Abstract
Mucin genes, both secreted (MUC2, MUC5AC, MUC5B, MUC7) and membrane associated (MUC1, MUC4, MUC16), have been reported to be expressed by ocular surface epithelia. The purpose of this study was to comprehensively assay the mucin content of human tear fluid using multiple antibodies for each mucin and to develop a sensitive, semi-quantitative method for the assay of mucins in tears. Tear washes were obtained by instillation of saline onto the ocular surface, followed by collection from the inferior fornix. Tear proteins were separated in 1% agarose gels, transferred to nitrocellulose membrane by vacuum blotting and probed with multiple antibodies recognizing MUC1, MUC2, MUC4, MUC5AC, MUC5B, MUC7 and MUC16. Binding was detected using chemiluminescence, and quantity was determined by densitometry. Serial dilutions of pooled tears from normal individuals were assayed to determine the linear range of detectability. MUC1, MUC4, MUC16, MUC5AC and low levels of MUC2 were consistently detected in human tear fluid, while MUC5B and MUC7 were not. Use of several antibodies recognizing different epitopes on the same mucin confirmed these findings. The antibodies to mucins bound to serial dilutions of tears in a linear fashion (r2 >0.9), indicating the feasibility of semi-quantitation. MUC5AC in tear fluid had an increased electrophoretic mobility compared to MUC5AC isolated from conjunctival tissue. This study provides clear evidence that the mucin component of tears is a mixture of secreted and shed membrane-associated mucins, and for the first time demonstrates MUC16 in tear fluid. Immunoblots of tears using agarose gel electrophoresis and chemiluminescence detection provide a semi-quantitative assay for mucin protein that will be useful for comparisons with tears from diseased eyes or after pharmacological intervention.
Keywords: Tears, ocular surface, mucins, membrane mucins, MUC16, MUC1, MUC5AC, MUC4
1. Introduction
Maintenance of the tear film on the ocular surface epithelia is facilitated by the presence of mucins secreted on its surface as well as by membrane-associated mucins in the apical cell glycocalyx. Mucins are defined as glycoproteins, hydrophilic in nature, that have at least 50–80% of their mass as carbohydrate, O-linked to serine and threonine residues present within tandem repeats of amino acids in their protein backbone (Gendler and Spicer, 1995; Moniaux et al., 2001; Hollingsworth and Swanson, 2004). To date, at least 20 distinct human mucin genes have been cloned (Gendler and Spicer, 1995; Lapensee et al., 1997; Williams et al., 1999; Williams et al., 2001; Yin and Lloyd, 2001; Gum et al., 2002; Pallesen et al., 2002; Chen et al., 2004; Higuchi et al., 2004; Hollingsworth and Swanson, 2004). Of these, MUCs 1, 3A, 3B, 4, 12, 13, 15, 16, 17 and 20 have been characterized as membrane associated. These mucins have a transmembrane domain, a short cytoplasmic tail, and an extended extracellular domain that forms the glycocalyx of epithelial cells (Gum, 1995). Many of the membrane-associated mucins are shed from the epithelial surface and are present as soluble forms in extracellular fluids (Moniaux et al., 2001). MUCs 2, 5AC, 5B, 6, 7 and 19 have been classified as secreted mucins. These mucins are secreted by goblet cells or other secretory cells and, with the exception of the monomeric MUC7, are gel-forming mucins, which form large oligomers through cysteine-cysteine interactions that contribute to the formation of a mucus gel (Hollingsworth and Swanson, 2004).
Mucins for which mRNA and proteins have been demonstrated in human ocular surface epithelia include the membrane-associated mucins MUC1, MUC4 and MUC16 in the stratified epithelial cells and the secreted, gel-forming mucin MUC5AC in the conjunctival goblet cells (Gipson, 2004). MUC2 mRNA has been detected at low levels (5,900-fold lower than MUC5AC) in human conjunctival tissue, and MUC2 protein was detected by immunoblot of conjunctival tissue (McKenzie et al., 2000). Lacrimal gland epithelia have been shown to produce the small soluble secreted mucin MUC7 (Jumblatt et al., 2003), as well as MUC1, 5AC, and 5B (Paulsen et al., 2004), but it is not clear whether MUC7 and 5B are present in the tear fluid. Previous studies have demonstrated the presence of MUC1, 2, 4, and 5AC protein in human preocular tear fluid (Ellingham et al., 1997; Garcher et al., 1998; Jumblatt et al., 1999; McKenzie et al., 2000; Pflugfelder et al., 2000; Zhao et al., 2001; Argueso et al., 2002; Jumblatt et al., 2002). Most of these studies examined tears for only one mucin; six used a single antibody for detection (Garcher et al., 1998; Jumblatt et al., 1999; Zhao et al., 2001; Argueso et al., 2002; Jumblatt et al., 2002) and one used two antibodies that recognize different epitopes on the same mucin (Pflugfelder et al., 2000). Two of the studies investigated more than one mucin (MUC1, MUC2, MUC5AC), using one antibody for each mucin examined (Ellingham et al., 1997; McKenzie et al., 2000). While these two studies concurred regarding the presence of MUC5AC, they obtained contradictory results regarding the presence of MUC2 in tears. There was also some disagreement among the published reports investigating the presence of MUC1 in tears; two identified MUC1 in tears (Garcher et al., 1998; Jumblatt et al., 2002) while the third did not (McKenzie et al., 2000). Furthermore, no quantitative assay has been developed for membrane-associated mucins in tear fluid.
Although one report has identified mRNAs for MUCs 13, 15 and 17 in conjunctival epithelial cells using reverse transcription-polymerase chain reaction (RT-PCR) (Corrales et al., 2003), no confirmatory data exists regarding the presence of these proteins in conjunctiva. We were only able to confirm the expression of MUC15 mRNA in conjunctiva (data not shown) and, since no antibodies specific for human MUC15 are currently available, we chose not to investigate the presence of these mucins in tears.
Since alterations in both membrane-associated and gel-forming mucins have been shown to occur in dry eye syndrome using impression cytology (Danjo et al., 1998; Argueso et al., 2002), it would be advantageous to develop sensitive, non-invasive, quantitative assays to measure tear fluid mucins. An ELISA assay has demonstrated decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren’s Syndrome (Argueso et al., 2002); although quantitative, the method cannot detect molecular characteristics (e.g., electrophoretic mobility or molecular weight) of MUC5AC. By immunolocalization there is an alteration in the distribution of a carbohydrate epitope on MUC16 designated H185 (Argueso et al., 2003a) along apical cells of conjunctiva of aqueous tear deficient, non-Sjögren’s dry eye syndrome patients (Danjo et al., 1998). It is not clear whether this alteration is due to decreased MUC16 expression, altered glycosylation or its shedding rate. More recently an immunohistochemical study revealed an alteration in the pattern of expression of the family of glycosyltransferases that act at the initial stage of mucin glycosylation in conjunctival biopsies of patients with ocular cicatricial pemphigoid as compared to normal subjects (Argueso et al., 2003b). Thus, the glycosylation of mucins may be altered by ocular surface diseases. The glycosylation status as well as proteolysis of mucins can affect their electrophoretic mobility within separating gels; such changes are not detectable with ELISA or immunohistochemistry.
The purpose was, therefore, to do a systematic study using multiple antibodies, each of which recognizes a different epitope on a mucin, to establish conclusively which mucins are present in tears. Once the mucin repertoire was identified, we sought to develop a single, sensitive chemiluminescence Western blot method that could be used on individual tear samples to quantitate the repertoire of ocular surface mucins secreted or shed into the tear fluid while allowing simultaneous detection of variation in molecular character that leads to changes in electrophoretic mobility. We anticipate that this method would be useful to detect mucin alterations in ocular surface disease, e.g., dry eye or after pharmacological intervention.
2. Materials and Methods
2.1. Subject selection
All samples used in his study were obtained in compliance with good clinical practice, institutional review board regulations, informed consent regulations, and the tenets of the Declaration of Helsinki. All prospective subjects completed an IRB approved questionnaire regarding history of ocular allergies, disease, surgery, or contact lens wear, current medications and the presence, type and frequency of symptoms of dry eye and dry mouth, and the use of dry eye therapy. Only normal subjects (defined as those with no allergies, eye diseases, surgery, contact lens wear, or dry eye symptoms) were recruited for this study. Human cervical mucus and saliva were available for use from a previous study (Gipson et al., 2001). Human colon tumor, fundus and gall bladder proteins were isolated with 2% SDS plus protease inhibitors from discarded tissues.
2.2. Tear collection
Tear washes were collected from 24 normal subjects as previously described (Argueso et al., 2002); 60 μl of sterile saline was instilled onto the unanesthetized ocular surface, and subjects were asked to move their eyes, without blinking, to mix the tear fluid content. Washes were then collected from the inferior fornix of each eye by micropipette. Cellular debris was removed by centrifugation at 14 000 rpm for 30 min at 4°C. Protein concentration was determined using Pierce Micro-BCA protein assay reagent kit (Rockford, IL). As previously reported, only minor amounts of protein (insufficient for analysis) were extractable from the pellets obtained after centrifugation (Argueso et al., 2002).
2.3. Agarose electrophoresis and immunoblot analysis of tear mucins
Due to their extremely large size and heavy glycosylation, mucin proteins barely enter traditional SDS-polyacrylamide electrophoresis gels (see Figure 10 in Gipson et al., 2003 (Gipson et al., 2003)). It has been shown that SDS-agarose gels are an excellent medium for separation and transfer of very large proteins (Preobrazhensky, 1993; Thornton et al., 1995). Using a modification of the protocol reported by Thornton et al. (Thornton et al., 2000), we have developed an SDS-agarose gel electrophoresis method for the separation of mucin proteins in tears.
Total protein from tears of single individuals or pooled from up to 21 individuals was denatured in reducing or non-reducing Laemmli sample buffer (Laemmli, 1970). When possible, non-reduced samples were examined in order to determine tear mucin content under more native conditions. However, the published conditions for the antibodies used for MUC2, 5B and 7 (see Table 1) required that these samples be run reduced. Proteins were separated at 4 °C for 2 h (50 volts constant current) under non-reducing conditions in a horizontal 1% (w/v) agarose gel (14.5 × 10.2 cm) cast and run in electrophoresis buffer (25 mM Tris, 192 mM glycine, pH 8.3, 0.1% SDS). Precision Plus Dual Color prestained molecular weight standards (Bio-Rad, Hercules, CA) were run in each gel. Alternatively, HiMark prestained high molecular weight standards (Invitrogen, Carlsbad, CA) can be used. For immunoblot detection of mucin protein, proteins were transferred to nitrocellulose membrane by vacuum blotting in transfer buffer (0.6M NaCl, 60 mM sodium citrate) using model 785 vacuum blotter with regulator (Bio-Rad) for 1 hour 30 minutes under a constant vacuum of 22 mm Hg. The efficiency of protein transfer was consistently confirmed by the absence of the prestained protein molecular weight standards in the agarose gels. Detection of mucins in tear fluid on blots was by well-characterized antibodies (2–3 per mucin) known to specifically recognize individual mucins. Multiple antibodies were used to detect each mucin in order to identify the mucins present in tears with a high level of confidence. The details of these primary antibodies, their epitopes, blocking solution and incubation conditions used are described in Table 1. Briefly, primary antibodies, diluted in the appropriate blocking solution (see Table 1), were incubated with the nitrocellulose membrane for 1.5 h, followed by 1 h in horseradish peroxidase-conjugated goat anti-mouse IgG (anti-chicken IgY for 791 and 799) secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) diluted in the same blocking solution used for the primary antibody. The optimal concentration for each antibody was empirically determined for each lot of antibody obtained. Extensive washes were performed after each antibody incubation with Tris-buffered saline (TBS) containing 0.1% Tween 20 (TTBS). Exceptions to this procedure were that Tween 20 was deleted from all steps with antibody H185, antibody 1G8 was washed with TBS containing 0.5% Tween 20, and antibodies PH1417 and PH1491 were washed with PBS plus 0.1% Tween 20 (TPBS). Antibody binding was then detected using Pierce SuperSignal West Pico Chemiluminescent Substrate, followed by exposure to Hyperfilm ECL (Amersham, Buckinghamshire, UK). Films were photographed and densitometric analyses performed using a Kodak Digital Science camera and 1D Image Analysis Software, Version 2.0.2 (Eastman Kodak Co., Rochester, NY). Band density was defined as the sum of the background subtracted pixels within a rectangle encompassing the entire band. If multiple bands were present with increased protein loading, densitometry was performed on the band present over the broadest range of protein concentrations. To determine the relative amount of individual mucins, densitometry of each mucin band was plotted against total protein concentration values and regression analyses were performed to determine linearity of assay.
Table 1.
Primary antibodies used for immunoblot analysis.
| MUCIN | ANTIBODY | BLOCKING SOLUTION | EPITOPE | SOURCE/REFERENCE |
|---|---|---|---|---|
| MUC1 | HMFG-1 | 5% BLOTTO* | TR† (APDTR), glycosylation sensitive | Biodesign, Saco ME (Taylor-Papadimitriou et al., 1981; Burchell J and Taylor-Papadimitriou, 1993; Price et al., 1998) |
| MUC1 | HMFG-2 | 5% BLOTTO | TR (DTR), glycosylation sensitive | Biodesign, Saco ME (Taylor-Papadimitriou et al., 1981; Burchell J and Taylor-Papadimitriou, 1993) |
| MUC1 | 214D4 | 5% BLOTTO in PBS | TR (PDTR), glycosylation independent | J. Hilkens, Netherlands Cancer Institute, Amsterdam, The Netherlands (Wesseling et al., 1995; Price et al., 1998) |
|
| ||||
| MUC2 | PMH1 | 5% BLOTTO in TBS | GalNAC-TR | U. Mandel, University of Copenhagen, Denmark (Reis et al., 1998) |
| MUC2 | PH1417 | 10% BLOTTO in TPBS | C-terminus(aa 4995–5013) | G. Hansson, Göteburg University, Sweden (Axelsson et al., 1998) |
| MUC2 | PH1491 | 10% BLOTTO in TPBS | N-terminus(aa 1167–1180) | G. Hansson, Göteburg University, Sweden (Asker et al., 1998) |
|
| ||||
| MUC4 | 1G8 | 3% BSA/0.5% TTBS | Ectodomain of ASGP-2 | Zymed, San Francisco, CA (Zhang et al., 2005) |
| MUC4 | 8G7 | 5% BLOTTO in PBS | TR | S.K. Batra, University of Nebraska Medical Center, Omaha, Nebraska(Moniaux et al., 2004) |
|
| ||||
| MUC5AC | 791 | 3% Fish Gel | D3 domain | I.K. Gipson (Argueso et al., 2002) |
| MUC5AC | CLH2 | 5% BLOTTO | TR | U. Mandel, University of Copenhagen, Denmark (Reis et al., 1997); Vector Labs, Burlingame, CA |
| MUC5AC | 1-13M1 | 5% BLOTTO | N-terminus: cys-2, cys-4 domains | NeoMarkers, Fremont, CA (Nollet et al., 2004b; Nollet et al., 2004a) |
|
| ||||
| MUC5B | 799 | 3% Fish Gel | D4 domain | I.K. Gipson (Gipson et al., 2001) |
| MUC5B | Eu-MUC5Ba | 5% BLOTTO in PBS | Cysteine-rich domain | D.M. Swallow, University College, London, UK (Rousseau et al., 2003) |
|
| ||||
| MUC7 | PANH3 | 5% BLOTTO | N-terminus(aa 72–92) | U. Mandel, University of Copenhagen, Denmark (Nielsen et al., 1996) |
| MUC7 | Eu-MUC7a | 5% BLOTTO in PBS | Histatin-like domain | D.M. Swallow, University College, London, UK (Rousseau et al., 2003) |
|
| ||||
| MUC16 | OC125 | 5% BLOTTO | TR | Dako Corporation, Carpenteria, CA (Bast et al., 1981) |
| MUC16 | M11 | 5% BLOTTO | TR (Different from OC125) | NeoMarkers, Fremont, CA (Nustad et al., 1996) |
| MUC16 | H185 | 5% BLOTTO in TBS | Carbohydrate epitope | I.K. Gipson (Watanabe et al., 1995; Argueso et al., 2003a) |
BLOTTO = Non-fat Dry Milk diluted (w/v) in TTBS, except as noted above.
TR = Tandem repeat
2.4. Investigation of differences in MUC5AC mobility
In preliminary experiments, we found a difference in electrophoretic mobility between MUC5AC isolated from conjunctival epithelial cells and that in tears. Thus, we sought to determine if proteases in tears were responsible. Conjunctival epithelial cells were collected by impression cytology as previously described (Argueso et al., 2002). Protein was extracted from the impression cytology filters by homogenization in 2% SDS or RIPA buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS) without protease inhibitors. To determine if the observed difference in mobility of MUC5AC in tears was due to the proteolytic action of a component present in human tears, complete protein inhibitor cocktail (Roche Biochemical, Indianapolis, IN) was immediately added to the tear washes collected from one eye only of two individuals, while the tear washes from the contralateral eyes were left untreated.
3. Results
3.1. Selection of specific mucin antibodies for detection of mucins in human tear fluid
Antibodies to mucins are notoriously difficult to characterize and often lack clear demonstration of specificity (Moniaux et al., 2004). Their binding can be affected by post-translational modifications (Rousseau et al., 2003) such as glycosylation or proteolysis. Thus, we used several antibodies per mucin (each recognizing a different epitope) on immunoblots of tear proteins pooled from up to 21 normal individuals to investigate the mucin’s presence and whether the binding was to a protein of high molecular weight.
MUCs 1, 4, 16 and 5AC were consistently detected in pooled tear samples from normal individuals with all the antibodies used (Fig. 1). Differences in electrophoretic mobility in agarose gels were detected, however, with the antibodies used to detect MUCs 1 and 4. Although all three MUC1 antibodies recognize the highly glycosylated tandem repeat region of MUC1 (Table 1), the binding of these antibodies are differentially affected by glycosylation status (Burchell J and Taylor-Papadimitriou, 1993; Price et al., 1998). 214D4 and HMFG-2 detect a less mobile species of MUC1 than does HMFG-1 (Fig. 1A). For further studies, we chose to use the two antibodies that recognize similarly migrating bands (HMFG-2, 214D4). In the case of the MUC4-specific antibodies, 8G7 recognizes a less mobile species of MUC4 than does 1G8 (Fig. 1A). Because of this variability, both antibodies (8G7, 1G8) were used in further studies.
Fig. 1.
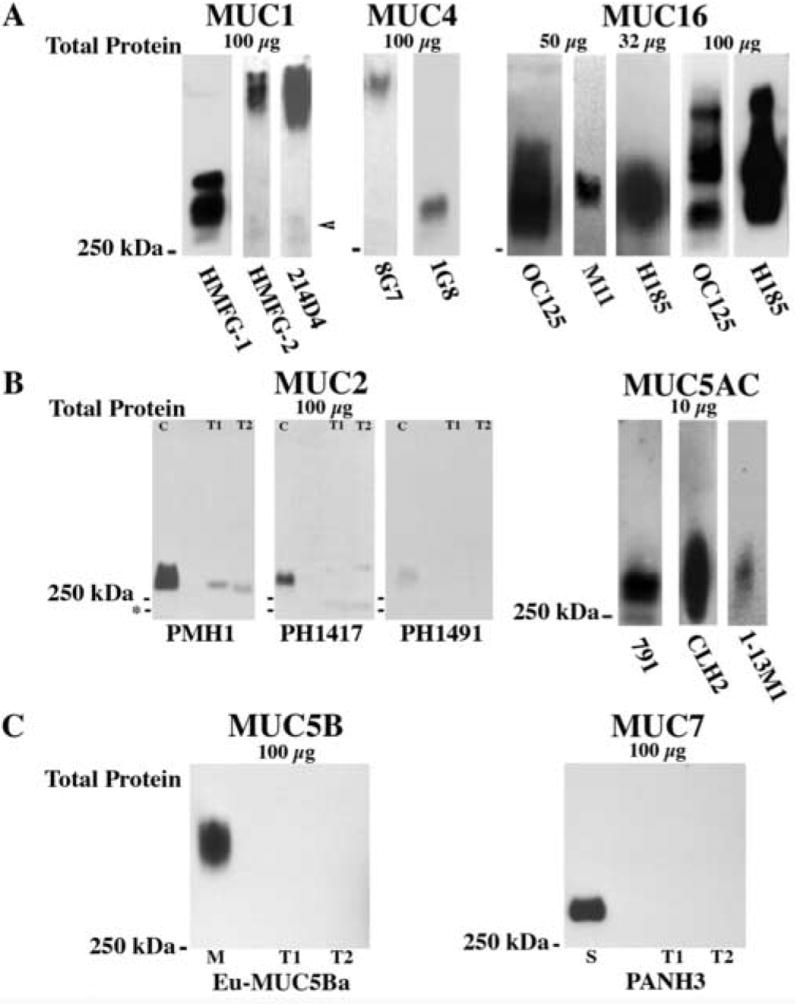
Detection of MUCs 1, 2, 4, 16 and 5AC in tear fluid was determined through the use of multiple mucin-specific antibodies on samples of pooled human tears (n=4, unless noted). In order to compare their binding patterns and determine which would be best for use in quantitative analyses, 2-to-3 antibodies with differing epitopes per membrane-associated mucin (A) and per secreted mucin (B, C) were screened. (A) Major differences in electrophoretic mobility were seen with the antibodies used to detect MUC1 and MUC4. Note, however, that faint bands with the same mobility as that detected with HMFG-1 can be seen in the HMFG-2 and 214D4 blots (arrowhead). No major differences were noted between any of the MUC16 antibodies. Note that multiple bands were detected for MUC16 and its carbohydrate epitope H185 when 100 μg of total tear proteins, pooled from 17 subjects, was examined. (B) Three MUC2-specific antibodies were tested on 100 μg of total protein per lane from tears pooled from multiple collections of a single individual (T1) or single collections of 21 normal individuals (T2). Weak binding (as compared to that of the positive control colon, (C)) was seen with 2 of the 3 antibodies (PMH1, PH1417), although the number and electrophoretic mobility of the positive bands was different (*-marks 75 kDa). In contrast, strong positive binding was seen with 10 μg of total protein loaded per lane for MUC5AC, with no major differences in electrophoretic mobility. (C) MUC5B and MUC7 were not detected in human tears, even with maximal loading of tear proteins (100 μg). One μg of purified human cervical mucins (M) was used as positive control for MUC5B and 10 μg of human saliva (S) as positive control for MUC7.
No major difference in binding patterns was found with the three antibodies used to detect MUC16 (OC125, M11, H185) in tears; all antibodies bound proteins just greater than 250 kDa (Fig. 1A). OC125 and H185 were chosen for further studies of quantitation of MUC16 in tears. When 100 μg of total protein from a pool of 17 individuals was examined with these two antibodies, additional bands were detected with OC125 and with H185, which recognizes a carbohydrate epitope on MUC16; the binding was to a smear encompassing the bands seen with OC125 (Fig. 1A, far right). These data suggest that MUC16 is polymorphic within the population (as is known to occur in MUC genes (Fowler et al., 2001)), that several sites for sheddases (protease) action on MUC16 occur within the extracellular domain, or that multiple glycoforms of MUC16 lead to multiple forms of MUC16 shed into tears.
The three antibodies used for MUC5AC (791, CLH2, 1-13M1) bound in similar patterns to tear proteins run under non-reducing conditions (Fig. 1B), although CLH2 recognizes a more polydisperse band than 791, and 1-13M1 bound less intensely. Antibodies 791 and CLH2 were selected for further studies of quantitation of MUC5AC in tears. Interestingly, antibody 791, which recognizes a region in the D3 domain of MUC5AC, recognizes MUC5AC in both reduced and non-reduced gels. CLH2, which recognizes a region in the tandem repeat domain, and 1-13M1, which recognizes the cys2 and cys4 domains in the N-terminus of MUC5AC, only recognize non-reduced MUC5AC (data not shown).
A previous report using a single antibody demonstrated that MUC2 protein is present in tears (McKenzie et al., 2000). In order to substantiate this finding and since message levels for the mucin are low (McKenzie et al., 2000), three different antibodies (PMH1, PH1417, PH1491; Table 1) were used in Western blot analysis to examine a maximal amount of tear protein (100 μg) from tear washes pooled from 21 individuals or from a single individual. MUC2 was detected in both tear preparations with two (PMH1 and PH1417) of the three antibodies examined (Fig. 1B). PMH1 bound to a protein band that co-migrated with the positive control; however, PH1417 bound to two bands in tears—one with slightly lower mobility than that seen with PMH1 and one migrating to ~75 kDa (Fig. 1B). No differences in binding were seen between 100 μg of tear proteins from a single individual and a pooled sample of 21 individuals (data not shown). Although PH1491 bound to the positive control (human colon tumor extract), there was no detectable binding to the two tear samples. The intensity of the binding to tear protein was much lower than that seen with equivalent loading of positive control protein, indicating that MUC2 is less prevalent in tears than in colon tumor extract. The binding of PH1491 to the positive control was less intense than that of the other two antibodies, so perhaps the lack of binding to tears is due to differences in antibody sensitivity. This could not be overcome by increasing the concentration of PH1491 antibody because increased background occurred, obscuring positive binding. No difference in binding pattern was found among these antibodies for the positive control, they all bound proteins greater than 250 kDa. Because 100 μg of total tear protein only yielded a weak positive signal with the MUC2 antibodies tested, a semi-quantitative assay for MUC2 was not pursued.
MUC5B and MUC7 message and protein have been found in human lacrimal gland tissue (Jumblatt et al., 2003; Paulsen et al., 2004); however, previous assays did not detect the mucins in tears (Gipson et al., 2001; Jumblatt et al., 2003). To more conclusively demonstrate their presence or absence in tears, two additional antibodies specific for each of these mucins were used to probe immunoblots of maximally loaded gels (100 μg of total tear protein) from a sample of pooled tears (N = 17). Antibodies Eu-MUC5Ba (Fig. 1C) and 799 were used for detection of MUC5B; PANH3 (Fig. 1C) and Eu-MUC7a were used for MUC7. Neither the gel-forming mucin MUC5B nor the small soluble mucin MUC 7 was detected with any of the antibodies tested, even with pooled samples and loading of high concentrations (100 μg) of total protein (Fig. 1C).
3.2. Antibody binding for quantitative assays of mucins in tears
Based on the results obtained with the battery of mucin antibodies examined initially, two antibodies per mucin were chosen to determine their suitability in a quantitative assay. Antibodies were chosen based on the results illustrated in Figure 1 as well as widespread availability for general use (i.e., commercially available). Two-fold serial dilutions of tear proteins were separated by agarose gel electrophoresis, transferred to nitrocellulose, probed with the mucin antibodies and band density measured by densitometry. Regression analyses performed on the plots for MUC1, MUC4, MUC16, H185 and MUC5AC antibodies (Fig. 2) resulted in regression coefficient (r2) values >0.9, validating the quantitative aspect of the assay over a discrete range of protein concentrations. Thus, a single concentration in the mid-linear range of detectability can be chosen for examination of mucin content in tears and allows semi-quantitative comparisons of mucin protein levels between tears from different subjects or conditions (e.g., disease, drug efficacy). Differences were found between the optimal amounts of total protein to be examined for reliable detection of individual mucins, i.e., detection of MUC1 and MUC4 mucin in tears required examination of more total protein than for MUC16 or MUC5AC. Substantially more total protein (50 μg versus 10 – 16 μg) is required for reliable detection of MUC1 and MUC4 (with 8G7) than for MUC4 (with 1G8) or MUC5AC or MUC16.
Fig. 2.
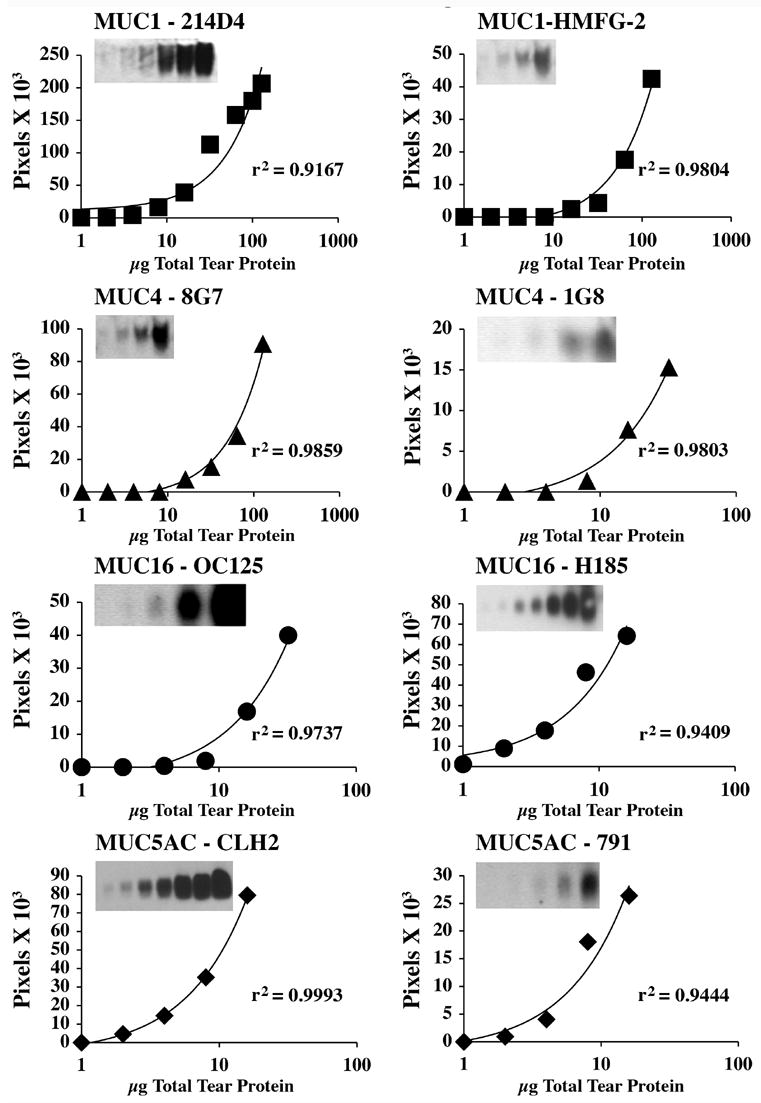
The feasibility of quantitating mucins in tears with the immunoblot assay developed in this study is confirmed by the regression analysis of the antibody binding over a discrete range of protein concentrations. Tears were run non-reduced in these assays. Densitometric analyses of immunoblots (insets) of two-fold serial dilutions of tear proteins pooled from 2-to-4 normal subjects are illustrated for each MUC antibody considered for further studies. The lower limit of detectability was reached for each mucin (see insets). Density in background subtracted pixels was determined and plotted against protein concentration. In these analyses the regression coefficients (r2) confirmed the linearity of the responses.
3.3. Assay of individual tear samples
In order to validate the usefulness of the assay for future studies, the protein concentration in the mid-linear range determined above was chosen for assaying mucin content in tear samples from individual normal subjects (Fig. 3). The greatest variability in mucin content in tears was observed for MUC1 (Fig. 3A). Thirty-two μg of total tear protein from twelve individuals were examined for MUC1; 4 out of 6 were positive with each of the MUC1 antibodies tested (214D4 and HMFG-2). Ten individuals were examined for MUC4 protein; 5 out of 5 were positive when ≥50 μg of total protein was probed with the 8G7 antibody, and 5 out of 5 were positive when 16 μg of total protein was probed with the 1G8 antibody (Fig. 3B). MUC16 mucin was detected in all 11 of the normal individuals examined with OC125 antibody at 10 μg of total protein (Fig. 3C). MUC5AC was detected in the tears of 15 normal individuals—9 out of 9 with CLH2 at 10 or 12 μg of total protein (Fig. 3D) and 6 out of 6 with 791 at 10 μg (data not shown). Upon assay of protein from 42 eyes (24 individuals), a range of 20 – 45 μl was recovered per eye, with an average protein concentration of 1.7 μg/μl. Thus, the average yield of total protein per eye ranged from 34 to 76.5 μg. When the amount of protein collected from an individual is maximal, e.g., 75 μg per eye, it is feasible to assay for all mucins present in tear fluid from the same sample (MUC1, 4, 16 and 5AC). In individuals with lower protein yield, it is necessary to pool tears from additional collections to assay all mucins.
Fig. 3.
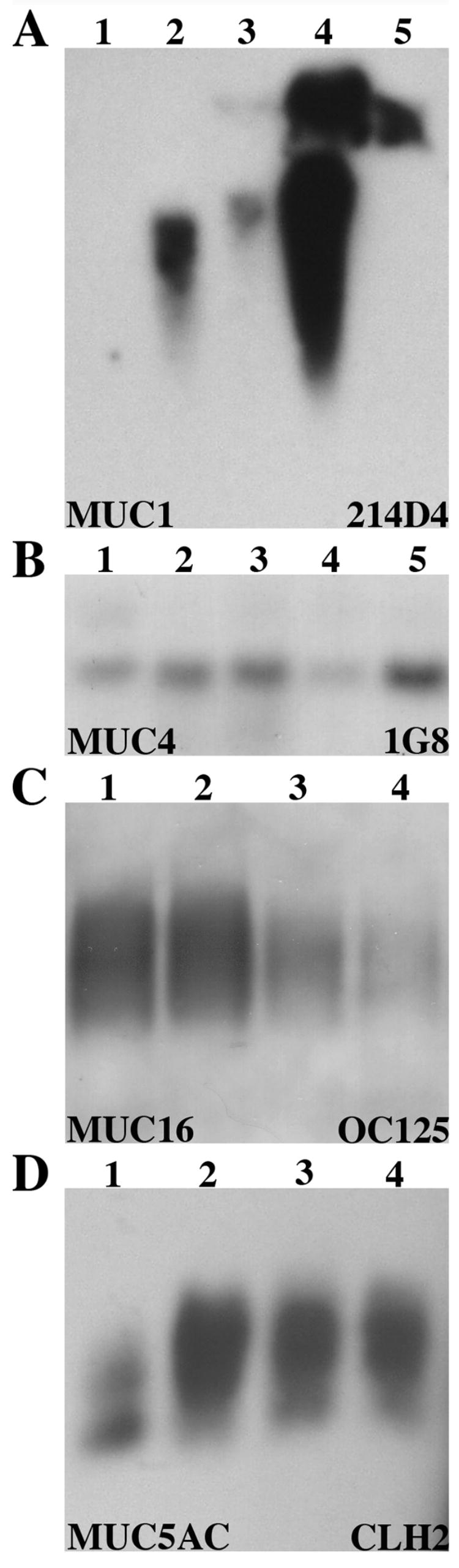
Pilot experiment to show feasibility that single collections of tears from individuals can be used to perform semi-quantitative analyses of the mucin content per μg total protein. Immunoblots of human tears from each of 4 to 5 individuals were probed with antibodies specific for MUCs 1, 4, 16 and 5AC. Note that differences in band density can be discerned between individuals when equivalent amounts of total protein are loaded per lane and that MUC1 showed the greatest variability between individuals. Blot (A) was probed for MUC1 with antibody 214D4, 32 μg of total protein was loaded per sample with 4 of 5 positive. (B) MUC4 was detected with antibody 1G8, 16 μg loaded per sample with 5 of 5 positive. (C) Antibody OC125 was used to detect MUC16 on 10 μg of total protein per sample, with 4 of 4 positive. (D) MUC5AC was detected with antibody CLH2, 12 μg of total protein, with 4 of 4 positive.
3.4. Variations in size of MUC5AC mucin protein in tears compared to conjunctival tissue
The MUC5AC present in tears was found to migrate further in SDS-agarose than that from human stomach and gall bladder tissue or in secreted human cervical mucus (samples used as positive controls for MUC5AC, Fig. 4A), raising the question of whether MUC5AC in tears has been proteolytically degraded. To directly compare tear MUC5AC mobility to that of conjunctival goblet cells of the same individual, protein was extracted from conjunctival epithelium collected by impression cytology using 2% SDS or RIPA buffer. Since protease inhibitors had not been added to tear washes, no protease inhibitors were added during extraction of cellular proteins. No difference in mobility was seen between samples extracted with 2% SDS or RIPA buffer (data not shown). In all paired samples examined to date (n=4), the MUC5AC that is present in the pre-ocular tear fluid of normal subjects was lower in molecular weight than that isolated from the conjunctival cells of the same individual, although a very faint band can be seen co-migrating with tears in some samples (Fig. 4B). To determine whether proteases in tears could be responsible for MUC5AC degradation post collection, tear samples were collected separately from both eyes of two individuals and protease inhibitors immediately added to one sample of each. No difference was seen in MUC5AC mobility between the two samples with or without protease inhibitors (Fig. 4C). This observation suggests that the difference observed between cellular and tear fluid MUC5AC mobility is not an artifact of proteolytic degradation post collection.
Fig. 4.
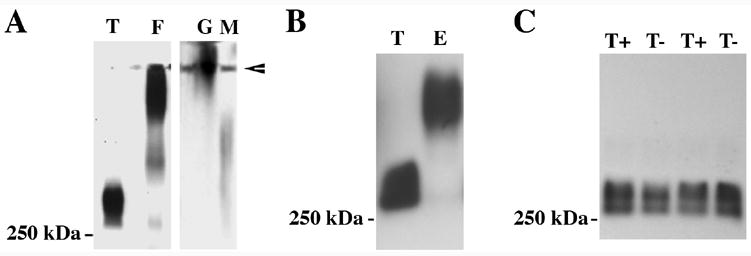
MUC5AC mucin collected from tears and conjunctival epithelial cells have different electrophoretic mobility. (A) MUC5AC secreted into tear fluid (T) is of higher mobility than that isolated from fundus (F) or gall bladder (G) and in secreted human cervical mucus (M). To ensure visualization of any minor components of MUC5AC in tears, 200 μg of total protein were examined in this blot. Arrowhead indicates the loading wells at the top of the gel. (B) MUC5AC in tears (T) is also of higher mobility than that isolated from conjunctival epithelial cells (E) of the same subject. (C) The addition of protease inhibitors (T+) to tears in two individuals did not affect MUC5AC electrophoretic mobility as compared to tears from the contralateral eye with no inhibitors (T−). These results indicate lack of proteolytic cleavage post-collection. In these experiments, MUC5AC mucin was detected on immunoblots of non-reduced proteins with the MUC5AC-specific antibodies 791 (A) and CLH2 (B, C).
4. Discussion
The systematic study reported herein provides more comprehensive and reliable data on the complex mucin composition of tears and demonstrates for the first time the presence of shed, membrane-associated MUC16 in individual human tear film samples. The use of multiple antibodies for each mucin—each one recognizing different epitopes on the mucin—confers a high level of confidence to the data presented herein. The data also show the feasibility of semi-quantitative Western blot assays of mucin content per μg of total protein directly from tear samples without prior fractionation or isolation of mucins. The method of collection is non-invasive, easily tolerated and performed. Parameters for the Western blot assays have been determined for reliable semi-quantitative detection of the prevalent mucins in tear fluid. Due to the large number of samples that can be run in a single agarose gel (e.g., 60–80 for a 14.5 × 10.2 cm gel), the straightforward method may be adapted to high throughput screening of individual tear samples for tear mucin quantitation. To ensure that comparisons were made between identically treated samples, comparisons in this study were made within a single blot. Addition of a mucin standard or reference sample to each gel in future studies would permit quantitative comparisons between samples examined over a large number of blots.
Previous reports that MUCs 1, 4 and 5AC are present in human tear fluid (Ellingham et al., 1997; Garcher et al., 1998; Jumblatt et al., 1999; Pflugfelder et al., 2000; Zhao et al., 2001; Argueso et al., 2002) have been corroborated by this study. This report also corroborates the presence of low levels of MUC2 in tears (McKenzie et al., 2000), with weak binding of 2 of the 3 antibodies tested on 100 μg of total tear protein, an amount 10 times greater than the mid-linear range for MUC5AC detection. This is consistent with the finding that message levels for MUC2, when found, are minor compared to those for MUC5AC (McKenzie et al., 2000). Our data using chemiluminescence detection and a newly developed antibody specific to a different region of human MUC5B (Rousseau et al., 2003) confirm our previous ELISA results that MUC5B protein, a mucin described in human lacrimal gland (Paulsen et al., 2004), is not detectable in human tears (Gipson et al., 2001), even when examining maximally loaded gels and 100-fold more total protein than necessary to detect MUC5B in a positive control secretion (purified human cervical mucin). Similarly, our use of 2 different MUC7-specific antibodies on maximally loaded gels, with 10-fold more total tear protein than necessary for detection in the control secretion (saliva), confirms the findings of Jumblatt et al. (Jumblatt et al., 2003) that the small soluble mucin MUC7, which is produced by lacrimal gland epithelia, is not present in human tear film. Since MUC5B and MUC7 proteins have been detected in lacrimal gland tissue (Paulsen et al., 2004), our data suggest that these two mucins are retained in the lacrimal gland where they may be produced and locally degraded.
The observation that the antibodies used for detection of MUC1, MUC2 and MUC4 bound to protein bands of different electrophoretic mobility may have several explanations. Thornton et al. have demonstrated glycoforms of MUC5AC in respiratory mucus using SDS-agarose gels and have shown a correlation between charge density on mucins and increasing mobility within these gels (Thornton et al., 1995). In tear samples, charge density may depend on the content of negatively charged sialic acid residues on mucins (Argueso et al., 1998). Access of the antibodies to their epitopes may be impeded by charge density, and so the differences in mobility noted between the three MUC1-specific antibodies may be due to recognition of different glycoforms of MUC1. In fact, it has been shown that HMFG-1 recognizes fully glycosylated MUC1 and its binding is sensitive to sialic acid residues, whereas the binding of HMFG-2 is sensitive to the length of the oligosaccharide side chains present on MUC1 (Burchell J and Taylor-Papadimitriou, 1993). 214D4, on the other hand, is generally considered to recognize MUC1 independent of its glycosylation status (Price et al., 1998). The three MUC2 antibodies used are directed against very different epitopes. PMH1 recognizes glycoforms of MUC2 (GalNAc residues attached to the tandem repeat domain) (Reis et al., 1998), while PH1417 and PH1491 recognize the C-terminus and N-terminus of MUC2, respectively. The band recognized by PH1417 that migrates around 75 kDa is consistent with the autocatalytic cleavage product of MUC2 that is identified with PH1417 (Lidell et al., 2003). A different explanation may account for the differences in electrophoretic mobility observed with the MUC4 antibodies. MUC4 is a heterodimeric molecule (Moniaux et al., 1999) consisting of two non-covalently linked subunits. MUC4α is the mucin-like subunit, containing the highly glycosylated tandem repeat region, the epitope for 8G7. MUC4β is the human homologue of ASGP-2 and is comprised of two EGF-like domains, two domains rich in N-glycosylation sites, two cysteine-rich domains, a putative GDPH cleavage site, a transmembrane sequence and a short cytoplasmic tail. The epitope recognized by 1G8 is between the 53rd amino acid on the N-terminus and the transmembrane domain of ASGP-2 and it has been shown to cross react with MUC4β (Zhang et al., 2005). Thus, the antibodies may be recognizing separate subunits of MUC4. Products of similar mobility have been observed in other systems with these two antibodies. The mobility of the products recognized in tears is consistent with that shown in previous reports using a MUC4-expressing pancreatic tumor cell line for 8G7 (Moniaux et al., 2004) and human milk for 1G8 (Zhang et al., 2005).
The quantitative assay for mucins in tears reported in this study has several advantages over previously reported ELISA and Schirmer strip collection assays (Garcher et al., 1998; Zhao et al., 2001; Argueso et al., 2002). Garcher et al. (Garcher et al., 1998) assayed tears eluted from impression cytology samples using an ELISA for the tumor marker CA19-9, which has since been identified as a marker for MUC1/Y (Akagi et al., 2001), a splice variant of MUC1 that is missing the tandem repeat domain (Zrihan-Licht et al., 1994). The interpretation of these results is complicated by the inability to exclude mucin contamination from cells in the impression cytology samples. Two previous quantitative assays have been reported for MUC5AC in tears (Zhao et al., 2001; Argueso et al., 2002). Zhao et al. (Zhao et al., 2001) presented the first quantitative analysis of MUC5AC in the human tear film with a direct immunochemical assay of MUC5AC absorbed to Schirmer strips. There are several drawbacks to this method. Due to the migration of the tears onto the Schirmer strip, the antibody binding is often seen as a smear rather than a discrete band, no differences in molecular size can be elucidated, and only one mucin can be detected per tear sample. Argüeso et al. (Argueso et al., 2002) developed a semi-quantitative ELISA assay for the amount of MUC5AC mucin present in human tear protein samples collected, as in this study. Although valuable for quantitation, ELISA assays do not allow for detection of changes in molecular character upon secretion or shedding from cell surfaces. The assay described herein overcomes several of the problems of the previously described techniques. Firstly, the tear collection method is simple to perform and provides sufficient sample to assay multiple mucins from a single individual without extensive processing prior to the assay. Secondly, samples can be centrifuged prior to use to exclude cellular contaminants. Thirdly, separation by agarose electrophoresis allows detection of differences in electrophoretic mobility, an indicator of modifications to the molecular character of the mucin. Finally, since the linear range of protein concentration for antibody binding to mucins in human tears has been defined for the mucins expressed by the ocular surface, and, since we have data from this as well as a previous study (Argueso et al., 2002) on the typical protein yield from several individuals using this collection method, we are confident that sufficient protein can be collected for the assays described herein. Thus, direct semi-quantitative comparisons can be made between samples from different individuals or within an individual before and after therapeutic treatments.
The major drawback of the assay is that it is semi-quantitative; standards do not exist for ocular surface mucins that would allow an absolute quantitation of mucin content. Although this assay can be used to directly compare the mucin content of specific mucins in different samples, comparisons cannot be made between tear content of different mucins. Thus, definitive conclusions cannot be made about the relative amounts of specific mucins in the tear fluid based on the differences in the amount of total protein required for the linear range of antibody binding. The differences could either be due to the efficiency of antibody binding or relative amounts of individual mucins in tears. It is interesting to note, however, that the increased amount of total protein necessary for detection of MUC1 and the larger degree of variability between individuals correlates to our previous findings that MUC1 mRNA is less prevalent in human conjunctival epithelial cells than that of MUC4, 16 or 5AC (Gipson et al., 2004).
Another intriguing finding of our study relates to the differences in electrophoretic mobility in agarose gels observed for MUC5AC between cellular and tear protein under non-reducing conditions. The data corroborates that of Berry et al. (Berry et al., 2004), who recently found the molecular weight of MUC5AC in N-acetyl cysteine washes of the ocular surface to be smaller than that of conjunctival tissue protein. It is interesting to note that the MUC5AC detected in secreted cervical mucus is larger in size than that seen in tears. Perhaps these differences in secreted MUC5AC are a tissue-specific variation in MUC5AC processing upon secretion that reflects the different requirements of mucus on each epithelium.
There are several possible explanations for the observed difference in mobility of tear and cellular conjunctival MUC5AC. The first possibility is cleavage of the molecule to smaller components. Lidell and Hansson have recently shown that a non-enzymatic, intracellular cleavage event occurs in the C-terminal cysteine-rich domain of MUC5AC, with the cleavage products held together by disulphide bonds (Lidell and Hansson, 2006). Wickström at al. have suggested that an enzymatic cleavage event occurs within the C-terminus of MUC5B, another gel-forming mucin with similar structure to MUC5AC (Wickstrom et al., 1998) and that a second, undefined cleavage event occurs in the N-terminus of MUC5B (Wickstrom and Carlstedt, 2001). In two of these studies, the cleavage products are only found under reducing conditions (Lidell and Hansson, 2006), whereas in the third, the cleavage products are not affected by reduction (Wickstrom and Carlstedt, 2001). Our data agrees with the later of these studies in that the smaller size of MUC5AC is seen only after secretion into tears and under non-reducing conditions. Use of site-specific N- and C-terminal antibodies in immunoblots of tear and conjunctival samples may help in determining if cleavage of the MUC5AC molecule occurs upon secretion.
A second possible explanation for the difference in MUC5AC size after secretion is change in glycosylation of the mucins by glycosidase activity in tears (Kitaoka et al., 1985; Matthews et al., 2001). Mucins by definition are 50–60% carbohydrate, so differences in the carbohydrate composition of the molecule could result in changes in size and thus increased mobility within an agarose gel. Often changes in glycosylation of mucins over time yield long smears within electrophoretic gels, thus the lack of a long smear for the MUC5AC in tears would argue that a very specific rapid change (in the protein backbone or the carbohydrate composition) occurs immediately upon secretion. Obviously, further study is needed to elucidate the exact mechanism responsible for the difference observed in MUC5AC after secretion into the tear fluid by the conjunctival goblet cells. The physiologic significance of smaller MUC5AC in the tears is also not clear.
In summary, a mixture of shed membrane-associated and secreted mucins accounts for the mucin content of tears. The development of the semi-quantitative assay for mucins in the tear film reported herein will permit further studies of alterations in mucin content in the tears and ocular surface of individual patients with ocular surface diseases. Such studies have the potential to provide clues for improved management of these diseases.
Acknowledgments
The authors gratefully acknowledge the generous gift of antibodies from Dr. J. Hilkens, Netherlands Cancer Institute, Amsterdam, The Netherlands; Dr. U. Mandel, University of Copenhagen, Denmark; Dr. G. Hansson, Göteburg University, Gothenburg, Sweden; Dr. S. K. Batra, University of Nebraska Medical Center, Omaha, Nebraska; and Dr. D. M. Swallow, University College, London, U.K. The authors also thank Cindy Leigh Russo, MS for expert technical assistance and Drs. Stefano Barabino, Vinodh Kakkassery and Antony Poothullil for collection of tears and impression cytology samples. This work was supported by NIH/NEI grants R01 EY03306 to IKG and R01 EY014847 to PA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akagi J, Takai E, Tamori Y, Nakagawa K, Ogawa M. CA19-9 epitope a possible marker for MUC-1/Y protein. Int J Oncol. 2001;18:1085–1091. doi: 10.3892/ijo.18.5.1085. [DOI] [PubMed] [Google Scholar]
- Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren’s syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–1011. [PubMed] [Google Scholar]
- Argueso P, Herreras J, Calonge M, Citores L, Pastor J, Girbes T. Analysis of human ocular mucus: effects of neuraminidase and chitinase enzymes. Cornea. 1998;17:200–207. doi: 10.1097/00003226-199803000-00015. [DOI] [PubMed] [Google Scholar]
- Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003a;44:2487–2495. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- Argueso P, Tisdale A, Mandel U, Letko E, Foster CS, Gipson IK. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Invest Ophthalmol Vis Sci. 2003b;44:86–92. doi: 10.1167/iovs.02-0181. [DOI] [PubMed] [Google Scholar]
- Asker N, Axelsson M, Olofsson S, Hansson G. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem. 1998;273:18857–18863. doi: 10.1074/jbc.273.30.18857. [DOI] [PubMed] [Google Scholar]
- Axelsson M, Asker N, Hansson G. O-glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J Biol Chem. 1998;273:18864–18870. doi: 10.1074/jbc.273.30.18864. [DOI] [PubMed] [Google Scholar]
- Bast RJ, Feeney M, Lazarus H, Nadler L, Colvin R, Knapp R. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M, Ellingham R, Corfield A. Human preocular mucins reflect changes in surface physiology. Br J Ophthalmol. 2004;88:377–383. doi: 10.1136/bjo.2003.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell J, Taylor-Papadimitriou J. Effect of modification of carbohydrate side chains on the reactivity of antibodies with core-protein epitopes of the MUC1 gene product. Epithelial Cell Biol. 1993;2:155–162. [PubMed] [Google Scholar]
- Chen Y, Zhao Y, Kalaslavadi T, Hamati E, Nehrke K, Le A, Ann D, Wu R. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol. 2004;30:155–165. doi: 10.1165/rcmb.2003-0103OC. [DOI] [PubMed] [Google Scholar]
- Corrales R, Galarreta D, Herreras J, Calonge M, Chaves F. Normal human conjunctival epithelium expresses MUC13, MUC15, MUC16 and MUC17 mucin genes. Arch Soc Esp Oftalmol. 2003;78:375–381. [PubMed] [Google Scholar]
- Danjo Y, Watanabe H, Tisdale AS, George M, Tsumura T, Abelson MB, Gipson IK. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–2609. [PubMed] [Google Scholar]
- Ellingham RB, Myerscough N, Gout I, Berry M, Corfield AP. Soluble mucins in human aqueous tears. Biochem Soc Trans. 1997;25:12S. doi: 10.1042/bst025012s. [DOI] [PubMed] [Google Scholar]
- Fowler J, Vinall L, Swallow D. Polymorphism of the human muc genes. Front Biosci. 2001;6:D1207–D1215. doi: 10.2741/A674. [DOI] [PubMed] [Google Scholar]
- Garcher C, Bron A, Baudouin C, Bildstein L, Bara J. CA 19-9 ELISA test: a new method for studying mucus changes in tears. Br J Ophthalmol. 1998;82:88–90. doi: 10.1136/bjo.82.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- Gipson I, Hori Y, Argüeso P. Character of Ocular Surface Mucins and Their Alteration in Dry Eye Disease. The Ocular Surface. 2004;2:131–148. doi: 10.1016/s1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- Gipson I, Moccia R, Spurr-Michaud S, Argueso P, Gargiulo A, Hill Jr, Offner G, Keutmann H. The amount of MUC5B mucin in cervical mucus peaks at midcycle. J Clin Endocrinol Metab. 2001;86:594–600. doi: 10.1210/jcem.86.2.7174. [DOI] [PubMed] [Google Scholar]
- Gipson I, Spurr-Michaud S, Argueso P, Tisdale A, Ng T, Russo C. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78:379–388. doi: 10.1016/s0014-4835(03)00204-5. [DOI] [PubMed] [Google Scholar]
- Gum J. Human mucin glycoproteins: varied structures predict diverse properties and specific functions. Biochem Soc Trans. 1995;23:795–799. doi: 10.1042/bst0230795. [DOI] [PubMed] [Google Scholar]
- Gum JR, Jr, Crawley SC, Hicks JW, Szymkowski DE, Kim YS. MUC17, a novel membrane-tethered mucin. Biochem Biophys Res Commun. 2002;291:466–475. doi: 10.1006/bbrc.2002.6475. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Orita T, Nakanishi S, Katsuya K, Watanabe H, Yamasaki Y, Waga I, Nanayama T, Yamamoto Y, Munger W, et al. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J Biol Chem. 2004;279:1968–1979. doi: 10.1074/jbc.M304558200. [DOI] [PubMed] [Google Scholar]
- Hollingsworth M, Swanson B. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Jumblatt J, Cunningham L, Li Y, Jumblatt M. Characterization of human ocular mucin secretion mediated by 15(S)-HETE. Cornea. 2002;21:818–824. doi: 10.1097/00003226-200211000-00018. [DOI] [PubMed] [Google Scholar]
- Jumblatt MM, McKenzie RW, Jumblatt JE. MUC5AC mucin is a component of the human precorneal tear film. Invest Ophthalmol Vis Sci. 1999;40:43–49. [PubMed] [Google Scholar]
- Jumblatt MM, McKenzie RW, Steele PS, Emberts CG, Jumblatt JE. MUC7 expression in the human lacrimal gland and conjunctiva. Cornea. 2003;22:41–45. doi: 10.1097/00003226-200301000-00010. [DOI] [PubMed] [Google Scholar]
- Kitaoka M, Nakazawa M, Hayasaka S. Lysosomal enzymes in human tear fluid collected by filter paper strips. Exp Eye Res. 1985;41:259–265. doi: 10.1016/s0014-4835(85)80015-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapensee L, Paquette Y, Bleau G. Allelic polymorphism and chromosomal localization of the human oviductin gene (MUC9) Fertil Steril. 1997;68:702–708. doi: 10.1016/s0015-0282(97)00317-8. [DOI] [PubMed] [Google Scholar]
- Lidell M, Hansson G. Cleavage in the GDPH sequence of the C-terminal cysteine-rich part of the human MUC5AC mucin. Biochem J. 2006 doi: 10.1042/BJ20060443. Jun 21:Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell M, Johansson M, Hansson G. An autocatalytic cleavage in the C terminus of the human MUC2 mucin occurs at the low pH of the late secretory pathway. J Biol Chem. 2003;278:13944–13951. doi: 10.1074/jbc.M210069200. [DOI] [PubMed] [Google Scholar]
- Matthews E, Dukes E, Hicks S, Gierow P, Erwin J, Corfield T, Carrington S. Lacrimal glycosidases degrade ocular surface mucins. Invest Ophthalmol Vis Sci. 2001;42:S264 (Abstr). [Google Scholar]
- McKenzie R, Jumblatt J, Jumblatt M. Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Invest Ophthalmol Vis Sci. 2000;41:703–708. [PubMed] [Google Scholar]
- Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192–1206. doi: 10.2741/moniaux. [DOI] [PubMed] [Google Scholar]
- Moniaux N, Nollet S, Porchet N, Degand P, Laine A, Aubert J. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem J. 1999;338:325–333. [PMC free article] [PubMed] [Google Scholar]
- Moniaux N, Varshney GC, Chauhan SC, Copin MC, Jain M, Wittel UA, Andrianifahanana M, Aubert JP, Batra SK. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52:253–261. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- Nielsen PA, Mandel U, Therkildsen MH, Clausen H. Differential expression of human high-molecular-weight salivary mucin (MG1) and low-molecular-weight salivary mucin (MG2) J Dent Res. 1996;75:1820–1826. doi: 10.1177/00220345960750110201. [DOI] [PubMed] [Google Scholar]
- Nollet S, Escande F, Buisine M, Forgue-Lafitte M, Kirkham P, Okada Y, Bara J. Mapping of SOMU1 and M1 epitopes on the apomucin encoded by the 5′ end of the MUC5AC gene. Hybrid Hybridomics. 2004a;23:93–99. doi: 10.1089/153685904774129694. [DOI] [PubMed] [Google Scholar]
- Nollet S, Escande F, Buisine M, Forgue-Lafitte M, Kirkham P, Okada Y, Bara J. Mapping of SOMU1 and M1 epitopes on the apomucin encoded by the 5′ end of the MUC5AC gene. Hybrid Hybridomics. 2004b;23:93–99. doi: 10.1089/153685904774129694. [DOI] [PubMed] [Google Scholar]
- Nustad K, Bast RJ, Brien T, Nilsson O, Seguin P, Suresh M, Saga T, Nozawa S, Bormer O, de Bruijn H, et al. Specificity and affinity of 26 monoclonal antibodies against the CA 125 antigen: first report from the ISOBM TD-1 workshop. International Society for Oncodevelopmental Biology and Medicine. Tumour Biol. 1996;17:196–219. doi: 10.1159/000217982. [DOI] [PubMed] [Google Scholar]
- Pallesen LT, Berglund L, Rasmussen LK, Petersen TE, Rasmussen JT. Isolation and characterization of MUC15, a novel cell membrane-associated mucin. Eur J Biochem. 2002;269:2755–2763. doi: 10.1046/j.1432-1033.2002.02949.x. [DOI] [PubMed] [Google Scholar]
- Paulsen F, Langer G, Hoffmann W, Berry M. Human lacrimal gland mucins. Cell Tissue Res. 2004;316:167–177. doi: 10.1007/s00441-004-0877-7. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Liu Z, Monroy D, Li DQ, Carvajal ME, Price-Schiavi SA, Idris N, Solomon A, Perez A, Carraway KL. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest Ophthalmol Vis Sci. 2000;41:1316–1326. [PubMed] [Google Scholar]
- Preobrazhensky A. Electrophoresis of neurochordins, a family of high molecular weight neural tissue glycoproteins, on horizontal submerged agarose gels in the presence of dodecyl sulfate. Anal Biochem. 1993;209:315–317. doi: 10.1006/abio.1993.1125. [DOI] [PubMed] [Google Scholar]
- Price M, Rye P, Petrakou E, Murray A, Brady K, Imai S, Haga S, Kiyozuka Y, Schol D, Meulenbroek M, et al. Summary report on the ISOBM TD-4 Workshop: analysis of 56 monoclonal antibodies against the MUC1 mucin. San Diego, Calif., November 17–23, 1996. Tumour Biol. 1998;19:1–20. doi: 10.1159/000056500. [DOI] [PubMed] [Google Scholar]
- Reis C, David L, Nielsen P, Clausen H, Mirgorodskaya K, Roepstorff P, Sobrinho-Simoes M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997;74:112–121. doi: 10.1002/(sici)1097-0215(19970220)74:1<112::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Reis C, Sorensen T, Mandel U, David L, Mirgorodskaya E, Roepstorff P, Kihlberg J, Hansen J, Clausen H. Development and characterization of an antibody directed to an alpha-N-acetyl-D-galactosamine glycosylated MUC2 peptide. Glycoconj J. 1998;15:51–62. doi: 10.1023/a:1006939432665. [DOI] [PubMed] [Google Scholar]
- Rousseau K, Wickstrom C, Whitehouse D, Carlstedt I, Swallow D. New monoclonal antibodies to non-glycosylated domains of the secreted mucins MUC5B and MUC7. Hybrid Hybridomics. 2003:22. doi: 10.1089/153685903322538818. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J, Peterson J, Arklie J, Burchell J, Ceriani R, Bodmer W. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981;28:17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- Thornton D, Howard M, Devine P, Sheehan J. Methods for separation and deglycosylation of mucin subunits. Anal Biochem. 1995;227:162–167. doi: 10.1006/abio.1995.1266. [DOI] [PubMed] [Google Scholar]
- Thornton D, Khan N, Sheehan J. Separation and identification of mucins and their glycoforms. Methods Mol Biol. 2000;125:77–85. doi: 10.1385/1-59259-048-9:077. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Fabricant M, Tisdale AS, Spurr-Michaud SJ, Lindberg K, Gipson IK. Human corneal and conjunctival epithelia produce a mucin-like glycoprotein for the apical surface. Invest Ophthalmol Vis Sci. 1995;36:337–344. [PubMed] [Google Scholar]
- Wesseling J, van der Valk S, Vos H, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–265. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom C, Carlstedt I. N-terminal cleavage of the salivary MUC5B mucin. Analogy with the Van Willebrand propolypeptide? J Biol Chem. 2001;276:47116–47121. doi: 10.1074/jbc.M106593200. [DOI] [PubMed] [Google Scholar]
- Wickstrom C, Davies J, Eriksen G, Veerman E, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem J. 1998;334:685–693. doi: 10.1042/bj3340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SJ, McGuckin MA, Gotley DC, Eyre HJ, Sutherland GR, Antalis TM. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 1999;59:4083–4089. [PubMed] [Google Scholar]
- Williams SJ, Wreschner DH, Tran M, Eyre HJ, Sutherland GR, McGuckin MA. Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J Biol Chem. 2001;276:18327–18336. doi: 10.1074/jbc.M008850200. [DOI] [PubMed] [Google Scholar]
- Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Perez A, Yasin M, Soto P, Rong M, Theodoropoulos G, Carothers Carraway CACK. Presence of MUC4 in human milk and at the luminal surfaces of blood vessels. J Cell Physiol. 2005;204:166–177. doi: 10.1002/jcp.20277. [DOI] [PubMed] [Google Scholar]
- Zhao H, Jumblatt JE, Wood TO, Jumblatt MM. Quantification of MUC5AC protein in human tears. Cornea. 2001;20:873–877. doi: 10.1097/00003226-200111000-00019. [DOI] [PubMed] [Google Scholar]
- Zrihan-Licht S, Vos H, Baruch A, Elroy-Stein O, Sagiv D, Keydar I, Hilkens J, Wreschner D. Characterization and molecular cloning of a novel MUC1 protein, devoid of tandem repeats, expressed in human breast cancer tissue. Eur J Biochem. 1994;224:787–795. doi: 10.1111/j.1432-1033.1994.00787.x. [DOI] [PubMed] [Google Scholar]


