Abstract
Objective
To evaluate the cost-effectiveness of a prize-based intervention as an addition to usual care for stimulant abusers.
Methods
This cost-effectiveness analysis is based on a randomized clinical trial implemented within the National Drug Abuse Treatment Clinical Trials Network. The trial was conducted at eight community-based outpatient psychosocial drug abuse treatment clinics. 415 stimulant abusers were assigned to usual care (n = 206) or usual care plus abstinence-based incentives (n=209) for 12 weeks. Participants randomized to the incentive condition earned the chance to draw for prizes for submitting substance negative samples; the number of draws earned increased with continuous abstinence time. Incremental cost-effectiveness ratios were estimated to compare prize-based incentives relative to usual care. The primary patient outcome was longest duration of confirmed stimulant abstinence (LDA). Unit costs were obtained via surveys administered at the eight participating clinics. Resource utilizations and patient outcomes were obtained from the clinical trial. Acceptability curves are presented to illustrate the uncertainty due to the sample and to provide policy relevant information.
Results
The incremental cost to lengthen the LDA by one week was $258 (95% confidence interval, $191 - $401). Sensitivity analyses on several key parameters show that this value ranges from $163 to $269.
Conclusions
Compared with the usual care group, the incentive group had significantly longer LDAs and significantly higher costs.
Keywords: Cost-effectiveness, contingency management, motivational incentives, substance abuse treatment, stimulant abuse, MIEDAR
1. Introduction
This study analyzes the cost-effectiveness of a prize-based contingency management intervention implemented within the National Institute on Drug Abuse Clinical Trials Network (CTN). The clinical trial, known within the CTN as Motivational Incentives for Enhanced Drug Abuse Recovery (MIEDAR), was implemented at eight community-based outpatient psychosocial substance abuse treatment clinics and included 415 stimulant abusers randomly assigned within each clinic to usual care or usual care plus prize-based contingency management (CM). Petry et al. (2005a) analyzed patient outcomes from the trial but did not report on costs of treatment nor on cost-effectiveness. The primary target drugs in the trial were cocaine, amphetamine, methamphetamine, and alcohol.
In voucher-based CM, participants receive vouchers exchangeable for goods and services whenever they abstain from using drugs according to objective criteria such as urine toxicology screens (Higgins et al., 1994; Higgins et al., 2000a; Higgins et al., 2003). This intervention is efficacious in treating a range of substance use disorders, including cocaine (Higgins et al., 1994; Higgins et al., 2000a; Higgins et al., 2003), opioids (Preston et al., 1998; Rawson et al., 2002; Silverman et al., 1996), and marijuana (Budney et al., 2000) dependence. However, the costs of CM may hinder wide adoption. In many voucher-based CM studies, participants could earn over $1,000 in vouchers during a 12-week treatment period, and average earnings in the above studies were around $600.
Prize-based CM was initially designed as a possibly lower cost alternative to the voucher system (Petry et al., 2000). In prize-based CM, participants earn opportunities to draw from a prize bowl. The bowl contains chips with prizes of varying magnitudes ($1-$100) listed on some, and ‘good job, try again’ listed on others. Thus, the participants earn chances to win prizes, contingent on being abstinent. The number of chips drawn escalates with increasing durations of continuous abstinence.
Recently, prize-based CM has been shown to be feasible to implement in community-based psychosocial outpatient substance abuse treatment (SAT) clinics and efficacious for a number of substance abusing populations, including stimulant-abusers (Petry et al., 2004; Petry et al., 2006), alcohol-dependent patients (Petry et al., 2000), and polydrug using methadone patients (Petry and Martin, 2002; Petry et al., 2005b). The CTN trial on which this cost-effectiveness study is based (Petry et al., 2005a) was the first study to evaluate the effectiveness of prize-based CM in geographically-diverse community clinics.
Although prize-based interventions may reduce the cost of CM compared to vouchers, they still increase costs to a financially constrained treatment system. In the prize-based CTN trial, average prize winnings were around $200. At issue is whether the extra expense is cost-effective in terms of the additional value gained. Without knowing the cost-effectiveness of CM interventions in community-based settings, policy and decision makers have little guidance in determining whether the additional expenditures on CM are worthwhile investments. Unfortunately, few studies have examined the cost-effectiveness of CM in general, and only Sindelar et al. (under review) have analyzed the cost-effectiveness of prize-based CM in community settings. Compared to the Sindelar et al. (under review) study, the present study uses a much larger sample size, more clinics, and the clinics comprise a greater range of treatment styles, patient mixes, and geographic locations.
This study adds to the small but growing literature on the cost-effectiveness of CM by conducting a cost-effectiveness analysis of a prize-based CM intervention targeted at stimulant abusers in community clinics. We present incremental cost-effectiveness ratios (ICERs) and conduct sensitivity analyses varying several key implementation parameters. We also present acceptability curves to illustrate the statistical uncertainty inherent in the ICERs and to provide policy relevant information. The emphasis on value per dollar spent on treatment is important for policy decisions related to the future expansion of CM interventions. We also add to the growing literature on cost-effectiveness of treatment for substance abuse in general (Barnett et al., 2001; French et al., 1996; Sindelar et al., 2004; Jofre-Bonet and Sindelar, 2004; Cartwright, 1998; Cartwright 2000). Finally, our results are likely to be widely generalizeable inasmuch as the clinics included in the MIEDAR study encompass considerable variation in usual care content, patient mix, and geographic location.
2. Methods
We conduct cost-effectiveness analyses of prize-based CM using patient outcomes and resource utilization data collected by the MIEDAR effectiveness study (Petry et al., 2005a). To these we add data from a cost survey that we administered to each of the clinics participating in MIEDAR. Methods and results of the MIEDAR effectiveness study are described in an earlier publication (Petry et al., 2005a) and summarized briefly below. Then we present our analytical methods.
2.1. MIEDAR Effectiveness Study
The MIEDAR study evaluated the effectiveness of prize-based CM as compared to usual care in each of eight outpatient psychosocial community-based SAT clinics that were members of the CTN. Individuals entering into treatment at the clinics were enrolled in the study between April 30, 2001 and February 28, 2003. The study intervention lasted for 12 weeks. Only patients who disclosed stimulant use during their initial evaluation, or who submitted a stimulant-positive urine sample, were offered the possibility of study participation. The final study sample comprised 415 stimulant-abusing participants who were randomly assigned to either usual care or usual care plus prize-based incentives. Random assignment was conducted at each site independently by a computer program using a dynamic balanced optimization procedure (Signorini et al., 1993). Neither demographics nor substance use variables differed between conditions based on between group comparisons using t-tests for continuous variables and Chi-square tests for categorical variables (Petry et al., 2005a).
2.1.1. Treatment
Usual Care
Usual care consisted primarily of group and possibly some individual and family counseling, depending on the clinic. As part of the study, participants in both conditions also received immediate feedback on their twice-weekly urinalysis and breathalyzer results. Study participation (or lack thereof) did not affect usual care at the clinics; participants could continue receiving treatment without continuing in the study, and after completing study participation.
Testing
Participants were asked to provide two urine samples per week on non-consecutive days (as opposed to once weekly), both to ensure detection of drug use and to increase the number of feedback/reinforcement opportunities. Study schedules were individualized to coincide with clinic attendance. Urine samples were tested for amphetamine, methamphetamine, cocaine, THC, and morphine. Participants also provided a breath sample at each visit that was tested for alcohol using a desktop or handheld breathalyzer.
Prize-based CM
Participants assigned to the prize-based CM condition, in addition to receiving usual care, earned the chance to win prizes each time they tested negative for cocaine, amphetamine, methamphetamine and alcohol (i.e., the primary target drugs). Those who tested negative for all primary target drugs were invited to draw between one and 12 square plastic chips from an opaque container containing 500 chips. Each chip was marked with an incentive reward value: 250 (50%) were marked “Good Job” and had no monetary value; 209 (41.8%) were marked “Small” and worth about $1 (e.g., toiletries, snacks, bus tokens); 40 (8%) were marked “Large” and worth about $20 (e.g., telephone, CD player); and 1 (0.2%) was marked “Jumbo” and worth about $100 (e.g., television, stereo).
The number of draws increased by one for each week of successful testing, but was reset to one draw after an unexcused absence or submission of a positive sample. This escalating reward system was designed to reinforce long durations of abstinence. At each study visit, participants could earn bonus draws if their sample also tested negative for opioids and marijuana (i.e., the secondary target drugs). Bonus draws required total abstinence from both primary and secondary drugs and did not escalate over time. In addition, to offset the low rate of reinforcement early in the study when the number of draws was low, a single large prize was awarded when a participant first achieved two consecutive weeks of abstinence from the primary target drugs. Participants in the incentives condition who provided all scheduled urine and breath samples throughout the study and who were negative for all primary and secondary drugs earned 204 draws, resulting in an expected value of approximately $400 in prizes.
2.1.2. Results
The MIEDAR study determined that participants assigned to prize-based CM had significantly better patient outcomes than participants assigned to usual care alone. As shown in Table 1, participants assigned to prize-based CM achieved significantly longer durations of continuous stimulant and alcohol abstinence, remained in the study significantly longer, and submitted significantly more stimulant-negative urine samples. In addition, 18.7% of the participants assigned to prize-based CM achieved the maximum possible duration of continuous stimulant and alcohol abstinence (12 weeks), compared to 4.9% of the participants assigned to usual care alone. The MIEDAR study found no significant difference between the two groups in the percentages of samples that tested positive for opioids and marijuana (i.e., the secondary target drugs). Given that sample characteristics were similar across the treatment arms, the differences in patient outcomes were likely due to the prize-based CM intervention.
Table 1.
Average and Incremental Patient Outcomes in MIEDAR Studya
| CM (N = 208) | UC (N = 204) | CM - UC | |
|---|---|---|---|
| Longest duration abstinent (weeks) | 4.3 (4.6) | 2.6 (3.4) | 1.7* |
| Number of negative urines | 12.6 (9.0) | 9.6 (8.0) | 3.0* |
| Length of stay in study (weeks) | 8.1 (4.2) | 7.0 (4.4) | 1.1* |
MIEDAR = Motivational incentives for enhanced drug abuse recovery
CM = prize-based contingency management
UC = usual care
Values represent means and standard deviations (parentheses) and are based on a sample comprising 412 MIEDAR participants (three of the 415 participants in the MIEDAR study were excluded in the present study due to missing data).
p-value < .05
2.2. Cost-effectiveness analysis
Incremental cost-effectiveness analysis is the appropriate approach to use in this study as we are comparing usual care to CM which incrementally adds costs (Drummond et al., 1997; Gold et al., 1996). To calculate incremental cost-effectiveness ratios (ICERs), we first calculate the unit costs using our cost survey data. Then, for each study participant, we multiply the resources used by the unit costs. Data on resources used (e.g. number of tests and counseling sessions) come from the original MIEDAR study. We are then able to calculate the total variable cost for each participant in each arm of treatment, and finally the incremental cost of prize-based CM over the cost of usual care. All cost-effectiveness analyses are based on a sample comprising 412 MIEDAR participants (three of the 415 participants in the MIEDAR study were excluded from the present study due to missing data on resource utilization).
2.2.1. Incremental Costs of Prize-Based CM
Unit costs
Unit cost data necessary to estimate the incremental cost of prize-based CM compared to usual care were collected via survey administered to each clinic in the MIEDAR study. CTN research associates at each clinic were responsible for filling in the survey. They were asked to seek information from the Chief Financial Officer, Chief Executive Officer, lead counselors and/or accountants to obtain the necessary cost information. Instructions to the research associates accompanied the survey. The clinics were each paid $100 to ensure participation.
Costs are calculated from the perspective of the clinic and include only those costs that vary by treatment condition. Such costs include those related to counseling sessions, urine and breath sample testing, and the prize system. All labor costs include fringe benefits, where appropriate (i.e., not all clinics provide benefits). Because implementing a prize-based CM intervention may require additional staff in the long run, all labor costs are multiplied by the overhead rate reported by each clinic (the overhead rate averaged 29.9% across the eight clinics in the study).
Unit counseling costs
Unit counseling costs measure the average per participant cost of a counseling session and were estimated for individual, group and family therapies. These unit costs include the time spent by the counselor both in treatment and in administration (e.g., taking notes before or after the session) and are prorated by the average number of patients in a session.
Unit testing costs
Unit testing costs measure the average cost per urine and breath test and include material costs (breathalyzer tubes and urine test cups) and time spent by staff administering the test.
Unit prize system costs
Prize system costs comprise three components: drawing session costs, prize costs, and costs of administering the prize system (e.g. taking inventory of prizes, shopping for prizes and restocking the system). For simplicity, we refer to the costs of administering the prize system as ‘restocking costs’ even though this component also includes the broader costs of taking inventory and shopping for prizes. Unit drawing costs measure the average cost of a drawing session; this is the time spent by staff administering each drawing valued at counselor salary plus fringe benefits and overhead. Unit prize costs are the value of the prizes won during the drawing sessions and are assumed to be $0 for “Good Job,” $1 for “Small,” $20 for “Large,” and $100 for “Jumbo.” Costs of administering the system are calculated by measuring the amount of time spent by staff taking inventory, purchasing, and restocking the prizes for the incentives condition; this time is then valued at counselor salary plus fringe benefits and overhead. Since staff typically perform these duties on a weekly or bi-weekly basis, there are several ways to apportion these costs on a per participant basis. In the present study, unit administrative costs refer to the administrative cost per prize won (not including “good jobs” which do not contribute to the need for restocking).
Resources used
In order to calculate the total variable costs, we need to multiply the above calculated unit costs by the number of units of each resource used. Resource utilizations for each participant were obtained from the MIEDAR study (Petry et al., 2005a). Data on the number of each of the following were collected: counseling sessions of each type (i.e., individual, group, and family), urinalysis tests, drawing sessions for prizes, and prizes won of each value. Variable costs per participant are then estimated straightforwardly by multiplying unit costs by corresponding resource utilizations.
Finally, the incremental cost of prize-based CM compared to usual care is estimated by subtracting the average per participant cost of the usual care group from the average per participant cost of the prize-based CM group.
2.2.2. Incremental Cost-Effectiveness Analysis
We conduct incremental cost-effectiveness analyses (ICEA) to answer the question of value per dollar spent on prize-based CM over usual care. The primary patient outcome used in the ICEA is the longest duration of abstinence (LDA) from primary target drugs. LDA is defined as the longest span of consecutive weeks in which all samples delivered under the twice-weekly testing schedule indicated abstinence from primary target drugs.
The LDA was chosen as the primary patient outcome for the ICEA both because (1) the escalating draw feature of the incentive condition was designed specifically to reinforce long durations of abstinence, and (2) the longest duration of abstinence achieved during treatment is among the best predictors of improved outcomes at follow-up periods (Higgins et al., 2003; Petry et al., 2006; Higgins et al., 2000b). As a check on the robustness of our results, we also consider two secondary patient outcome measures: the length of stay (LOS) in the study and the number of stimulant-negative urine samples submitted.
For each of the three patient outcome measures, we calculate incremental cost-effectiveness ratios (ICERs). The ICER is defined as the incremental cost divided by the incremental effect. We use incremental costs estimated as described above and incremental effects obtained from the MIEDAR study. The ICERs measure the incremental cost of using the prize-based CM intervention, compared to usual care, to produce an extra unit of effect for each of the patient outcomes.
Bootstrapping (with 1,000 replicates) is used to estimate confidence intervals for each of the ICERs and to produce an acceptability curve for LDA (Briggs, 2001). The acceptability curve illustrates the statistical uncertainty in our study due to our sample and provides policy relevant information (Briggs, 2001; Fenwick et al., 2001; Lothgren and Zethraeus, 2000). Finally, we conduct sensitivity analyses on several key parameters to assess how the ICERs would likely change if MIEDAR had been implemented under different realistic conditions.
3. Results
3.1. Incremental costs of prize-based CM
Unit costs were estimated following the methods described above using the data collected via the cost survey administered to the clinics in the MIEDAR study. Table 2 presents the weighted average of the unit costs, where the average is taken across all clinics and weighted by the sample size at each clinic. For example, the average per participant cost of a group counseling session was $6.25 (the low cost is due to approximately ten patients sharing the total cost of the group session). Each test comprising a urinalysis and breathalyzer cost an average of $16.73, the majority of which was the material cost of the urine sample test cups (breathalyzer tubes are only $0.21 apiece). The administrative time to conduct a drawing session was valued at an average of $3.25, and each monetary prize (i.e., not the “good jobs”) incurred an average administrative cost of $2.87 (including inventorying, shopping, and restocking).
Table 2.
Average Unit Costs of Resources at MIEDAR Clinicsa (N = 412)
| Unit Cost ($/unit) | |
|---|---|
| Counselingb | |
| Group | 6.25 (3.44) |
| Individual | 28.71 (4.20) |
| Family | 15.21 (12.91) |
| Testingc | |
| Materials | 13.06 (1.69) |
| Labor | 3.67 (0.99) |
| Incentives | |
| Drawingd | 3.25 (1.78) |
| Good job | 0.00 (0.0) |
| Prize-small | 1.00 (0.0) |
| Prize-large | 20.00 (0.0) |
| Prize-jumbo | 100.00 (0.0) |
| Restockinge | 2.87 (1.48) |
MIEDAR = Motivational incentives for enhanced drug abuse recovery
Values represent means and standard deviations (parentheses). Unit costs are averaged across all sites and weighted by sample size at each site. All labor includes fringe benefits and overhead.
$ per participant per session. Includes session time plus administrative time (e.g., taking notes). Prorated by average number of session participants.
$ per test. Includes both alcohol and drug testing.
$ per drawing session. Includes time to administer drawing.
$ per prize won. Includes time to inventory, shop, and restock prizes (typically done weekly, prorated by number of prizes won).
Resource utilizations per participant were obtained from the MIEDAR study and are summarized in Table 3. On average, compared to the usual care group, participants in the prize-based CM group attended significantly more individual and group counseling sessions and submitted significantly more urine samples to be tested. Participants in the prize-based CM group had an average of 11.6 drawing sessions and earned an average of 76.4 draws, resulting in an average of 36.8 “good job” chips, 32.1 small prizes, 7.4 large prizes, and 0.24 jumbo prizes.
Table 3.
Average and Incremental Resources Consumed Per MIEDAR Participanta
| CM (N = 208) | UC (N = 204) | CM - UC | |
|---|---|---|---|
| Counseling Sessions | |||
| Group | 15.7 (14.9) | 12.8 (12.5) | 2.9* |
| Individual | 2.2 (2.7) | 1.7 (2.1) | 0.5* |
| Family | 1.3 (3.0) | 1.2 (2.6) | 0.1 |
| Tests | 13.0 (8.0) | 9.9 (7.0) | 3.1* |
| Incentives | |||
| Drawing sessions | 11.6 (8.5) | 0 | 11.6* |
| Good jobs | 36.8 (34.9) | 0 | 36.8* |
| Prizes-small | 32.1 (31.4) | 0 | 32.1* |
| Prizes-large | 7.4 (7.6) | 0 | 7.4* |
| Prizes-jumbo | 0.24 (0.5) | 0 | 0.24* |
MIEDAR = Motivational incentives for enhanced drug abuse recovery
CM = prize-based contingency management
UC = usual care
Values represent means and standard deviations (parentheses).
p-value < .05
Table 4 presents the average variable cost per participant for both prize-based CM and usual care, as well as the incremental costs of prize-based CM compared to usual care. As shown in Table 4, participants in the prize-based CM group incurred significantly higher counseling, testing, and incentives costs. The incremental cost of prize-based CM compared to usual care was $438 per participant. Most of this incremental cost is due to the incentives ($349), of which $213 is the value of the prizes (including the value of the single large prize awarded when participants in prize-based CM first achieved two consecutive weeks of abstinence from primary target drugs), $101 is for administering the prize system (i.e., taking inventory of prizes, shopping for prizes, and restocking the system), and $35 is for administering the drawing sessions.
Table 4.
Average and Incremental Variable Cost Per MIEDAR Participanta
| CM (N = 208) ($) | UC (N = 204) ($) | CM - UC ($) | |
|---|---|---|---|
| Counseling | |||
| Group | 86 (90) | 66 (69) | 20* |
| Individual | 65 (83) | 47 (59) | 18* |
| Family | 13 (30) | 12 (27) | 1 |
| Subtotal | 164 (145) | 125 (113) | 39* |
| Testing | 217 (139) | 167 (125) | 50* |
| Incentives | |||
| Drawing | 35 (35) | 0 | 35* |
| Prizesb | 213 (218) | 0 | 213* |
| Restocking | 101 (114) | 0 | 101* |
| Subtotal | 349 (340) | 0 | 349* |
| Total | 730 (552) | 292 (217) | 438* |
MIEDAR = Motivational incentives for enhanced drug abuse recovery
CM = prize-based contingency management
UC = usual care
Values represent means and standard deviations (parentheses). Average variable costs were determined by first estimating the variable cost of each participant using that participant’s resource utilization and clinic-specific unit costs, and then averaging across all participants in each condition.
Includes the single large prize awarded when participants in prize-based CM first achieved two consecutive weeks of abstinence from primary target drugs (i.e., cocaine, amphetamine, methamphetamine, alcohol). One-half (n = 104) of the participants in prize-based CM qualified for this prize.
p-value < .05
3.2. Incremental cost-effectiveness of prize-based CM
Column 1 of Table 5 presents incremental cost-effectiveness ratios (ICERs) for the comparison of prize-based CM with usual care. These ICERs were calculated using the incremental effects from Table 1 and the incremental costs from Table 4. Compared to usual care, the incremental cost of using prize-based CM to lengthen the LDA by one week was $258 (95% confidence interval [CI], $191 - $401), to obtain an additional stimulant-negative urine sample was $146 (95% CI, $106 - $269), and to extend the length of stay in the study by one week was $398 (95% CI, $257 - $1074).
Table 5.
Incremental Cost-Effectiveness Ratios—Base Case and Sensitivity Analyses
| Base Casea ($) | Unfavorable Scenariob ($) | Conservative Scenarioc ($) | Favorable Scenariod ($) | |
|---|---|---|---|---|
| Cost of extending LDA by 1 week | 258 (191 - 401) | 269 | 229 | 163 |
| Cost of an additional negative urine | 146 (106 - 269) | 153 | 130 | 78 |
| Cost of extending LOS by 1 week | 398 (257 - 1074) | 416 | 354 | 163 |
LDA = longest duration abstinent during study; LOS = length of stay in study
Base case corresponds to actual implementation of MIEDAR. 95% confidence intervals in parentheses.
Unfavorable scenario assumes (1) $4.80 test cups, (2) usual care group gets tested half as often but retains 100% of their patient outcomes, (3) full-scale implementation has no effect on the unit cost of administering the prize system, and (4) full overhead rate is applied to labor costs.
Conservative scenario assumes (1) $4.80 test cups, (2) usual care group gets tested half as often but retains 100% of their patient outcomes, (3) full-scale implementation reduces the unit cost of administering the prize system by 50%, and (4) one-half (50%) of the overhead rate is applied to labor costs.
Favorable scenario assumes (1) $4.80 test cups, (2) usual care group gets tested once per week and retains 85% of their patient outcomes, (3) full-scale implementation reduces the unit cost of administering the prize system by 75%, and (4) one-quarter (25%) of the overhead rate is applied to labor costs.
Acceptability curves are a relatively new approach that is used to illustrate the statistical uncertainty inherent in estimates of ICERs due to the use of a single sample. They also provide policy relevant information to decision makers (Briggs, 2001; Fenwick et al., 2001; Lothgren and Zethraeus, 2000). Figure 1 is the acceptability curve associated with the ICER for the patient outcome LDA. The acceptability curve indicates the likelihood that an intervention will be ‘acceptable’ to decision makers given a large set of alternative values that decision makers could place on the incremental outcome. For example, if the threshold value (perhaps determined by society’s willingness to pay) to extend the LDA by one week were $205, then the prize-based CM intervention would be only 10% likely to be cost-effective. On the other hand, if the threshold value to extend the LDA by one week were $325, then the prize-based CM intervention would be 90% likely to be cost-effective.
Figure 1.
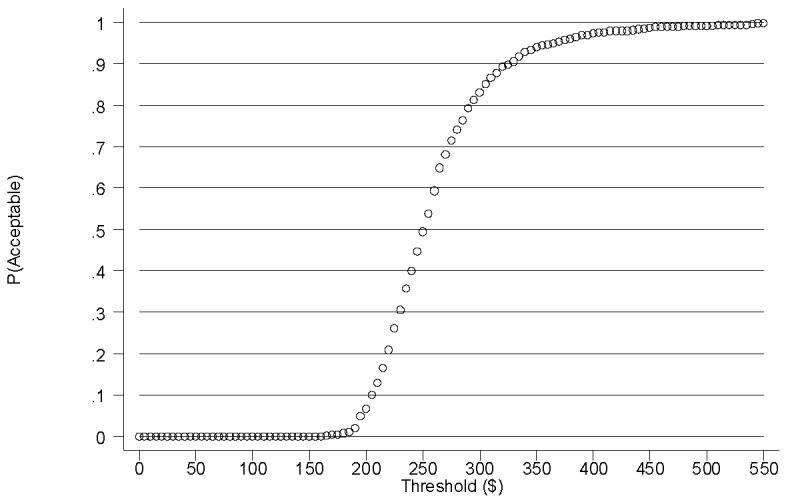
Acceptability Curve for Longest Duration Abstinent (LDA) - Base Case
3.2.1. Sensitivity analysis
In order to determine how sensitive our results are to alternative assumptions, we conduct additional analyses. We focus on what the costs and outcomes of prize-based CM might be if MIEDAR had been implemented in 2005 and on a large scale. Three realistic scenarios were modeled that reflect different assumptions about (1) the unit cost of urine sample test cups, (2) the testing frequency of participants in the usual care group and associated treatment effects, (3) the unit cost of administering the prize system, and (4) the amount of overhead included in labor costs.
The unit cost of the test cups used in the MIEDAR study averaged $12.85. Due to technological improvements since the time of the MIEDAR study, the unit cost of test cups capable of detecting amphetamine, methamphetamine, cocaine, THC, and morphine is now only $4.80 (e.g., EZ Split Key 5-Panel Test Cup, Medical Disposables, Ft. Lauderdale, FL). Thus, all scenarios below assume the unit cost of test cups is $4.80.
The MIEDAR protocol scheduled two tests per week for participants assigned to usual care. In practice, tests are typically scheduled only once per week at the clinics in the MIEDAR study. Since participants in the usual care group averaged 1.4 tests per week in the MIEDAR study, they probably experienced higher testing costs than would occur in normal practice, thereby understating the ICERs. At the same time, more frequent feedback from the twice-weekly tests may have improved patient outcomes in the usual care group more than would have been the case with testing once per week, thereby overstating the ICERs. Thus, we compare the base case to alternative cases that vary both the testing frequency and the concomitant effectiveness of usual care.
The costs of administering the prize system (taking inventory, shopping, and restocking prizes) were spread out over a relatively small number of prizes won. If the prize-based CM intervention had been implemented on a larger scale within the clinics—only a small fraction of clinic patients were enrolled in the MIEDAR study—then clinics would be able to take advantage of economies of scale in running the prize system, thereby reducing the ICERs. Thus, we consider alternative unit costs of administrating the prize system.
Labor costs were multiplied by the clinic overhead rate to account for long-run costs that might vary due to additional staff required by the prized-based CM intervention. In practice, it is unlikely that all elements of overhead would vary proportionally with staff (e.g., clinic director, water for the lawn, etc.). Thus, we consider alternative cases that vary the amount of overhead included in the labor costs.
We modeled three scenarios that reflect different assumptions—favorable, conservative, and unfavorable—about the cost-effectiveness of prize-based CM compared to usual care. Table 6 summarizes the specific assumptions made for each of the three scenarios.
Table 6.
Assumptions Used in Sensitivity Analyses
| Parameter | Unfavorable Scenario | Conservative Scenario | Favorable Scenario |
|---|---|---|---|
| Unit cost of test cupa | $4.80 | $4.80 | $4.80 |
| Frequency of testing usual care groupb | 0.7 times/week | 0.7 times/week | 1 time/week |
| Effect of treatment on usual care group | 100% of base | 100% of base | 85% of base |
| Unit cost of administering prize system in full-scale implementation | 100% of base | 50% of base | 25% of base |
| Portion of overhead rate applied to labor costsc | 100% | 50% | 25% |
In MIEDAR, the unit cost of test cups averaged $12.85.
In MIEDAR, the usual care group was tested an average of 1.4 times per week.
In base case, the full overhead rate was applied to labor costs.
Columns 2-4 of Table 5 present the results of these sensitivity analyses that examine how the ICERs would likely change if MIEDAR had been implemented under different realistic conditions. The ICER for the patient outcome LDA, for example, ranges from a low of $163 in the favorable scenario to a high of $269 in the unfavorable scenario. In general, the ICERs in the unfavorable (conservative) scenario are only slightly higher (lower) than the base case, whereas the ICERs in the favorable scenario are much lower than the base case.
4. Discussion
In a population of stimulant abusers treated in community-based psychosocial SAT clinics, prized-based contingency management produced significant gains in patient outcomes, as well as significant increases in treatment costs. That is, prize-based CM provided better patient outcomes than usual care but required additional costs. Compared to usual care, we found that the incremental cost of using prize-based CM to lengthen the longest duration of continuous stimulant and alcohol abstinence (LDA) by one week was $258 (95% CI, $191 - $401), to obtain an additional stimulant-negative urine sample was $146 (95% CI, $106 - $269), and to extend the length of stay in the study by one week was $398 (95% CI, $257 - $1074).
Determining whether prize-based CM is cost-effective for improving patient outcomes depends on the existence of threshold values against which to compare the estimated ICERs (one threshold value for each patient outcome). At this time, however, no threshold values exist for any of the patient outcomes in the MIEDAR study (or any other outcome from substance abuse treatment). To address this issue, we present an acceptability curve for the longest duration of abstinence, our primary patient outcome measure. For any given threshold value for LDA, the acceptability curve presents decision makers with the likelihood that prize-based CM is cost-effective. For example, if decision makers believe that $205 is an acceptable threshold to extend the LDA by one week, then the prize-based CM intervention is only 10% likely to be cost-effective. On the other hand, if decision makers believe that $325 is an acceptable threshold to extend the LDA by one week, then the prize-based CM intervention is 90% likely to be cost-effective. Decision makers can use the information from the acceptability curve in combination with their own evaluation of the value of outcomes to make policy decisions. As an illustrative example, the link between illicit drug use and crime is well established (Sindelar and Olmstead, 2006; Jofre-Bonet and Sindelar, 2001), and French et al. (under review) estimate the per-offense cost of a robbery (involving force or the threat of force) and a theft (without force or the threat of force) at $48,095 and $1,583, respectively. Based on the acceptability curve in Figure 1, if decision makers believe that extending the LDA by one week would reduce the probability of a single robbery by at least 0.7% (i.e., 325/48,095) or of a single theft by at least 21% (i.e., 325/1,583), then prize-based CM would achieve savings in avoided crime costs that would be 90% likely to outweigh its incremental costs. Thus, in this scenario, prize-based CM would be 90% likely to dominate usual care (i.e., lead to better patient outcomes at lower cost) due to reductions in crime alone (not to mention the value of potential improvements in other negative externalities associated with drug abuse such as disease and welfare). Although the societal costs of substance abuse may be lower if prize-based CM were implemented widely (due to reductions in crime, disease, and welfare), we recognize that it may be difficult in practice for policy makers to distribute additional resources to substance abuse treatment to realize these savings.
Sensitivity analyses were conducted to determine how the ICERs would likely change if MIEDAR had been implemented under different realistic conditions. In general, the ICERs in the unfavorable (conservative) scenario are only slightly higher (lower) than the base case, whereas the ICERs in the favorable scenario are much lower than the base case. Thus, it seems reasonable to conclude that had MIEDAR been implemented under alternative realistic conditions, the ICERs would probably be no higher and may well be lower than the base case.
The present study has several strengths. First, it is based on a clinical trial from the National Drug Abuse Treatment CTN that used a large sample size, relied on objective indicators of patient outcomes, and included eight community-based treatment programs with a diverse set of usual care practices and clienteles (Petry et al., 2005a). Thus, the cost-effectiveness results of the present study are likely to be widely generalizable to stimulant-abusing populations. Second, the present study conducted sensitivity analyses to examine how the estimated ICERs would likely change if MIEDAR had been implemented under different realistic scenarios. Third, in the absence of consensus threshold values against which to compare the estimated ICER for the primary patient outcome, LDA, we present an acceptability curve to aid decision makers in determining whether prize-based CM is cost-effective. Finally, the ICERs estimated in the present study can be used as thresholds for future studies that evaluate the cost-effectiveness of interventions designed to improve the LDA, number of stimulant-negative urine samples, and length of stay.
There are several limitations as well. First, inasmuch as MIEDAR targeted stimulant (i.e., cocaine, amphetamine, methamphetamine) and alcohol abusers, the ICERs may not generalize to populations using other types of drugs. Second, MIEDAR’s twice-weekly testing protocol was more frequent than typically occurred for usual care in most of the clinics in the study, and may have reduced differences between groups in both testing costs and patient outcomes. However, sensitivity analyses in the present study suggest that testing the usual care group less frequently during MIEDAR would not have had a serious adverse affect on the ICERs, and possibly would have improved them if patient outcomes in the usual care group declined as well. Third, given that 18.7% of participants assigned to prize-based CM were abstinent from the primary target drugs for the duration of the study (12 weeks), compared to 4.9% of participants assigned to usual care alone, it is possible that the incremental LDA would have been larger had twice-weekly drug testing extended beyond the 12 weeks of the study; if so, this would lower the ICERs for the LDA outcome.
This study adds to the growing literature on cost-effectiveness in substance abuse treatment by providing one of the first cost-effectiveness analyses of prize-based CM. Cost-effectiveness analysis of CM interventions is critical because CM adds clear and certain costs to usual care. We are also among the first in SAT research to use an acceptability curve to provide decision makers with information on when prize-based CM would be considered cost-effective. Further research is necessary to determine consensus threshold values against which to compare ICERs for various patient outcomes in SAT, and to determine which treatments, including prize-based CM interventions with alternative incentive parameters (e.g., higher/lower-valued prizes, more/less frequent drawing sessions), provide the greatest value.
Acknowledgements
The authors gratefully acknowledge research support from the National Institute on Drug Abuse (Grant Nos. RO1-DA14471, U10DA13034, R01-DA13444, RO1-DA016855, RO1-DA14618, R01-DA018883, P50-DA09241, and P50-AA03510). We thank Maxine Stitzer, PhD, for use of the MIEDAR clinical trial data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barnett PG, Zaric GS, Brandeau ML. The cost-effectiveness of buprenorphine maintenance therapy for opiate addiction in the United States. Addiction. 2001;96:1267–1278. doi: 10.1046/j.1360-0443.2001.96912676.x. [DOI] [PubMed] [Google Scholar]
- Briggs A. Handling uncertainty in economic evaluation and presenting the results. In: Drummond M, McGuire A, editors. Economic Evaluation in Health Care: Merging Theory with Practice. Oxford University Press; Oxford, UK: 2001. pp. 172–214. [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Cartwright WS. Cost benefit and cost-effectiveness analysis of drug abuse treatment services. Eval Rev. 1998;22:609–636. doi: 10.1177/0193841X9802200503. [DOI] [PubMed] [Google Scholar]
- Cartwright WS. Cost-benefit analysis of drug treatment services: Review of the literature. J Ment Health Policy Econ. 2000;3:11–26. doi: 10.1002/1099-176x(200003)3:1<11::aid-mhp66>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programs. 2nd ed. Oxford University Press; Oxford, UK.: 1997. [Google Scholar]
- Fenwick E, Claxton K, Schulpher M. Representing uncertainty: The role of cost-effectiveness acceptability curves. Health Econ. 2001;10:779–787. doi: 10.1002/hec.635. [DOI] [PubMed] [Google Scholar]
- French MT, Mausopf JA, Teague JL, Roland J. Estimating the dollar value of health outcomes from drug abuse interventions. Med Care. 1996;34:890–910. doi: 10.1097/00005650-199609000-00003. [DOI] [PubMed] [Google Scholar]
- French MT, McCollister KE, Reznik D. The cost of crime to society: new crime-specific estimates for policy and program evaluation. Journal of Research on Crime and Delinquency. doi: 10.1016/j.drugalcdep.2009.12.002. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford University Press; Oxford, UK.: 1996. [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer-term cocaine abstinence. Exp Clin Psychopharmacol. 2000;8(b):377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg F, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, Dantona RL, Anthony S. Community reinforcement therapy for cocaine-dependent outpatients. Arch Gen Psychiatry. 2003;60:1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden D, Dantona RI. Contingent reinforcement increases cocaine abstinence during outpatient treatment and one year of follow-up. J Consult Clin Psychol. 2000;68(a):64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Jofre-Bonet M, Sindelar JL. Drug treatment as a crime fighting tool. J Ment Health Policy Econ. 2001;4:175–188. [PubMed] [Google Scholar]
- Jofre-Bonet M, Sindelar JL. Creating an aggregate outcome index: cost-effectiveness analysis of substance abuse treatment. J Behav Health Serv Res. 2004;31:229–241. doi: 10.1007/BF02287287. [DOI] [PubMed] [Google Scholar]
- Lothgren M, Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Econ. 2000;9:623–630. doi: 10.1002/1099-1050(200010)9:7<623::aid-hec539>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Marx J, Austin M, Tardif M. Vouchers versus prizes: Contingency management treatment of substance abusers in community settings. J Consult Clin Psychol. 2005;73:1005–1014. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Lower-cost contingency management for treating cocaine-abusing methadone patients. J Consult Clin Psychol. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes and they will come: Contingency management for the treatment of alcohol dependence. J Consult Clin Psychol. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Simcic F. Prize reinforcement contingency management for cocaine dependence: Integration with group therapy in a methadone clinic. J Consult Clin Psychol. 2005;73(b):354–359. doi: 10.1037/0022-006X.73.2.354. [DOI] [PubMed] [Google Scholar]
- Petry NM, Pierce J, Stitzer ML, Blaine J, Roll JM, Cohen A, Obert J, Killeen T, Saladin M, Cowell M, Kirby K, Sterling R, Royer-Malvestuto C, Hamilton J, Booth R, Macdonald M, Liebert M, Rader L, Burns R, DiMaria J, Copersino M, Stabile PQ, Kolodner K, Li R. Prize-based incentives improve outcomes of stimulant abusers in outpatient psychosocial treatment programs: A national drug abuse treatment clinical trials network study. Arch Gen Psychiatry. 2005;62(a):1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll K, Rounsaville B. Prize reinforcement contingency management for treating cocaine user: how low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Higgins ST, Brooner RK, Montoya I, Schuster CR, Cone EJ. Cocaine use early in treatment predicts outcome in a behavioral treatment program. J Consult Clin Psychol. 1998;66:691–696. doi: 10.1037//0022-006x.66.4.691. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Signorini DF, Leung O, Simes RJ, Beler E, Gebski VJ, Callaghan T. Dynamic balanced randomization for clinical trials. Stat Med. 1993;30:2343–2350. doi: 10.1002/sim.4780122410. [DOI] [PubMed] [Google Scholar]
- Silverman K, Higgins ST, Brooner RK, Montoya ID. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry. 1996;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Sindelar JL, Elbel B, Petry NM. Do we get what we pay for? Cost-effectiveness of adding contingency management. Addiction. doi: 10.1111/j.1360-0443.2006.01689.x. Under review. [DOI] [PubMed] [Google Scholar]
- Sindelar JL, Jofre-Bonet M, French MT, McLellan TA. Cost-effectiveness analysis of treatments for illicit drug dependence: Paradoxes with multivariate outcomes. Drug Alcohol Depend. 2004;73:41–50. doi: 10.1016/j.drugalcdep.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Sindelar JL, Olmstead TA. Illicit drug use and drug-related crime. In: Jones A, editor. Elgar Companion to Health Economics. Edward Elgar Publishing; Brookfield, US: 2006. pp. 83–94. [Google Scholar]


