, IL-6, IL-12p40, TNFα, MCP-1 | [78] |
| Activated macrophages | PPARα, γ | iNOS, heme ogygenase (COX-2, HSP70 not affected) | [70] |
| Activated macrophages | PPARγ | iNOS, MMP-9, scavenger receptor A | [80] |
| Activated human monocytes | PPARγ | IL-1β, IL-6, TNFα | [81] |
| Activated macrophages | PPARγ | Reactive oxigen species | [77] |
| LPS-stimulated microglia | PPARγ | IL-1β, IL-6, TNFα, MCP-1, iNOS | [87] |
| LPS-stimulated microglia | PPARγ | IL-6, TNFα, iNOS, COX-2 | [85] |
| Aβ-stimulated microglia | PPARγ | IL-6, TNFα, COX-2 | [82] |
| LPS-stimulated astrocytes | PPARα, RXR | IL-1β, IL-6, TNFα, iNOS, MCP-1 | [88] |
| LPS-stimulated astrocytes | PPARγ | IL-6, TNFα, iNOS, COX-2 | [85] |
| LPS-stimulated astrocytes | PPARγ | IL-1β, IL-6, TNFα, MCP-1, iNOS | [87] |
| Cerebellar injection of IFNγ, LPS | PPARγ | iNOS, cell death | [89] |
| MPTP-treated mice | PPARγ | iNOS, IκB, cell death | [86] |
| APPV717I transgenic mice | PPARγ | iNOS, COX-2, β-amyloid | [83] |
| Rat spinal cord injury | PPARγ | IRF-1, IL-1β, IL-6, MCP-1, ICAM, Egr-1 | [106] |
| Rat model for stroke | PPARγ | IL-1β, iNOS, COX-2 | [107] |
Such effects were observed with natural ligands, for example, prostaglandins, with artificial PPAR activators, for example, antidiabetic thiadiazolidinones, and with nonsteroid anti-inflammatory drugs [83]. A very efficient endogenous agonist of PPARγ is the prostaglandin 15d-PGJ2 [70]. However, this drug also interferes with the transcriptional activity of NFκB independently of PPARγ activation because overexpression of PPARγ or an antagonist of PPARγ did not alter the 15d-PGJ2 effect on LPS/IFNγ-dependent inflammatory reaction. It was concluded that 15d-PGJ2 inhibits the inflammatory response by directly regulating the NFκB and PI3K-Akt pathways [79, 84].
With respect to microglia effects on nerve cells, PPAR activation with synthetic ligands can be neuroprotective. When cortical neurons were exposed to the cell-free supernatant from activated microglia, neurons survived better when microglia had also been incubated with PPARγ agonists [85]. Some experiments in vivo also support this concept. Oral administration of pioglitazone or the nonsteroidal anti-inflammatory drug ibuprofen, which is also a ligand for PPARγ, reduced the number of activated microglia and reactive astrocytes in the hippocampus and cerebral cortex. This was observed in 10-month-old APPV717I mice, an inflammation model for Alzheimer's disease [83]. Pioglitazone protected mice also from MPTP-induced dopaminergic neuronal cell loss and mitigated inflammatory response by microglia in an animal model of Parkinson's disease [86].
As mentioned before, astrocytes participate in the secretion of cytokines and form a glial scar around the damaged tissue. Since the production of microglia primary cultures also involves isolation of astrocytes, it was a straightforward approach to test PPAR agonists on this type of glia as well (Figure 3) [87–89]. Results, which were generally in line with the anti-inflammatory effects on microglia, are also included in Table 1.
5. APPLICATION OF RETINOIDS AND PPAR LIGANDS AFTER SPINAL CORD AND PERIPHERAL NERVE INJURY
Retinoic acid is a well-characterized morphogenetic factor in embryonic development. Stem cells and several cell lines can be induced with RA to differentiate to a neuronal phenotype. A number of experiments with developing sympathetic neurons and with sensory neurons from dorsal root ganglia revealed that RA has neurotrophic effects or induces neurite growth. The question arises whether these properties can be exploited to support axonal regeneration [5, 90].
5.1. Retinoic acid-induced axonal growth in vitro and in vivo
While the most convincing demonstration that RA can act as an axon-guiding chemoattractant was recently published using the snail Lymnaea stagnalis [91], a number of earlier studies indicated that retinoids may fulfill similar functions in vertebrates. Neurites extending from chick neural tube cells responded to a gradient of RA [92]. Positive effects on axon outgrowth were also reported using explants from murine embryonic spinal cord [93], embryonic cerebellum [94], and amphibian spinal cord [95]. In a chick retinal explant assay, RA enhanced neurite outgrowth under the condition that the neurotrophin BDNF was subsequently added in vitro [96]. RA supported survival and morphological differentiation of cultured rat spinal cord neurons and increased neurite density. In this context, astrocytes were implicated as regulators of local RA concentration [16, 17].
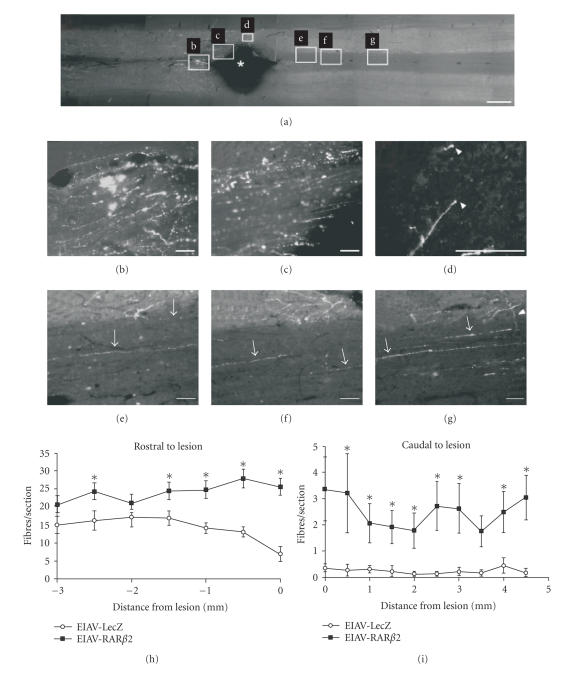
Corcoran, Maden, and coworkers suggested that the loss of regeneration in the adult mammalian CNS is related to developmental changes in retinoid signaling. They observed that the RARβ2 receptor, which is present when neurite outgrowth occurs from embryonic spinal cord explants, was not detectable in the adult spinal cord [97]. A supporting result was that specifically RARβ mediated the induction of neurite outgrowth from subpopulations of sensory neurons [24]. In addition to the inhibitory glial environment [1], intrinsic changes in CNS neurons have been assumed to be responsible for the loss of regenerative ability during CNS development [98]. The declining expression of RARβ2 may be one of those changes. To test this hypothesis, the RARβ2 gene was transfected into spinal cord explants with a lentiviral vector. This treatment allowed the outgrowth of many neurites, implying the involvement of RARβ2 in axonal regeneration [97]. Consequently, to demonstrate that a renewed expression of the appropriate receptor mechanism might induce axonal regeneration into the spinal cord in vivo, Wong and others [99] transfected RARβ2 in adult rats. They then analyzed regeneration of sensory axons into the spinal cord and performed behavioral experiments. Following complete dorsal root transections by means of a crush injury, axons regenerated across the dorsal root entry zone into the CNS. Both myelinated and nonmyelinated fibers were found to have grown in the spinal cord where they projected into the gray matter, formed functional connections and improved sensorimotor recovery of the animals [99]. In another study, artificially induced RARβ2 expression also supported regeneration of descending corticospinal tract fibers after midcervical spinal cord injury (Figure 4). Again, the induced regeneration of fibers was accompanied by improved sensory and locomotor behavior [100]. These data suggest not only that RARβ is involved in activating a physiological program of regeneration but also that retinoic acid signaling may be used as a therapeutic tool in spinal cord injury.
Figure 4.
Transfection of RARβ induces regeneration of corticospinal axons in vivo. (a) Longitudinal sections of the adult rat spinal cord were traced with BDA to determine fibre regeneration in the corticospinal tract; overview of the lesion site in an animal injected with the RARβ2 expressing construct (EIAV-RARβ2, Rostral: left). (b) Animals with RARβ2-transfected cells displayed less fiber degeneration than control rats. (c) Labeled axons were detected up to the edge of the lesion, (d) at the distal edge of the lesion, and (e–g) at various distances caudal to the lesion. Growth cone-like endings were detected at the tips of some axons (white arrowheads in d). Axons appear to send collaterals (white arrowhead in g) from white matter to grey matter. (Scale bars in (a): 1mm, in (b–g): 100μ m.) (h–i) Quantification of BDA-labeled fibres in the corticospinal tract after spinal cord lesion. EIAV-RARβ2-treated animals displayed increased fiber numbers rostral and caudal to the lesion compared to control animals (P < .05, two-way ANOVA) (source: [100]).
5.2. Manipulation of RAR/RXR signaling in the peripheral nervous system
As mentioned above, it has been known for some time that retinoids act as neurotrophic factors for sympathetic and sensory neurons of the peripheral nervous system [20–24]. However, very few studies have addressed the role of RA in peripheral nerve regeneration in vivo. Taha and coworkers tested whether local RA injections improved morphological and functional regeneration of tibial nerves undergoing anastomosis. They showed that animals injected locally with RA exhibited increased axonal density when compared to vehicle-treated animals [101]. Several cell culture experiments suggested that RA effects may involve an upregulation of neurotrophin receptors, and molecular studies indicate mutually synergistic influences of the RA- and neurotrophin-signaling pathways. Nerve growth factor induced the RA-synthesizing enzyme RALDH in dorsal root ganglia [23] and activated the RARβ promoter [30]. RA, on the other hand, caused expression of various neurotrophin receptors (see [5, 90] for review). That synergistic interactions between RA and neurotrophins take place in vivo was revealed in a study using a mouse model for diabetes [102]. Treatment of the animals with all-trans RA via subcutaneous injection in a dose of 20mg/kg was able to prevent the NGF depletion normally seen in diabetic mice. Microscopic analysis subsequently showed that diabetes-associated loss of Schwann cells, of myelinated, and of nonmyelinated axons was strongly reduced by RA. This beneficial effect was also measurable in behavioral tests of sensory-motor functions [102].
5.3. Retinoids and PPAR ligands in pain signaling
In a different line of research about spinal cord sensitization, some contrasting results were obtained recently. To investigate whether retinoids might be involved in the processing of nociceptive information, especially in situations of hyperalgesia due to inflammation, rats were given 10–15mg/kg all-trans RA orally for 4 days. Electrophysiological recordings revealed that treated animals had decreased thresholds to mechanical and electrical stimulation of the paws and increased cutaneous receptive fields. This increased responsiveness caused by RA was similar to hyperalgesia induced by intraplantar administration of carrageenan (which leads to local inflammation) [103]. On the molecular level, RA induced changes of gene expression in the spinal cord, for instance, an increase of COX-2 and IL-1. Inhibition of these pathways with IL-1 receptor antagonist and the COX-inhibitor dexketoprofen reduced responses to mechanical or thermal stimulation when those had been sensitized with RA [104]. In these studies, it was concluded that all-trans RA induced changes in the spinal cord that are similar to inflammation. At this point, it is difficult to reconcile this conclusion with the anti-inflammatory properties when RA was combined with stimuli of bacterial infection or Aβ deposition.
In contrast to RA, administration of the PPARα agonists GW7647, Wy14643, perfluorooctanoic acid, and PEA reduced nocifensive behaviors in various animal models of hyperalgesia, implicating a mitigating role of PPAR in the regulation of pain signaling [43, 44]. GW7647 and PEA also prevented firing of spinal cord nociceptive neurons in rats after peripheral exposure to formalin [44].
5.4. Use of PPAR ligands as anti-inflammatory treatment after spinal cord injury
The knowledge about anti-inflammatory properties of PPAR ligands obtained in cell cultures soon prompted animal experiments. In these, PPARα ligands were indeed shown to exert neuroprotective effects after traumatic brain injury [105]. Recently, it was discovered that synthetic PPARγ ligands rosiglitazone and pioglitazone are also neuroprotective by reducing the inflammation in the spinal cord. Treatment of rats directly after SCI resulted in a decreased lesion site, better survival of motor neurons, sparing of myelin, reduced astrogliosis, and less microglial activation. These features were accompanied by enhanced functional motor recovery and reduced hyperalgesia [106]. Investigation of gene expression revealed that pioglitazone lowered levels of transcription factors IRF-1, NFκB, transmembrane proteins Egr-1, ICAM, and cytokine/chemokines IL-1β, IL-6, MCP-1, but increased expression of neuroprotective genes and antioxidant enzymes. In a rat model for stroke (middle cerebral artery occlusion), PPARγ ligands were able to improve neurological outcome, decreased infarct size, and reduced inflammation. Fewer microglia/macrophages appeared to be present, and transcript and protein levels of IL-1, COX-2, and iNOS were also lower [107].
6. CONCLUSION
The studies discussed here demonstrate that RAR/RXR and PPAR/RXR signaling may be therapeutic targets after spinal cord injury. The main potential of PPAR ligands derives from their anti-inflammatory capacity, which has been corroborated in rodent models of inflammation involving various peripheral organs. After spinal cord injury, inflammatory reactions account for a large proportion of the secondary damage to neurons and oligodendrocytes. In this context, first experiments show that PPAR ligands are neuroprotective. Since PPAR activators are already in use to treat diabetes, clinical studies after stoke or other kinds of CNS damage are to be expected. While retinoids also have anti-inflammatory properties, they exert more direct effects on the nerve tissue. In several populations of neurons, axonal regeneration can be induced or supported by the activation of RAR/RXR signaling. Since systemic application of retinoic acid influences neural physiology within the CNS, therapeutic possibilities arise here as well. As in the case of cancer treatment, an important consideration is the expression of retinoid receptors. After spinal cord injury the induction of RARβ appears to be a requirement. In addition to the classical transcriptional effects, retinoids and PPAR ligands can act via receptor-independent mechanisms. This, however, is a largely uncharted territory where therapeutic benefits remain to be discovered.
ACKNOWLEDGMENTS
S. van Neerven is supported by a Marie Curie Fellowship of the EURON Graduate School of Neuroscience. The authors thank Eric Kampmann for helpful discussions and proofreading of the manuscript.
ABBREVIATIONS
- Aβ:
Beta amyloid peptide
- ADH:
Alcohol dehydrogenase
- AP:
Activating protein
- BDA:
Biotinylated dextran amine
- BDNF:
Brain-derived neurotrophic factor
- CNS:
Central nervous system
- CNTF:
Ciliary neurotrophic factor
- COX:
Cyclooxygenase
- CRABP:
Cellular retinoic acid binding protein
- CRBP:
Cellular retinol binding protein
- dpo:
dies post operationem
- EGF:
Epidermal growth factor
- Egr:
EGF receptor
- ErbB:
Tyrosine kinase related to the EGF recaptor
- GAP43:
Growth associated protein 43
- ICAM:
Intercellular cell adhesion molecule
- IFN:
Interferon, IL—interleukin
- iNOS:
Inducible NO synthase
- IRF:
Interferon regulatory factor
- LPS:
Lipopolysaccharide
- LTB4:
Leukotriene B4
- MCP:
Macrophage chemoattractant protein
- MIP:
Macrophage inflammatory protein
- MMP:
Matrix metalloproteinase
- MPTP:
1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine
- NFκB:
Nuclear factor κB NGF Nerve growth factor
- NR(plus number):
Nuclear receptor
- PEA:
Palmitoyl ethanolamide
- 15d-PGJ2:
15-deoxy-delta prostaglandin J2
- PI3-K:
Phosphoinositide 3-kinase
- PNS:
Peripheral nervous system
- PPAR:
Peroxisome proliferator-activated receptor
- PPRE:
PPAR response element
- RA:
Retinoic acid
- RALDH:
Retinaldehyde dehydrogenase
- RAR:
Retinoic acid receptor
- RARE:
Retinoic acid response element
- RXR:
Retinoid X receptor
- SCI:
Spinal cord injury
- STAT:
Signal transducer and activator of transcription
- TGF:
Transforming growth factor
- TNF:
Tumor necrosis factor
- TR:
Thyroid hormone receptor
- TTNPB:
4-[(E)-2-(5,6,7,8-tetrahydro- 5,5,8,8-tetramethyl-2- naphthalenyl)-1-propenyl] benzoic acid
- 2,4-TZD:
2,4-thiazolidinedione
- VCAM:
Vascular cell adhesion molecule
References
- 1.Silver J, Miller JH. Regeneration beyond the glial scar. Nature Reviews Neuroscience. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 2.Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. Journal of Neurochemistry. 2004;89(5):1092–1100. doi: 10.1111/j.1471-4159.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- 3.Makwana M, Raivich G. Molecular mechanisms in successful peripheral regeneration. FEBS Journal. 2005;272(11):2628–2638. doi: 10.1111/j.1742-4658.2005.04699.x. [DOI] [PubMed] [Google Scholar]
- 4.Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. Journal of Neurotrauma. 2006;23(3-4):318–334. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- 5.Mey J. New therapeutic target for CNS injury? The role of retinoic acid signaling after nerve lesions. Journal of Neurobiology. 2006;66(7):757–779. doi: 10.1002/neu.20238. [DOI] [PubMed] [Google Scholar]
- 6.Kielian T, Drew PD. Effects of peroxisome proliferator-activated receptor-γ agonists on central nervous system inflammation. Journal of Neuroscience Research. 2003;71(3):315–325. doi: 10.1002/jnr.10501. [DOI] [PubMed] [Google Scholar]
- 7.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. Journal of Neurobiology. 2006;66(7):606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 8.Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- 9.Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 10.Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328(1-2):1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Lane MA, Bailey SJ. Role of retinoid signalling in the adult brain. Progress in Neurobiology. 2005;75(4):275–293. doi: 10.1016/j.pneurobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Mey J, Morassutti DJ, Brook G, et al. Retinoic acid synthesis by a population of NG2-positive cells in the injured spinal cord. European Journal of Neuroscience. 2005;21(6):1555–1568. doi: 10.1111/j.1460-9568.2005.03928.x. [DOI] [PubMed] [Google Scholar]
- 13.Kern J, Schrage K, Koopmans GC, Joosten EA, McCaffery P, Mey J. Characterization of retinaldehyde dehydrogenase-2 induction in NG2-positive glia after spinal cord contusion injury. International Journal of Developmental Neuroscience. 2007;25(1):7–16. doi: 10.1016/j.ijdevneu.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Schrage K, Koopmans G, Joosten EAJ, Mey J. Macrophages and neurons are targets of retinoic acid signaling after spinal cord contusion injury. European Journal of Neuroscience. 2006;23(2):285–295. doi: 10.1111/j.1460-9568.2005.04534.x. [DOI] [PubMed] [Google Scholar]
- 15.Sockanathan S, Perlmann T, Jessell TM. Retinoid receptor signaling in postmitotic motor neurons regulates rostrocaudal positional identity and axonal projection pattern. Neuron. 2003;40(1):97–111. doi: 10.1016/s0896-6273(03)00532-4. [DOI] [PubMed] [Google Scholar]
- 16.Wuarin L, Sidell N. Differential susceptibilities of spinal cord neurons to retinoic acid-induced survival and differentiation. Developmental Biology. 1991;144(2):429–435. doi: 10.1016/0012-1606(91)90435-6. [DOI] [PubMed] [Google Scholar]
- 17.Wuarin L, Sidell N, de Vellis J. Retinoids increase perinatal spinal cord neuronal survival and astroglial differentiation. International Journal of Developmental Neuroscience. 1990;8(3):317–326. doi: 10.1016/0736-5748(90)90038-4. [DOI] [PubMed] [Google Scholar]
- 18.Vermot J, Schuhbaur B, Le Mouellic H, et al. Retinaldehyde dehydrogenase 2 and Hoxc8 are required in the murine brachial spinal cord for the specification on Lim1+ motoneurons and the correct distribution of Islet1+ motoneurons. Development. 2005;132(7):1611–1621. doi: 10.1242/dev.01718. [DOI] [PubMed] [Google Scholar]
- 19.Liu J-P, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32(6):997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- 20.Holst AV, Lefcort F, Rohrer H. TrkA expression levels of sympathetic neurons correlate with NGF-dependent survival during development and after treatment with retinoic acid. European Journal of Neuroscience. 1997;9(10):2169–2177. doi: 10.1111/j.1460-9568.1997.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 21.Wyatt S, Andres R, Rohrer H, Davies AM. Regulation of neurotrophin receptor expression by retinoic acid in mouse sympathetic neuroblasts. Journal of Neuroscience. 1999;19(3):1062–1071. doi: 10.1523/JNEUROSCI.19-03-01062.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Tebar A, Rohrer H. Retinoic acid induces NGF-dependent survival response and high-affinity NGF receptors in immature chick sympathetic neurons. Development. 1991;112(3):813–820. doi: 10.1242/dev.112.3.813. [DOI] [PubMed] [Google Scholar]
- 23.Corcoran J, Maden M. Nerve growth factor acts via retinoic acid synthesis to stimulate neurite outgrowth. Nature Neuroscience. 1999;2(4):307–308. doi: 10.1038/7214. [DOI] [PubMed] [Google Scholar]
- 24.Corcoran J, Shroot B, Pizzey J, Maden M. The role of retinoic acid receptors in neurite outgrowth from different populations of embryonic mouse dorsal root ganglia. Journal of Cell Science. 2000;113(14):2567–2574. doi: 10.1242/jcs.113.14.2567. [DOI] [PubMed] [Google Scholar]
- 25.Zhelyaznik N, Schrage K, McCaffery P, Mey J. Activation of retinoic acid signalling after sciatic nerve injury: up-regulation of cellular retinoid binding proteins. European Journal of Neuroscience. 2003;18(5):1033–1040. doi: 10.1046/j.1460-9568.2003.02834.x. [DOI] [PubMed] [Google Scholar]
- 26.Belyaeva OV, Korkina OV, Stetsenko AV, Kim T, Nelson PS, Kedishvili NY. Biochemical properties of purified human retinol dehydrogenase 12 (RDH12): catalytic efficiency toward retinoids and C9 aldehydes and effects of cellular retinol-binding protein type I (CRBPI) and cellular retinaldehyde-binding protein (CRALBP) on the oxidation and reduction of retinoids. Biochemistry. 2005;44(18):7035–7047. doi: 10.1021/bi050226k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budhu AS, Noy N. Direct channeling of retinoic acid between cellular retinoic acid-binding protein II and retinoic acid receptor sensitizes mammary carcinoma cells to retinoic acid-induced growth arrest. Molecular and Cellular Biology. 2002;22(8):2632–2641. doi: 10.1128/MCB.22.8.2632-2641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhelyaznik N, Mey J. Regulation of retinoic acid receptors α, β and retinoid X receptor α after sciatic nerve injury. Neuroscience. 2006;141(4):1761–1774. doi: 10.1016/j.neuroscience.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 29.So P-L, Yip PK, Bunting S, et al. Interactions between retinoic acid, nerve growth factor and sonic hedgehog signalling pathways in neurite outgrowth. Developmental Biology. 2006;298(1):167–175. doi: 10.1016/j.ydbio.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 30.Cosgaya JM, Aranda A. Nerve growth factor activates the RARβ2 promoter by a Ras-dependent mechanism. Journal of Neurochemistry. 2001;76(3):661–670. doi: 10.1046/j.1471-4159.2001.00078.x. [DOI] [PubMed] [Google Scholar]
- 31.Johann V, Jeliaznik N, Schrage K, Mey J. Retinoic acid downregulates the expression of ciliary neurotrophic factor in rat Schwann cells. Neuroscience Letters. 2003;339(1):13–16. doi: 10.1016/s0304-3940(02)01427-1. [DOI] [PubMed] [Google Scholar]
- 32.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 33.Kliewer SA, Forman BM, Blumberg B, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kota BP, Huang TH-W, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacological Research. 2005;51(2):85–94. doi: 10.1016/j.phrs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Gervois P, Chopin-Delannoy S, Fadel A, et al. Fibrates increase human REV-ERBα expression in liver via a novel peroxisome proliferator-activated receptor response element. Molecular Endocrinology. 1999;13(3):400–409. doi: 10.1210/mend.13.3.0248. [DOI] [PubMed] [Google Scholar]
- 36.Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacology and Therapeutics. 2006;110(3):371–385. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Braissant O, Wahli W. Differential expression of peroxisome proliferator-activated receptor-α, -β, and -γ during rat embryonic development. Endocrinology. 1998;139(6):2748–2754. doi: 10.1210/endo.139.6.6049. [DOI] [PubMed] [Google Scholar]
- 38.Benani A, Krémarik-Bouillaud P, Bianchi A, Netter P, Minn A, Dauça M. Evidence for the presence of both peroxisome proliferator-activated receptors alpha and beta in the rat spinal cord. Journal of Chemical Neuroanatomy. 2003;25(1):29–38. doi: 10.1016/s0891-0618(02)00093-5. [DOI] [PubMed] [Google Scholar]
- 39.Cimini A, Benedetti E, Cristiano L, et al. Expression of peroxisome proliferator-activated receptors (PPARs) and retinoic acid receptors (RXRs) in rat cortical neurons. Neuroscience. 2005;130(2):325–337. doi: 10.1016/j.neuroscience.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 40.Bernardo A, Levi G, Minghetti L. Role of the peroxisome proliferator-activated receptor-γ (PPAR-γ) and its natural ligand 15-deoxy-Δ12,14-prostaglandin J2 in the regulation of microglial functions. European Journal of Neuroscience. 2000;12(7):2215–2223. doi: 10.1046/j.1460-9568.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- 41.Moreno S, Farioli-Vecchioli S, Cerù MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123(1):131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 42.Genovese T, Mazzon E, Di Paola R, et al. Role of endogenous ligands for the peroxisome proliferators activated receptors alpha in the secondary damage in experimental spinal cord trauma. Experimental Neurology. 2005;194(1):267–278. doi: 10.1016/j.expneurol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Taylor BK, Dadia N, Yang CB, Krishnan S, Badr M. Peroxisome proliferator-activated receptor agonists inhibit inflammatory edema and hyperalgesia. Inflammation. 2002;26(3):121–127. doi: 10.1023/a:1015500531113. [DOI] [PubMed] [Google Scholar]
- 44.LoVerme J, Russo R, La Rana G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-α . Journal of Pharmacology and Experimental Therapeutics. 2006;319(3):1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- 45.Raivich G, Bohatschek M, Kloss CUA, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Research Reviews. 1999;30(1):77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 46.Popovich PG, Stuckman S, Gienapp IE, Whitacre CC. Alterations in immune cell phenotype and function after experimental spinal cord injury. Journal of Neurotrauma. 2001;18(9):957–966. doi: 10.1089/089771501750451866. [DOI] [PubMed] [Google Scholar]
- 47.Orfanos CE, Bauer R. Evidence for anti-inflammatory activities of oral synthetic retinoids: experimental findings and clinical experience. British Journal of Dermatology. 1983;109(supplement 25):55–60. [PubMed] [Google Scholar]
- 48.Fang H, Jin H, Wang H. Effect of all-trans retinoic acid on airway inflammation in asthmatic rats and its mechanism. Journal of Huazhong University of Science and Technology—Medical Science. 2004;24(3):229–232. doi: 10.1007/BF02831997. [DOI] [PubMed] [Google Scholar]
- 49.Nozaki Y, Yamagata T, Sugiyama M, Ikoma S, Kinoshita K, Funauchi M. Anti-inflammatory effect of all-trans-retinoic acid in inflammatory arthritis. Clinical Immunology. 2006;119(3):272–279. doi: 10.1016/j.clim.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Zhu L, Bisgaier CL, Aviram M, Newton RS. 9-cis retinoic acid induces monocyte chemoattractant protein-1 secretion in human monocytic THP-1 cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(9):2105–2111. doi: 10.1161/01.atv.19.9.2105. [DOI] [PubMed] [Google Scholar]
- 51.Ertesvag A, Engedal N, Naderi S, Blomhoff HK. Retinoic acid stimulates the cell cycle machinery in normal T cells: involvement of retinoic acid receptor-mediated IL-2 secretion. Journal of Immunology. 2002;169(10):5555–5563. doi: 10.4049/jimmunol.169.10.5555. [DOI] [PubMed] [Google Scholar]
- 52.Na S-Y, Kang BY, Chung SW, et al. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFκB. Journal of Biological Chemistry. 1999;274(12):7674–7680. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- 53.Dheen ST, Jun Y, Yan Z, Tay SSW, Ling EA. Retinoic acid inhibits expression of TNF-α and iNOS in activated rat microglia. GLIA. 2005;50(1):21–31. doi: 10.1002/glia.20153. [DOI] [PubMed] [Google Scholar]
- 54.Xu J, Drew PD. 9-cis-retinoic acid suppresses inflammatory responses of microglia and astrocytes. Journal of Neuroimmunology. 2006;171(1-2):135–144. doi: 10.1016/j.jneuroim.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi W-H, Ji K-A, Jeon S-B, et al. Anti-inflammatory roles of retinoic acid in rat brain astrocytes: suppression of interferon-γ-induced JAK/STAT phosphorylation. Biochemical and Biophysical Research Communications. 2005;329(1):125–131. doi: 10.1016/j.bbrc.2005.01.110. [DOI] [PubMed] [Google Scholar]
- 56.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. Journal of Neuroscience. 2002;22(17):7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. American Journal of Physiology. 1998;274(5 part 2):R1203–R1211. doi: 10.1152/ajpregu.1998.274.5.R1203. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen J, Dumont J, Bauvois B. Comparative effects of interferon-gamma and all-trans retinoic acid on secreted and surface-associated matrix metalloproteinase-9 expression of human monocytes. Cellular and Molecular Biology. 2006;52(1):51–58. [PubMed] [Google Scholar]
- 59.Ho L-J, Lin L-C, Hung L-F, et al. Retinoic acid blocks pro-inflammatory cytokine-induced matrix metalloproteinase production by down-regulating JNK-AP-1 signaling in human chondrocytes. Biochemical Pharmacology. 2005;70(2):200–208. doi: 10.1016/j.bcp.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 60.Delerive P, Martin-Nizard F, Chinetti G, et al. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circulation Research. 1999;85(5):394–402. doi: 10.1161/01.res.85.5.394. [DOI] [PubMed] [Google Scholar]
- 61.Delerive P, De Bosscher K, Besnard S, et al. Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. Journal of Biological Chemistry. 1999;274(45):32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 62.Jové M, Laguna JC, Vázquez-Carrera M. Agonist-induced activation releases peroxisome proliferator-activated receptor β/δ from its inhibition by palmitate-induced nuclear factor-κB in skeletal muscle cells. Biochimica et Biophysica Acta. 2005;1734(1):52–61. doi: 10.1016/j.bbalip.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Okamoto H, Iwamoto T, Kotake S, Momohara S, Yamanaka H, Kamatani N. Inhibition of NK-κB signaling by fenofibrate, a peroxisome proliferator-activated receptor-α ligand, presents a therapeutic strategy for rheumatoid arthritis. Clinical and Experimental Rheumatology. 2005;23(3):323–330. [PubMed] [Google Scholar]
- 64.Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARα-leukotriene B4 pathway to inflammation control. Nature. 1996;384(6604):39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 65.Spencer NFL, Poynter ME, Im S-Y, Daynes RA. Constitutive activation of NF-κB in an animal model of aging. International Immunology. 1997;9(10):1581–1588. doi: 10.1093/intimm/9.10.1581. [DOI] [PubMed] [Google Scholar]
- 66.Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor α activation modulates cellular redox status, represses nuclear factor-κB signaling, and reduces inflammatory cytokine production in aging. Journal of Biological Chemistry. 1998;273(49):32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- 67.Cuzzocrea S, Mazzon E, Di Paola R, et al. The role of the peroxisome proliferator-activated receptor-α (PPAR-α) in the regulation of acute inflammation. Journal of Leukocyte Biology. 2006;79(5):999–1010. doi: 10.1189/jlb.0605341. [DOI] [PubMed] [Google Scholar]
- 68.Delayre-Orthez C, Becker J, Guenon I, et al. PPARα downregulates airway inflammation induced by lipopolysaccharide in the mouse. Respiratory Research. 2005;6(1):91. doi: 10.1186/1465-9921-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuzzocrea S, Di Paola R, Mazzon E, et al. Role of endogenous and exogenous ligands for the peroxisome proliferators activated receptors alpha (PPAR-α) in the development of inflammatory bowel disease in mice. Laboratory Investigation. 2004;84(12):1643–1654. doi: 10.1038/labinvest.3700185. [DOI] [PubMed] [Google Scholar]
- 70.Colville-Nash PR, Qureshi SS, Willis D, Willoughby DA. Inhibition of inducible nitric oxide synthase by peroxisome proliferator-activated receptor agonists: correlation with induction of heme oxygenase 1. Journal of Immunology. 1998;161(2):978–984. [PubMed] [Google Scholar]
- 71.Nakajima A, Wada K, Miki H, et al. Endogenous PPARγ mediates anti-inflammatory activity in murine ischemia-reperfusion injury. Gastroenterology. 2001;120(2):460–469. doi: 10.1053/gast.2001.21191. [DOI] [PubMed] [Google Scholar]
- 72.Wada K, Nakajima A, Takahashi H, et al. Protective effect of endogenous PPARγ against acute gastric mucosal lesions associated with ischemia-reperfusion. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2004;287(2):G452–G458. doi: 10.1152/ajpgi.00523.2003. [DOI] [PubMed] [Google Scholar]
- 73.Sung B, Park S, Yu BP, Chung HY. Amelioration of age-related inflammation and oxidative stress by PPARγ activator: suppression of NF-κB by 2,4-thiazolidinedione. Experimental Gerontology. 2006;41(6):590–599. doi: 10.1016/j.exger.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 74.Lee S, Kim W, Moon S-O, et al. Rosiglitazone ameliorates cisplatin-induced renal injury in mice. Nephrology Dialysis Transplantation. 2006;21(8):2096–2105. doi: 10.1093/ndt/gfl194. [DOI] [PubMed] [Google Scholar]
- 75.Li AC, Palinski W. Peroxisome proliferator-activated receptors: how their effects on macrophages can lead to the development of a new drug therapy against atherosclerosis. Annual Review of Pharmacology and Toxicology. 2006;46:1–39. doi: 10.1146/annurev.pharmtox.46.120604.141247. [DOI] [PubMed] [Google Scholar]
- 76.Chinetti G, Griglio S, Antonucci M, et al. Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. Journal of Biological Chemistry. 1998;273(40):25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 77.von Knethen A, Brüne B. Delayed activation of PPARγ by LPS and IFN-γ attenuates the oxidative burst in macrophages. FASEB Journal. 2001;15(2):535–544. doi: 10.1096/fj.00-0187com. [DOI] [PubMed] [Google Scholar]
- 78.Xu J, Storer PD, Chavis JA, Racke MK, Drew PD. Agonists for the peroxisome proliferator-activated receptor-α and the retinoid X receptor inhibit inflammatory responses of microglia. Journal of Neuroscience Research. 2005;81(3):403–411. doi: 10.1002/jnr.20518. [DOI] [PubMed] [Google Scholar]
- 79.Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-Δ12,14-prostaglandin J2 . Proceedings of the National Academy of Sciences of the United States of America. 1999;96(8):4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 81.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 82.Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer's disease: inhibition of β- amyloid-stimulated proinflammatory responses and neurotoxicity by PPARγ agonists. Journal of Neuroscience. 2000;20(2):558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heneka MT, Sastre M, Dumitrescu-Ozimek L, et al. Acute treatment with the PPARγ agonist pioglitazone and ibuprofen reduces glial inflammation and Aβ1-42 levels in APPV717I transgenic mice. Brain. 2005;128(6):1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 84.Giri S, Rattan R, Singh AK, Singh I. The 15-deoxy-δ12,14-prostaglandin J2 inhibits the inflammatory response in primary rat astrocytes via down-regulating multiple steps in phosphatidylinositol 3-kinase-akt-NF-κB-p300 pathway independent of peroxisome proliferator-activated receptor γ . Journal of Immunology. 2004;173(8):5196–5208. doi: 10.4049/jimmunol.173.8.5196. [DOI] [PubMed] [Google Scholar]
- 85.Luna-Medina R, Cortes-Canteli M, Alonso M, Santos A, Martínez A, Perez-Castillo A. Regulation of inflammatory response in neural cells in vitro by thiadiazolidinones derivatives through peroxisome proliferator-activated receptor γ activation. Journal of Biological Chemistry. 2005;280(22):21453–21462. doi: 10.1074/jbc.M414390200. [DOI] [PubMed] [Google Scholar]
- 86.Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson's disease correlates with IκBα induction and block of NFκB and iNOS activation. Journal of Neurochemistry. 2004;88(2):494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- 87.Storer PD, Xu J, Chavis J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. Journal of Neuroimmunology. 2005;161(1-2):113–122. doi: 10.1016/j.jneuroim.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 88.Xu J, Chavis JA, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-α and retinoid X receptor agonists inhibit inflammatory responses of astrocytes. Journal of Neuroimmunology. 2006;176(1-2):95–105. doi: 10.1016/j.jneuroim.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 89.Heneka MT, Klockgether T, Feinstein DL. Peroxisome proliferator-activated receptor-γ ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. Journal of Neuroscience. 2000;20(18):6862–6867. doi: 10.1523/JNEUROSCI.20-18-06862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clagett-Dame M, McNeill EM, Muley PD. Role of all-trans retinoic acid in neurite outgrowth and axonal elongation. Journal of Neurobiology. 2006;66(7):739–756. doi: 10.1002/neu.20241. [DOI] [PubMed] [Google Scholar]
- 91.Dmetrichuk JM, Carlone RL, Spencer GE. Retinoic acid induces neurite outgrowth and growth cone turning in invertebrate neurons. Developmental Biology. 2006;294(1):39–49. doi: 10.1016/j.ydbio.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 92.Maden M, Keen G, Jones GE. Retinoic acid as a chemotactic molecule in neuronal development. International Journal of Developmental Neuroscience. 1998;16(5):317–322. doi: 10.1016/s0736-5748(98)00046-x. [DOI] [PubMed] [Google Scholar]
- 93.Quinn SDP, De Boni U. Enhanced neuronal regeneration by retinoic acid of murine dorsal root ganglia and of fetal murine and human spinal cord in vitro. In Vitro Cellular and Developmental Biology - Animal. 1991;27(1):55–62. doi: 10.1007/BF02630895. [DOI] [PubMed] [Google Scholar]
- 94.Yamamoto M, McCaffery P, Dräger UC. Influence of the choroid plexus on cerebellar development: analysis of retinoic acid synthesis. Developmental Brain Research. 1996;93(1-2):182–190. doi: 10.1016/0165-3806(96)00038-7. [DOI] [PubMed] [Google Scholar]
- 95.Hunter K, Maden M, Summerbell D, Eriksson U, Holder N. Retinoic acid stimulates neurite outgrowth in the amphibian spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(9):3666–3670. doi: 10.1073/pnas.88.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mey J, Rombach N. Retinoic acid increases BDNF-dependent regeneration of chick retinal ganglion cells in vitro. NeuroReport. 1999;10(17):3573–3577. doi: 10.1097/00001756-199911260-00020. [DOI] [PubMed] [Google Scholar]
- 97.Corcoran J, So P-L, Barber RD, et al. Retinoic acid receptor β 2 and neurite outgrowth in the adult mouse spinal cord in vitro. Journal of Cell Science. 2002;115(19):3779–3786. doi: 10.1242/jcs.00046. [DOI] [PubMed] [Google Scholar]
- 98.Mey J, Thanos S. Ontogenetic changes in the regenerative ability of chick retinal ganglion cells as revealed by organ explants. Cell and Tissue Research. 1991;264(2):347–355. doi: 10.1007/BF00313973. [DOI] [PubMed] [Google Scholar]
- 99.Wong L-F, Yip PK, Battaglia A, et al. Retinoic acid receptor β 2 promotes functional regeneration of sensory axons in the spinal cord. Nature Neuroscience. 2006;9(2):243–250. doi: 10.1038/nn1622. [DOI] [PubMed] [Google Scholar]
- 100.Yip PK, Wong L-F, Pattinson D, et al. Lentiviral vector expressing retinoic acid receptor β 2 promotes recovery of function after corticospinal tract injury in the adult rat spinal cord. Human Molecular Genetics. 2006;15(21):3107–3118. doi: 10.1093/hmg/ddl251. [DOI] [PubMed] [Google Scholar]
- 101.Taha MO, Rosseto M, Fraga MM, et al. Effect of retinoic acid on tibial nerve regeneration after anastomosis in rats: histological and functional analyses. Transplantation Proceedings. 2004;36(2):404–408. doi: 10.1016/j.transproceed.2004.01.100. [DOI] [PubMed] [Google Scholar]
- 102.Arrieta O, García-Navarrete R, Zúñiga S, et al. Retinoic acid increases tissue and plasma contents of nerve growth factor and prevents neuropathy in diabetic mice. European Journal of Clinical Investigation. 2005;35(3):201–207. doi: 10.1111/j.1365-2362.2005.01467.x. [DOI] [PubMed] [Google Scholar]
- 103.Romero-Sandoval EA, Molina C, Alique M, Moreno-Manzano V, Lucio FJ, Herrero JF. Vitamin A active metabolite, all-trans retinoic acid, induces spinal cord sensitization. I. Effects after oral administration. British Journal of Pharmacology. 2006;149(1):56–64. doi: 10.1038/sj.bjp.0706829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alique M, Lucio FJ, Herrero JF. Vitamin A active metabolite, all-trans retinoic acid, induces spinal cord sensitization. II. Effects after intrathecal administration. British Journal of Pharmacology. 2006;149(1):65–72. doi: 10.1038/sj.bjp.0706826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Besson VC, Chen XR, Plotkine M, Marchand-Verrecchia C. Fenofibrate, a peroxisome proliferator-activated receptor α agonist, exerts neuroprotective effects in traumatic brain injury. Neuroscience Letters. 2005;388(1):7–12. doi: 10.1016/j.neulet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 106.Park S-W, Yi J-H, Miranpuri G, et al. Thiazolidinedione class of peroxisome proliferator-activated receptor γ agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. Journal of Pharmacology and Experimental Therapeutics. 2007;320(3):1002–1012. doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- 107.Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-γ ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130(3):685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]