Abstract
We investigated whether compensatory reinnervation in the corticospinal tract (CST) and the corticorubral tract (CRT) is enhanced by administration of bone marrow stromal cells (BMSCs) after experimental stroke. Adult male Wistar rats were subjected to permanent right middle cerebral artery occlusion (MCAo). Phosphate-buffered saline (PBS, control, n=7) or 3 × 106 BMSCs in PBS (n=8) were injected into a tail vein at 1 day postischemia. The CST of the left sensorimotor cortices was labeled with DiI 2 days prior to MCAo. Functional recovery was measured. Rats were sacrificed at 28 days after MCAo. The brain and spinal cord were removed and processed for vibratome sections for laser-scanning confocal analysis and paraffin sections for immunohistochemistry. Normal rats (n=4) exhibited a predominantly unilateral pattern of innervation of CST and CRT axons. After stroke, bilateral innervation occurred through axonal sprouting of the uninjured CRT and CST. Administration of BMSCs significantly increased the axonal restructuring on the de-afferented red nucleus and the denervated spinal motoneurons (p<0.05). BMSC treatment also significantly increased synaptic proteins in the denervated motoneurons. These results were highly correlated with improved functional outcome after stroke (r>0.81, p<0.01). We conclude that the transplantation of BMSCs enhance axonal sprouting and rewiring into the denervated spinal cord which may facilitate functional recovery after focal cerebral ischemia.
Keywords: axonal sprouting, bone marrow stromal cells, corticospinal tract, corticorubral tract, middle cerebral artery occlusion, rats, stroke
1. Introduction
In contrast to axons of the peripheral nervous system, axons in the central nervous system (CNS) of adult mammals display a limited ability for self-repair after stroke or injury, often leading to long-term devastating and irreversible disability. However, both experimental study and clinical observation indicate that, depending on the site or the degree of damage, some functional recovery may occur spontaneously with time, and can be enhanced by rehabilitation training and therapies (Rijntjes, 2006). This recovery may in part be due to the reorganization of the neural network in the remaining compromised brain tissue (Carmichael, 2006).
The mechanisms underlying motor function recovery depend on the extent of the injury. Following damage to only part of the motor cortex or the pyramidal tract, motor recovery is mediated by reorganization of motor function immediately around the lesion site (Bastings et al., 2002). If the motor cortex is extensively damaged, an alternative network outside the damaged area either in the same hemisphere or in the opposite hemisphere occurs in addition to recruitment of the cortex within the damaged system (Seitz et al., 1998). Three major mechanisms for the plastic reorganization of connections between motor cortex and target motoneurons have been considered: unmasking of existing but functionally inactive pathways, sprouting of fibers from surviving neurons and formation of new synapses, and redundancy of CNS circuitry allowing alternative pathways to take over functions (Lee and van Donkelaar, 1995). Unfortunately, the direct neuroanatomical mechanisms for plastic compensatory reorganization of neural connective pathways to rewire the denervated spinal cord from the brain during the motor control recovery phase after stroke are not well understood.
Using a model of middle cerebral artery occlusion (MCAo) in rodents, we have demonstrated that bone marrow stromal cell (BMSC) administration significantly improves functional outcome after stroke (Chen et al., 2000). Previous studies show that the BMSCs survive in the ischemic brain, and specifically migrate into the ischemic boundary zone (IBZ) after transplantation (Chen et al., 2001; Irons et al., 2004; Lee et al., 2003; Li et al., 2002). Administration of BMSCs reduces neuronal apoptosis (Chen et al., 2003a), promotes angiogenesis (Chen et al., 2003b), synaptogenesis (Zhang et al., 2006) and neurogenesis (Chen et al., 2004) associated with cerebral ischemia in adult rat brain. Hence, transplantation of BMSCs may play an important integrative role in the neural plastic reorganization needed for neurological functional recovery.
Because neural rewiring of the denervated side of spinal cord from the brain must be the neuroanatomical foundation of functional recovery after stroke, we focused on the descending axons of the corticospinal tract (CST), the only direct pathway from the motorsensory cortex to the spinal cord and the major neural pathway for control of voluntary movement (Heffner and Masterton, 1983), and the corticorubral tract (CRT), a well-established subcortical target of the forelimb motor cortex and an important nucleus for coordinating sensations and movements of the whole upper body (Cooper et al., 2000). We therefore designed a cortical anterograde tracing experiment to label the cortex pyramidal neurons and their long axons, to investigate whether the compensatory reinnervation from the intact side onto the denervated red nucleus and spinal cord is enhanced by BMSC treatment in a rat MCAo model.
2. Results
2.1. Neurological functional test and lesion volume
In this study, the adhesive-removal test was used to measure the sensorimotor performance. Rats with BMSC treatment had significantly improved functional recovery at 14, 21, and 28 days compared to the control group (Figure 1A, p<0.05). The lesion volume showed no difference between the control and treatment groups at four weeks after stroke (Figure 1B).
Figure 1. Neurological functional test and lesion volume.
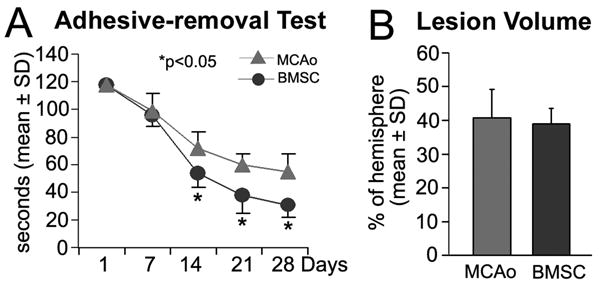
Compared to the control animals (n=7), BMSC treatment (n=8) significantly improved the functional recovery measured by the adhesive-removal test after stroke (A, *p<0.05). The lesion volume showed no difference between the control and BMSC treatment groups at four weeks after stroke (B).
2.2. CRT structural remodeling on the denervated red nucleus
To investigate the structural reorganization of the neural pathways from the sensorimotor cortex to the red nuclei and spinal cord during the recovery phase after experimental stroke in the rat, we labeled the CRT and the CST anterogradely with the fluorescent tracer DiI injected into laminae V of the left intact motorsensory cortex (Figure 2A). Because we injected a small dye volume through a finely drawn glass capillary, no leakage was observed outside of the cortex at the injection site. The restructuring of the CRT after ischemic stroke was assessed by the ratio of DiI density in the red nuclei of the intact and injured sides. After stroke, reorganization of the intact CRT to the lesioned red nucleus is apparent (Figure 2C), while the normal corticorubral pathway is primarily an ipsilateral projection with a minor contralateral component (Figure 2B). However, rats with BMSC treatment after stroke showed significantly higher density of DiI-labeled fibers in the de-afferented red nucleus than controls (Figure 2D and E, p<0.05), and high correlation with improved functional recovery (r=0.82, p<0.01).
Figure 2. CRT and CST tracing with DiI cortical injections.
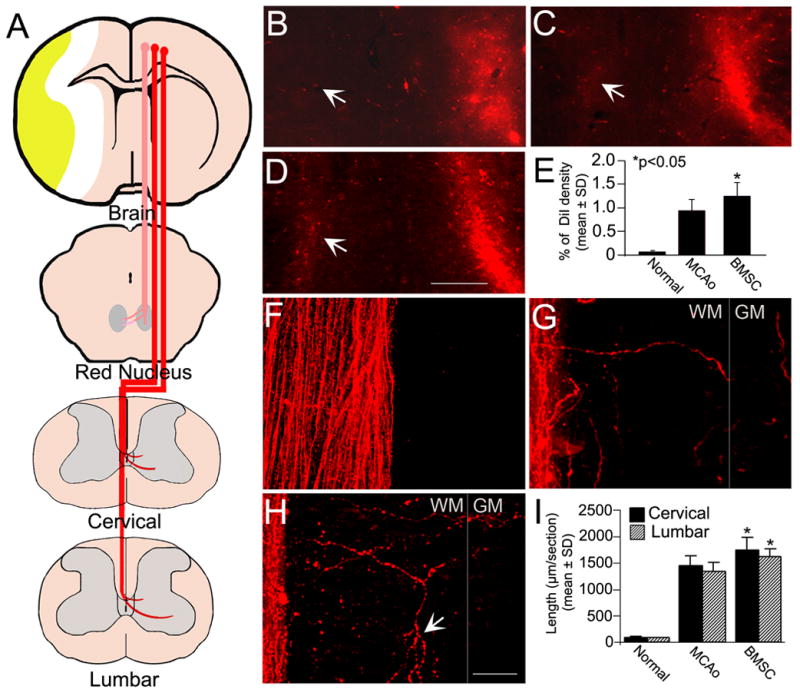
The CRT and CST arising from the left intact hemisphere were labeled by DiI cortical injections as shown in the schematic diagram (A). In normal rats, the CRT innervation was unilateral (B). Some CRT fibers changed their pathway to the lesioned red nucleus after stroke (C). Rats with BMSC treatment after stroke had an increased density of DiI-labeled fibers in the contralateral de-afferented red nucleus (D). Quantitation shows a significant CRT restructuring in BMSC treated animals as compared with controls (E, *p<0.05). In normal rats, no obvious axons crossed the midline into the opposite part of dorsal funiculus in a representative cervical longitudinal section (F). Four weeks after stroke, some DiI-labeled CST fibers were observed in the denervated side of the dorsal funiculus extending toward to the gray matter (G). The sprouting of branched CST axons from the intact side was enhanced by BMSC treatment postischemia (arrows in H). Compared with controls, the length of CST fibers was significantly increased in BMSC treated animals both in the cervical and lumbar cord (I, *p<0.05). WM: white matter, GM: gray matter. Scale Bar=250 μm in B–D, =25 μm in F–H.
2.3. CST sprouting into the denervated dorsal funiculus of the spinal cord
As early as two weeks after cortical injection, the dye diffused over the entire length of CST through the decussation, and extended to the ventral-most part of the contralateral dorsal funiculus. There were very few uncrossed CST fibers running in the ventral funiculus close to the midline (data not shown). In normal animals, the dorsal CST axons show a nearly complete unilateral innervation pattern (Figure 2F). Four weeks after stroke, some CST axons of the intact side spontaneously sprouted throughout the opposite half part of dorsal funiculus into the gray matter of the spinal cord (Figure 2G), in where the endogenous CST axons were degenerated (data not shown). The BMSC treatment significantly increased the number and length of sprouting axons in the denervated dorsal funiculus compared to controls (Figure 2H and I, p<0.05). Statistical analysis showed a significant correlation between the axonal sprouting in the white matter and the functional outcome enhanced by BMSC treatment (r=0.87, p<0.01). In addition, increased axonal branching was observed on growing fibers in the BMSC treatment group (arrows in Figure 2H).
2.4. CST reorganization crossing over the midline into the denervated side of spinal gray matter
At the cervical and lumbar levels, the descending CST motor fibers extend into the same side of gray matter to form neural circuits from spinal interneurons to spinal motoneurons for the control of forelimb and hindlimb movement (Yang and Lemon, 2003). No obvious DiI-labeled CST fibers crossing over the midline were found in the opposite side of the spinal cord on transverse sections in normal rats (Figure 3A). In MCAo control rats, a remarkable axonal sprouting from the intact CST was observed in the denervated side of the gray matter (Figure 3B), without conspicuous increase of axonal outgrowth in the uninjured gray matter. However, the CST sprouting was further enhanced in the BMSC treated animals (Figure 3C). Axonal reorganization was evaluated by the ratio of fluorescent density in the denervated side and the intact side, which was interpreted as axonal innervation arising from the cortical pyramidal cells in the intact hemisphere. Our data indicate that the treatment of stroke with BMSCs significantly promoted axonal reorganization in the denervated spinal cord at both cervical and lumbar levels after experimental stroke (Figure 3D, p<0.05), which were highly correlated to the functional recovery (r=0.81, p<0.01).
Figure 3. Transverse confocal images showing the axonal sprouting into the spinal gray matter of the cervical cord.

In normal rats, the CST showed a unilateral innervation manner (A). In rats after MCAo, DiI-labeled intact CST axons extended into the denervated side of the gray matter (B). BMSC treatment significantly increased the intact CST axonal sprouting into the denervated side in both the cervical and lumbar levels (C and D, *p<0.05). Insert of A, schematic drawing of the spinal gray matter showing the position of the photomicrograph appearing in A, B and C. cc: central canal. Scale Bar=25 μm in A–C.
2.5. Immunohistochemical analysis of synapse formation on motoneurons in the denervated side of the spinal cord
To confirm the rewiring of denervated spinal motoneurons, we further examined whether synaptic structure of denervated motoneurons were changed after ischemic stroke with or without BMSC treatment at the light microscopic level with immunoreactivity for the presynaptic marker synaptophysin and postsynaptic marker PSD-95. In the ventral horn of spinal transverse sections from normal rats, immunostaining for the synaptophysin proteins yielded punctated signals, which collectively encircled spinal motoneurons, including their especially large cell bodies and dendritic trees (Figure 4A). After ischemic stroke, the positive synaptophysin density around neuronal somata and along dendrites was greatly reduced in the denervated side of the spinal cord (Figure 4B). Nevertheless, substantial enhancement in the staining for synaptophysin was observed in the motor pool of denervated ventral horn after BMSC treatment (Figure 4C). Because the synapses between CST axons and motoneurons cannot be specifically identified, we measured the abatement of positive staining density in the denervated ventral horn compared to that in the intact side on the same sections. Quantitation showed that in control rats, synaptophysin was reduced by 44.7% and 42.5% in the cervical and lumbar enlargements, respectively (Figure 4D). Although there was a significant increase of 9.0% in the cervical cord and 7.2% in the lumbar cord in the BMSC treatment group compared with the MCAo control group (Figure 4D, p<0.05), the density of synaptophysin remained lower than normal level. Statistical data showed that the increased synaptophysin expression was highly correlated to the functional outcome enhanced by BMSCs (r=0.91, p<0.01).
Figure 4. Immunohistochemical staining of synaptophysin and PSD-95 on spinal motoneurons in the denervated side of the spinal cord.

In normal rats, synaptophysin positive staining encircled spinal motoneurons, including their large cell bodies and dendritic trees (A). After stroke, the synaptophysin density was reduced (B). Synaptophysin staining was increased in the motor pool of the denervated ventral horn after BMSC treatment (C). Quantitative data show that the expression of synaptophysin was significantly enhanced in the cervical and lumbar cord by BMSC administration compared to controls (D, *p<0.05). PSD-95 staining was observed in the cytoplasm with thick proximal dendrites of spinal motoneurons in normal rats (E). PSD-95 expression was reduced postischemia (F). BMSC treatment significantly increased the PSD-95 level in the denervated spinal motoneurons (G and H, *p<0.05). Insert of A, schematic drawing of the spinal gray matter showing the position of the photomicrograph appearing in A–C and E–G. Scale Bar=25 μm in A–C and E–G.
PSD-95 staining was observed in the cytoplasm and thicker proximal dendrites of motoneurons in the spinal ventral horn (Figure 4E). Similar to the staining pattern of synaptophysin, PSD-95 density was reduced to 58.1% and 60.8% of normal level in the cervical and lumber cord postischemia, respectively (Figure 4F and H). BMSC treatment increased the PSD-95 level in the denervated spinal motoneurons to 67.9% and 69.8% of that in normal side of the cervical and lumbar cord, respectively, significantly greater than MCAo controls (Figure 4G and H, p<0.05). A high correlation coefficient was also observed between the BMSCs enhanced PSD-95 expression and functional recovery (r=0.88, p<0.01).
3. Discussion
To our knowledge, we, for the first time demonstrated that BMSC treatment of ischemic stroke increase axonal sprouting and rewiring of the CST emanating from the uninjured motor cortex onto denervated spinal cord. In normal rodents, innervation of the dorsal CST axons was predominantly unilateral. After stroke, bilateral innervation occurs through the intact CST sprouting to rewire the denervated spinal cord. Furthermore, our data showed that BMSC treatment of ischemic stroke in the rat significantly increased the axonal connections from the intact motor cortex to the denervated spinal cord at both the cervical and lumbar levels. In light of high correlation coefficients (r>0.81) relating the functional outcome to neuroanatomical measurements and immunostaining data, we propose that stroke recovery enhanced by BMSC treatment may in part be attributed to structural reorganization of the intact CST neurites. However, the increased compensatory innervation from the intact CST projections crossing over onto the denervated spinal cord is unlikely to be the only aspect of axonal plasticity responsible for the improved function. Neuroanatomical plasticity is also observed on the de-afferent red nucleus from the contralateral intact hemisphere postischemia. Although a few of the uncrossed CST fibers were found in the ventromedial funiculus, consistent with a previous report (Brosamle and Schwab, 2000), their terminal arbors were not detected with this tracing method due to the limited labeling efficiency. Further studies are needed to identify synaptic connections through additional pathways such as the uncrossed CST axons arising from the contralateral intact hemisphere, the CST arising from the area around the lesion site in the same hemisphere, the rubrospinal tract and other descending projections adaptively regulated by BMSC treatment after stroke.
Labeling of the CST system is a useful experimental model for investigating CNS regeneration and degeneration. Compared with Biotinylated Dextran Amine, another commonly used neural tracer for CST labeling, with which the final reaction product is more stable and provides effective dendrite filling, the lipophilic fluorescent dye DiI is an excellent anterograde corticospinal tracer due to its high fluorescence intensity, resistance to photo bleaching, and non-cytotoxicity (Vercelli et al., 2000). Furthermore, the CST labeling method is technically simple, both in tracer application and tissue processing. The panorama of the axonal structure can be readily observed from a thick vibratome section by using a confocal microscope without additional processing for tissue visualization, or labor-intensive outlining of axons. However, with this tracing method, there may be variations in the number of labeled CST fibers due to slight differences in the location or amount of dye applied. To reduce inter-animal variation in the evaluation of axonal sprouting, we therefore used the ratio of DiI density in the denervated side to that in the intact side on same sections.
In the adult mammal, injured CNS is a highly inhibitory environment for axonal regeneration, severely limiting functional recovery after damage, although neurons in the CNS are capable of neurite outgrowth. This limitation may be attributed to several factors, including lack of neurotrophins, formation of glial scars, and the presence of growth-inhibitory molecules (Yiu and He, 2006). The outgrowth of lesioned CST axons can be promoted by application of neurotrophic growth factors, such as vascular endothelial growth factor (VEGF) (Facchiano et al., 2002), brain-derived neurotrophic factor (BDNF) (Vavrek et al., 2006), or by knockout or neutralization of inhibitory signals such as Nogo (Schwab, 2004) and Nogo receptors (GrandPre et al., 2002). Following ischemic lesions, the adult CNS can induce cellular responses needed for neurite growth and synaptic formation (Cramer and Chopp, 2000), including the expression of growth-promoting factors, such as BDNF (Comelli et al., 1993) and basic fibroblast growth factor (bFGF) (Lin et al., 1997) that could lead to partial spontaneous functional recovery. Related studies have indicated that functional recovery in rodent ischemic stroke models is associated with collateral sprouting of the uninjured CST to the denervated side of spinal cord and is enhanced by treatment of inosine (Chen et al., 2002), bFGF (Kawamata et al., 1997), Nogo antibody (Papadopoulos et al., 2002) and Nogo receptor antagonist (Lee et al., 2004). Based on our and other studies, the BMSCs transplanted into the ischemic brain environment may provide their beneficial effects by secretion of neurotrophins and growth factors, including BDNF (Dormady et al., 2001; Li et al., 2002) and VEGF (Chen et al., 2003a). BMSC treatment can also reduce the gliosis and scar formation in the ischemic brain (Li et al., 2005). However, until now there have been no studies on the effect of cell-based therapies on the spinal cord after stroke. The BMSC potentiation of axonal outgrowth-promoting factors secreted by BMSCs or by endogenous parenchymal cells in response to BMSC stimulation may be, at least in part, responsible for the increased compensatory axonal plasticity and functional recovery from experimental stroke in rodents. In addition, there are a large number of parallel pathways for controlling manual dexterity in the CNS (Darian-Smith et al., 1999). After stroke, in the inhibitory CNS environment, there may be an advantage to use these available pathways for formation of new circuits via short-range axonal sprouting from neighboring uninjured fibers rather than from long-distance outgrowth of injured fibers (Cramer et al., 1997).
The transplantation of BMSCs has an important neuroprotective effect in the IBZ after stroke (Chen et al., 2003a). Enhanced functional recovery by BMSC treatment may also be due to the rescue of pyramidal neurons in the IBZ. Another possibility is that, sprouting of CST fibers in the region around the lesion area occurs in the ischemic hemisphere. However, the technical limitations of tracing methods make it difficult to distinguish newly growing sprouts in the denervated side. In this study, some DiI-labeled CST fibers were detected in the motor pool of denervated ventral horn (data not shown). Although the direct synaptic connection between CST and spinal motoneurons was reported with using CST tracing method (Liang et al., 1991), an electron microscopy study showed that no evidence of direct cortico-motoneuronal synaptic connections present in the rat (Yang and Lemon, 2003). Further studies are needed to identify the terminal innervations of contralateral CST sprouting enhanced by BMSC treatment after ischemic stroke. Because spinal motoneurons depend on synaptic transmission to receive information from propriospinal and supraspinal fibers, synaptic protein loss is also likely to contribute to the motor dysfunction (Meng et al., 2004). Synaptophysin, a presynaptic marker localized on the membrane of synaptic vesicles, is commonly used for measurement of synapse plasticity (Thiel, 1993). PSD-95, another synaptic marker used in this study, is necessary for excitatory glutamatergic postsynaptic responses, in anchoring and clustering N-methyl-D-aspartate receptors to the synapse (Kennedy, 2000). Our data show that the BMSC treatment in this model of experimental stroke in rats significantly increased both the presynaptic and postsynaptic proteins on denervated spinal motoneurons, suggesting that the denervated motoneurons have been rewired from the cortical pyramidal cells, although further electrophysiological studies are needed to directly confirm synaptic formation.
In conclusion, our direct axonal tracing data indicate that, the BMSC treatment of stroke enhances restructuring of CST and CRT axons arising from the intact cerebral hemisphere to rewire onto the opposite denervated side of the spinal cord. This BMSC treatment induced plasticity may contribute to the improvement of functional outcome after stroke. Moreover, the results observed in this study suggest that the reorganization of neural pathways may be a key element for the development of rehabilitative therapeutic strategies of stroke or other CNS damage.
4. Experimental Procedure
Adult male Wistar rats (n=19, 2 month-old, weighting 225–275 g) were used throughout this study. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
4.1. Animal model
Permanent right side MCAo was induced using a method of intraluminal vascular occlusion, modified in our laboratory (Chen et al., 1992).
4.2. Intravenous administration of BMSCs
Primary cultures of BMSCs were provided from Theradigm, Inc. (Baltimore, MD, USA). BMSCs were obtained from donor young adult Wistar rats (2–3 month-old) and separated, as previously described (Chen et al., 2001). At one day postischemia, randomly selected rats (n=8) received MSC transplantation. Rats were initially anesthetized with 2% Forane (Isoflurane) and maintained with 1.5% Forane in 70% N2O and 30% O2 using a face mask. Approximately 3 × 106 BMSCs in 1 ml total fluid volume of phosphate-buffered saline (PBS) were injected into a tail vein. Immunosuppressants were not used in any animal. Rats injected with PBS alone were employed as MCAo control (n=7). A third group of naive rats without surgery and treatment was employed as normal controls (n=4).
4.3. Anterograde tracing for the contralateral CST
For corticospinal tracing, the dye injection was performed 2 days before MCAo. Under deep anesthesia with an intraperitoneal injection of Ketamine (44 mg/kg) and Xylazine (13 mg/kg), rats underwent a left unilateral craniotomy at a region of 3–4 mm lateral to the midline and 0–4 mm posterior to the bregma. A high-speed drill (Foredom Electric, Bethel, CT, USA) was carefully used to reduce the skull thickness by about 80%. Residual skull was completely removed by gently scraping with microsurgical forceps. 2.5% DMSO solution of 1,1″-Dioleyl-3,3,3″,3″-tetramethylindocarbocyanine methanesulfonate (Delta 9-DiI; AnaSpec, San Jose, CA, USA) was injected through a finely drawn glass capillary with an electric injection system into eight points in the left motorsensory cortex (Paxinos and Watsson, 1986) (80 nl per injection; stereotaxic coordinates: 0, 1, 2 and 3 mm posterior to the bregma, 3 and 4 mm lateral to the midline, 1.5 mm depth from the surface of the cortex). The micropipette remained in place for 4 min after completion of the injection. If cortical vessels were encountered at the intended incision site, the site was moved immediately rostral or caudal to avoid the cortical vessel.
4.4. Neurological functional test
In all animals, a series of adhesive-removal tests (Schallert and Whishaw, 1984) were performed at 1, 7, 14, 21 and 28 days after MCAo to evaluate the sensorimotor functional recovery. All functional tests, fluorescent tracers and immunohistochemistry analyses were performed blindly.
4.5. Tissue preparation and measurements
Animals were sacrificed under deep Ketamine anesthesia at 28 days after MCAo. Rats were perfused transcardialy with saline, followed by 4% paraformaldehyde. The entire brain and spinal cord were immersed in 4% paraformaldehyde overnight. The first part of brain anterior to the bregma -5 mm was cut into five equally spaced (2 mm) coronal blocks using a rat brain matrix (Activational System, Warren, MI, USA). The brain blocks and C7-8 and L5-6 spinal cord were embedded in paraffin. A series of adjacent 6 μm-thick sections were cut from each block in the coronal plane and stained with Hematoxylin and Eosin. The sections were traced using a Global Laboratory image-analysis system (Data Translation, Marlboro, MA, USA). The indirect lesion area was calculated as the intact area of the ipsilateral hemisphere subtracted from the area of the contralateral hemisphere (Swanson et al., 1990). Lesion volume is presented as a volume percentage of the lesion compared with the contralateral hemisphere.
The other part of brain and spinal cord segments of C3-7 and L1-5 were processed for vibratome sections (100 μm). The fluorescent labeling in the red nucleus on brain sections, spinal longitudinal sections (C3-4 and L1-2) and transverse sections (C5-6 and L3-4) were analyzed with a Bio-Rad MRC 1024 (argon and krypton) laser-scanning confocal imaging system mounted onto a Zeiss microscope (Bio-Rad, Cambridge, MA, USA). Microscopic data were acquired with a 10x objective or a 40x oil-immersion objective lens.
4.6. Immunohistochemistry
Following deparaffinization, 6 μm-thick paraffin sections were boiled in 0.1 mol/L sodium citrate buffer (pH 6.0) for 6 minutes. Then the sections were exposed to 3% H2O2 for 10 minutes to bleach endogenous peroxidases. Sections were selectively incubated using primary antibodies against synaptophysin (1:1000, Chemicon, Temecula, CA, USA) and postsynaptic density protein of 95 kDa (PSD-95, 1:1000, Synaptic Sys, Goettingen, Germany) for 1 hour at 37°C, and then incubated with a biotinylated secondary antibody for 30 minutes at 37°C. Tissues were visualized with 3,3′-diaminobenzidine tetrahydrochloride for light microscopy examination.
4.7. Quantification
The length or density of DiI-labeled axons was analyzed with NIH image software (Image J) based on the average of three histology slides, which showed the highest density of labeled axons, from 40 continuous sections for each experimental site. To correct for inter-animal variation in the tracing efficiency reduced by any subtle difference of injection location or volume, the ratio of the DiI-labeled fiber density in the denervated side and intact side on same sections was used as an index of axonal reorganization, while the mean length of crossing axons in the spinal white matter of the denervated side was determined directly from three sections. The density ratio of the positive staining in the close proximity of spinal motoneurons between bilateral sides of the ventral horn was also used to correct the immunostaining nuance.
4.8 Statistics
The behavioral outcome was tested between the treated and control rats at 1, 7, 14, 21 and 28 days after MCAo by one-way ANOVA. Two-sample t-test was used to test the difference in lesion volume, DiI-labeled axon length, optical density and positive immunostaining density between two groups. Pearson’s correlation coefficients were calculated between functional recovery and anatomical reorganization or synaptic immunohistochemistry. All data are presented as mean ± SD. A value of p<0.05 was taken as significant.
Acknowledgments
The authors wish to thank Yisheng Cui, Cindi Roberts, Sutapa Santra and Qinge Lu for technical assistance. This work was supported by NINDS grants PO1 NS23393 and RO1 NS45041.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bastings EP, Greenberg JP, Good DC. Hand motor recovery after stroke: a transcranial magnetic stimulation mapping study of motor output areas and their relation to functional status. Neurorehabil Neural Repair. 2002;16:275–82. doi: 10.1177/154596802401105207. [DOI] [PubMed] [Google Scholar]
- Brosamle C, Schwab ME. Ipsilateral, ventral corticospinal tract of the adult rat: ultrastructure, myelination and synaptic connections. J Neurocytol. 2000;29:499–507. doi: 10.1023/a:1007297712821. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–42. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- Chen H, Chopp M, Zhang ZG, Garcia JH. The effect of hypothermia on transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1992;12:621–8. doi: 10.1038/jcbfm.1992.86. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Chopp M. Intracerebral transplantation of bone marrow with BDNF after MCAo in rat. Neuropharmacology. 2000;39:711–6. doi: 10.1016/s0028-3908(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–11. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003a;73:778–86. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003b;92:692–9. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y, Lu M, Zhang Z, Chopp M. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004;1005:21–8. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031–6. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comelli MC, Guidolin D, Seren MS, Zanoni R, Canella R, Rubini R, Manev H. Time course, localization and pharmacological modulation of immediate early inducible genes, brain-derived neurotrophic factor and trkB messenger RNAs in the rat brain following photochemical stroke. Neuroscience. 1993;55:473–90. doi: 10.1016/0306-4522(93)90517-j. [DOI] [PubMed] [Google Scholar]
- Cooper SE, Martin JH, Ghez C. Effects of inactivation of the anterior interpositus nucleus on the kinematic and dynamic control of multijoint movement. J Neurophysiol. 2000;84:1988–2000. doi: 10.1152/jn.2000.84.4.1988. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–27. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23:265–71. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I, Burman K, Darian-Smith C. Parallel pathways mediating manual dexterity in the macaque. Exp Brain Res. 1999;128:101–8. doi: 10.1007/s002210050824. [DOI] [PubMed] [Google Scholar]
- Dormady SP, Bashayan O, Dougherty R, Zhang XM, Basch RS. Immortalized multipotential mesenchymal cells and the hematopoietic microenvironment. J Hematother Stem Cell Res. 2001;10:125–40. doi: 10.1089/152581601750098372. [DOI] [PubMed] [Google Scholar]
- Facchiano F, Fernandez E, Mancarella S, Maira G, Miscusi M, D'Arcangelo D, Cimino-Reale G, Falchetti ML, Capogrossi MC, Pallini R. Promotion of regeneration of corticospinal tract axons in rats with recombinant vascular endothelial growth factor alone and combined with adenovirus coding for this factor. J Neurosurg. 2002;97:161–8. doi: 10.3171/jns.2002.97.1.0161. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–51. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Masterton RB. The role of the corticospinal tract in the evolution of human digital dexterity. Brain Behav Evol. 1983;23:165–83. doi: 10.1159/000121494. [DOI] [PubMed] [Google Scholar]
- Irons H, Lind JG, Wakade CG, Yu G, Hadman M, Carroll J, Hess DC, Borlongan CV. Intracerebral xenotransplantation of GFP mouse bone marrow stromal cells in intact and stroke rat brain: graft survival and immunologic response. Cell Transplant. 2004;13:283–94. doi: 10.3727/000000004783983990. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Dietrich WD, Schallert T, Gotts JE, Cocke RR, Benowitz LI, Finklestein SP. Intracisternal basic fibroblast growth factor enhances functional recovery and up-regulates the expression of a molecular marker of neuronal sprouting following focal cerebral infarction. Proc Natl Acad Sci U S A. 1997;94:8179–84. doi: 10.1073/pnas.94.15.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–4. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Lee J, Kuroda S, Shichinohe H, Ikeda J, Seki T, Hida K, Tada M, Sawada K, Iwasaki Y. Migration and differentiation of nuclear fluorescence-labeled bone marrow stromal cells after transplantation into cerebral infarct and spinal cord injury in mice. Neuropathology. 2003;23:169–80. doi: 10.1046/j.1440-1789.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–17. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RG, van Donkelaar P. Mechanisms underlying functional recovery following stroke. Can J Neurol Sci. 1995;22:257–63. doi: 10.1017/s0317167100039445. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–23. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–17. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- Liang FY, Moret V, Wiesendanger M, Rouiller EM. Corticomotoneuronal connections in the rat: evidence from double-labeling of motoneurons and corticospinal axon arborizations. J Comp Neurol. 1991;311:356–66. doi: 10.1002/cne.903110306. [DOI] [PubMed] [Google Scholar]
- Lin TN, Te J, Lee M, Sun GY, Hsu CY. Induction of basic fibroblast growth factor (bFGF) expression following focal cerebral ischemia. Brain Res Mol Brain Res. 1997;49:255–65. doi: 10.1016/s0169-328x(97)00152-6. [DOI] [PubMed] [Google Scholar]
- Meng Z, Li Q, Martin JH. The transition from development to motor control function in the corticospinal system. J Neurosci. 2004;24:605–14. doi: 10.1523/JNEUROSCI.4313-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos CM, Tsai SY, Alsbiei T, O'Brien TE, Schwab ME, Kartje GL. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol. 2002;51:433–41. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watsson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- Rijntjes M. Mechanisms of recovery in stroke patients with hemiparesis or aphasia: new insights, old questions and the meaning of therapies. Curr Opin Neurol. 2006;19:76–83. doi: 10.1097/01.wco.0000203886.28068.38. [DOI] [PubMed] [Google Scholar]
- Schallert T, Whishaw IQ. Bilateral cutaneous stimulation of the somatosensory system in hemidecorticate rats. Behav Neurosci. 1984;98:518–40. doi: 10.1037//0735-7044.98.3.518. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–24. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol. 1998;55:1081–8. doi: 10.1001/archneur.55.8.1081. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Thiel G. Synapsin I, synapsin II, and synaptophysin: marker proteins of synaptic vesicles. Brain Pathol. 1993;3:87–95. doi: 10.1111/j.1750-3639.1993.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Vavrek R, Girgis J, Tetzlaff W, Hiebert GW, Fouad K. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129:1534–45. doi: 10.1093/brain/awl087. [DOI] [PubMed] [Google Scholar]
- Vercelli A, Repici M, Garbossa D, Grimaldi A. Recent techniques for tracing pathways in the central nervous system of developing and adult mammals. Brain Res Bull. 2000;51:11–28. doi: 10.1016/s0361-9230(99)00229-4. [DOI] [PubMed] [Google Scholar]
- Yang HW, Lemon RN. An electron microscopic examination of the corticospinal projection to the cervical spinal cord in the rat: lack of evidence for cortico-motoneuronal synapses. Exp Brain Res. 2003;149:458–69. doi: 10.1007/s00221-003-1393-9. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–27. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li Y, Chen J, Gao Q, Zacharek A, Kapke A, Chopp M. Bone marrow stromal cells upregulate expression of bone morphogenetic proteins 2 and 4, gap junction protein connexin-43 and synaptophysin after stroke in rats. Neuroscience. 2006;141:687–95. doi: 10.1016/j.neuroscience.2006.04.054. [DOI] [PubMed] [Google Scholar]


