Abstract
Traumatic spinal cord injury (SCI) is accompanied by a dramatic inflammatory response, which escalates over the first week post-injury and is thought to contribute to secondary pathology after SCI. Peroxisome proliferator-activated receptors (PPAR) are widely expressed nuclear receptors whose activation has led to diminished pro-inflammatory cascades in several CNS disorders. Therefore, we examined the efficacy of the PPARγ agonist Pioglitazone in a rodent SCI model. Rats received a moderate mid-thoracic contusion and were randomly placed into groups receiving vehicle, low dose or high dose Pioglitazone. Drug or vehicle was injected i.p. at 15 min post-injury and then every 12h for the first 7d post-injury. Locomotor function was followed for 5 weeks using the BBB scale. BBB scores were greater in treated animals at 7d post-injury and significant improvements in BBB subscores were noted, including better toe clearance, earlier stepping and more parallel paw position. Stereological measurements throughout the lesion revealed a significant increase in rostral spared white matter in both Pioglitazone treatment groups. Spinal cords from the high dose group also had significantly more gray matter sparing and motor neurons rostral and caudal to epicenter. Thus, our results reveal that clinical treatment with Pioglitazone, an FDA-approved drug used currently for diabetes, may be a feasible and promising strategy for promoting anatomical and functional repair after SCI.
Keywords: inflammation, locomotion, motor neurons, white matter sparing, gray matter, spinal contusion
Peroxisome proliferator-activated receptors (PPARs), which exist as α, δ and γ isotypes, are ligand-activated transcription factors found in most eukaryotic cells (Desvergne and Wahli, 1999; Hihi et al., 2002). Upon ligand binding, the receptor/ligand complex can up-regulate or down-regulate transcription of genes containing a peroxisome proliferator response element (PPRE). Most ligands identified to date for PPARs are long-chain fatty acids and, accordingly, many PPAR gene targets are involved in fatty acid metabolism (Desvergne and Wahli, 1999; Hihi et al., 2002). PPAR functions extend beyond fatty acid metabolism, however, and include regulation of other important processes, such as inflammation (for reviews, see Landreth and Heneka, 2001; Moraes et al., 2006). PPARγ agonists in particular have been examined for their anti-inflammatory and anti-oxidant properties in several models of central nervous system (CNS) injury and disease, such as amyotrophic lateral sclerosis (Kiaei et al., 2006; Schutz et al., 2005), Parkinson’s disease (Breidert et al, 2002;Dehmer et al., 2004), cerebral ischemia or hemorrhage (Ou et al., 2006; Victor et al., 2006; Zhao et al., 2006), and experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (Niino et al., 2001; Feinstein et al., 2002; Diab et al., 2002). These and other studies have shown that activation of PPARγ in CNS disorders consistently reduces iNOS levels, improves neuronal survival, and decreases expression of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNFα) and interleukin-1β (IL-1β), (Combs et al., 2000; Heneka et al., 2000; Niino et al., 2001; Breidert et al., 2002; Schutz et al., 2005; Zhao et al., 2006). A direct action within the brain or spinal cord is possible since upregulated PPARγ expression has been detected within the injured CNS (Diab et al., 2002; Ou et al., 2006; Victor et al., 2006). However, PPARγ agonists also can act directly on monocytes and T-cells to reduce pro-inflammatory cytokine release and decrease proliferation (Jiang et al., 1998; Ricote et al., 1998; Combs et al., 2000; Diab et al., 2002; Feinstein et al., 2002). Thus, activation of the PPARγ pathway appears to function as a potent anti-inflammatory mechanism in circumstances of CNS injury or disease.
Many of the inflammatory-related molecules reduced by PPARγ activation are also thought to contribute to pathology following spinal cord injury (SCI). Because of the success of PPARγ agonists in multiple studies of CNS injury, we hypothesized that PPARγ activation would be beneficial in an animal model of spinal contusion that mimics a large portion of human SCI. Thus, we examined the effect of Pioglitazone given during the first week post-injury on chronic recovery from spinal contusion in rats. Pioglitazone is an FDA approved PPARγ agonist used clinically for the treatment of diabetes; it is also currently being tested in multiple sclerosis patients (Pershadsingh et al., 2004). Importantly, if it proved advantageous to the outcome of experimental SCI, the drug would be readily available to SCI patients. Indeed, our results reveal that Pioglitazone treatment during the first week post-injury promoted several aspects of anatomical repair, including white matter and gray matter sparing and motor neuron survival at the lesion margins. In addition, several aspects of locomotion were improved in animals receiving Pioglitazone treatment. Thus, this therapeutically available agent appears to hold promise for the treatment of spinal cord injury.
Methods and materials
These studies were divided into three main experiments: Study I, II and III. The purpose of Study I was to compare two different doses of Pioglitazone on anatomical and locomotor outcome at 5 weeks post-injury. Study II was a replication study using the more efficacious dose of Pioglitazone. The third study was an acute experiment in which rats were treated with Pioglitazone as in Study II then sacrificed on day 7 at the end of treatment. Adult female Sprague-Dawley rats (214–245g) were used for all studies. All procedures were carried out in accordance to the Ohio State University IUCAC guidelines.
Study I
Rats received a moderate contusion injury using the OSU electromagnetic spinal cord injury device as previously described (McTigue et al., 2006). Briefly, rats were anesthetized with ketamine (80mg/kg) and xylazine (10mg/kg), and then a partial laminectomy was performed at vertebral level T8. A computer controlled impact probe was slowly lowered to the dural surface. Next, the probe rapidly impacted the spinal cord to a depth of 0.7mm over 23 msec, producing a closed-dural contusion injury. The injury site was surgically closed and the animal was hydrated and placed in a warmed recovery cage. Animals received antibiotics for 5 days post-injury (dpi) and manual bladder expression until return of automatic voiding, typically 10–14dpi.
Rats were randomly assigned to one of three groups (n=6/group): 10mg/kg Pioglitazone, 1mg/kg Pioglitazone or vehicle (sterile phosphate buffered saline (PBS)). The high dose Pioglitazone solution was prepared as a suspension by dissolving one pulverized 45mg Pioglitazone tablet (Takeda Pharmaceutical Company, Japan; purchased at OSU Hospital pharmacy) into 9ml of PBS (37°) for a concentration of 5mg/ml. The low dose was prepared by diluting 1ml of the high dose to 0.5 mg/ml. Drug solutions were made fresh for each administration; drug or vehicle injections (i.p.) were given 15 min post-injury and, beginning on day 1 post-injury, every 12 hr for 7d. Rats survived for 5 weeks post-injury.
Study II
Rats underwent laminectomy surgery as above. Because a faulty force transducer on the injury device was replaced between Study I and II, the displacement for injuries in Study II had to be increased to 0.8mm in attempt to match the injury severity of the first study. Rats were randomly assigned to a Pioglitazone group (10mg/kg; n=6) or vehicle group (PBS; n=6), and injections were delivered as in Study I. Rats survived for 5 weeks post-injury.
Study III
Rats underwent laminectomy surgery as above and an 0.8mm displacement spinal contusion injury was delivered as in Study II. Animals were randomly divided into a Pioglitazone group (10mg/kg; n=7) or vehicle group (PBS; n=7) as in Study II. Rats survived for 7dpi.
Behavioral Evaluation
BBB
All animals were gentled prior to injury so that they were comfortable being handled and walking in the open field apparatus. Using the Basso-Beattie-Bresnahan (BBB) locomotor rating scale (Basso et al., 1995), rats were pre-tested prior to injury to ensure that all animals began with a normal score of 21. Then on days 1, 3, 5, 7, 10, 14, 21, 28 and 35 post-injury rats were scored by two observers blinded to the treatment groups. Scores for each hind-limb were averaged, and then used to create group means at each day. Groups were compared using two-way repeated measures ANOVA followed by Bonferroni post-hoc analysis.
BBB subscores
BBB scores were further analyzed by calculating subscores (Popovich et al., 1999; Lankhorst et al., 2001; Basso, 2004), which allows for characterization of the individual aspects of locomotion, alone or in combination. To calculate subscores, individual categories of BBB outcomes were quantified as shown in Table 1. For stepping, toe clearance and paw placement, each limb was scored separately and then summed for a final score in each category. For example, the maximum possible score for stepping is 6, which equates to consistent stepping (score of 3) on both sides. To compute the overall subscore, all individual scores were summed to produce a maximum possible score of 23. Because the subscore only quantifies characteristics of locomotion that are present once the animal can step, this measure allows a more targeted and expanded evaluation of stepping quality than the basic BBB score. Each category within the subscore was also examined independently to determine which specific aspects of locomotion were altered by drug treatment.
Table 1.
BBB Subscore Tabulation
| None | Occasional | Frequent | Consistent | |
|---|---|---|---|---|
| Stepping | 0 | 1 | 2 | 3 |
| Coordination | 0 | 1 | 2 | 3 |
| Toe Clearance | 0 | 1 | 2 | 3 |
| Rotated/Rotated | Parallel/Rotated | Parallel/Parallel | ||
| Paw Placement | 0 | 1 | 2 | |
| Down | Up & Down | Up | ||
| Tail | 0 | 1 | 2 | |
| Yes | No | |||
| Instability | 0 | 1 | ||
For each category, scores for right and left feet were summed. For overall subscore, the scores in each category were summed; the maximum possible overall subscore is 23.
Tissue processing and immunohistochemistry
At the designated time post-injury (35dpi for Studies I and II; 7dpi for Study III), rats were deeply anesthetized with ketamine/xylazine and perfused intracardially with cold PBS followed by 4% paraformaldehyde. Spinal cords were dissected, placed into fixative for two hours and then into 0.2M PB overnight. The next day, spinal cords were immersed in a 30% sucrose solution then stored at 4°C for 3–4 d. Next, spinal cords were cut into a 6 mm piece centered on the injury site and frozen with powdered dry ice. Four to five spinal cords were aligned in a cryomold and then frozen in OCT compound (Electron Microscopy Sciences). Cords were sectioned on a cryostat at 10μm and mounted serially on slides such that 10 complete sets of sections spanning the entire tissue block were produced.
Antibodies used in this study were 1:2000 mouse anti-neurofilament (Boehringer Mannheim) which was combined with EC (Eriochrome Cyanine) to label myelin; 1:10,000 rabbit anti-Caspase-3 (R&D Systems); 1:50,000 mouse anti-NeuN (Chemicon); 1:2000 mouse anti-OX42 (Serotec) and 1:3000 mouse anti-ED1 (Serotec). On the first day of immunohistochemistry, sections rinsed with 0.1M PBS and incubated in 4%BSA/0.1%Tx-100/0.1M PBS for one hour at room temperature. Next, primary antibodies were applied and incubated overnight at 4°C. The following day, sections were rinsed with 0.1M PBS 4x for a total of 20 min and then incubated with biotinylated anti-rabbit IgG (1:800) or biotinylated anti-mouse IgG (1:800) followed by Elite-ABC (horseradish peroxidase conjugated-avidin-biotin complex) for one hour each at room temperature. The sections were rinsed with 0.1M PBS and incubated with the DAB substrate (Vector Laboratories). Finally, sections were rinsed with dH2O, dehydrated and coverslipped.
Tissue Analysis
Stereological Measurements of Spared White and Gray Matter – Studies I and II
The Cavalieri method was used to estimate the average area per section of regions of interest from the injured spinal cords (Howard and Reed, 1998; Ankeny et al., 2004). Regions of interest included spared white matter, spared grey matter, lesioned tissue and total cross-section area. To identify regions of interest, cross-sections were labeled for myelin (EC) and axons (neurofilament). Using the epicenter as a central point of reference, color images of every third section rostral and caudal to the epicenter were captured with a scale bar and printed (sections were spaced 100μm apart). A total of 11 sections per animal were analyzed so that the total distance examined was 3mm, or 1.5mm rostral and caudal to the epicenter. The perimeters of spared white and grey matter and lesion were manually outlined on the print-outs; sections were concomitantly viewed on the microscope to verify line placement. Uniform point grids were placed with a random orientation on the print-outs and the points that fell within each region of interest were summed. A point grid that resulted in at least 200 points falling within the target volume along the entire length of the lesion was chosen (Howard and Reed, 1998). Volume estimates were derived from point sums using the formula: estimated volume = T·a/p·11Σi=1Pi, where T=distance between sections (300μm), a/p=calculated area represented by each point and 11Σi=1Pi=sum of points sampled for the 11 sections. Area per section was determined by multiplying the point sums by a/p.
Motor neuron counts – Study I
Sections spanning the lesion site were immunolabeled with the neuronal marker NeuN. The total number of ventral horn motor neurons in each section was quantified in every third section by a blinded observer (sections were spaced 100μm apart), beginning 2.4mm rostral to epicenter and spanning to 2.4mm caudal to epicenter. Sections were viewed using Nomarski optics to visualize tissue topography, and motor neurons were identified based on location in ventral horn, size and morphology. For each distance, group means were calculated and compared using two-way repeated measures ANOVA followed by Bonferroni post-hoc comparisons.
Caspase-3 positive cell counts – Study III
Cross-sections labeled for cleaved caspase-3 were digitized using the MCID Elite image analysis system (Imaging Research, St. Catherines, Ontario). Analysis was conducted on cross-sections located 0.9 – 2.4mm rostral to the lesion epicenter. A standardized sampling box was placed in the region of the ventral horn, corticospinal tract, dorsal edge of the dorsal funiculus, and pial edges of the lateral and ventral funiculi. The MCID software was used to automatically count the number of caspase-3+ cells within each sampling box; the threshold intensity was set such that only positively-labeled cells were counted. Data for left and right ventral horns, dorsal funiculus, lateral funiculi and ventral funiculi were averaged and group means for each location determined. The mean values for each region were compared between groups using unpaired t-tests.
Ox42 and ED-1 quantification – Study III
Adjacent sections spanning the lesion site were immunolabeled with Ox42 and ED1 antibodies. Ox42 labels the CD11b receptor on microglia and macrophages and provides an assessment of overall microglial/macrophage activation; ED1 labels the rat homolog of human CD68, which is found on the lysosomal membranes of myeloid cells. The area of tissue positively labeled in each section was quantified by a blinded observer in every sixth section (sections spaced 100μm apart), beginning at 2.4mm rostral to epicenter and spanning to 2.4mm caudal. The area of Ox42 or ED1 was divided by the total cross-section area to determine the proportional area of immunolabeling. Group means were determined at each distance examined and data were compared between groups using two-way repeated measures ANOVA.
Results
Study I
Pioglitazone treatment improved multiple features of locomotion after SCI
Treated rats tended to have higher BBB scores than controls at 35dpi (15.4 ± 1.1 for high dose group vs. 13 ± 1.3 for controls) although scores did not reach significance (Fig 1A). Comparison of the total subscores, however, revealed that the high dose scores were significantly greater than vehicle at 14, 21, and 28dpi (Fig. 1B). Thus, treatment with Pioglitazone improved certain aspects of locomotor behavior.
Fig 1.
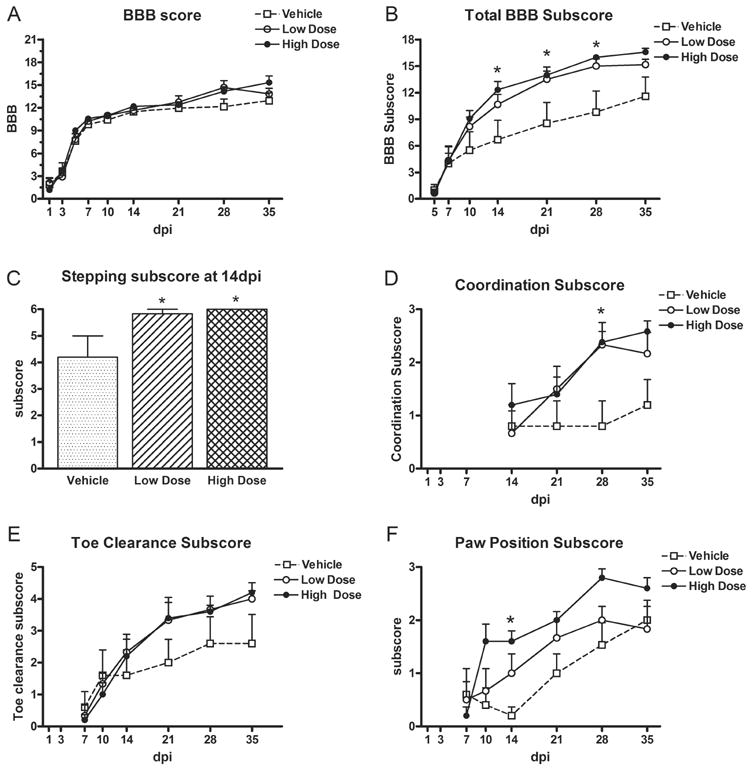
BBB related measures after SCI and treatment with vehicle, low dose (1mg/kg) or high dose (10mg/kg) of Pioglitazone. (A) Rats in each group recovered to a similar extent on the BBB scale. (B) Subscores were tallied for each category (see Table 1) and summed, which revealed that the high dose group attained significantly better total BBB subscores at 14d, 21d and 28d post-injury (dpi) compared to controls. (C) Significantly more rats in the high dose and low dose Pioglitazone groups recovered the ability to step by 14dpi compared to vehicle treated rats. (D) Both Pioglitazone groups displayed more frequent forelimb-hindlimb coordination than vehicle rats at 28dpi. (E) There was a trend for improved toe clearance over time in treated rats (Interaction X Treatment p<0.05). (F) Paw position subscores increased over time in all groups. At 14dpi, the high dose Pioglitazone group had a greater paw position subscore compared to vehicle controls due to more frequent parallel paw placements. All data are expressed as mean ± SEM. *p<0.05
To determine which categories within the BBB scale were improved by drug treatment, each subscore category was examined individually. This revealed that animals in both Pioglitazone groups recovered the ability to consistently step sooner (14dpi) than control animals (p<0.05; Fig 1C). Indeed, at 14dpi 6/6 high dose and 5/6 low dose animals could consistently step compared to only 2/6 vehicle animals. By 35dpi, all Pioglitazone treated rats were consistently stepping (12/12) compared to only half of the vehicle treated rats (3/6; data not shown). Both Pioglitazone groups also displayed more frequent coordination compared to vehicle rats at 28dpi (p<0.05; Fig 1D), a trend that continued until the last day of testing (p=0.051). For toe clearance, there was a significant Interaction X Treatment effect (p<0.05) suggesting that treated animals had better overall toe clearance than controls (Fig. 1E). Rats receiving the higher dose of Pioglitazone also had better paw position at 14dpi, reflected by their ability to achieve parallel paw placement more often than low dose rats or controls (Fig. 1F). Lastly, high dose Pioglitazone rats were able to lift their tail during locomotion earlier than controls, which implies the treated group may have had better overall stability (data not shown). Thus, Pioglitazone-treated rats stepped earlier and displayed a more normal stepping pattern compared to controls.
Pioglitazone treatment improved white matter and grey matter sparing after SCI
Using stereological measurements, spared white matter (SWM), spared grey matter (SGM), lesion area and cross-sectional area were estimated in sections spanning 1.5mm rostral and caudal from the lesion epicenter. For SWM, a significant Distance X Treatment effect was noted (p<0.001; Fig 2A-C). The high dose Pioglitazone group had significantly more white matter compared to vehicle-treated animals at 0.9 – 1.5mm rostral, and compared to low dose treated animals at 1.5mm rostral (Fig. 2C). In the low dose group, SWM was increased compared to controls at 1.5mm rostral to epicenter (Fig. 2C).
Fig 2.
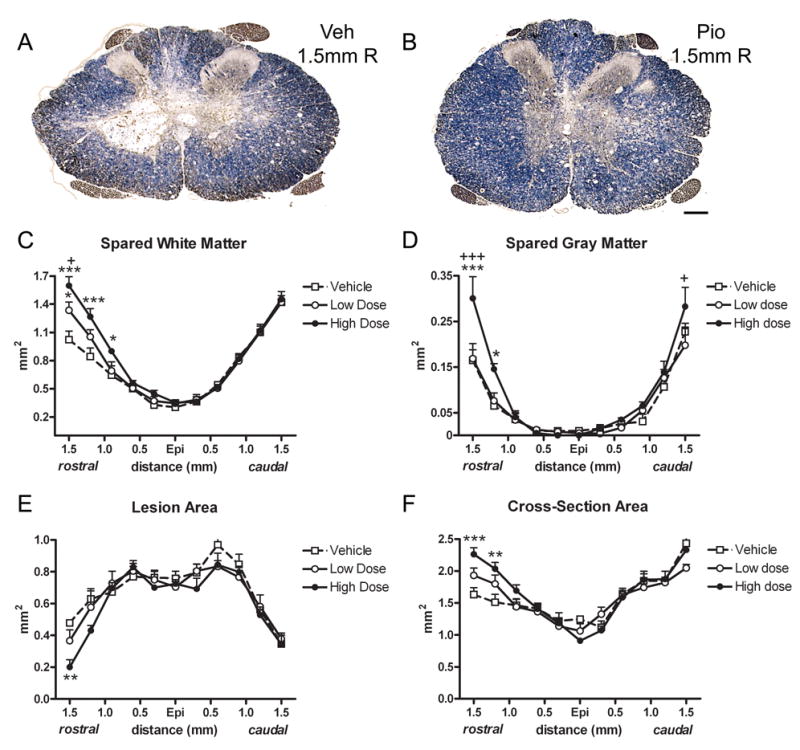
Pioglitazone treatment increased tissue sparing after SCI. (A,B) Cross-sections 1.5mm rostral (R) to epicenter labeled for myelin (blue) and axons (brown) from the vehicle group (A) and high dose Pioglitazone group (B). (C) Stereological measurement of white matter area revealed that spinal cords from Pioglitazone-treated rats had more white matter rostral to epicenter compared to controls. (D) Spinal cords sections from the high dose Pioglitazone group also contained more gray matter 1.5mm rostral to epicenter compared to control and low dose group, 1.2mm rostral compared to control and 1.5mm caudal compared to low dose group. (E) Tissue area identified as lesioned (e.g., absence of myelin, disrupted morphology, presence of cavities) was reduced compared to control at 1.5mm rostral to epicenter in the high dose group. (F) Total cross-section area was greater in high dose spinal cords rostral to epicenter compared to controls. All data are expressed as ± SEM. * p<0.05, **p<0.01, ***p<0.001 vs. vehicle; +p<0.05, +++p<0.001 vs. low dose Pioglitazone. Scale = 200μm (A–B).
Gray matter sparing was also improved in the high dose Pioglitazone group, which displayed more gray matter both rostrally and caudally (Distance X Treatment effect p<0.001; Fig. 2D). Sections 1.5 and 1.2mm rostral contained more SGM compared to vehicle sections at the same distances, while sections 1.5mm caudal to epicenter contained more SGM than the low dose group (Fig. 2D).
Comparisons of lesion area and total cross-sectional area revealed that lesion area was smaller 1.5mm rostral to epicenter in the high dose Pioglitazone group compared to vehicle (p<0.01; Fig. 2E). Total cross-section area was greater in the high dose group at 1.5 and 1.2mm rostral compared to controls (Fig. 2E), which is likely due to the combination of greater tissue sparing and reduced lesioned area.
Pioglitazone increased the number of spared motor neurons after SCI
Cross-sections labeled for NeuN were used to quantify the number of motor neurons in the ventral horns of all animals. Examples of ventral horn labeling from a vehicle and high dose Pioglitazone spinal cord are shown in Fig. 3A and B. Analysis of motor neuron counts revealed that the high dose Pioglitazone group had significantly more motor neurons rostral (2.1mm) and caudal (1.8 and 2.1mm) to epicenter compared to vehicle-treated rats (p<0.05; Fig 3C).
Fig 3.
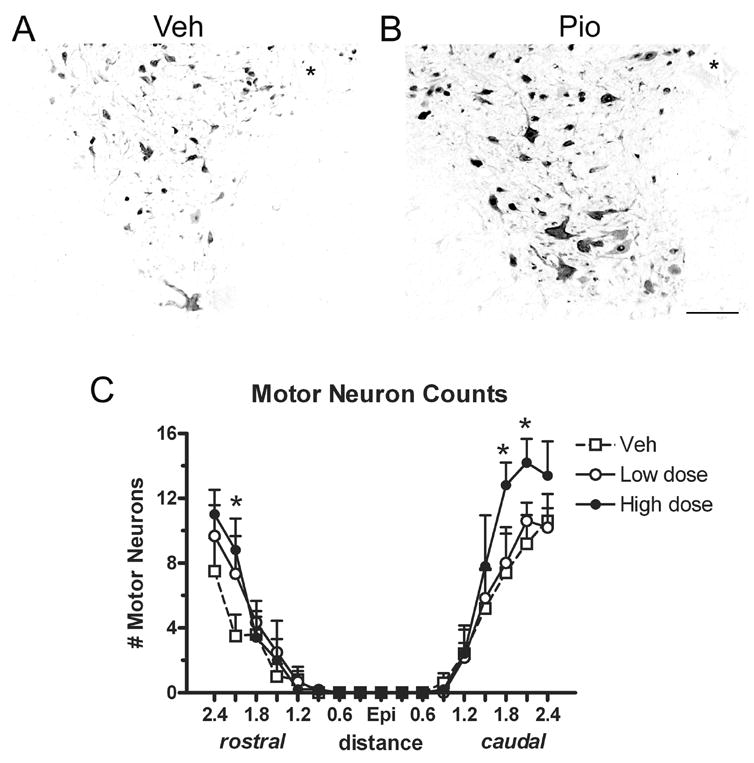
Rats receiving the high dose of Pioglitazone had more motor neurons rostral and caudal to the injury site (Epi). (A,B) Ventral horns from spinal cord sections 2.4mm caudal to epicenter immunolabeled with NeuN for neurons from vehicle (Veh) and high dose Pioglitazone (Pio) groups. (C) Blinded motor neurons counts revealed that sections from the high dose Pio group contained more motor neurons rostral (2.1mm) and caudal (1.8mm and 2.1mm) to epicenter compared to vehicle controls. All data are expressed as ± SEM. *p<0.05 vs. vehicle. Scale = 100μm (A,B)
Study II – Replication Study
Pioglitazone treatment again improved locomotion after SCI
Based on results of Study I, only the 10mg/kg dose of Pioglitazone was used in Study II. Also, as stated in the methods section, a slightly higher injury severity was used in this study to compensate for a change in the injury device between studies. Consequently, overall BBB scores were slightly lower than those in Study I. Interestingly, Pioglitazone treatment now caused a significant increase in BBB scores at 7dpi (p<0.001; Fig 4A). Total overall subscores were also greater in animals treated with Pioglitazone compared to vehicle at 21, 28 and 35dpi (Fig 4B). As before, more Pioglitazone treated rats could step at 14dpi compared to controls (Fig 4C). Coordination subscores did not reach significance, likely reflecting the greater injury severity, but both toe clearance and paw position were again improved by Pioglitazone treatment (Fig 4D–F).
Fig 4.
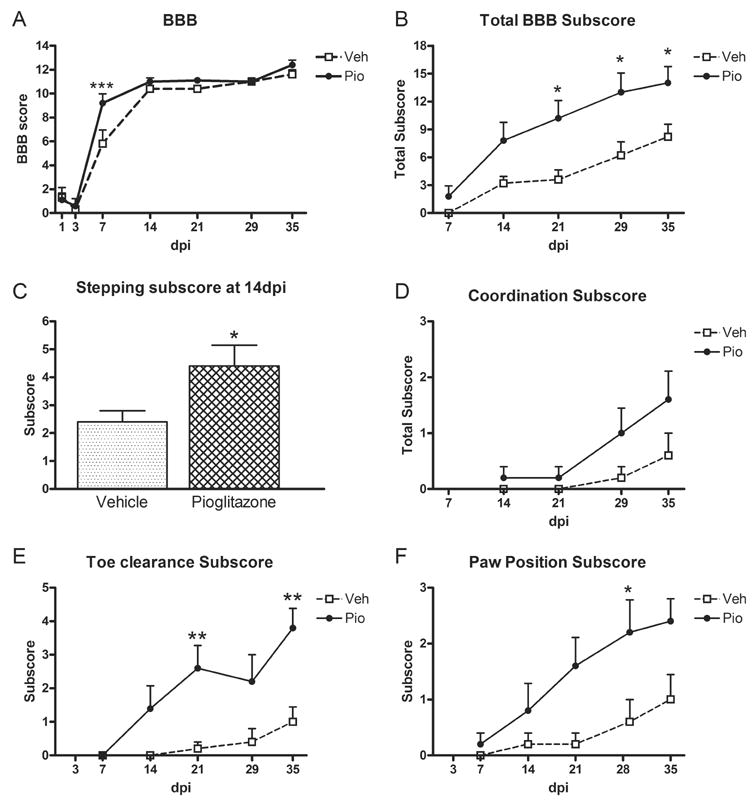
Locomotor scores in Study II (replication of first study) were greater in rats receiving Pioglitazone (Pio) and mimicked those from Study I. (A) BBB scores of treated and control rats over time post-injury. Pio treated rats had significantly higher BBB scores at 7d post-injury (dpi). (B) BBB subscores were higher in treated rats compared to controls at 21–35 dpi. (C) Significantly more Pio-treated rats were able to plantar step at 14dpi. (D) There was a non-significant trend for treated rats to display more forelimb-hindlimb coordination than controls. (E) Pio-treated animals achieved significantly better toe clearance at 21 and 35dpi than vehicle controls. (F) Rats receiving Pio also had more parallel paw placements than controls. All data are expressed as ± SEM. *p<0.05, **p<0.01 vs. control
Pioglitazone treatment again improved grey matter sparing after SCI
Spinal cord sections rostral to the epicenter again displayed more spared gray matter in Pioglitazone-treated rats compared to controls, similar to Study I (p<0.05; data not shown). In these sections, white matter sparing was slightly higher but not significantly different in treated rats compared to controls, perhaps due to the modest increase in injury severity. These results indicate that the effect on gray matter appears more robust than that on white matter.
Study III – 7dpi
Pioglitazone treatment did not alter apoptosis or macrophage density at 7dpi
Apoptosis contributes to cell loss after SCI and may be exacerbated by pro-inflammatory mechanisms. Therefore, we quantified the number of cleaved casapase-3 immunoreactive cells in sections rostral and caudal to the epicenter. Analysis was limited to distal sections since the greatest tissue sparing was noted distally. Using a standardized sampling box, caspase-3+ cells were counted in regions of white matter (corticospinal tract, dorsal funiculus, lateral and ventral white matter) and gray matter (ventral horn). The number of caspase-3+ cells was comparable between treated and control spinal cords in each region (data not shown).
Because one proposed mechanism for Pioglitazone-induced effects is a reduction in macrophage activation, tissue sections were analyzed for the presence of microglia and macrophages using the Ox42 antibody against the CD11b complement receptor. Digitized images of equally spaced cross-sections spanning the lesion were used to measure the proportional area of Ox42 labeling, which reflects the percent of cross-sectional area occupied by Ox42 immunoreactivity. This measurement revealed no difference in Ox42 levels throughout the extent of the lesion (data not shown).
To measure the level of phagocytic macrophages present within the sections, ED1 immunohistochemistry was quantified as above for Ox42. Although Pioglitazone-treated spinal cord sections typically contained lower amounts of ED1 immunoreactivity, there was no significant difference between treated and vehicle groups (Fig 5A–C).
Fig 5.

ED1 immunolabeling was used to measure the level of phagocytic macrophages within spinal cord sections at 7d post-injury. Example of ED1 immunolabeling from a vehicle control group (A) and Pioglitazone group (B) in cross-sections 1.5mm from epicenter. (C) The area of ED1 immunoreactivity was quantified in sections spanning the rostral-caudal extent of the lesion. All data are expressed as mean ± SEM. Scale = 200μm (A,B)
Discussion
In two independent studies, our work demonstrates that treatment with the PPARγ agonist Pioglitazone improves locomotor ability and tissue sparing in a rodent model of SCI. Compared to controls, rats given Pioglitazone during the first 7dpi recovered earlier stepping ability and attained overall locomotor patterns that were more similar to those of uninjured rats, including improved forelimb-hindlimb coordination, better toe clearance and more normal paw position during locomotion. Since most treated rats did not achieve consistent coordination, improved stepping abilities were not reflected in BBB scores at the end of the study; improvements were detected, however, in BBB subscores. This reveals the utility of the subscoring system for detecting changes in stepping ability, as has been noted previously (Popovich et al., 1999; Lankhorst et al., 2001; Basso, 2004; Basso et al., 2006). Given that differences between the groups were detected as early as 7–14dpi, it is unlikely that the improved walking behavior was due to altered regeneration, but most likely resulted from enhanced tissue sparing at the lesion poles in rats given Pioglitazone. Notably, our results confirm those of a very recent study which also examined the effects of Pioglitazone after SCI in rats (Park et al., 2006).
Increased white matter sparing in this study was restricted to sections rostral to the epicenter, while enhanced gray matter and motor neuron sparing were observed both rostrally and caudally. No anatomical improvements were noted at the epicenter, which suggests that damage created at the impact site was too immediate and/or severe to be modulated by PPARγ activation. In contrast, tissue pathology at the lesion extensions may develop over a more protracted period, in which case a therapeutic window exists and intervention is possible. A similar phenomenon has been noted in rodent SCI studies using the anti-inflammatory drug minocycline. When given for the first 5dpi, minocycline promoted functional recovery concomitant with white matter sparing at the lesion poles but not the epicenter (Teng et al., 2004). Typically, the extent of locomotor recovery achievable after rodent SCI is thought to correlate to the amount of intact white matter at the epicenter. However, data from our study and others suggest that tissue at the lesion poles can also mediate functional recovery after SCI. Indeed, a study by the Schwab group revealed a possible explanation for this phenomenon (Bareyre et al., 2004). Their work demonstrated that descending tracts maintained rostral to an SCI site undergo significant sprouting and can successfully form new synapses with local propriospinal neurons whose axons span the lesion and remain connected to caudal segments below the injury. This injury-induced plasticity allowed descending information to be carried by a new tract (i.e., the propriospinal tract) which effectively transmitted motor signals to the caudal spinal cord. In our model, increased tissue sparing rostral to the injury may have preserved more descending axons and propriospinal targets, which thereby allowed more descending motor signals to reach the lower spinal cord and contribute to locomotor control. Treated animals in our study also had significantly more motor neurons rostral and caudal to the injury epicenter. Although these were located in the thoracic region and unlikely to have directly modulated hindlimb ability, they may have contributed to recovery by providing more targets for descending relays. Their presence also may have promoted trunk stability in treated rats, which would aid in recovery of a more normal stepping pattern.
Neuroprotective actions of Pioglitazone may be a general characteristic of PPARγ activation, as this phenomenon has been noted in other models of CNS gray matter injury. For instance, Pioglitazone treatment in murine models of Parkinson’s disease promoted neuron sparing within the substantia nigra (Breidert et al., 2002; Dehmer et al., 2004). Potent neuroprotection of motor neurons was also induced by Pioglitazone in transgenic mouse models of amyotrophic lateral sclerosis (Kiaei et al., 2005; Schutz et al., 2005). Additionally, several studies have detected enhanced neuroprotection and decreased lesion sizes in animal models of stroke and intracerebral hemorrhage (Ou et al., 2006; Victor et al., 2006; Zhao et al., 2006). Part of the effects in our study and others may be due to a direct action of Pioglitazone on PPARγ expressed by neurons. For instance, a recent study showed that a PPARγ agonist acted directly on cultured neurons to protect them against excitotoxicity (Zhao et al., 2006). Thus, enhancing PPARγ activation in the injured CNS may function as a mechanism for gray matter preservation, and Pioglitazone appears to be a feasible clinical agent with which to activate this pathway in multiple disorders. PPARγ activation also increased white matter preservation in experimental autoimmune encephalomyelitis (EAE) which is an animal model of multiple sclerosis (MS). Feinstein et al. (2002) administered PPARγ agonists to mice with EAE and revealed a marked improvement in axon preservation and myelination, which was accompanied by decreased CNS infiltration of leukocytes, reduced pro-inflammatory cytokines and attenuated clinical signs. Similar results have been noted in several other reports testing PPARγ agonists in EAE (Niino et al., 2001; Diab et al., 2002; Diab et al., 2004). Notably, because multiple studies have shown the efficacy of Pioglitazone in EAE, a phase one clinical trial was recently performed which tested the safety and tolerability of Pioglitazone in patients with relapsing remitting MS (www.clinicaltrials.gov/ct/show/NCT00242177?order=1).
The mechanism of action for promoting tissue sparing by Pioglitazone could include several possible pathways. First, as stated above, a direct effect on CNS parenchyma is possible. When given peripherally, moderate levels of Pioglitazone (~18%) enter the CNS within 6h of administration (Maeshiba et al., 1997). Since SCI leads to blood vessel disruption and prolonged opening of the blood-brain barrier, access of Pioglitazone to the spinal parenchyma is likely enhanced following trauma. Importantly, CNS parenchymal cells express PPARγ and display detectable changes in activity following PPARγ activation (Roth et al., 2003; Moreno et al., 2004; Bernardo et al., 2005; Cimini et al., 2005). Although the clinical route of administration is oral delivery, in our study administration by intraperitoneal injection was chosen since SCI rats tend to have poor appetites for 1–2d post-injury. The oral route of administration may lead to higher levels of circulating drug than the ip route used herein. However, our injected doses are comparable to similar studies which have seen advantageous results (Cuzzocrea et al., 2004; Victor et al., 2006) and also are within the range of those using oral administration of Pioglitazone (Niino et al., 2001; Breidert et al., 2002; Feinstein et al., 2002; Schutz et al., 2005).
PPARγ-induced neuroprotection also may have been mediated by regulation of local and peripheral inflammatory cells, which enter spinal cord lesions during the first week post-injury (Dusart and Schwab, 1994; Popovich et al., 1997). For instance, proliferation of T-cells, which enter the SCI site during the first 7dpi (Sroga et al., 2003), can be inhibited by application of 15-deoxy-prostaglandin J2, which is thought to be an endogenous ligand for PPARγ (Diab et al., 2002). Microglia and macrophages are also targets of PPARγ activation. Studies have shown that activated macrophages upregulate expression of PPARγ (Ricote et al., 1998) and that Pioglitazone and other PPARγ agonists potently reduce pro-inflammatory cytokine release by these cells, including tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β) and IL-6 – three pro-inflammatory cytokines upregulated early after SCI (Wang et al., 1996; Jiang et al., 1998; Streit et al., 1998; Lee et al., 2000; Niino et al., 2001; Pineau and Lacroix, 2007). Anti-inflammatory effects also are achieved in part by blocking the action of the transcription factors AP-1 and NFKB, resulting in an inhibition of iNOS and COX-2 (Landreth and Heneka, 2001; Feinstein et al., 2002; Dehmer et al., 2004). Indeed, decreased levels of the pro-inflammatory molecules such as TNFα and iNOS have been detected in the damaged CNS of animals treated with PPARγ activators (Heneka et al., 2000; Niino et al., 2001; Dehmer et al., 2004; Schutz et al., 2005). Thus, multiple mechanisms acting in concert may have led to the improved outcomes in our study. Determining the mediators affected by Pioglitazone treatment after SCI is the objective of ongoing investigations in our laboratory.
In addition to altering secretion of inflammatory mediators, PPARγ activation can induce apoptosis of activated macrophages (Chinetti et al., 1998). In our study, apoptosis as measured by caspase-3 activation was not different between groups at 7dpi; however, since Pioglitazone was given systemically, it is possible that only peripheral cells were affected, or that a difference in apoptotic cells would have been detected at different times post-injury. We also detected no measurable difference in the level of CD11b or ED1 between treated and control groups at 7dpi, suggesting that the overall level of macrophage infiltration and activation was similar between groups. However, it should be noted that an earlier time point may have revealed differences in macrophage numbers within the lesion. For instance, Pioglitazone treatment in a mouse model of Parkinson’s disease led to reduced microglial activation 2d after toxin treatment but not at later time points (Breidert et al., 2002). Additionally, anti-CD11d antibody given after SCI reduced macrophage markers within 2d of injury but not later (Saville et al., 2004). Similar to our study, functional outcome measures were improved by the anti-CD11d antibody despite no long-term change in macrophage number in the lesion. The comparable overall number of macrophages present in each experimental group does not rule out the possibility, however, that the cells were functionally distinct in treated and non-treated animals.
The presence of inflammatory cells within the injured spinal cord can lead to deleterious consequences, which is illustrated by the improved behavioral and/or anatomical outcomes in several SCI studies testing anti-inflammatory treatments (Giulian and Robertson, 1990; Bethea et al., 1999; Nesic et al., 2001; Gonzalez et al., 2003; Gris et al., 2004). Furthermore, depletion of peripheral monocytes in rats during the first week after SCI resulted in locomotor improvements similar to those seen in our current study (Popovich et al., 1999). Since peripheral monocytes infiltrate the lesions and transition to activated macrophages, it is likely that the improved tissue sparing and locomotor recovery following macrophage depletion was due to a reduction in number and/or activation state of the infiltrating monocytes.
Collectively these reports suggest that anti-inflammatory agents may be potent therapeutics for CNS disorders. Other studies, however, reveal the complexity and necessity of inflammation in CNS repair. For instance, using a model of CNS demyelination that typically undergoes spontaneous remyelination, the anti-inflammatory treatments minocycline and methylprednisolone both reduced oligodendrocyte remyelination (Li et al., 2005; Chari et al., 2006). Furthermore, unlike SCI in which depletion of peripheral macrophages improved white matter sparing, the same treatment reduced remyelination in a demyelination model (Kotter et al., 2005). These studies suggest that a certain level and/or type of inflammation is needed for CNS wound repair and that different injuries or diseases may require different aspects of inflammation for wound repair to proceed.
Our data showing improved tissue sparing and locomotor recovery after SCI demonstrate promising results from a clinically available drug. Given that only one FDA-approved treatment for SCI currently exists, studies examining other treatment strategies for spinal trauma are urgently needed. Our treatment paradigm began with a Pioglitazone injection at 15 min post-injury. This time-frame was chosen to maximize the possible down-regulation of early pro-inflammatory cytokines, which are elevated within the first 4–12h post-injury. It is recognized that administering any treatment clinically at 15 min post-injury would be difficult. Therefore, ongoing studies are examining the efficacy of delaying the initial treatment beyond 15 min. Encouragingly, methylprednisolone shows beneficial effects when given within the first eight hours post-injury. Therefore, it is likely that the therapeutic window for other anti-inflammatory strategies will extend beyond 15 min post-injury. Future studies will be directed toward further evaluation of the potential use of PPAR agonists for spinal cord trauma.
Acknowledgments
The authors gratefully acknowledge the assistance of Patricia Walters and David Schonberg with surgeries and animal care. We also thank Dr. Phillip Popovich for critical review of the manuscript. This work was funded by NIH NS043494 and P30-NS045758.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ankeny DP, McTigue DM, Jakeman LB. Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp Neurol. 2004;190:17–31. doi: 10.1016/j.expneurol.2004.05.045. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997;9:1422–1438. doi: 10.1111/j.1460-9568.1997.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Basso DM. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J Neurotrauma. 2004;21:395–404. doi: 10.1089/089771504323004548. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Bernardo A, jmone-Cat MA, Gasparini L, Ongini E, Minghetti L. Nuclear receptor peroxisome proliferator-activated receptor-gamma is activated in rat microglial cells by the anti-inflammatory drug HCT1026, a derivative of flurbiprofen. J Neurochem. 2005;92:895–903. doi: 10.1111/j.1471-4159.2004.02932.x. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J Neurochem. 2002;82:615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- Chari DM, Zhao C, Kotter MR, Blakemore WF, Franklin RJ. Corticosteroids delay remyelination of experimental demyelination in the rodent central nervous system. J Neurosci Res. 2006;83:594–605. doi: 10.1002/jnr.20763. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- Cimini A, Benedetti E, Cristiano L, Sebastiani P, D’Amico MA, D’Angelo B, Di LS. Expression of peroxisome proliferator-activated receptors (PPARs) and retinoic acid receptors (RXRs) in rat cortical neurons. Neuroscience. 2005;130:325–337. doi: 10.1016/j.neuroscience.2004.09.043. [DOI] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPAR gamma agonists. J Neurosci. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Maffia P, Patel NSA, Di Paolo R, Ialenti A, Genovise T, Chatterjee PK, Di Rosa M, Caputi AP, Thiemermann C. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-γ, induces acute inflammation. Eur J Neurosci. 2004;483:79–93. doi: 10.1016/j.ejphar.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with IkappaBalpha induction and block of NFkappaB and iNOS activation. J Neurochem. 2004;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- Diab A, Hussain RZ, Lovett-Racke AE, Chavis JA, Drew PD, Racke MK. Ligands for the peroxisome proliferator-activated receptor-gamma and the retinoid X receptor exert additive anti-inflammatory effects on experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:116–126. doi: 10.1016/j.jneuroim.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994;6:712–724. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Gavrilyuk V, Brosnan CF, Whitacre CC, Dumitrescu-Ozimek L, Landreth GE, Pershadsingh HA, Weinberg G, Heneka MT. Peroxisome proliferator-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis. Ann Neurol. 2002;51:694–702. doi: 10.1002/ana.10206. [DOI] [PubMed] [Google Scholar]
- Giulian D, Robertson C. Inhibition of mononuclear phagocytes reduces ischemic injury in the spinal cord. Ann Neurol. 1990;27:33–42. doi: 10.1002/ana.410270107. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. 2003;184:456–463. doi: 10.1016/s0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Klockgether T, Feinstein DL. Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. J Neurosci. 2000;20:6862–6867. doi: 10.1523/JNEUROSCI.20-18-06862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hihi AK, Michalik L, Wahli W. PPARs: transcriptional effectors of fatty acids and their derivatives. Cell Mol Life Sci. 2002;59:790–798. doi: 10.1007/s00018-002-8467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology: Three-dimensional Measurement in Microscopy. New York; Springer-Verlag: 1998. [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Kiaei M, Kiiani K, Chen J, Calingasan NY, Beal MF. Peroxisome prolifertor-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2005;191:331–336. doi: 10.1016/j.expneurol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Zhao C, van Rooijen N, Franklin RJ. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol Dis. 2005;18:166–175. doi: 10.1016/j.nbd.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Landreth GE, Heneka MT. Anti-inflammatory actions of peroxisome proliferator-activated receptor gamma agonists in Alzheimer’s disease. Neurobiol Aging. 2001;22:937–944. doi: 10.1016/s0197-4580(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Lankhorst AJ, ter Laak MP, van Laar TJ, van Meeteren NL, de Groot JC, Schrama LH, Hamers FP, Gispen WH. Effects of enriched housing on functional recovery after spinal cord contusive injury in the adult rat. J Neurotrauma. 2001;18:203–215. doi: 10.1089/08977150150502622. [DOI] [PubMed] [Google Scholar]
- Lee SM, Yune TY, Kim SJ, Park W, Lee YK, Kim YC, Oh YJ, Markelonis GJ, Oh TH. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. 2003;20:1017–1027. doi: 10.1089/089771503770195867. [DOI] [PubMed] [Google Scholar]
- Lee YB, Yune TY, Baik SY, Shin YH, Du S, Rhim H, Lee EB, Kim YC, Shin ML, Markelonis GJ, Oh TH. Role of tumor necrosis factor-alpha in neuronal and glial apoptosis after spinal cord injury. Exp Neurol. 2000;166:190–195. doi: 10.1006/exnr.2000.7494. [DOI] [PubMed] [Google Scholar]
- Li WW, Setzu A, Zhao C, Franklin RJ. Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. J Neuroimmunol. 2005;158:58–66. doi: 10.1016/j.jneuroim.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Maeshiba Y, Kiyota Y, Yamashita K, Yoshimura Y, Motohashi M, Tanayama S. Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneimittelforschung. 1997;47:29–35. [PubMed] [Google Scholar]
- McTigue DM, Tani M, Krivacic K, Chernosky A, Kelner GS, Maciejewski D, Maki R, Ransohoff RM, Stokes BT. Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. J Neurosci Res. 1998;53:368–376. doi: 10.1002/(SICI)1097-4547(19980801)53:3<368::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi R, Wei P. NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. J Neuropathol Exp Neurol. 2006;65:406–420. doi: 10.1097/01.jnen.0000218447.32320.52. [DOI] [PubMed] [Google Scholar]
- Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther. 2006;110:371–385. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Nesic O, Xu GY, McAdoo D, High KW, Hulsebosch C, Perez-Pol R. IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma. 2001;18:947–956. doi: 10.1089/089771501750451857. [DOI] [PubMed] [Google Scholar]
- Niino M, Iwabuchi K, Kikuchi S, Ato M, Morohashi T, Ogata A, Tashiro K, Onoe K. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by an agonist of peroxisome proliferator-activated receptor-gamma. J Neuroimmunol. 2001;116:40–48. doi: 10.1016/s0165-5728(01)00285-5. [DOI] [PubMed] [Google Scholar]
- Ou Z, Zhao X, Labiche LA, Strong R, Grotta JC, Herrmann O, Aronowski J. Neuronal expression of peroxisome proliferator-activated receptor-gamma (PPAR gamma) and 15d-prostaglandin J2--mediated protection of brain after experimental cerebral ischemia in rat. Brain Res. 2006;1096:196–203. doi: 10.1016/j.brainres.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Park S-W, Yi J-H, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R. Thiazolidinedione class of PPARγ agonists prevent neuronal damage, motor dysfunction, myelin loss, neuropathic pain and inflammation following spinal cord injury in adult rats. J Pharm Exp Ther, Fast Forward Published online. 2006 doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- Pershadsingh HA, Heneka MT, Saini R, Amin NM, Broeske DJ, Feinstein DL. Effect of pioglitazone treatment in a patient with secondary multiple sclerosis. J Neuroinflammation. 2004;1:3. doi: 10.1186/1742-2094-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague- Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Roth AD, Leisewitz AV, Jung JE, Cassina P, Barbeito L, Inestrosa NC, Bronfman M. PPAR gamma activators induce growth arrest and process extension in B12 oligodendrocyte-like cells and terminal differentiation of cultured oligodendrocytes. J Neurosci Res. 2003;72:425–435. doi: 10.1002/jnr.10596. [DOI] [PubMed] [Google Scholar]
- Saville LR, Pospisil CH, Mawhinney LA, Bao F, Simedrea FC, Peters AA, O’Connell PJ, Weaver LC, Dekaban GA. A monoclonal antibody to CD11d reduces the inflammatory infiltrate into the injured spinal cord: a potential neuroprotective treatment. J Neuroimmunol. 2004;156:42–57. doi: 10.1016/j.jneuroim.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Schutz B, Reimann J, Dumitrescu-Ozimek L, Kappes-Horn K, Landreth GE, Schurmann B, Zimmer A, Heneka MT. The oral antidiabetic pioglitazone protects from neurodegeneration and amyotrophic lateral sclerosis-like symptoms in superoxide dismutase-G93A transgenic mice. J Neurosci. 2005;25:7805–7812. doi: 10.1523/JNEUROSCI.2038-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME, Friedlander RM. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A. 2004;101:3071–3076. doi: 10.1073/pnas.0306239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Wang CX, Nuttin B, Heremans H, Dom R, Gybels J. Production of tumor necrosis factor in spinal cord following traumatic injury in rats. J Neuroimmunol. 1996;69:151–156. doi: 10.1016/0165-5728(96)00080-x. [DOI] [PubMed] [Google Scholar]
- Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- Yune TY, Chang MJ, Kim SJ, Lee YB, Shin SW, Rhim H, Kim YC, Shin ML, Oh YJ, Han CT, Markelonis GJ, Oh TH. Increased production of tumor necrosis factor-alpha induces apoptosis after traumatic spinal cord injury in rats. J Neurotrauma. 2003;20:207–219. doi: 10.1089/08977150360547116. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Strong R, Grotta JC, Aronowski J. 15d-Prostaglandin J2 activates peroxisome proliferator-activated receptor-gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2006;26:811–820. doi: 10.1038/sj.jcbfm.9600233. [DOI] [PubMed] [Google Scholar]


