Abstract
The function of the S phase kinase cyclin A/Cdk2 in maintaining and regulating cell cycle kinetics is well established. However an alternative role in the regulation of progesterone receptor (PR) signaling is emerging. PR and its coactivators are phosphoproteins. Cyclin A/Cdk2 phosphorylates several of the PR phosphorylation sites in vitro and there is evidence that it participates in PR phosphorylation in vivo. Cyclin A/Cdk2 also functions as a PR coactivator. Overexpression increases PR transcriptional activity independent of PR phosphorylation. In the presence of hormone, cyclin A/Cdk2 is recruited to PR bound to DNA of target genes. Inhibition of Cdk activity prevents recruitment of the p160 coactivator SRC-1 (steroid receptor coactivator-1), suggesting that Cdk2 phosphorylates SRC-1. Consistent with this finding, phosphatase treatment of SRC-1 reduces its ability to interact with PR in vitro. Moreover, PR transcriptional activity is highest in S phase where cyclin A is expressed. In G1, PR activity is reduced and the capacity to recruit SRC-1 to a progestin responsive promoter is diminished. Future studies will focus on the importance of cyclin A/Cdk2 phosphorylation of other components of the PR transcription complex, such as the p160 coactivator SRC-1, and the specific role of Cdk2 target sites in the regulation of PR activity.
Keywords: Progesterone receptor, Cyclin A, Cdk2, Kinase, Phosphorylation
1. Introduction
The progesterone receptor (PR), a member of the steroid hormone receptor superfamily, mediates the action of progesterone, which has critical roles in the regulation of female reproductive function (reviewed in [1,2]). Human PR is expressed as two isoforms with PRA lacking the first 164 amino acids of PRB [3,4]. They are expressed from unique mRNAs transcribed from alternate estrogen inducible promoters within a single gene [4]. Although structurally similar, the A and B isoforms have distinctly different activities in vitro and in vivo. The B isoform is generally a stronger activator of transcription while the A isoform has been shown to repress transcription of PRB as well as the estrogen, androgen, glucocorticoid and mineralocorticoid receptors [5–7]. Additionally, the B and A isoforms regulate expression of different subsets of target genes [8]. Analysis of isoform specific receptor knockout mice demonstrated that loss of PRA causes abnormal uterine and ovarian function and loss of PRB leads to defective mammary gland development during pregnancy [9,10]. Both A and B forms of the PR have three major functional domains (Fig. 1) – a poorly conserved amino terminal transactivation domain (NTD) containing transcriptional activation function-1 (AF-1), a zinc finger containing DNA binding domain (DBD) which also mediates receptor dimerization and a C-terminal ligand binding domain (LBD) containing a second, ligand dependent, activation function, AF-2, as well as a strong dimerization interface (reviewed in [2]). A flexible hinge region (H) separates the DBD and LBD and also contains the nuclear localization signal. The B isoform contains an additional activation function (AF-3) [11].
Fig. 1.
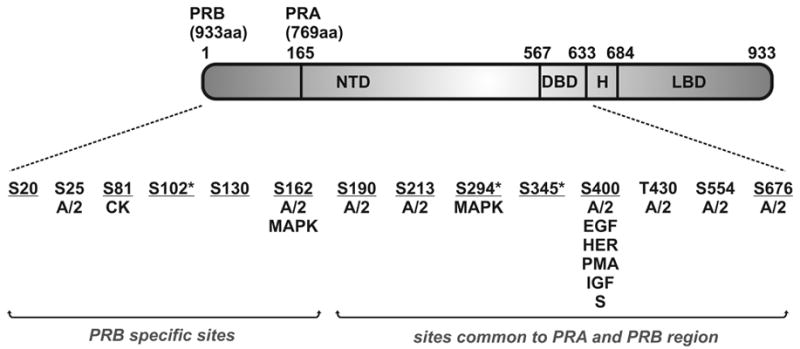
Schematic showing major functional domains of the human PR with phosphorylation sites identified. Kinases or activators of kinases that have been reported to cause phosphorylation in vitro or in vivo are indicated (A/2 = cyclin A/Cdk2, CK = casein kinase II, MAPK = mitogen activated protein kinase, EGF = epidermal growth factor, HER = heregulin, PMA = phorbol myristate acetate, IGF = insulin like growth factor, S = fetal bovine serum). Sites confirmed as authentic in vivo targets are underlined. Asterisks denote sites identified as strongly hormone dependent. The remaining sites are basally phosphorylated at least to some extent in the absence of hormone, or their hormone dependence has not yet been determined.
In the classical model of PR action [12–14] (Fig. 2), progestins diffuse across the cell membrane and bind to PR located within the cytoplasm, where they induce a conformational change in the receptor that leads to release of associated chaperone proteins (including heat shock proteins such as HSP70 and HSP90 as well as p23 and immunophilins such as FKBP51 and FKBP52), dimerization and nuclear translocation. Inside the nucleus, PR dimers bind to progesterone response elements situated in the regulatory regions of target genes where they recruit coactivator molecules such as those of the p160 family and other components of the transcriptional machinery, leading to reorganization of chromatin structure and the initiation of transcription. Although a consensus palindromic DNA binding site has been identified, many natural binding sites contain half sites and likely require additional protein/protein interactions for strong PR binding. More recently, alternate mechanisms of action have been described where PRs can interact with and modulate other transcription factors, such as Sp1 and AP-1, influencing gene transcription without binding directly to DNA [15–19] (Fig. 2). While this mode of action of PR is also likely to involve coactivators, and the transcriptional regulating protein of 132 kDa (TReP-132) has recently been identified as a coactivator of PR tethered to Sp1 sites in the p21 and p27 genes [20], the precise role and contribution of other specific coactivators has not yet been characterized.
Fig. 2.
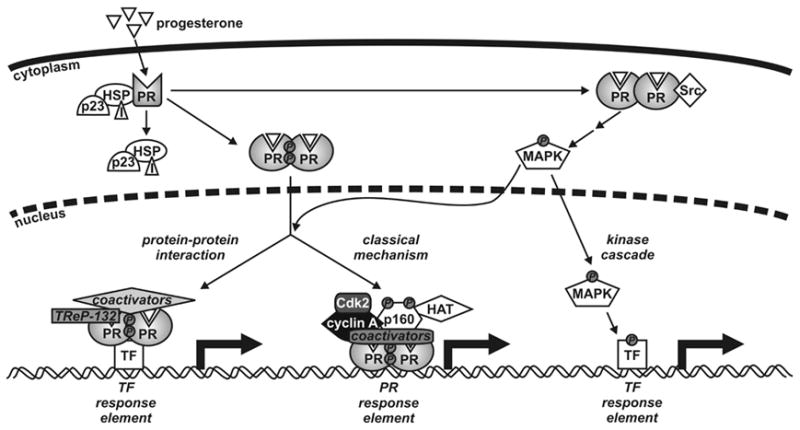
Model for progesterone receptor (PR) genomic and non-genomic actions. Progesterone diffuses across the cell membrane and binds to the PR in the cytoplasm of target cells, inducing conformational changes in the receptor, dissociation of molecular chaperones (including heat shock proteins (HSP), p23 and immunophilins (I)), dimerization and nuclear translocation. In the classical mechanism of action, the PR dimer binds to specific DNA response elements situated in the regulatory regions of target genes and recruits coactivators, such as p160s, histone acetyltransferases and the cyclin A/Cdk2 complex, and other components of the general transcription machinery enabling RNA synthesis. PRs also regulate transcriptional activity of other transcription factors (TFs) through protein-protein interactions and coactivator recruitment rather than direct DNA binding. Moreover, progestins activate kinase cascades such as Src and MAPK, which leads to phosphorylation of a variety of transcription factors.
In addition to steroid hormone ligands, another important mechanism for regulating PR activity is through cell signaling pathways (Fig. 2). PR and its coactivators are extensively phosphorylated [21–26]. Kinases such as cyclin dependent kinase 2 (Cdk2), mitogen activated protein kinase (MAPK), cyclic AMP dependent protein kinase, okadaic acid (an inhibitor of phosphatases 1 and 2a) and tetradecanoyl 12-phorbol 12-acetate (a protein kinase C activator) can enhance PR activity in the presence of ligand [27–32]. However, in contrast to other steroid receptors, ligand independent activation of human PR by kinase cascades is not consistently observed, although it has been described in some reports [29–31,33–35]. In turn, progestins and PR localized in the cytoplasm or the cell membrane can rapidly induce cell signaling cascades, such as the Src/MAPK pathway, that lead to phosphorylation of a variety of proteins, which may or may not be involved in transcription [36,37].
2. Phosphorylation sites identified in PR
The key role of kinases in the regulation of genomic and non-genomic actions of PR has prompted numerous biochemical and functional studies on the phosphorylation of PR. Like other steroid receptors, PR is a phosphoprotein (reviewed in [21,38,39]), with phosphorylation observed in response to hormone and activation of various kinases. Evidence to date suggests that individual phosphorylation sites are likely to be candidates for multiple kinases in vivo permitting integration of signals from multiple signaling pathways. Fourteen candidate phosphorylation sites, summarized in Fig. 1, have been identified to date in the human PR, the majority of which lie in the NTD suggesting that they play roles in protein-protein interactions. Most of these are found in Ser/Thr-Pro motifs which suggests that proline-directed kinases such as the Cdks, glycogen synthase kinase 3 or MAPKs are responsible for PR phosphorylation [40]. There are 15 Ser/Thr-Pro motifs in PRB and 10 in PRA and 13 of these have been confirmed as candidate Cdk2 or MAPK targets in vitro or in vivo. However not all Ser/Thr-Pro motifs in PR are phosphorylated by Cdks and MAPKs (Ser202 and Thr351 have not been identified as targets to date), suggesting that kinases target PR in a context-specific manner, allowing highly specific regulation by different kinase signaling pathways.
PR phosphorylation appears to occur in three phases – basal phosphorylation in the absence of hormone, rapid (within a few minutes) hormone dependent phosphorylation and delayed hormone dependent phosphorylation (up to two hours) [32,41]. Some, if not all, of the delayed hormone dependent phosphorylations are also dependent on PR binding to DNA [32,42].
Basal or hormone induced phosphorylation sites were originally identified by in vivo [32P] incorporation into endogenous PR in T47D human breast cancer cells. The first sites to be confirmed in this manner were Ser81 and Ser162 [43]. These exhibit basal phosphorylation in the absence of hormone, with further increases in response to hormone [41]. Sites targeted by specific kinases have been identified via in vitro phosphorylation of a baculovirus expressed hPR and in this manner Ser81 was found to be a target for casein kinase II [43]. Ser81 is the only site reported to date which does not lie in a Ser/Thr-Pro motif. Ser102, Ser294 and Ser345 are optimally phosphorylated in vivo in response to longer-term (2 hours) treatment with hormone [41]. Hormone dependent phosphorylation of PR is associated with a decrease in mobility on SDS-PAGE gels, and this characteristic has been attributed at least in part to phosphorylation at Ser345 [41]. Cyclin A/Cdk2, discussed in further detail below, phosphorylates PR in vitro at Ser25, Ser162, Ser190, Ser213, Ser400, Thr 430, Ser554 and Ser676 [22,44]. The use of baculovirus expressed hPR in Sf9 insect cells enabled additional sites to be identified due to the higher amounts obtainable compared to native PR from T47D cells. Phosphorylation of hPR isolated from Sf9 insect cells was found to be qualitatively similar to that of endogenous PR in T47D cells [45]. Enhanced sensitivity was also achieved using modified trypsin, which digests with increased efficiency, as well as mass spectrometry, and Ser20, Ser676 and a peptide containing the Ser-Pro motif at Ser130 were identified as in vivo sites using these techniques [22]. Ser294 was verified as a target of the MAPK pathway firstly by mutation of this site to an unphosphorylatable alanine [46] and then by the use of an antibody that exclusively recognizes PR phosphorylated at Ser294 [29,47]. However, phosphorylation of Ser294 induced by R5020 is not inhibited by the MEK inhibitor U0126, implicating an additional kinase in hormone dependent phosphorylation [47]. Phosphorylation of Ser400 is also increased in vivo by treatment with epidermal growth factor, heregulin, phorbol myristate acetate, insulin-like growth factor and fetal bovine serum in T47D cells and is rapidly (within 15 minutes) phosphorylated in response to hormone [33].
3. Cyclin A/Cdk2 and PR
Of the kinases tested in vitro, Cyclin A/Cdk2 phosphorylates the largest number of candidate sites (8 of the 14 identified authentic and candidate phosphorylation sites) (Fig. 1). As discussed below, PR signaling and Cdk2 activity are intricately connected with multiple mechanisms of cell cycle-associated feedback regulation.
3.1. Cyclin A/Cdk2 and the cell cycle
Cyclin A/Cdk2 is an S phase kinase composed of the regulatory cyclin A subunit and the catalytic kinase subunit, Cdk2, and its role in cell cycle control is well accepted. There are two forms of cyclin A. Cyclin A1 is expressed during meiosis and embryonic development as well as in some cancers while cyclin A2 is expressed in all proliferating cells in adult tissues [48]. This review focuses exclusively on the action of the cyclin A2/Cdk2 complex as the role of cyclin A1 in steroid receptor function has received little attention. Cyclin A2 appears essential as knockout of this gene results in embryonic lethality [49]. However, at least from a developmental perspective, Cdk2 seems redundant as Cdk2−/− mice, although sterile, develop normally and with only a minor delay in cell cycle kinetics observed [50,51]. Cyclin A levels increase during late G1 phase and remain high throughout the S and G2 phases until degradation occurs during mitosis [52]. The level of Cdk2 remains relatively constant throughout the cell cycle but its activity is regulated by interaction with its cyclin A partner during S phase as well as by other regulatory proteins such as cyclin E (which peaks during G1), Cdk activating kinases, Cdk inhibiting kinases and Cdk inhibitors (for example p21 and p27) [53]. The active cyclin A/Cdk2 complex subsequently regulates its target proteins via phosphorylation principally during the S phase of the cell cycle.
3.2. Phosphorylation of PR by cyclin A/Cdk2 and the functional significance of target sites
Cyclin A/Cdk2 was initially found to phosphorylate PR at Ser162, Ser190 and Ser400 in vitro, sites which are also authentic in vivo targets (Fig. 1) [43,44]. In vitro phosphorylation of baculovirus PR with [32P]-ATP in combination with an alternative modified tryptic digest strategy and edman degradation identified additional cyclin A/Cdk2 sites, including Ser25, Ser213, Thr430, Ser554, and Ser676 [22]. Subsequently, mass spectrometry was used to show Ser20 and Ser676 as in vivo targets in baculovirus PR expressed in Sf9 cells [22]. The development of antibodies recognizing specific phosphorylations on PR has facilitated the task of determining which signaling pathways target PR. An antibody recognizing phospho-PR-Ser190 has previously been described [54]. As shown in Fig. 3, this residue is an in vivo Cdk2 site, although it is also a substrate for other kinases. In HeLa cells transiently expressing hPRB, basal phosphorylation of Ser190 was observed and this increased 5.1-fold in cells co-transfected with plasmids coding for cyclin A and Cdk2. In contrast, hormone dependent Ser190 phosphorylation was not further enhanced by cyclin A/Cdk2 and may even be reduced. Thus there are likely multiple kinases that phosphorylate this site. The lower level of hormone dependently phosphorylated PR observed in the presence of cyclin A/Cdk2 in this experiment might be due to increased ubiquitin-mediated receptor degradation in response to phosphorylation in the absence of proteasome inhibitors (see below).
Fig. 3.
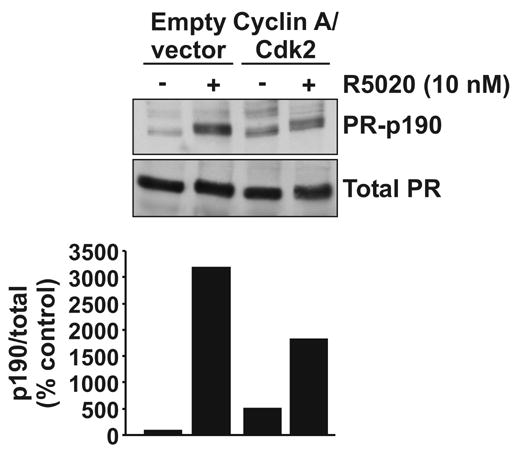
PR phosphorylation by cyclin A/Cdk2. HeLa cells were transiently transfected with an expression plasmid for PRB, cyclin A and Cdk2 as shown and 24 hours later cells were treated with R5020 (10 nM) or vehicle (0.1% ethanol). Cell lysates were harvested after a further 24 hours and 30 μg was electrophoresed on an SDS-PAGE gel. Proteins were transferred to nitrocellulose and blotted for phosphorylated PR-190 or total PR (upper panel). Band intensity was measured by densitometry, corrected for background and expressed graphically as p190/total PR signal relative to the empty vector vehicle treated control (lower panel). Materials and methods are as previously described [27,47].
Numerous studies have attempted to find a functional effect of these phosphorylations by site directed mutagenesis of Ser/Thr targets to alanine, which is unable to be phosphorylated. Mutation of Ser190, Ser676 as well as a cluster of serine residues just upstream of the DBD (including Ser549, Ser552, Ser554, Ser558, Ser561) have modest inhibitory effects on PRA and PRB transcriptional activity measured using transiently transfected receptor and reporter plasmids that contain one or more consensus progesterone response elements, although the effects are cell and promoter specific [55]. However mutation of other serines in the region common to both A and B forms of PR have no effect on ligand dependent transactivation or DNA binding and PRB mutants lacking phosphorylation sites in the N-terminal B-specific region have comparable transcriptional activity to the wild type PRB under these conditions [33,55]. This study included mutation of all known Cdk2 target sites with the exception of Ser213 and Thr430. Interestingly, an increase in ligand-independent PRB activity is observed in the presence of a dominant active Cdk2 (in cells expressing low levels of the Cdk2 inhibitor p27) which was abolished upon mutation of Ser400 to alanine [33,56], suggesting a role for this residue in ligand-independent PR activity. However in a different study, an increase in wild type PRA activity in the absence of ligand in cells overexpressing wild type Cdk2 was not observed [27], therefore the precise effects of Cdk2 on PR activity in the absence of ligand requires further clarification.
PRs undergo hormone induced degradation which is mediated by the 26S proteasome and this process is augmented by phosphorylation [29,33,46,57]. The Cdk2 site Ser400 lies within a consensus motif, known as the destruction box, also found in other proteins (including cyclin A) which are degraded by the ubiquitin-proteasome pathway [58,59]. In transiently transfected HeLa cells, ligand induced degradation of PRB is enhanced by overexpression of a dominant active Cdk2 [33]. PRB with an alanine substitution at position 400 also undergoes ligand induced degradation at a similar rate to wild type PRB however when a dominant active Cdk2 is overexpressed in this system, mutation at Ser400 abrogates degradation of ligand-bound PR and the receptor accumulates. This suggests an important role for Ser400 in regulating the hormone dependent degradation of PR in the presence of Cdk2 by the proteasome. A further role for Ser400 phosphorylation in regulating PR nuclear translocation is supported by studies showing that mutation of Ser400 to alanine results in delayed nuclear translocation following treatment with R5020 in transiently transfected HeLa cells [33]. Moreover, activated Cdk2 induces nuclear translocation of wild type PR, but not PR mutated at Ser400, suggesting that phosphorylation of this residue by Cdk2 mediates, at least in part, ligand-independent nuclear translocation of PR.
3.3. Cyclin A/Cdk2 regulation of PR activity
Studies in our laboratory and others have shown that Cdk2 stimulates agonist dependent PR activity [27,33,56]. As cyclin A/Cdk2 is an S phase kinase, this is consistent with data showing that PR is regulated in a cell cycle dependent manner, with PR transcriptional activity peaking in the S phase [47]. Interestingly, the ability of cyclin A/Cdk2 to increase PR activity is independent of its ability to phosphorylate PR as mutation of all the Ser/Thr-Pro motifs in PRA does not prevent coactivation. Instead, PR recruits cyclin A/Cdk2 via direct interaction to the transcription complex where it can phosphorylate other associated factors leading to increased transcription. Components of the PR transcription complex that have been shown to be targets of Cdk2 include SRC-1, histone H1 and CREB-binding protein (CBP). SRC-1 is phosphorylated in vitro by cyclin A/Cdk2 [27] while histone H1 is phosphorylated by Cdk2 in vitro and in vivo [60] and studies using the Cdk2 inhibitor p21 imply that CBP is also phosphorylated by Cdk2 [61]. These data suggest that, in addition to the direct effects cyclin A/Cdk2 has on PR, it also has indirect effects on other proteins which influence PR activity. This mechanism is in contrast to that by which cyclin A/Cdk2 increases estrogen receptor activity [62], which appears dependent on Cdk2 phosphorylation at Ser104 and Ser106, as mutation of these sites abrogates coactivation by cyclin A/Cdk2 [63]. The glucocorticoid receptor (GR) is also a substrate for cyclin A/Cdk2 [64]. Using yeast strains deficient in the mammalian homologs of cyclin A (Clb proteins) or Cdk (Cdc28), activity of exogenous rat GR was diminished compared to wild type strains [64], suggesting that GR activity is dependent on cyclin/Cdk complexes. Although the specific effect of Cdk2 phosphorylation sites on GR activity in the presence of cyclin A/Cdk2 has not been investigated, the importance of phosphorylation for GR function is unclear as mutation of reported sites in human or mouse GR has led to conflicting results for their effect on transactivation activity [65–67].
The role of Cdk2 in regulating PR phosphorylation and activity has been further investigated through the use of roscovitine, an inhibitor of Cdk2. Roscovitine is a purine analogue that binds to the ATP binding pocket of Cdk2 (as well as Cdk1 and Cdk5), preventing phosphate transfer from ATP to the protein substrate [68]. Roscovitine induces cell cycle arrest, apoptosis and differentiation and is currently undergoing clinical trials as a cancer therapeutic [69]. We have found in our laboratory that roscovitine completely abolishes ligand induced PR activity [27]. As roscovitine also inhibits Cdk1 and Cdk5, a siRNA approach was used to specifically knock down Cdk2, again resulting in a marked reduction in PR activity. These results strongly suggest that Cdk2 is fundamental to the regulation of PR action. The mechanism by which this occurs may be explained by the inhibition of SRC-1 recruitment and resulting histone H4 acetylation at the PR transcription complex in response to roscovitine [27]. An alternative Cdk2 inhibitor (Cdk2 inhibitor II) has been used to demonstrate that Cdk2 is a key kinase mediating hormone dependent phosphorylation of PR at Ser400, as the R5020 induced phosphorylation of this site was almost completely abolished by the inhibitor [33].
Interestingly, while the PR antagonist RU486 inhibits PR activity alone or in the presence of PR agonists, we have demonstrated that in the presence of cyclin A/Cdk2 the response of PR to RU486 is altered, causing it to act as an agonist (Wardell et al, manuscript in preparation). This change of RU486 to a PR agonist has also been observed in the presence of 8-bromo-cAMP [31,70–72]. Although the mechanisms for this agonist-antagonist switch are yet to be elucidated, cyclin A/Cdk2 may, through increased phosphorylation and recruitment of SRC-1 to the PR transcription complex, promote assembly of a coactivator complex rather than the inactive corepressor complex which is typically formed upon binding of antagonists to receptors. This observation provides further evidence that cyclin A/Cdk2 has profound effects on PR activity and may have clinical implications for patients receiving antiprogestin therapies.
3.4. Progestin regulation of cyclins and Cdks
While the above studies demonstrate potent regulation of PR signaling by cyclin A/Cdk2, there are several studies supporting potential feedback regulation of cyclin A and Cdk2 by PR. It has been reported that a single dose of natural or synthetic progestin stimulates one round of the cell cycle in T47D cells but that cells arrest in late G1 of the second cycle and this arrest is maintained following subsequent doses of progesterone [73,74]. This biphasic response to progesterone is accompanied by a rise and fall of cyclin A protein and Cdk2 activity as well as a sequential increase in p21 followed by p27 levels and their association with cyclin/Cdk complexes. An increase in Cdk2 protein levels in response to progestins is also observed [33] and experiments using the kinase inhibitor UO126 demonstrated that the Erk 1/2 MAPK pathway is required for this regulation [56]. Moreover, progesterone regulates transcription of the Cdk2 inhibitor p21 [16]. Progestin regulation of cell cycle molecules, particularly Cdk2 which enhances PR activity, may represent an added mechanism by which progestins maintain PR activation and further support a close functional association between PR and molecules involved in cell cycle control.
4. Concluding remarks
While recent studies have shown that Cdk2 is not essential in a developmental context, the studies presented here provide strong evidence for the importance of Cdk2 in the regulation of PR signaling pathways. Cdk2 coactivates PR via a mechanism that involves direct phosphorylation of a fundamental component of the PR transcription complex, the p160 coactivator SRC-1 [27]. Cdk2 also phosphorylates PR at eight Ser/Thr-Pro motifs (Fig 1), although this does not appear to influence PR activity in the presence of Cdk2. Despite the number of phosphorylation sites identified and intensive efforts to define their functional significance, little effect of these sites (perhaps with the exception of Ser400) on PR function in the presence or absence of Cdk2 has been observed. It has been proposed that the use of transient overexpression systems, which have been employed in many studies, may not be optimal for detecting functional changes upon mutation of phosphorylation targets, and that the use of stably expressed mutant PRs may be more informative. Using this approach, a role for the MAPK target Ser294 in ligand-induced ubiquitin mediated PR degradation [29,46] and ligand independent EGF induced nuclear translocation of PR [28,75] has been demonstrated using cells stably expressing a PR-S294A mutant. Thus the generation of cell lines stably expressing PRs with mutations in other Cdk2 sites will likely enable a more thorough analysis of the functional consequences of PR phosphorylation. Moreover, most functional studies to date have been restricted to systems using transiently transfected reporters with promoters that contain multiple progestin response elements. These promoters don’t require the extensive chromatin remodeling of endogenous targets nor do they require binding and interaction with other site specific transcription factors to induce transcription. Thus an analysis of endogenous gene expression likely will reveal additional functions for the phosphorylation sites.
Cdk2 partners two cyclins, cyclin A and cyclin E, and it is currently unknown which of these exerts a greater influence on PR signaling. Studies from the Weigel lab [27] have overexpressed Cdk2 in conjunction with cyclin A to show that this complex coactivates PR while the Lange lab [33] demonstrated increased PR activity in cells only overexpressing a dominant active Cdk2 mutant. Endogenous cyclin A and E partners in the cell lines used in these experiments can interact with Cdk2 and as such the possibility of both cyclins contributing to the effect of Cdk2 cannot be excluded. A mutant cyclin A that cannot bind to Cdk2 does not enhance PR activity [27], implying that the interaction between cyclin A and Cdk2 is essential for coactivation. However, cyclin E may potentially interact instead with Cdk2 and enable it to continue affecting PR in vivo. The observation that PR activity peaks during S phase, and not during G1 when cyclin E levels peak, may suggest that cyclin A is the predominant cyclin in regulating PR activity. Experiments using siRNA against cyclin A and cyclin E are required to confirm their individual effects. Furthermore, the abilities of cyclin A2 versus cyclin A1 to regulate PR activity are unknown. A role for cyclin A1 in regulating the stimulatory effect of Cdk2 on PR activity may be of significance in the subset of PR positive breast and ovarian cancers that overexpress cyclin A1.
The indirect effects of cyclin A/Cdk2 on PR function are not completely understood. In the classical model of PR action, PR binds to the regulatory regions of target genes and recruits a specific set of coactivator molecules which operate together to stimulate transcription. Cyclin A/Cdk2 may be recruited to the PR early in this process and direct further recruitment of other components of the transcription complex through its kinase activity. This is suggested firstly by studies where roscovitine inhibited recruitment of SRC-1 and histone acetylation but not PR or cyclin A recruitment and secondly by the increased interaction between PR and SRC-1 in the presence of active cyclin A/Cdk2 [27]. It was proposed that recruitment of cyclin A/Cdk2 increases kinase activity in the PR transcription complex and that this can influence the activity of other associated factors. Indeed, cyclin A/Cdk2 enhances the intrinsic activity of SRC-1 [27]. Presumably this occurs via direct phosphorylation of SRC-1 by cyclin A/Cdk2 and identification of the targeted sites in SRC-1 may reveal the mechanisms involved. Moreover, Cdk2 may also have the potential to phosphorylate other proteins in the PR transcription complex although alternative targets are yet to be confirmed. Taken together, the direct consequences of Cdk2 phosphorylation of PR and the indirect effects mediated by its phosphorylation of other associated factors imply a multifaceted role for Cdk2 in the regulation of PR transcriptional activity.
Acknowledgments
These studies were supported by NIH R01 CA57539.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18:502–19. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- 2.Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68:771–8. doi: 10.1016/s0039-128x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz KB, Alexander PS. In situ photolinked nuclear progesterone receptors of human breast cancer cells: subunit molecular weights after transformation and translocation. Endocrinology. 1983;113:2195–201. doi: 10.1210/endo-113-6-2195. [DOI] [PubMed] [Google Scholar]
- 4.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–14. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer ME, Quirin-Stricker C, Lerouge T, Bocquel MT, Gronemeyer H. A limiting factor mediates the differential activation of promoters by the human progesterone receptor isoforms. J Biol Chem. 1992;267:10882–7. [PubMed] [Google Scholar]
- 6.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–55. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 7.McDonnell DP, Shahbaz MM, Vegeto E, Goldman ME. The human progesterone receptor A-form functions as a transcriptional modulator of mineralocorticoid receptor transcriptional activity. J Steroid Biochem Mol Biol. 1994;48:425–32. doi: 10.1016/0960-0760(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 8.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–18. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 9.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–4. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 10.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sartorius CA, Melville MY, Hovland AR, Tung L, Takimoto GS, Horwitz KB. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol Endocrinol. 1994;8:1347–60. doi: 10.1210/mend.8.10.7854352. [DOI] [PubMed] [Google Scholar]
- 12.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–86. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 13.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld JM, Kao HY, Evans RM. The Nuclear Receptor Superfamily. In: Henderson BE, Ponder B, Ross RK, editors. Hormones, Genes and Cancer. New York: Oxford University Press; 2003. pp. 38–98. [Google Scholar]
- 15.Bamberger AM, Bamberger CM, Gellersen B, Schulte HM. Modulation of AP-1 activity by the human progesterone receptor in endometrial adenocarcinoma cells. Proc Natl Acad Sci U S A. 1996;93:6169–74. doi: 10.1073/pnas.93.12.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21(WAF1) cyclin-dependent kinase inhibitor gene through Sp1 and CBP/p300. J Biol Chem. 1998;273:10696–701. doi: 10.1074/jbc.273.17.10696. [DOI] [PubMed] [Google Scholar]
- 17.Gao J, Mazella J, Seppala M, Tseng L. Ligand activated hPR modulates the glycodelin promoter activity through the Sp1 sites in human endometrial adenocarcinoma cells. Mol Cell Endocrinol. 2001;176:97–102. doi: 10.1016/s0303-7207(01)00450-6. [DOI] [PubMed] [Google Scholar]
- 18.Tseng L, Tang M, Wang Z, Mazella J. Progesterone receptor (hPR) upregulates the fibronectin promoter activity in human decidual fibroblasts. DNA Cell Biol. 2003;22:633–40. doi: 10.1089/104454903770238102. [DOI] [PubMed] [Google Scholar]
- 19.Tang M, Mazella J, Gao J, Tseng L. Progesterone receptor activates its promoter activity in human endometrial stromal cells. Mol Cell Endocrinol. 2002;192:45–53. doi: 10.1016/s0303-7207(02)00111-9. [DOI] [PubMed] [Google Scholar]
- 20.Gizard F, Robillard R, Gross B, Barbier O, Revillion F, Peyrat JP, Torpier G, Hum DW, Staels B. TReP-132 is a novel progesterone receptor coactivator required for the inhibition of breast cancer cell growth and enhancement of differentiation by progesterone. Mol Cell Biol. 2006;26:7632–44. doi: 10.1128/MCB.00326-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weigel NL. Steroid hormone receptors and their regulation by phosphorylation. Biochem J. 1996;319:657–67. doi: 10.1042/bj3190657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knotts TA, Orkiszewski RS, Cook RG, Edwards DP, Weigel NL. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J Biol Chem. 2001;276:8475–83. doi: 10.1074/jbc.M009805200. [DOI] [PubMed] [Google Scholar]
- 23.Rowan BG, Garrison N, Weigel NL, O’Malley BW. 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol Cell Biol. 2000;20:8720–30. doi: 10.1128/mcb.20.23.8720-8730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowan BG, Weigel NL, O’Malley BW. Phosphorylation of steroid receptor coactivator-1. Identification of the phosphorylation sites and phosphorylation through the mitogen-activated protein kinase pathway. J Biol Chem. 2000;275:4475–83. doi: 10.1074/jbc.275.6.4475. [DOI] [PubMed] [Google Scholar]
- 25.Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, O’Malley BW. Selective Phosphorylations of the SRC-3/AIB1 Coactivator Integrate Genomic Reponses to Multiple Cellular Signaling Pathways. Mol Cell. 2004;15:937–49. doi: 10.1016/j.molcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Wu RC, Smith CL, O’Malley BW. Transcriptional regulation by steroid receptor coactivator phosphorylation. Endocr Rev. 2005;26:393–9. doi: 10.1210/er.2004-0018. [DOI] [PubMed] [Google Scholar]
- 27.Narayanan R, Adigun AA, Edwards DP, Weigel NL. Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Mol Cell Biol. 2005;25:264–77. doi: 10.1128/MCB.25.1.264-277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu M, Lange CA. MAP kinases couple multiple functions of human progesterone receptors: degradation, transcriptional synergy, and nuclear association. J Steroid Biochem Mol Biol. 2003;85:147–57. doi: 10.1016/s0960-0760(03)00221-8. [DOI] [PubMed] [Google Scholar]
- 29.Shen T, Horwitz KB, Lange CA. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol Cell Biol. 2001;21:6122–31. doi: 10.1128/MCB.21.18.6122-6131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weigel NL, Zhang Y. Ligand-independent activation of steroid hormone receptors. J Mol Med. 1998;76:469–79. doi: 10.1007/s001090050241. [DOI] [PubMed] [Google Scholar]
- 31.Edwards DP, Weigel NL, Nordeen SK, Beck CA. Modulators of cellular protein phosphorylation alter the trans-activation function of human progesterone receptor and the biological activity of progesterone antagonists. Breast Cancer Res Treat. 1993;27:41–56. doi: 10.1007/BF00683192. [DOI] [PubMed] [Google Scholar]
- 32.Beck CA, Weigel NL, Edwards DP. Effects of hormone and cellular modulators of protein phosphorylation on transcriptional activity, DNA binding, and phosphorylation of human progesterone receptors. Mol Endocrinol. 1992;6:607–20. doi: 10.1210/mend.6.4.1316549. [DOI] [PubMed] [Google Scholar]
- 33.Pierson-Mullany LK, Lange CA. Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol Cell Biol. 2004;24:10542–57. doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labriola L, Salatino M, Proietti CJ, Pecci A, Coso OA, Kornblihtt AR, Charreau EH, Elizalde PV. Heregulin induces transcriptional activation of the progesterone receptor by a mechanism that requires functional ErbB-2 and mitogen-activated protein kinase activation in breast cancer cells. Mol Cell Biol. 2003;23:1095–111. doi: 10.1128/MCB.23.3.1095-1111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kazmi SM, Visconti V, Plante RK, Ishaque A, Lau C. Differential regulation of human progesterone receptor A and B form-mediated trans-activation by phosphorylation. Endocrinology. 1993;133:1230–8. doi: 10.1210/endo.133.3.8365365. [DOI] [PubMed] [Google Scholar]
- 36.Boonyaratanakornkit V, Edwards DP. Receptor mechanisms of rapid extranuclear signalling initiated by steroid hormones. Essays Biochem. 2004;40:105–20. doi: 10.1042/bse0400105. [DOI] [PubMed] [Google Scholar]
- 37.Watson CS, Lange CA. Steadying the boat: integrating mechanisms of membrane and nuclear-steroid-receptor signalling. EMBO Rep. 2005;6:116–9. doi: 10.1038/sj.embor.7400336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weigel NL, Bai W, Zhang Y, Beck CA, Edwards DP, Poletti A. Phosphorylation and progesterone receptor function. J Steroid Biochem Mol Biol. 1995;53:509–14. doi: 10.1016/0960-0760(95)00098-k. [DOI] [PubMed] [Google Scholar]
- 39.Lange CA. Making sense of cross-talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol Endocrinol. 2004;18:269–78. doi: 10.1210/me.2003-0331. [DOI] [PubMed] [Google Scholar]
- 40.Biondi RM, Nebreda AR. Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem J. 2003;372:1–13. doi: 10.1042/BJ20021641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Beck CA, Poletti A, Edwards DP, Weigel NL. Identification of a group of Ser-Pro motif hormone-inducible phosphorylation sites in the human progesterone receptor. Mol Endocrinol. 1995;9:1029–40. doi: 10.1210/mend.9.8.7476977. [DOI] [PubMed] [Google Scholar]
- 42.Takimoto GS, Tasset DM, Eppert AC, Horwitz KB. Hormone-induced progesterone receptor phosphorylation consists of sequential DNA-independent and DNA-dependent stages: analysis with zinc finger mutants and the progesterone antagonist ZK98299. Proc Natl Acad Sci U S A. 1992;89:3050–4. doi: 10.1073/pnas.89.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Beck CA, Poletti A, Edwards DP, Weigel NL. Identification of phosphorylation sites unique to the B form of human progesterone receptor. In vitro phosphorylation by casein kinase II. J Biol Chem. 1994;269:31034–40. [PubMed] [Google Scholar]
- 44.Zhang Y, Beck CA, Poletti A, Clement JP, Prendergast P, Yip TT, Hutchens TW, Edwards DP, Weigel NL. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol Endocrinol. 1997;11:823–32. doi: 10.1210/mend.11.6.0006. [DOI] [PubMed] [Google Scholar]
- 45.Beck CA, Zhang Y, Altmann M, Weigel NL, Edwards DP. Stoichiometry and site-specific phosphorylation of human progesterone receptor in native target cells and in the baculovirus expression system. J Biol Chem. 1996;271:19546–55. doi: 10.1074/jbc.271.32.19546. [DOI] [PubMed] [Google Scholar]
- 46.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97:1032–7. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narayanan R, Edwards DP, Weigel NL. Human progesterone receptor displays cell cycle-dependent changes in transcriptional activity. Mol Cell Biol. 2005;25:2885–98. doi: 10.1128/MCB.25.8.2885-2898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yam CH, Fung TK, Poon RY. Cyclin A in cell cycle control and cancer. Cell Mol Life Sci. 2002;59:1317–26. doi: 10.1007/s00018-002-8510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy M, Stinnakre MG, Senamaud-Beaufort C, Winston NJ, Sweeney C, Kubelka M, Carrington M, Brechot C, Sobczak-Thepot J. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat Genet. 1997;15:83–6. doi: 10.1038/ng0197-83. [DOI] [PubMed] [Google Scholar]
- 50.Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, Barbero JL, Malumbres M, Barbacid M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 51.Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol. 2003;13:1775–85. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 52.Fung TK, Poon RY. A roller coaster ride with the mitotic cyclins. Semin Cell Dev Biol. 2005;16:335–42. doi: 10.1016/j.semcdb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–91. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 54.Clemm DL, Sherman L, Boonyaratanakornkit V, Schrader WT, Weigel NL, Edwards DP. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol Endocrinol. 2000;14:52–65. doi: 10.1210/mend.14.1.0413. [DOI] [PubMed] [Google Scholar]
- 55.Takimoto GS, Hovland AR, Tasset DM, Melville MY, Tung L, Horwitz KB. Role of phosphorylation on DNA binding and transcriptional functions of human progesterone receptors. J Biol Chem. 1996;271:13308–16. doi: 10.1074/jbc.271.23.13308. [DOI] [PubMed] [Google Scholar]
- 56.Faivre E, Skildum A, Pierson-Mullany L, Lange CA. Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: role of mitogen-activated protein kinases and cell cycle regulators. Steroids. 2005;70:418–26. doi: 10.1016/j.steroids.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 57.Nardulli AM, Katzenellenbogen BS. Progesterone receptor regulation in T47D human breast cancer cells: analysis by density labeling of progesterone receptor synthesis and degradation and their modulation by progestin. Endocrinology. 1988;122:1532–40. doi: 10.1210/endo-122-4-1532. [DOI] [PubMed] [Google Scholar]
- 58.Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–8. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 59.King RW, Glotzer M, Kirschner MW. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol Biol Cell. 1996;7:1343–57. doi: 10.1091/mbc.7.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhattacharjee RN, Banks GC, Trotter KW, Lee HL, Archer TK. Histone H1 phosphorylation by Cdk2 selectively modulates mouse mammary tumor virus transcription through chromatin remodeling. Mol Cell Biol. 2001;21:5417–25. doi: 10.1128/MCB.21.16.5417-5425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Redeuilh G, Attia A, Mester J, Sabbah M. Transcriptional activation by the oestrogen receptor alpha is modulated through inhibition of cyclin-dependent kinases. Oncogene. 2002;21:5773–82. doi: 10.1038/sj.onc.1205753. [DOI] [PubMed] [Google Scholar]
- 62.Trowbridge JM, Rogatsky I, Garabedian MJ. Regulation of estrogen receptor transcriptional enhancement by the cyclin A/Cdk2 complex. Proc Natl Acad Sci U S A. 1997;94:10132–7. doi: 10.1073/pnas.94.19.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rogatsky I, Trowbridge JM, Garabedian MJ. Potentiation of human estrogen receptor alpha transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-CDK2 complex. J Biol Chem. 1999;274:22296–302. doi: 10.1074/jbc.274.32.22296. [DOI] [PubMed] [Google Scholar]
- 64.Krstic MD, Rogatsky I, Yamamoto KR, Garabedian MJ. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol. 1997;17:3947–54. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Almlof T, Wright AP, Gustafsson JA. Role of acidic and phosphorylated residues in gene activation by the glucocorticoid receptor. J Biol Chem. 1995;270:17535–40. doi: 10.1074/jbc.270.29.17535. [DOI] [PubMed] [Google Scholar]
- 66.Mason SA, Housley PR. Site-directed mutagenesis of the phosphorylation sites in the mouse glucocorticoid receptor. J Biol Chem. 1993;268:21501–4. [PubMed] [Google Scholar]
- 67.Webster JC, Jewell CM, Bodwell JE, Munck A, Sar M, Cidlowski JA. Mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein. J Biol Chem. 1997;272:9287–93. doi: 10.1074/jbc.272.14.9287. [DOI] [PubMed] [Google Scholar]
- 68.Senderowicz AM. Small-molecule cyclin-dependent kinase modulators. Oncogene. 2003;22:6609–20. doi: 10.1038/sj.onc.1206954. [DOI] [PubMed] [Google Scholar]
- 69.Meijer L, Raymond E. Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials. Acc Chem Res. 2003;36:417–25. doi: 10.1021/ar0201198. [DOI] [PubMed] [Google Scholar]
- 70.Beck CA, Weigel NL, Moyer ML, Nordeen SK, Edwards DP. The progesterone antagonist RU486 acquires agonist activity upon stimulation of cAMP signaling pathways. Proc Natl Acad Sci U S A. 1993;90:4441–5. doi: 10.1073/pnas.90.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sartorius CA, Tung L, Takimoto GS, Horwitz KB. Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonists by cAMP. J Biol Chem. 1993;268:9262–6. [PubMed] [Google Scholar]
- 72.Sartorius CA, Groshong SD, Miller LA, Powell RL, Tung L, Takimoto GS, Horwitz KB. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors: only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 1994;54:3868–77. [PubMed] [Google Scholar]
- 73.Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1) Mol Endocrinol. 1997;11:1593–607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- 74.Musgrove EA, Swarbrick A, Lee CS, Cornish AL, Sutherland RL. Mechanisms of cyclin-dependent kinase inactivation by progestins. Mol Cell Biol. 1998;18:1812–25. doi: 10.1128/mcb.18.4.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu M, Olsen A, Faivre E, Horwitz KB, Lange CA. Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol. 2003;17:628–42. doi: 10.1210/me.2002-0378. [DOI] [PubMed] [Google Scholar]


