Abstract
Purpose
Elevated intraocular pressure (IOP), the major causal risk factor for glaucoma, often decreases after cataract removal by phacoemulsification ultrasound. In this study, the hypothesis that ultrasound energy propagated through a fluid medium induces a stress response with the potential to lower IOP was investigated.
Methods
Normal and glaucomatous trabecular meshwork (TM) cell culture lines were initiated from tissue isolated from human cadaveric eyes or trabeculectomy specimens. Cultured cells were treated for 60 seconds with a phacoemulsification ultrasound probe set to a power of 70%. Activation of the TM cell-specific stress response was assayed by enzyme-linked immunosorbent assay (ELISA) and immunolocalization.
Results
Normal TM cell cultures did not release detectable levels of the stress response protein, IL-1α, into their culture medium. In contrast, IL-1α was easily detected after treatment with ultrasound energy. Consistent with earlier findings, glaucomatous TM cells produced IL-1α constitutively, and the level of expression was increased after treatment with phacoemulsification ultrasound. As was previously demonstrated, the stressregulated transcription factor NF-κB was present in the cytoplasm of normal cells, but in the nucleus of glaucomatous cells. After treatment with ultrasound energy, NF-κB translocated to the nucleus in the normal cells. Endothelial leukocyte-adhesion molecule (ELAM)-1 was not detected in normal TM cells, but was constitutively present on glaucomatous TM cells, consistent with findings in previous work. ELAM-1 expression was induced in normal cells by ultrasound treatment.
Conclusions
A potentially IOP-lowering stress response is induced in TM cells by ultrasound. The findings suggest that this response may be induced clinically during cataract removal by phacoemulsification, and may be one mechanism responsible for the reduction in IOP that often follows this procedure.
The glaucomas, characterized by cupping of the optic nerve head and loss of retinal ganglion cells, are the leading cause of irreversible blindness, affecting approximately 70 million people worldwide.1 Commonly, glaucoma begins with a defect in the aqueous outflow pathway or “drain” in the front of the eye, located in the angle of the anterior chamber. The consequence of this impairment is an elevation in the intraocular pressure (IOP) that leads to irreversible optic nerve damage and ultimately, blindness.
The eye’s aqueous outflow pathway consists of a series of structures for conducting fluid into the episcleral vasculature. Fluid first enters the trabecular meshwork (TM), a spongelike filter that acts as the major site for outflow resistance. Like the vasculature, the cells of the TM are a form of endothelium; however, they have some distinct differences that reflect their developmental origin in the neural crest.2,3
Recently, we reported that expression of the vascular endothelial leukocyte-adhesion molecule (ELAM)-1 by TM cells is diagnostic of glaucoma.4 We found that ELAM-1 expression in glaucomatous TM cells is due to sustained activation of an interleukin (IL)-1 autocrine feedback loop through the transcription factor nuclear factor (NF)- κB. In contrast, IL-1 is not synthesized by normal TM cells, but synthesis can be stimulated by treatment with exogenous IL-1.
IL-1 has been reported to decrease aqueous outflow facility when administered exogenously.5 Studies in vascular endothelium have revealed that acute changes in fluid pressure dynamics, including shear stress and pulsatile pressure gradients, activate a tissue-specific stress response that involves the induction of IL-1 and expression of ELAM-1.6 Putting these two observations together has led us to propose that stimulation of IL-1 synthesis by TM cells may be a natural response to elevated IOP, acting to bring the pressure down.
A clinical observation is that IOP is commonly elevated after intracapsular or extracapsular cataract extraction. In contrast, IOP typically decreases after the current technique of cataract removal by ultrasound phacoemulsification.7–13 The reason for the difference in outcome of the two surgical techniques is not known. It occurred to us that ultrasound creates a fluid pressure gradient14–17 and that this may have the same effect as elevated IOP or sheer stress in activating expression of IL- and may explain the IOP-lowering effects of ultrasound phacoemulsification. In the study reported herein, we began to examine this hypothesis by investigating the effects of phacoemulsification ultrasound treatment on activation of the IL-1/NF-κB pathway and expression of ELAM-1 by cultured TM cells.
Materials and Methods
Cell Culture and Phacoemulsification Treatment
Normal human cadaveric eyes with no history of glaucoma, ocular surgery, or trauma were obtained from National Disease Research Interchange (NDRI; Philadelphia, PA). Enucleations were performed within 6 hours of death. Glaucomatous TM was isolated from either human cadaveric eyes or trabeculectomy specimens.
Anterior segments were removed and divided into four segments after the lenses were removed. The TM was dissected from neighboring tissues, and incisions were made at the anterior and posterior edges of the TM. The explants were placed in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 1% 200 mM glutamine, 1% antibiotic-antimycotic, and 1% 1 M HEPES buffer. The explants were incubated in a 5% CO2 humidified atmosphere at 37°C. When the cells grew out sufficiently, the explants were removed, and the cells continued to grow under the same conditions. When confluence was reached, the cells were trypsinized and subcultured.
For experiments, cultured TM cells were plated into a 24-well culture dish and allowed to multiply until 80% to 90% confluent. Just before an experiment was initiated, the cell culture medium was removed and 1 mL of fresh medium was added to nearly fill each well. Cultures were then treated with ultrasound energy for 60 seconds, using a phacoemulsification probe set to a power of 70%. The probe tip was immersed in the cell culture medium, but not deep enough to touch the cells. Sham samples were exposed to the probe, but the power was not turned on. Untreated cells were not exposed to the probe. Cell-conditioned medium was collected from cultures at 4, 12, 24, and 40 hours after treatment.
Enzyme-Linked Immunosorbent Assay and Immunolocalization
IL-1α levels in samples of conditioned medium were determined by ELISA (R&D Systems Inc., Minneapolis, MN). Analysis for statistical significance of differences between treatment groups was performed by application of Student’s t-test. The α level for significance was set at P = 0.01.
Activation of transcription factor NF-κB was determined by monitoring its translocation to the nucleus by indirect immunolocalization, using a primary antibody specific for the p65 subunit of NF-κB (mouse monoclonal; Roche Molecular Biochemicals, Indianapolis, IN). The secondary antibody was a fluorescein isothiocyanate (FITC)–conjugated donkey anti-sheep IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Expression of ELAM-1 in cells was determined by indirect immunolocalization using anti-ELAM-1 as the primary antibody (human anti-mouse; PharMingen Inc., San Diego, CA). Antigen localization was visualized with a microscope equipped for epifluorescence (Nikon, Tokyo, Japan). The method for indirect immunolocalization was as we have described.4
Results
Production of IL-1α by cultured TM cells was determined by an ELISA measuring the amount of IL-1α that accumulated in the culture medium over a specific period (Fig. 1). Normal untreated TM cells did not release detectable amounts of IL-1α into their culture medium. In contrast, released IL-1α was easily detected in normal cell-conditioned culture medium after the cells were treated with phacoemulsification ultrasound. The IL-1α level in these cells was significantly increased at 4 hours after treatment, compared with the sham control. At 24 and 40 hours after treatment, IL-1α remained elevated, but did not increase significantly over the 4-hour time point. Treatment with phacoemulsification ultrasound did not disrupt TM cells, as revealed by phase contrast microscopy (data not shown), suggesting that release of IL-1α was the result of new synthesis and secretion.
Figure 1.
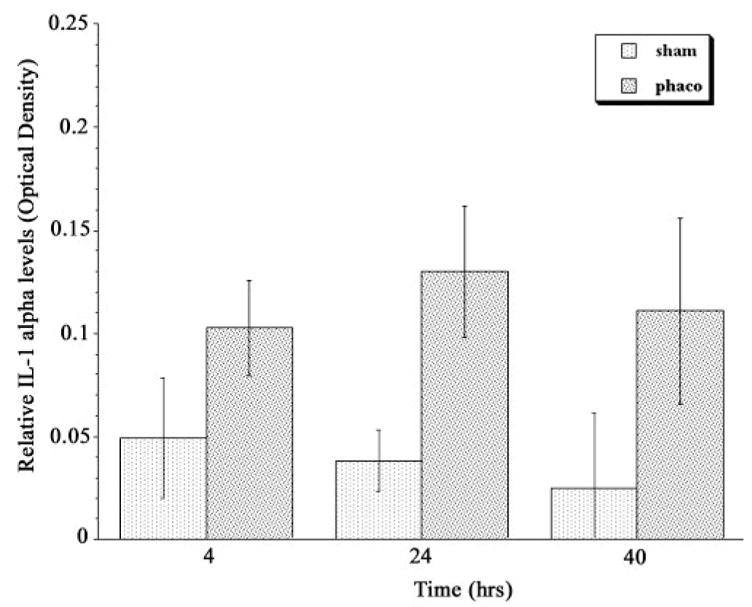
IL-1α accumulation in cell culture medium conditioned by normal TM cells after treatment with phacoemulsification ultrasound energy. Culture medium was collected at the indicated time points after phacoemulsification ultrasound energy (phaco) or sham treatment, and IL-1α content was quantified by ELISA. The error bars indicate SD from the mean. n = 8; P = 0.0006 at 4 hours, 0.0003 at 24 hours, and 0.0029 at 40 hours.
Consistent with our earlier findings, glaucomatous TM cells released IL-1α into the culture medium constitutively (Fig. 2). The level appeared to increase after treatment with ultrasound energy, but this did not achieve statistical significance at any time point.
Figure 2.
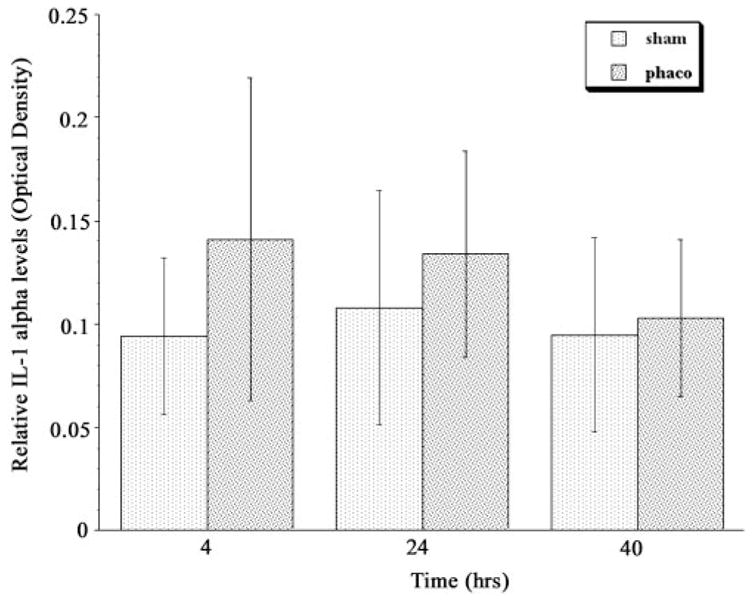
IL-1α accumulation in cell culture medium conditioned by glaucomatous TM cells after treatment with phacoemulsification ultrasound energy. Medium was examined as in Figure 1. The error bars indicate SD from the mean. n = 7; P = 0.11 at 4 hours, 0.29 at 24 hours, and 0.68 at 40 hours.
Expression of the gene for IL-1α is controlled by the transcription factor NF-κB. Activation of NF-κB was determined by monitoring its translocation from the cytoplasm to the nucleus of individual cells by indirect immunolocalization (Fig. 3). As we previously demonstrated, NF-κB was present in the cytoplasm of normal TM cells, but in the nucleus of glaucomatous TM cells. Four hours after treatment with ultrasound energy, NF-κB had translocated to the nucleus in some of the normal cells. At 12 hours after ultrasound treatment, most of the cell nuclei contained NF-κB protein. After 24 hours, there was a reduction in nuclear staining for NF-κB.
Figure 3.
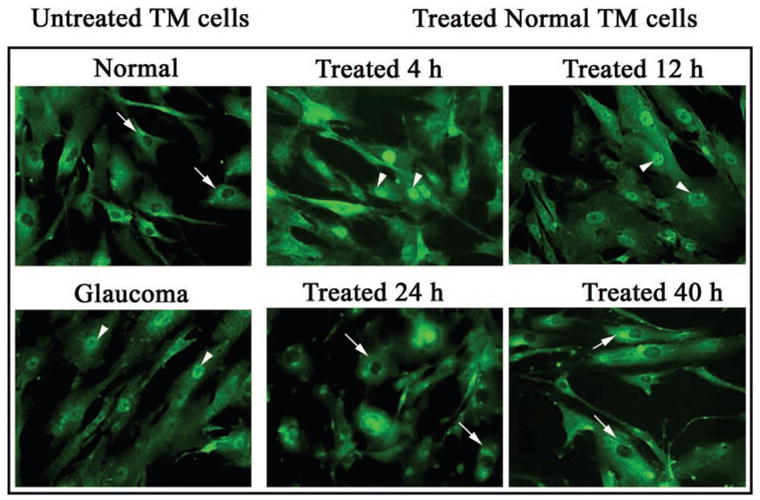
Activation of NF-κB in TM cells after treatment with phacoemulsification ultrasound energy. Arrows: concentration of the p65 subunit of NF-κB, as determined by indirect immunofluorescence localization.
ELAM-1 was not detected in normal TM cells, but was constitutively present on glaucomatous TM cells, consistent with our previous work (Fig. 4). Sham treatment did not induce expression of ELAM-1 in normal TM cells, but expression was induced by ultrasound treatment. In untreated cells, the protein was diffusely localized in treated cells, the protein was concentrated in a caplike structure around the cell nucleus, consistent with its active synthesis and secretion through the endoplasmic reticulum and Golgi apparatus. The number of treated cells that stained positively for ELAM-1 was at a maximum at 12 to 24 hours after treatment. No change in ELAM-1 expression occurred in glaucomatous TM cells after application of ultrasound energy.
Figure 4.
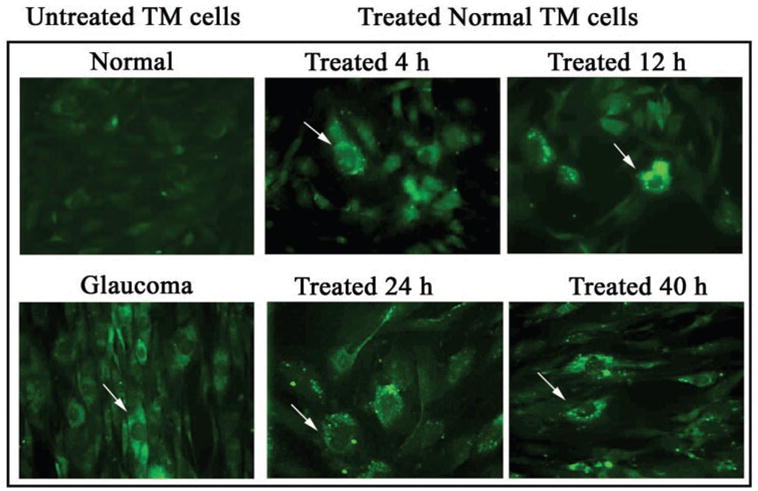
Expression of ELAM-1 by TM cells after treatment with phacoemulsification ultrasound energy. Arrows: concentration of ELAM-1, as determined by indirect immunofluorescence localization.
Discussion
In the current study, the IL-1/NF-κB/ELAM-1 pathway was activated in TM cells when cultures were challenged with phacoemulsification ultrasound. These findings are consistent with activation of this pathway in response to phacoemulsification clinically. We suggest that the release of IL-1 may be involved in the reduction in IOP that occurs after the procedure.
Propagation of power ultrasound through a liquid initiates acoustic cavitation and also induces fluid dynamics phenomena, such as free deformation, convection, and acoustic streaming.17 This effect has been harnessed in the phacoemulsification procedure. However, depending on its intensity and duration, ultrasound can cause undesirable side effects—in particular, damage to the corneal endothelium.15 In one study, it was found that the degree of lethal cell injury induced by ultrasound correlated with exposure intensity and duration.15 A causal factor in endothelial cell damage was the mechanical effects of the cavitation bubbles on endothelial cell permeability. Another damaging factor was identified as free radicals,14 which are induced when cavitation bubbles implode. At the lower intensity levels used in the study described herein, however, ultrasound treatment was not lethal, causing only transient changes in plasma membrane permeability.15
The corneal endothelium is continuous with the TM and shares a common embryonic origin. It is reasonable to propose that the phacoemulsification procedure would have similar effects on both cell types, although this has never been specifically examined. Mechanical stresses alter the structural and functional properties of cells at the cellular, molecular, and genetic levels, causing rapid responses in the adjacent tissues and slower adaptive changes to a sustained stressful environment.18 Cellular responses differ, depending on the type of stress and on dynamics such as duration and repetition.6 IL-1 may be induced with some stimuli; free radicals are well-known inducers of the IL-1/NF-κB pathway in a variety of cell types.
Vascular biologists have long recognized that cell culture provides an optimal model for investigating the specific effects of mechanical stress on cells, because its reductionist nature eliminates the multiple physiological factors that would confound analysis in vivo.6 More recently, cell culture models have been similarly used for studying fluid-pressure–related mechanical effects on TM cells.19–24 These studies have identified specific changes in gene expression, cytoskeletal organization, and signal-transduction pathway activation. In vascular endothelial cells, the IL-1/NF-κB pathway is activated and ELAM-1 expression is upregulated in response to specific types of fluid stress. However, this is the first study to our knowledge to show that a similar change can be produced in TM cells.
Endogenous production of IL-1 by TM cells may be a unifying mechanism that explains the beneficial effects of various procedures on enhancing aqueous outflow. An example of such a procedure is laser trabeculoplasty, a common, noninvasive treatment for glaucoma that consists of applying 50-μm burns to the trabecular meshwork using an argon-dye laser. This treatment restores aqueous outflow in a significant percentage of treated patients.25 Recent studies have demonstrated that the procedure stimulates production of IL-1 and TNF-α by the cells, which in turn stimulates synthesis of matrix metalloproteinases.26
Stress responses are typically protective in the short run, but when continued on a prolonged basis, they can become the disease entity itself. In addition to being upregulated in response to shear stress, IL-1 and ELAM-1 are also expressed in vascular disease.27 In fact, ELAM-1 is one of the earliest markers of the atherosclerotic plaque. Similarly, short-term activation of the IL-1 autocrine loop may help to control IOP and be cyto-protective, but it is also possible that a pathologic TM response may occur when activation is sustained. This may result from elevated fluid pressure gradients, or from other stimuli that induce IL-1 expression, such as oxidative stress.28,29
IL-1 causes changes in aqueous outflow when applied exogenously to eyes.5,26 How IL-1 facilitates aqueous outflow is not known. The changes related to IL-1 may be due to a number of factors, one being the production of matrix metalloproteinases (MMPs), enzymes that catalyze tissue remodeling. The hypothesis is that this reduces extracellular matrix resistance, increasing facility of outflow. Another possibility is that IL-1 affects ciliary muscle tone. IL-1 could also have direct effects on the TM, for example, stimulating phagocytotic activity. IL-1 induces expression of ELAM-1, and this could alter outflow by changing cell shape.
Our findings of the induction of the IL-1/NF-κB/ELAM-1 pathway by phacoemulsification ultrasound support the concept that this cascade represents a final common pathway in the response of the TM to stress. The activation of this pathway by a variety of stressors suggests the primacy of this route in the genetic programming of the TM. The functional implications of these findings and the importance of this pathway in the pathogenesis of glaucoma or its modulation in glaucoma therapy remains to be explored.
Acknowledgments
The authors are grateful to Maria A. Page for technical support.
Supported by National Eye Institute Grants R01-EY13178, R01-EY09828, and P30-EY13078; by a grant from the Massachusetts Lions Eye Research Fund Inc.; and by Research to Prevent Blindness.
Footnotes
Disclosure: N. Wang, None; S.K. Chintala, None; M.E. Fini, None; J.S. Schuman, None
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krohn J. Expression of factor VIII-related antigen in human aqueous drainage channels. Acta Ophthalmol Scand. 1999;77:9–12. doi: 10.1034/j.1600-0420.1999.770102.x. [DOI] [PubMed] [Google Scholar]
- 3.Foets B, van den Oord J, Engelmann K, Missotten L. A comparative immunohistochemical study of human corneotrabecular tissue. Graefes Arch Clin Exp Ophthalmol. 1992;230:269–274. doi: 10.1007/BF00176303. [DOI] [PubMed] [Google Scholar]
- 4.Wang N, Chintala SK, Fini ME, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001;7:304–309. doi: 10.1038/85446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kee C, Seo K. The effect of interleukin-1α on outflow facility in rat eyes. J Glaucoma. 1997;6:246–249. [PubMed] [Google Scholar]
- 6.Gimbrone MA, Jr, Nagel T, Topper JN. Biomechanical activation: an emerging paradigm in endothelial adhesion biology. J Clin Invest. 1997;100:S61–S65. [PubMed] [Google Scholar]
- 7.Pohjalainen T, Vesti E, Uusitalo RJ, Laatikainen L. Phacoemulsification and intraocular lens implantation in eyes with open-angle glaucoma. Acta Ophthalmol Scand. 2001;79:313–316. doi: 10.1034/j.1600-0420.2001.790322.x. [DOI] [PubMed] [Google Scholar]
- 8.Pohjalainen T, Vesti E, Uusitalo RJ, Laatikainen L. Intraocular pressure after phacoemulsification and intraocular lens implantation in nonglaucomatous eyes with and without exfoliation. J Cataract Refract Surg. 2001;27:426–431. doi: 10.1016/s0886-3350(00)00691-x. [DOI] [PubMed] [Google Scholar]
- 9.Shingleton BJ, Wadhwani RA, O’Donoghue MW, Baylus S, Hoey H. Evaluation of intraocular pressure in the immediate period after phacoemulsification. J Cataract Refract Surg. 2001;27:524–527. doi: 10.1016/s0886-3350(00)00641-6. [DOI] [PubMed] [Google Scholar]
- 10.Merkur A, Damji KF, Mintsioulis G, Hodge WG. Intraocular pressure decrease after phacoemulsification in patients with pseudoexfoliation syndrome. J Cataract Refract Surg. 2001;27:528–532. doi: 10.1016/s0886-3350(00)00753-7. [DOI] [PubMed] [Google Scholar]
- 11.Sacca S, Marletta A, Pascotto A, et al. Daily tonometric curves after cataract surgery. Br J Ophthalmol. 2001;85:24–29. doi: 10.1136/bjo.85.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong JT, Miller KM. Intraocular pressure change after sutureless phacoemulsification and foldable posterior chamber lens implantation. J Cataract Refract Surg. 1998;24:256–262. doi: 10.1016/s0886-3350(98)80208-3. [DOI] [PubMed] [Google Scholar]
- 13.Tong JT, Miller KM. Long-term intraocular pressure changes after phacoemulsification. J Cataract Refract Surg. 1999;25:1560. doi: 10.1016/s0886-3350(99)00313-2. [DOI] [PubMed] [Google Scholar]
- 14.Holst A, Rolfsen W, Svensson B, Ollinger K, Lundgren B. Formation of free radicals during phacoemulsification. Curr Eye Res. 1993;12:359–365. doi: 10.3109/02713689308999460. [DOI] [PubMed] [Google Scholar]
- 15.Saito K, Miyake K, McNeil PL, Kato K, Yago K, Sugai N. Plasma membrane disruption underlies injury of the corneal endothelium by ultrasound. Exp Eye Res. 1999;68:431–437. doi: 10.1006/exer.1998.0626. [DOI] [PubMed] [Google Scholar]
- 16.Lin L, Wu J, Ho KP, Qi S. Ultrasound-induced physiological effects and secondary metabolite (saponin) production in Panax ginseng cell cultures. Ultrasound Med Biol. 2001;27:1147–1152. doi: 10.1016/s0301-5629(01)00412-4. [DOI] [PubMed] [Google Scholar]
- 17.Laborde JL, Hita A, Caltagirone JP, Gerard A. Fluid dynamics phenomena induced by power ultrasounds. Ultrasonics. 2000;38:297–300. doi: 10.1016/s0041-624x(99)00124-9. [DOI] [PubMed] [Google Scholar]
- 18.Davies PF, Tripathi SC. Mechanical stress mechanisms and the cell: an endothelial paradigm. Circ Res. 1993;72:239–245. doi: 10.1161/01.res.72.2.239. [DOI] [PubMed] [Google Scholar]
- 19.Tumminia SJ, Mitton KP, Arora J, Zelenka P, Epstein DL, Russell P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1998;39:1361–1371. [PubMed] [Google Scholar]
- 20.Mitton KP, Tumminia SJ, Arora J, Zelenka P, Epstein DL, Russell P. Transient loss of alphaB-crystallin: an early cellular response to mechanical stretch. Biochem Biophys Res Commun. 1997;235:69–73. doi: 10.1006/bbrc.1997.6737. [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, Matsuo T, Ohtsuki H. A novel gene (oculomedin) induced by mechanical stretching in human trabecular cells of the eye. Biochem Biophys Res Commun. 1999;259:349–351. doi: 10.1006/bbrc.1999.0797. [DOI] [PubMed] [Google Scholar]
- 22.Booth A, Nguyen T, Polansky J. TIGR and stretch in the trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:1888–1889. [PubMed] [Google Scholar]
- 23.Tamm ER, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular mesh-work. Invest Ophthalmol Vis Sci. 1999;40:2577–2582. [PubMed] [Google Scholar]
- 24.Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42:1505–1513. [PubMed] [Google Scholar]
- 25.Van Buskirk EM. Pathophysiology of laser trabeculoplasty. Surv Ophthalmol. 1989;33:264–272. doi: 10.1016/0039-6257(82)90152-7. [DOI] [PubMed] [Google Scholar]
- 26.Bradley JM, Anderssohn AM, Colvis CM, et al. Mediation of laser trabeculoplasty-induced matrix metalloproteinase expression by IL-1β and TNFα. Invest Ophthalmol Vis Sci. 2000;41:422–430. [PubMed] [Google Scholar]
- 27.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 28.Green K. Free radicals and aging of anterior segment tissues of the eye: a hypothesis. Ophthalmic Res. 1995;27:143–149. doi: 10.1159/000267860. [DOI] [PubMed] [Google Scholar]
- 29.Flammer J, Haefliger IO, Orgul S, Resink T. Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma. 1999;8:212–219. [PubMed] [Google Scholar]


