Abstract
The consequences of inbreeding for host immunity to parasitic infection have broad implications for the evolutionary and dynamical impacts of parasites on populations where inbreeding occurs. To rigorously assess the magnitude and the prevalence of inbreeding effects on immunity, multiple components of host immune response should be related to inbreeding coefficient (f) in free-living individuals. We used a pedigreed, free-living population of song sparrows (Melospiza melodia) to test whether individual responses to widely used experimental immune challenges varied consistently with f. The patagial swelling response to phytohaemagglutinin declined markedly with f in both females and males in both 2002 and 2003, although overall inbreeding depression was greater in males. The primary antibody response to tetanus toxoid declined with f in females but not in males in both 2004 and 2005. Primary antibody responses to diphtheria toxoid were low but tended to decline with f in 2004. Overall inbreeding depression did not solely reflect particularly strong immune responses in outbred offspring of immigrant–native pairings or weak responses in highly inbred individuals. These data indicate substantial and apparently sex-specific inbreeding effects on immune response, implying that inbred hosts may be relatively susceptible to parasitic infection to differing degrees in males and females.
Keywords: cell-mediated immunity, conservation genetics, heterozygosity, humoral immunity, parasite-mediated selection
1. Introduction
The relationship between a host's inbreeding level and its immunity to parasitic infection is of broad interest in evolutionary and population ecology. A decline in immunity with inbreeding would indicate a dominance genetic component to parasite resistance, bearing on the evolutionary consequences of host–parasite interactions and the maintenance of genetic variation in natural populations (Potts & Wakeland 1990; Coltman et al. 1999; Westerdahl et al. 2005). Such declines would also suggest that parasites could mediate inbreeding depression in reproduction and survival, thereby threatening the persistence of small, inbred populations (Altizer et al. 2003; Tompkins et al. 2006; Whiteman et al. 2006). Quantifying the magnitude and the prevalence of inbreeding depression in immunity is therefore integral to predicting the genetic, evolutionary and population dynamic consequences of host–parasite interactions (Coltman et al. 1999; Reid et al. 2003; Spielman et al. 2004). Furthermore, in general, the evolutionary and dynamical impacts of parasites on host populations depend on whether the fitness cost of parasite exposure is constant or variable, and on the host sex, age or stage classes that are most affected (Murdoch et al. 1997; Coltman et al. 1999). Comprehensive studies of inbreeding effects on host immunity should therefore quantify whether effects act consistently or are exhibited only occasionally or in specific categories of population members (Giese & Hedrick 2003; Côté et al. 2005).
One common approach to measuring inbreeding effects on immunity is to relate host inbreeding level to the degree of infection or mortality after natural or experimental exposure to specific natural parasites (e.g. Cassinello et al. 2001; Haag et al. 2003; Spielman et al. 2004). A complementary approach is to relate inbreeding level to the host's response to a novel, experimental immune challenge (Giese & Hedrick 2003; Reid et al. 2003; Hawley et al. 2005). This approach has the advantage of eliminating confounding variation in a host's prior exposure to the focal parasite, thereby allowing inbreeding effects on immunity per se to be clearly distinguished from inbreeding effects on exposure (Norris & Evans 2000; Staszewski & Boulinier 2004). Such novel immune challenges are also ecologically and evolutionarily pertinent, since newly emerging parasites may exert particularly severe selection on naive host populations (Daszak et al. 2000; Altizer et al. 2003). Studies using this approach should apply multiple challenges targeting different components of host immunity, since different components may not show correlated responses (Norris & Evans 2000; Haag et al. 2003; Adamo 2004). Furthermore, since inbreeding effects on phenotypic traits are often less severe in captivity than in the wild (Hedrick & Kalinowski 2000; Joron & Brakefield 2003), studies should ideally be carried out in free-living individuals; the evolutionary and dynamical impacts of parasites on natural populations might otherwise be inaccurately inferred. However, despite their broad relevance, there remain few data describing relationships between host inbreeding per se and multiple components of immune response in free-living individuals, or the extent to which inbreeding effects vary among seasons or categories of population members.
We used a free-living, pedigreed population of song sparrows, Melospiza melodia, inhabiting Mandarte Island, Canada, to test whether individual responses to two widely used experimental immune challenges varied with an individual's coefficient of inbreeding (f). Specifically, we related individual variation in the patagial swelling response to phytohaemagglutinin (PHA), and in the humoral antibody response to diphtheria–tetanus vaccine (DTV), to individual f. We repeated each experimental challenge in two different years on different samples of males and females of varying ages, and thereby investigate whether inbreeding effects on immunity varied between years or with host age or sex.
2. Material and methods
(a) Study population
Mandarte Island, approximately 6 ha in size, lies 25 km northeast of Victoria, British Columbia, Canada. Its resident song sparrow population, which numbered 16–27 breeding pairs during 2002–2005, has been studied intensively since 1975 (Smith et al. 2006). During this long-term study, all song sparrows fledged on Mandarte have been individually colour-ringed, almost always before leaving their natal territory. All immigrants to the breeding population (1.1 yr−1 on average) have been colour-ringed soon after settling. All population members are therefore individually identifiable (Smith et al. 2006). Detailed observations of breeding behaviour have allowed a complete social pedigree to be constructed, covering all sparrows hatched since 1981 (Keller 1998). We used standard algorithms to estimate each individual's f directly from the pedigree (Falconer & Mackay 1996; Keller 1998). The value of f reflects the probability that two homologous alleles will be identical by descent and estimates an individual's genome-wide homozygosity relative to the baseline population (Falconer & Mackay 1996). While immigrants to Mandarte are themselves of unknown f, they are genetically distinguishable from Mandarte's existing population at the time of arrival (assignment test based on nine microsatellite loci, p<0.02 for all immigrants during 1993–1996, see also Keller et al. 2001). Offspring of immigrant–native pairings can therefore be defined as outbred (f=0.000, Marr et al. 2002). Extra-pair fertilizations (epfs) occur on Mandarte (approx. 25% of offspring hatched during 1993–1996, O'Connor et al. 2006) and in song sparrows more widely (e.g. 11% of offspring, Major & Barber 2004). These epfs introduce error into the social pedigree and therefore into estimates of f. However, there is no evidence that epfs occur systematically with respect to relatedness in song sparrows on Mandarte. Briefly, across 283 broods hatched during 1993–1996, epfs were not more frequent in females that were more closely related to their social mates (p=0.52, O'Connor et al. in preparation). Furthermore, females were no more or less closely related to their extra-pair mate than to their social mate (n=100 triads, p=0.65, O'Connor et al. in preparation). Epfs are therefore likely to introduce error but not substantial bias into estimates of f. Such error is expected to cause inbreeding depression in phenotypic traits to be underestimated (Keller et al. 2002; Kruuk et al. 2002; Marr et al. in press). Since f reflects the relatedness between an individual's parents rather than an individual's relatedness to its own offspring, epfs will not necessarily introduce more error into estimates of f in males than in females. Such sex-biased error in f, and consequently in the estimated magnitude of inbreeding depression in phenotypic traits, could only arise given a biased sex ratio in extra-pair young. On Mandarte, the sex ratio of extra-pair young identified during 1993–1996 did not differ from 1 : 1 (65 males, 54 females, χ21=0.4, p=0.53). Furthermore, sex ratio did not differ between within-pair and extra-pair young (p=0.61, Generalized linear mixed model controlling for year and brood). Therefore, although paternity of the song sparrows exposed to recent experimental immune challenges has not yet been fully verified, data from previous years do not lead us to expect sex-biased error in estimates of f or inbreeding depression.
(b) PHA response
The patagial (wing-web) swelling response to subcutaneous injection of PHA is a widely used measure of avian immune responsiveness (Goto et al. 1978; Tella et al. 2002; Martin et al. 2006). PHA response is often interpreted as a measure of ‘cell-mediated immunity’, but may in fact reflect multiple immunological processes manifested as the infiltration and proliferation of multiple cell types over varying time periods (Martin et al. 2006). PHA response is thought to trade-off against other life-history components and to vary with individual condition and ‘quality’ (Tella et al. 2002; Martin et al. 2006), is often positively correlated with survival (Møller & Saino 2003) and has been related to MHC genotype (Taylor et al. 1987; Bonneaud et al. 2005). Therefore, while the underlying immunological processes may be more complex than often recognized, PHA response remains a useful measure of immunological ‘condition’ in the context of immunoecology.
During February 2002, September 2002 and September 2003, we measured PHA response in song sparrows on Mandarte. Methodology followed Reid et al. (2003). Briefly, sparrows were mist-netted after 11.00 and wing length, tarsus length and mass were recorded. Patagium thicknesses were measured three times using a modified dial calliper (Mitutoyo, Japan). Sparrows were injected with 30 μl 2 mg ml−1 PHA (L9132, Sigma, St Louis, Missouri) in phosphate buffer solution (PBS, 9.7 gl−1 D5773, Sigma, St Louis, Missouri) in the right patagium and 30 μl PBS in the left patagium and roosted overnight in individual enclosures with ad libitum food and water. Left and right patagial thicknesses were remeasured approximately 18 h after injection and sparrows were released. PHA response was estimated as the difference in increase in thickness between right and left patagia over the experimental period. Patagium thickness measurements were highly repeatable within individuals (r>0.94, p<0.0001, Reid et al. 2003).
(c) Diphtheria–tetanus response
The specific humoral antibody response to foreign antigens constitutes one major component of avian acquired immunity (Roitt et al. 1998). During September 2004 and September 2005, we measured humoral immunity as a song sparrow's primary antibody response to tetanus and diphtheria toxoids (Hasselquist et al. 1999, 2001; Owen-Ashley et al. 2004). Sparrows were mist-netted, biometrics were recorded as above, and approximately 100 μl blood was collected by brachial venipuncture. Individuals were vaccinated with 70 μl human DTV(2 Lf diphtheria toxoid, 5 Lf tetanus toxoid adsorbed in aluminium phosphate, Aventis Pasteur Ltd, Toronto, Canada) in the pectoral muscle and released. Primary antibody responses peak 9–15 days after vaccination in song sparrows and other passerines (Hasselquist et al. 1999; Owen-Ashley et al. 2004). Correspondingly, we attempted to recapture sparrows 10–12 days after vaccination. Recaptured sparrows were reweighed, blood sampled as before and released. Since we could not control the exact timing of recapture of free-flying individuals and weather conditions impeded mist-netting on some days, we blood sampled all individuals recaptured 8–14 days after vaccination and subsequently controlled statistically for inter-sample period (see §2d). Blood samples were placed immediately on ice and centrifuged for 4 min at 3000rpm. within 5 h. Plasma was separated off, stored buried in ice and frozen at −20°C within 72 h. Enzyme-linked immunosorbent assays (ELISAs) were subsequently used to quantify tetanus and diphtheria antibody titres in pre- and post-vaccination plasma samples. Protocols followed those previously developed for song sparrows and other passerines (Hasselquist et al. 1999, 2001; Owen-Ashley et al. 2004). ELISA plates held an individual's pre- and post-vaccination plasma samples in duplicate. Titres were standardized for any among-plate variation in conditions or reagents within each year by reference to serially diluted standard samples that were included on each plate. Each individual's primary antibody response to tetanus and diphtheria toxoids was estimated as the difference between post- and pre-vaccination standardized antibody titres (Hasselquist et al. 1999; Owen-Ashley et al. 2004). Owing to within-year standardization of titres and slight between-year differences in ELISA assays, between-year differences in mean antibody response may not solely reflect true biological variation. In naive hosts, pre-vaccination antibody titres are expected to be low but non-zero due to natural antibodies that can bind antigens without previous exposure (Lee et al. 2006).
(d) Analysis
We used general linear models (GLMs) to test whether PHA, tetanus or diphtheria responses varied with individual f within each year in which each immune challenge was applied, or across all data combined. We additionally modelled effects of individual age, body condition, maternal f and paternal f as covariates and sex and year as fixed factors. Sexes were determined by observing adult breeding behaviour or PCR amplification of sex-linked CHD1 genes (Smith et al. 2006; Heinrich et al. in preparation). Body condition was estimated as the residual of mass on the cube of the first principal component of wing and tarsus length. Age was calculated to the nearest month from ringing data. We additionally modelled challenge date (within season) and inter-sample period as linear and quadratic covariates. Challenge date was eliminated from all models (all p>0.35). Some individuals vaccinated with DTV in 2005 were offspring of mothers that had been vaccinated in 2004. Since humoral immunity shows inter-generational effects of maternal vaccination in song sparrows (Reid et al. 2006), we included maternal vaccination history as a binary fixed factor (‘vaccinated’ or ‘unvaccinated’) in analyses of antibody responses in 2005. Since samples included sets of siblings, we initially modelled ‘family’ as a random factor. However, since family effects were not significant, models reduced to final GLMs.
A small number of individuals experienced the same immune challenge twice in consecutive years. Since repeat immune responses may not be independent (Roitt et al. 1998), we restricted analyses to each individual's first exposure to each challenge. We did not apply both PHA and DTV to the same individuals in the same year because mounting multiple simultaneous immune responses can reduce survival (Hansson et al. 2004). Ten individuals that experienced DTV during 2004–2005 had experienced PHA during 2002–2003. Diphtheria and tetanus responses did not differ between individuals that had and had not experienced PHA (p>0.6).
Since the Mandarte song sparrow pedigree is relatively deep, the only individuals that are considered completely outbred (f=0.000) are the offspring of immigrant–native pairings. Such F1 offspring of between-population crosses may show enhanced phenotypes due to heterosis (Falconer & Mackay 1996; Marr et al. 2002). Our samples also included some highly inbred offspring of pairings between first- or second-order relatives. Such pairings may involve a phenotypically non-random subset of parents (Reid et al. in preparation) and form relative outliers with respect to f. Therefore, to test whether overall declines in immune response with inbreeding were solely driven by heterosis in outbred offspring or by particularly weak responses in highly inbred individuals, we investigated whether final models or parameter estimates were altered by excluding outbred individuals, or individuals with f≥0.125.
Dependent variables were log transformed to reduce deviations from normality. Estimated slopes can consequently be directly interpreted as the inbreeding load or number of lethal equivalents (the slope of a log (trait) on f regression, Falconer & Mackay 1996). Bf, Bm and B are the estimated inbreeding loads for females, males and all individuals (assuming no sex×f interaction) and are presented with 95% confidence limits. η2 is the partial effect size for f. Independent variables were not tightly correlated (all r<0.4). Residuals were approximately normally distributed and were not correlated or heteroscedastic with respect to predicted values. Immune response data were collected blind to individual f and other characteristics. Analyses were run in Pedigree Viewer (http://www-personal.une.edu.au/~bkinghor/pedigree.htm), SPSS (v. 14.0) and R (v. 2.2.1). All tests were two tailed. Variables were retained in models if p≤0.10. Two- and three-way interactions involving individual f were tested and eliminated except where stated. Sample sizes vary among models because body condition was not calculated for two individuals in wing moult during September 2002 and f was unknown for immigrant parents. Analyses of PHA response in 2002 differ from those reported previously (Reid et al. 2003) in that data from adult and juvenile sparrows are combined. Fieldwork was approved by the University of British Columbia Animal Care Committee.
3. Results
Table 1 describes the samples of song sparrows whose PHA, tetanus and diphtheria responses were measured during 2002–2005.
Table 1.
Descriptive statistics of song sparrows challenged with phytohaemagglutinin (PHA) and diphtheria–tetanus vaccine (DTV) during 2002–2005. (Nt, Njuv and Nad are the total samples sizes of naive individuals, juveniles (individuals hatched in the current year) and adults (individuals hatched in previous years). Ranges are shown in parentheses. Means are shown ±1s.e. Mist-netting effort totalled approximately 26 000 net metre hours.)
| test | year | Nt | Njuv | Nad | median f | median age of adults (months) | mean inter-sample period (days) | mean immune response | difference of mean response from zero |
|---|---|---|---|---|---|---|---|---|---|
| PHA | 2002 | 72 | 35 | 37 | 0.053 | 16 | — | 0.32±0.02 | t1,71=19.1, p<0.001 |
| (0.000–0.303) | (9–93) | ||||||||
| PHA | 2003 | 44 | 37 | 7 | 0.052 | 52 | — | 0.25±0.02 | t1,43=13.6, p<0.001 |
| (0.000–0.289) | (16–88) | ||||||||
| DTV | 2004 | 38 | 26 | 12 | 0.04 | 16 | 9.7±0.3 | Tet: 76.7±14.6 | t1,37=5.2, p<0.001 |
| (0.000–0.289) | (16–52) | (8–14) | Dip: 13.2±4.3 | t1,37=3.0, p=0.004 | |||||
| DTV | 2005 | 48 | 46 | 2 | 0.04 | — | 9.5±0.2 | Tet: 235.1±38.0 | t1,47=6.2, p<0.001 |
| (0.000–0.285) | (40&52) | (8–13) | Dip: 4.1±2.3 | t1,47=1.7, p=0.090 |
(a) PHA response
Mean PHA response exceeded zero in both 2002 and 2003 (table 1). PHA response declined with increasing f in both 2002 and 2003 and across all data combined (table 2, figure 1). PHA response also increased with body condition in 2003 and across all data (table 2). There was also a significant sex×f interaction in 2003 and across all data; PHA response declined more markedly with f in males than in females (table 2). The final model remained quantitatively similar after excluding eight outbred individuals (Bf=−2.1 (95% CI −5.3 to 1.0), Bm=−4.3 (95% CI −5.8 to −2.8)). After excluding 12 highly inbred individuals, the sex×f interaction was no longer significant (p=0.47) but the estimated overall inbreeding load (B) increased to −4.3 (95% CI −5.7 to −2.9).
Table 2.
Models explaining variation in PHA response in song sparrows. (Nt, Nf and Nm are the total sample sizes of naive individuals, females and males included in final models. Variables retained in final models are indicated in bold. Age×f, year×f and year×sex×f interactions were not significant (p>0.20).)
| year | Nt | Nf | Nm | individual f | age | body condition | maternal f | paternal f | sex | sex×f | season or year | final model |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2002 | 72 | 29 | 43 | F=29.4 | F=1.7 | F=1.7 | F=0.04 | F=0.5 | F=0.4 | F=0.8 | F=0.8 | F1,71=29.4 |
| p<0.001 | p=0.20 | p=0.19 | p=0.85 | p=0.48 | p=0.55 | p=0.78 | p=0.37 | p<0.001 | ||||
| B=−2.7 (−3.6–−1.7) | R2=0.29 | |||||||||||
| η2=0.29 | ||||||||||||
| 2003 | 44 | 21 | 23 | F=64.9 | F=0.03 | F=5.1 | F=0.01 | F=0.1 | F=7.0 | F=19.4 | — | F4,43=22.9 |
| p<0.001 | p=0.87 | p=0.029 | p=0.93 | p=0.70 | p=0.012 | p<0.001 | p<0.001 | |||||
| Bf=−2.1 (−4.4–−0.1) | R2=0.67 | |||||||||||
| Bm=−7.0 (−9.1–−4.9) | ||||||||||||
| B=−2.7 (−3.7–−1.8) | ||||||||||||
| η2=0.63 | ||||||||||||
| all data | 114 | 49 | 65 | F=71.5 | F=0.7 | F=9.3 | F=0.1 | F=0.8 | F=0.4 | F=4.8 | F=9.7 | F6,113=16.8 |
| p<0.001 | p=0.42 | p=0.003 | p=0.74 | p=0.37 | p=0.52 | p=0.030 | p<0.001 | p<0.001 | ||||
| Bf=−2.3 (−3.8–−0.8) | R2=0.46 | |||||||||||
| Bm=−3.9 (−5.2–−2.7) | ||||||||||||
| B=−2.8 (−3.4–−2.1) | ||||||||||||
| η2=0.40 |
Figure 1.
Relationships between an individual song sparrow's coefficient of inbreeding (f) and (log) phytohaemagglutinin (PHA) response measured in 2002 (open symbols, dashed line) and 2003 (filled symbols, solid line).
(b) Tetanus response
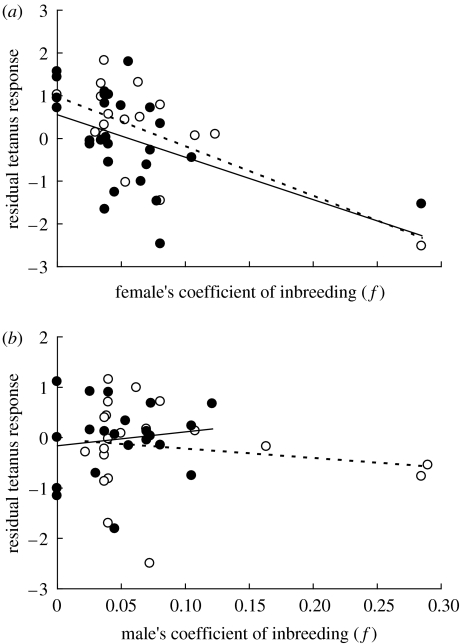
Mean tetanus response was substantial in both 2004 and 2005 (table 1). Tetanus response declined with increasing f in 2004 and 2005 (assuming no sex×f interaction) and across all data (table 3). However, the main effect of sex and the sex×f interaction were significant in both 2004 and 2005 and across all data; females mounted slightly higher mean tetanus responses than males, and tetanus response declined with f in females but not in males (table 3, figure 2). Tetanus response also varied with inter-sample period and maternal vaccination history and tended to vary with paternal f in 2005. Sparrows whose mothers had been vaccinated in 2004 or whose fathers were relatively inbred showed stronger antibody responses (although the latter effect was weak, table 3, see also Reid et al. 2006). The final model remained quantitatively similar after excluding nine outbred individuals (Bf=−7.4 (95% CI −12.8 to −2.0), Bm=−1.5 (95% CI −5.0 to 2.0)) and qualitatively similar after excluding five highly inbred individuals (although confidence intervals around estimated inbreeding loads increased substantially in this case, Bf=−11.2 (95% CI −20.5 to −1.9), Bm=2.3 (95% CI −5.6 to 10.3)).
Table 3.
Models explaining variation in tetanus response in song sparrows. (Nt, Nf and Nm are the total sample sizes of naive individuals, females and males included in final models. There was insufficient variance to include age as an explanatory variable in 2005 (table 1). Variables retained in final models are indicated in bold. Age×f, year×f and year×sex×f interactions were not significant (p>0.35).)
| year | Nt | Nf | Nm | individual f | inter-sample period | inter-sample period2 | age | body condition | maternal f | paternal f | sex | sex×f | maternal vaccination history | year | final model |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2004 | 38 | 18 | 20 | F=9.9 | F=7.0 | F=5.0 | F=2.4 | F=0.2 | F=0.01 | F=0.2 | F=5.5 | F=4.9 | — | — | F5,37=5.2 |
| p=0.003 | p=0.013 | p=0.033 | p=0.13 | p=0.66 | p=0.96 | p=0.62 | p=0.025 | p=0.033 | p=0.001 | ||||||
| Bf=−7.8 (−13.7–−2.0) | R2=0.36 | ||||||||||||||
| Bm=−1.5 (−5.0–2.1) | |||||||||||||||
| B=−3.8 (−6.8–−0.7) | |||||||||||||||
| η2=0.24 | |||||||||||||||
| 2005 | 47 | 27 | 20 | F=0.5 | F=6.1 | F=5.9 | — | F=0.01 | F=0.10 | F=3.5 | F=3.3 | F=4.4 | F=7.8 | — | F7,46=5.5 |
| p=0.49 | p=0.018 | p=0.020 | p=0.93 | p=0.77 | p=0.071 | p=0.076 | p=0.044 | p=0.008 | p<0.001 | ||||||
| Bf=−7.2 (−14.9–−0.3) | R2=0.41 | ||||||||||||||
| Bm=3.8 (−5.3–12.9) | |||||||||||||||
| B=−4.7 (−8.8–−0.5) | |||||||||||||||
| η2=0.12 | |||||||||||||||
| all data | 85 | 45 | 40 | F=14.9 | F=11.1 | F=8.8 | F=2.4 | F=1.1 | F=0.2 | F=2.8 | F=4.8 | F=7.5 | F=11.4 | F=37.2 | F8,84=20.8 |
| p<0.001 | p=0.001 | p=0.004 | p=0.13 | p=0.29 | p=0.67 | p=0.10 | p=0.031 | p=0.008 | p=0.001 | p<0.001 | p<0.001 | ||||
| Bf=−7.7 (−12.4–−3.0) | R2=0.65 | ||||||||||||||
| Bm=−1.1 (−4.5–2.3) | |||||||||||||||
| B=−4.5 (−7.1–−2.2) | |||||||||||||||
| η2=0.16 |
Figure 2.
Relationships between an individual song sparrow's coefficient of inbreeding (f) and residual (log) tetanus response (controlling for inter-sample period, maternal vaccination history and paternal f) for (a) females and (b) males, measured in 2004 (open symbols, dashed line) and 2005 (filled symbols, solid line).
(c) Diphtheria response
Mean diphtheria response exceeded zero in 2004 but not in 2005 (table 1). We therefore restricted analyses to 2004. Similarly low primary antibody responses to diphtheria toxoid have previously been reported in song sparrows (Owen-Ashley et al. 2004). Diphtheria and tetanus responses were correlated across individuals tested in 2004 (N=38, r=0.46, p=0.004).
Diphtheria response tended to decline with increasing f (figure 3) and increased then declined with age (age: F=18.2, p<0.001; age2: F=8.3, p=0.007). Females mounted marginally higher mean diphtheria responses than males (F=3.2, p=0.082), but the sex×f interaction was not significant (p=0.30). Other covariates were eliminated (all p>0.1; final model: F4,37=7.5, p<0.001, R2=0.41). The final model remained quantitatively similar after excluding a single outbred individual. After excluding four highly inbred individuals, the estimated inbreeding load increased to −4.4, although this effect no longer approached statistical significance due to increased confidence limits (95% CL −13.4 to 4.7, p=0.33).
Figure 3.
Relationship between an individual song sparrow's coefficient of inbreeding (f) and residual (log) diphtheria response (controlling for age and sex) measured in 2004. Diphtheria response tended to decline with f (F=3.7, p=0.065, B=−2.8 (95% CL −5.8 to 0.2), η2=0.10).
4. Discussion
In free-living song sparrows, PHA, tetanus and diphtheria responses all varied to some extent with individual f. Estimated inbreeding loads for PHA and tetanus responses did not vary markedly among years or sparrows of different ages, but apparently differed between females and males. PHA response declined with f in both sexes, but declined more markedly in males than in females in 2003 and across all data. Tetanus response declined with f in females but not in males in 2004 and 2005 and across all data. We had sufficient statistical power to detect the inbreeding load for tetanus response estimated in females had it also occurred in males (observed r≈0.56, n=40, power≈0.97, given that overall variance in f was similar in both sexes). There is no clear expectation that apparent female-specific inbreeding depression should have arisen because measurement error in tetanus response or f was consistently greater in males (e.g. due to paternity error, see §2), and collinearity of explanatory variables did not differ between the sexes. Therefore, while it remains possible that the repeatable sex-specific inbreeding depression observed in tetanus response simply reflects sampling variance or stochastic male-biased error in f, our data suggest that tetanus response declined more markedly with f in females than males. Song sparrows showed weak primary diphtheria responses, especially in 2005 (see also Owen-Ashley et al. 2004). However, diphtheria response tended to decline with f across individuals tested in 2004. With respect to all the three measures of immune response, overall models were broadly robust to the exclusion of outbred and highly inbred individuals. Although the sex×f interaction no longer explained a significant proportion of variation in PHA response after excluding inbred individuals, and confidence intervals around parameter estimates were in some cases increased, overall patterns of declining immune responses with increasing f remained clear. Apparent inbreeding depression in immune response was therefore not solely attributable to heterosis in offspring of immigrants–native pairings, or to particularly weak immunity in the few highly inbred individuals that were tested.
Inbreeding depression is thought to reflect increased genetic homozygosity and consequently increased expression of deleterious recessive alleles and reduced expression of overdominance (Charlesworth & Charlesworth 1999). Inbreeding depression in immune response might therefore reflect direct effects of increased homozygosity at key response loci. However, since immune responses can be energetically demanding and depend on an individual's physiological and hormonal state (Hasselquist et al. 1999; Ots et al. 2001; Owen-Ashley et al. 2004), inbreeding depression may also reflect more general detrimental effects of inbreeding on metabolic or endocrinological pathways. PHA response declined with body condition in song sparrows, indicating condition dependence in this component of immunity (see also Alonso-Alvarez & Tella 2001; Råberg & Stjernman 2003). Tetanus and diphtheria responses did not vary with body condition (see also Råberg et al. 2003). However, the apparent sex-specific inbreeding depression observed in tetanus response may suggest an indirect physiological mechanism. Direct phenotypic consequences of inbreeding should not differ between females and males unless key loci are sex linked (Falconer & Mackay 1996), and major MHC loci that mediate major aspects of humoral (and cell-mediated) immunity are autosomal in birds (Edwards et al. 1999, although it is possible that sex-linked loci are also involved). In contrast, sex-specific variation in immune response resulting from sex-specific variation in life-history allocation and physiology is widely predicted and documented (Zuk & McKean 1998; Hasselquist et al. 1999; Stoehr & Kokko 2006). Predicted variation, however, primarily relates to mean responses and patterns of seasonal variation. The apparent sex-specific inbreeding depression observed in immune response is therefore unexpected. It is not clear why inbreeding effects should differ between males and females or why the direction of sex-specific effects should differ among components of immunity. Since investment in immune responsiveness may trade-off against investment in other life-history components (Tella et al. 2002; Adamo 2004), one final possibility is that relatively inbred individuals strategically invest less in immunity. Such reduced allocation could be viewed in a life-history context as an adaptive response to increased intrinsic mortality rates in inbred individuals, and could conceivably be modulated in response to direct physiological or endocrinological effects of inbreeding. Such strategic investment patterns may provide one possible explanation for sex-specific allocation to immunity with respect to f.
Irrespective of the underlying mechanisms, our results broadly concur with an increasing body of evidence that parasite resistance commonly declines with reduced heterozygosity and/or genetic diversity in vertebrates (e.g. Acevedo-Whitehouse et al. 2005; MacDougall-Shackleton et al. 2005; Westerdahl et al. 2005; Whiteman et al. 2006, although see Giese & Hedrick 2003; Côté et al. 2005). However, most studies, particularly those on free-living individuals, have related parasite resistance or immunity to heterozygosity or genetic diversity measured across a few microsatellite or MHC loci rather than individual f. In many cases, it is therefore uncertain whether observed effects reflect genome-wide heterozygosity or inbreeding per se, as opposed to heterozygosity at specific focal or linked loci (Balloux et al. 2004; Slate et al. 2004). Furthermore, few studies have related any measure of inbreeding, heterozygosity or genetic diversity to an individual's response to any novel, non-specific immune challenge, and no general pattern has emerged. For example, one generation of sib–sib mating did not reduce encapsulation response in bumble-bees (Bombus terrestris, Gerloff et al. 2003). PHA response but not the antibody response to sheep red blood cells (SRBC response) increased with multilocus heterozygosity in captive house finches (Carpodacus mexicanus, Hawley et al. 2005). Neither PHA nor SRBC response varied with MHC diversity in captive house sparrows (Passer domesticus, Bonneaud et al. 2005). In contrast, PHA, tetanus and diphtheria responses all declined to some extent with increasing f in song sparrows. Further studies are required to characterize the patterns and causes of this variation and identify the circumstances under which inbreeding and/or genetic diversity is most likely to influence immune response.
It is likely to be simplistic to interpret an individual's response to any single immune challenge as a measure of general parasite resistance (Norris & Evans 2000; Adamo 2004). Since only ten song sparrows experienced both PHA and DTV and these individuals experienced these challenges in different years, we have little power to assess whether PHA, diphtheria and tetanus responses were correlated within individuals. Notwithstanding these limitations, individual PHA and tetanus responses tended to be negatively correlated after controlling for year, inter-sample period and maternal vaccination history (rs=−0.55, n=10, p=0.09). However, our repeated challenge experiments suggested that PHA, tetanus and diphtheria responses, which to some degree reflect the main components of avian immunity (Norris & Evans 2000; Martin et al. 2006), show broadly similar declines with increasing f in free-living song sparrows. Inbreeding loads were estimated as 2.1–7.0, 7.2–7.8 and 2.8 lethal equivalents per gamete for PHA, tetanus (in females) and diphtheria responses, respectively. Individual f explained a remarkably high proportion of variation, particularly in PHA response. Furthermore, given the likely paternity error in the Mandarte pedigree, these figures may underestimate the true magnitude of inbreeding depression (Keller et al. 2002; Kruuk et al. 2002; Marr et al. in press). We cannot quantitatively compare the inbreeding loads observed in PHA, diphtheria and tetanus responses, since these different components of immunity were measured in different individuals in different years. Among-component variation in inbreeding depression cannot therefore be distinguished from among-year variation, such as might result from among-year variation in environmental severity (e.g. Keller et al. 2002). However, our data indicate that the magnitude of inbreeding depression can be of the same order in multiple components of immunity, at least in females, and equals or exceeds that commonly observed in major fitness components, including in song sparrows on Mandarte (Keller 1998; Kruuk et al. 2002).
The substantial inbreeding depression observed in immune response seems likely to be biologically significant. Variation in PHA response has been linked to substantial changes in life-history allocation (Martin et al. 2006), and an average effect size of r=0.43 has been reported for the relationship between immune response and survival in birds (Møller & Saino 2003; see also Råberg & Stjernman 2003). Given this effect size and the inbreeding depression in immunity observed in song sparrows, even moderate inbreeding (f≈0.1) would equate to a substantial reduction in adult survival. Therefore, interpreted directly, our data indicate that even moderate inbreeding may impair immunity sufficiently to reduce fitness in free-living birds (assuming exposure to a relevant parasite and notwithstanding that fitness may not be maximized by maximizing immune responsiveness, Råberg & Stjernman 2003; Adamo 2004; Viney et al. 2005). These data support the general suggestion that host–parasite interactions can be influenced by host genotype and specifically highlight the likely importance of dominance genetic effects such that the parasite resistance declines with host genome-wide heterozygosity. Resulting parasite-mediated selection against inbred hosts may help maintain genetic diversity in natural populations (Potts & Wakeland 1990; Coltman et al. 1999), influence the evolution and maintenance of inter-sexual selection (Reid et al. 2005) and threaten the persistence of small populations (Altizer et al. 2003; Whiteman et al. 2006). The apparent sex-specific inbreeding depression observed in PHA response and particularly tetanus response is unexpected, and implies that further compound consequences of host–parasite interactions might arise. For example, given sex-specific inbreeding depression in immunity, parasites might cause biased sex ratios in small populations of inbred hosts, thereby reducing effective population size and further increasing inbreeding, genetic drift and stochastic extinction risk. Further studies are required to investigate the prevalence, magnitude and causes of sex-specific inbreeding depression in parasite resistance and explore the demographic and evolutionary implications.
Acknowledgments
We thank the Tsawout and Tseycum First Nations bands for access to Mandarte and Brad Fedy, Emily Gonzales, Franziska Heinrich, Michael Janssen, Kelly Jewell, Ursina Koller, Kathy Martin, Judy Myers, Kerstin Persson, Carol Ritland, Douglas Sejberg, Mark Sloan, Jamie Smith and Amy Wilson for their invaluable assistance with field and labwork. We thank NSERC, NSF, British Ecological Society, Killam Trusts, Jesus & Newnham Colleges (Cambridge), the UK Royal Society, the Swedish Research Council for the Environment, Agricultural Science and Spatial Planning (Formas), the Swedish Research Council (VR), the Carl Trygger and Crafoord Foundations and particularly Werner & Hildegard Hesse for their support. Robert Brunham kindly provided vaccines and Stuart Piertney and two anonymous referees helpfully commented on the manuscript.
References
- Acevedo-Whitehouse K, Vicente J, Gortazar C, Höfle U, Fernández-de-Mera I.G, Amos W. Genetic resistance to bovine tuberculosis in the Iberian wild boar. Mol. Ecol. 2005;14:3209–3217. doi: 10.1111/j.1365-294X.2005.02656.x. doi:10.1111/j.1365-294X.2005.02656.x [DOI] [PubMed] [Google Scholar]
- Adamo S.A. How should behavioural ecologists interpret measures of immunity? Anim. Behav. 2004;68:1443–1449. doi:10.1016/j.anbehav.2004.05.005 [Google Scholar]
- Alonso-Alvarez C, Tella J.L. Effects of experimental food restriction and body-mass changes on the avian T-cell-mediated immune response. Can. J. Zool. 2001;79:101–105. doi:10.1139/cjz-79-1-101 [Google Scholar]
- Altizer S, Harvell D, Friedle E. Rapid evolutionary dynamics and disease threats to biodiversity. Trends Ecol. Evol. 2003;18:589–596. doi:10.1016/j.tree.2003.08.013 [Google Scholar]
- Balloux F, Amos W, Coulson T. Does heterozygosity estimate inbreeding in real populations? Mol. Ecol. 2004;13:3021–3031. doi: 10.1111/j.1365-294X.2004.02318.x. doi:10.1111/j.1365-294X.2004.02318.x [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Richard M, Faivre B, Westerdahl H, Sorci G. An MHC class I allele associated to the expression of T-dependent immune response in the house sparrow. Immunogenetics. 2005;57:782–789. doi: 10.1007/s00251-005-0046-5. doi:10.1007/s00251-005-0046-5 [DOI] [PubMed] [Google Scholar]
- Cassinello J, Gomendio M, Roldan E.R.S. Relationship between coefficient of inbreeding and parasite burden in endangered gazelles. Conserv. Biol. 2001;15:1171–1174. doi:10.1046/j.1523-1739.2001.0150041171.x [Google Scholar]
- Charlesworth B, Charlesworth D. The genetic basis of inbreeding depression. Genet. Res. 1999;74:329–340. doi: 10.1017/s0016672399004152. doi:10.1017/S0016672399004152 [DOI] [PubMed] [Google Scholar]
- Coltman D.W, Pilkington J.G, Smith J.A, Pemberton J.M. Parasite-mediated selection against inbred Soay sheep in a free-living, island population. Evolution. 1999;53:1259–1267. doi: 10.1111/j.1558-5646.1999.tb04538.x. doi:10.2307/2640828 [DOI] [PubMed] [Google Scholar]
- Côté S.D, Stien A, Irvine R.J, Dallas J.F, Marshall F, Halvorsen O, Langvatn R, Albon S.D. Resistance to abomasal nematodes and individual genetic variability in reindeer. Mol. Ecol. 2005;14:4159–4168. doi: 10.1111/j.1365-294X.2005.02733.x. doi:10.1111/j.1365-294X.2005.02733.x [DOI] [PubMed] [Google Scholar]
- Daszak P, Cunningham A, Hyatt A.D. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. doi:10.1126/science.287.5452.443 [DOI] [PubMed] [Google Scholar]
- Edwards S.V, Hess C, Gasper J, Garrigan D. Toward an evolutionary genomics of the avian MHC. Immunol. Rev. 1999;167:119–132. doi: 10.1111/j.1600-065x.1999.tb01386.x. doi:10.1111/j.1600-065X.1999.tb01386.x [DOI] [PubMed] [Google Scholar]
- Falconer D.S, Mackay T.F.C. 4th edn. Longman; London, UK: 1996. Introduction to quantitative genetics. [Google Scholar]
- Gerloff C.U, Ottmer B.K, Schmid-Hempel P. Effects of inbreeding on immune response and body size in a social insect, Bombus terrestris. Funct. Ecol. 2003;17:582–589. doi:10.1046/j.1365-2435.2003.00769.x [Google Scholar]
- Giese A.R, Hedrick P.W. Genetic variation and resistance to a bacterial infection in the endangered Gila topminnow. Anim. Conserv. 2003;6:369–377. doi:10.1017/S1367943003003445 [Google Scholar]
- Goto N, Kodama H, Okada K, Fujimoto Y. Suppression of phytohemagglutinin skin response in thymectomized chickens. Poult. Sci. 1978;57:246–250. doi: 10.3382/ps.0570246. [DOI] [PubMed] [Google Scholar]
- Haag C.R, Sakwińska O, Ebert D. Test of synergistic interaction between infection and inbreeding in Daphnia magna. Evolution. 2003;57:777–783. doi: 10.1111/j.0014-3820.2003.tb00289.x. doi:10.1554/0014-3820(2003)057[0777:TOSIBI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hansson S.A, Hasselquist D, Folstad I, Erikstad K.E. Costs of immunity: immune responsiveness reduces survival in a vertebrate. Proc. R. Soc. B. 2004;271:925–930. doi: 10.1098/rspb.2004.2678. doi:10.1098/rspb.2004.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselquist D, Marsh J.A, Sherman P.W, Wingfield J.C. Is avian humoral immunocompetence suppressed by testosterone? Behav. Ecol. Sociobiol. 1999;45:167–175. doi:10.1007/s002650050550 [Google Scholar]
- Hasselquist D, Wasson M.F, Winkler D.W. Humoral immunocompetence correlates with date of egg-laying and reflects work load in female tree swallows. Behav. Ecol. 2001;12:93–97. doi:10.1093/beheco/12.4.457 [Google Scholar]
- Hawley D.M, Sydenstricker K.V, Kollias G.V, Dhondt A.A. Genetic diversity predicts pathogen resistance and cell-mediated immunocompetence in house finches. Biol. Lett. 2005;1:326–329. doi: 10.1098/rsbl.2005.0303. doi:10.1098/rsbl.2005.0303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick P.W, Kalinowski S.T. Inbreeding depression in conservation biology. Annu. Rev. Ecol. Syst. 2000;31:139–162. doi:10.1146/annurev.ecolsys.31.1.139 [Google Scholar]
- Joron M, Brakefield P. Captivity masks inbreeding effects on male mating success in butterflies. Nature. 2003;424:191–194. doi: 10.1038/nature01713. doi:10.1038/nature01713 [DOI] [PubMed] [Google Scholar]
- Keller L.F. Inbreeding and its fitness effects in an insular population of song sparrows (Melospiza melodia) Evolution. 1998;52:240–250. doi: 10.1111/j.1558-5646.1998.tb05157.x. doi:10.2307/2410939 [DOI] [PubMed] [Google Scholar]
- Keller L.F, Jeffery K.J, Arcese P, Beaumont M.A, Hochachka W.M, Smith J.N.M, Bruford M.W. Immigration and the ephemerality of a natural population bottleneck: evidence from molecular markers. Proc. R. Soc. B. 2001;268:1387–1394. doi: 10.1098/rspb.2001.1607. doi:10.1098/rspb.2001.1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L.F, Grant P.R, Grant B.R, Petren K. Environmental conditions affect the magnitude of inbreeding depression in survival of Darwin's finches. Evolution. 2002;56:1229–1239. doi: 10.1111/j.0014-3820.2002.tb01434.x. doi:10.1554/0014-3820(2002)056[1229:ECATMO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kruuk L.E.B, Sheldon B.C, Merilä J. Severe inbreeding depression in collared flycatchers (Ficedula albicollis) Proc. R. Soc. B. 2002;269:1581–1589. doi: 10.1098/rspb.2002.2049. doi:10.1098/rspb.2002.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.A, Martin L.B, Hasselquist D, Ricklefs R.E, Wikelski M. Contrasting adaptive immune defenses and blood parasite prevalence in closely related Passer sparrows. Oecologia. 2006;150:383–392. doi: 10.1007/s00442-006-0537-6. doi:10.1097/s00442-006-0537-6 [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton E.A, Derryberry E.P, Foufopoulos J, Dobson A.P, Hahn T.P. Parasite-mediated heterozygote advantage in an outbred songbird population. Biol. Lett. 2005;1:105–107. doi: 10.1098/rsbl.2004.0264. doi:10.1098/rsbl.2004.0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major D.L, Barber C.A. Extra-pair paternity in first and second broods of eastern song sparrows. J. Field Ornithol. 2004;75:152–156. [Google Scholar]
- Marr A.B, Keller L.F, Arcese P. Heterosis and outbreeding depression in descendants of natural immigrants to an inbred population of song sparrows (Melospiza melodia) Evolution. 2002;56:131–142. doi: 10.1111/j.0014-3820.2002.tb00855.x. doi:10.1554/0014-3820(2002)056[0131:HAODID]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Marr, A. B., Dallaire, L. C. & Keller, L. F. In press. Pedigree errors bias estimates of inbreeding depression. Anim. Genet
- Martin L.B, Han P, Lewittes J, Kuhlman J.R, Klasing K.C, Wikelski M. Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Funct. Ecol. 2006;20:290–299. doi:10.1111/j.1365-2435.2006.01094.x [Google Scholar]
- Møller A.P, Saino N. Immune response and survival. Oikos. 2003;104:299–304. doi:10.1111/j.0030-1299.2004.12844.x [Google Scholar]
- Murdoch W.W, Briggs C.J, Nisbet R.M. Dynamical effects of host size- and parasitoid state-dependent attacks by parasitoids. J. Anim. Ecol. 1997;66:542–556. doi:10.2307/5948 [Google Scholar]
- Norris K, Evans M.R. Ecological immunity: life history trade-offs and immune defense in birds. Behav. Ecol. 2000;11:19–26. doi:10.1093/beheco/11.1.19 [Google Scholar]
- O'Connor K.D, Marr A.B, Arcese P, Keller L.F, Jeffery K.J, Bruford M.W. Extra-pair fertilization and effective population size in the song sparrow Melospiza melodia. J. Avian Biol. 2006;37:572–578. doi:10.1111/j.2006.0908-8857.03681.x [Google Scholar]
- Ots I, Kerimov A.B, Ivankina E.V, Ilyina T.A, Hõrak P. Immune challenge affects basal metabolic activity in wintering great tits. Proc. R. Soc. B. 2001;268:1175–1181. doi: 10.1098/rspb.2001.1636. doi:10.1098/rspb.2001.1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Ashley N.T, Hasselquist D, Wingfield J.C. Androgens and the immunocompetence handicap hypothesis: unravelling direct and indirect pathways of immunosuppression in song sparrows. Am. Nat. 2004;164:490–505. doi: 10.1086/423714. doi:10.1086/423714 [DOI] [PubMed] [Google Scholar]
- Potts W.K, Wakeland E.K. Evolution of diversity at the major histocompatibility complex. Trends Ecol. Evol. 1990;5:181–187. doi: 10.1016/0169-5347(90)90207-T. doi:10.1016/0169-5347(90)90207-T [DOI] [PubMed] [Google Scholar]
- Råberg L, Stjernman M. Natural selection on immune responsiveness in blue tits Parus caeruleus. Evolution. 2003;57:1670–1678. doi: 10.1554/02-417. doi:10.1554/02-417 [DOI] [PubMed] [Google Scholar]
- Råberg L, Stjernman M, Hasselquist D. Immune responsiveness in adult blue tits: heritability and effects of nutritional status during ontogeny. Oecologia. 2003;136:360–364. doi: 10.1007/s00442-003-1287-3. doi:10.1007/s00442-003-1287-3 [DOI] [PubMed] [Google Scholar]
- Reid J.M, Arcese P, Keller L.F. Inbreeding depresses immune response in song sparrows (Melospiza melodia): direct and inter-generational effects. Proc. R. Soc. B. 2003;270:2151–2157. doi: 10.1098/rspb.2003.2480. doi:10.1098/rspb.2003.2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J.M, Arcese P, Cassidy A.L.E.V, Marr A.B, Smith J.N.M, Keller L.F. Hamilton & Zuk meet heterozygosity? Song repertoire size signals inbreeding and immunity in song sparrows (Melospiza melodia) Proc. R. Soc. B. 2005;272:481–487. doi: 10.1098/rspb.2004.2983. doi:10.1098/rspb.2004.2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J.M, Arcese P, Keller L.F, Hasselquist D. Long-term maternal effect on offspring immune response in song sparrows (Melospiza melodia) Biol. Lett. 2006;2:573–576. doi: 10.1098/rsbl.2006.0544. doi:10.1098/rsbl.2006.0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitt I, Brostoff J, Male D. 5th edn. Mosby; London, UK: 1998. Immunology. [Google Scholar]
- Slate J, David P, Dodds K.G, Veenvliet B.A, Glass B.C, Broad T.E, McEwen J.C. Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: theoretical expectations and empirical data. Heredity. 2004;93:255–265. doi: 10.1038/sj.hdy.6800485. doi:10.1038/sj.hdy.6800485 [DOI] [PubMed] [Google Scholar]
- Smith J.N.M, Keller L.F, Marr A.B, Arcese P. Oxford University Press; New York, NY: 2006. Conservation and biology of small populations: the song sparrows of Mandarte Island. [Google Scholar]
- Spielman D, Brook B.W, Briscoe D.A, Frankham R. Does inbreeding and loss of genetic diversity decrease disease resistance? Conserv. Genet. 2004;5:439–448. doi:10.1023/B:COGE.0000041030.76598.cd [Google Scholar]
- Staszewski V, Boulinier T. Vaccination: a way to address questions in behavioural and population ecology? Trends Parasitol. 2004;20:17–22. doi: 10.1016/j.pt.2003.11.005. doi:10.1016/j.pt.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Stoehr A.M, Kokko H. Sexual dimorphism in immunocompetence: what does life-history theory predict? Behav. Ecol. 2006;17:751–756. doi:10.1093/beheco/ark018 [Google Scholar]
- Taylor R.L.J, Cotter P.F, Wing T.L, Briles W.E. Major histocompatibility B complex and sex effects on the phytohemagglutinin wattle response. Anim. Genet. 1987;18:343–350. doi: 10.1111/j.1365-2052.1987.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Tella J.L, Scheuerlein A, Ricklefs R.E. Is cell-mediated immunity related to the evolution of life history strategies of birds? Proc. R. Soc. B. 2002;269:1059–1066. doi: 10.1098/rspb.2001.1951. doi:10.1098/rspb.2001.1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins D.M, Mitchell R.A, Bryant D.M. Hybridization increases measures of innate and cell-mediated immunity in an endangered bird species. J. Anim. Ecol. 2006;75:559–564. doi: 10.1111/j.1365-2656.2006.01076.x. doi:10.1111/j.1365-2656.2006.01076.x [DOI] [PubMed] [Google Scholar]
- Viney M.E, Riley E.M, Buchanan K.L. Optimal immune responses: immunocompetence revisited. Trends Ecol. Evol. 2005;20:665–669. doi: 10.1016/j.tree.2005.10.003. doi:10.1016/j.tree.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Westerdahl H, Waldenström J, Hansson B, Hasselquist D, von Schantz T, Bensch S. Association between malaria and MHC in a migratory songbird. Proc. R. Soc. B. 2005;272:1511–1518. doi: 10.1098/rspb.2005.3113. doi:10.1098/rspb.2005.3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman N.K, Matson K.D, Bollmer J.L, Parker P.G. Disease ecology in the Galápagos hawk (Buteo galapagoensis): host genetic diversity, parasite load and natural antibodies. Proc. R. Soc. B. 2006;273:797–804. doi: 10.1098/rspb.2005.3396. doi:10.1098/rspb.2005.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk M, McKean K. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 1998;26:1009–1023. doi:10.1016/S0020-7519(96)00086-0 [PubMed] [Google Scholar]