Abstract
Alien predators are widely considered to be more harmful to prey populations than native predators. To evaluate this expectation, we conducted a meta-analysis of the responses of vertebrate prey in 45 replicated and 35 unreplicated field experiments in which the population densities of mammalian and avian predators had been manipulated. Our results showed that predator origin (native versus alien) had a highly significant effect on prey responses, with alien predators having an impact double that of native predators. Also the interaction between location (mainland versus island) and predator origin was significant, revealing the strongest effects with alien predators in mainland areas. Although both these results were mainly influenced by the huge impact of alien predators on the Australian mainland compared with their impact elsewhere, the results demonstrate that introduced predators can impose more intense suppression on remnant populations of native species and hold them further from their predator-free densities than do native predators preying upon coexisting prey.
Keywords: invasive species, biodiversity conservation, predation impact, filter effect, prey naiveté
1. Introduction
Alien predators are considered to be one of the most important causes of declines and extinctions of species, and of biodiversity losses, worldwide (Vitousek et al. 1997). Few ecosystems have escaped their impact, and especially destructive effects have been reported from insular ecosystems (Courchamp et al. 2003; Blackburn et al. 2004). Prey in such systems are often naive to the hunting tactics of novel alien predators, which may be especially efficient in the small areas of islands. Once established, alien predators typically generate complex linkages with native biota, which then pose major challenges for management (Shea & Chesson 2002; Glen & Dickman 2005). Understanding how alien predators affect their prey is therefore a crucial conservation objective, not only to identify and protect prey species at risk, but also to ensure efficient and targeted management of the problem.
Early workers on predator–prey interactions considered that native vertebrate predators do not have large detrimental effects on the population sizes of their native prey, because these predators have coexisted with their prey for long periods and kill only non-reproductive or surplus individuals that are doomed to die in any case (Errington 1956). During the last 20 years, this traditional paradigm has changed slowly with the recognition that native vertebrate predators can limit or even regulate the population sizes of their prey (e.g. Korpimäki & Krebs 1996; Côté & Sutherland 1997; Sinclair et al. 1998; Gurevitch et al. 2000; Korpimäki et al. 2004) and, on occasion, locally obliterate them (e.g. Kavanagh 1988). Yet, the prevailing dogma is still that alien predators have far more detrimental effects on the population sizes and diversity of native animals than native predators. This assertion has been made many times (e.g. Wood Jones 1925; Troughton 1941; Diamond 1989) and underlies great concern about the role of alien predators in extinctions of birds and mammals (Blackburn et al. 2004), but it has not been critically evaluated (Gurevitch & Padilla 2004). To address this issue, we performed a worldwide search of available field experiments in which the population densities of vertebrate (mammalian and avian) predators had been manipulated, and conducted a meta-analysis on the responses of their vertebrate prey. We ask two main questions: (i) Do alien predators impact more than native predators on populations of vertebrate prey? (ii) Are the impacts of predation more intense in insular than in mainland ecosystems? We focus on direct impacts that could be demonstrated on prey population size and reproduction as these have the most immediate consequences for conservation.
2. Material and methods
To obtain a comprehensive set of studies for our meta-analysis, we searched online databases of Web of Science, Biosis Previews and Biological Abstracts using combinations of the following keywords: introduced; alien; feral; predator; predation; experiment; manipulation; removal; reduction; control; effect; and impact. We also used the bibliographies of earlier reviews (Côté & Sutherland 1997; Newton 1998) and of papers already retrieved. Data searches ended in January 2006. A preliminary search yielded 159 articles, from which 84 were discarded as they did not meet our criteria (see below). This left 45 replicated studies (at least two control and two treatment plots or a before-and-after design) and 30 unreplicated studies (only one sample for either treatment or control or both; 30 studies describing 35 experiments) that were included in the final dataset (see Appendix S1 in the electronic supplementary material). Most of these were published in international scientific journals on ecology, conservation and wildlife, and we have also included book chapters and one unpublished Ph.D. thesis. Articles were published between 1939 and 2006, with most originating from the last 10 years.
We selected publications that described the effect of reduction or addition of avian or mammalian predators on avian or mammalian prey, excluding livestock and other non-native prey species. Studies that had removed both native and introduced predators were excluded, if the effects of these predator groups could not be separated. Acceptable prey responses to predator manipulations were classified as either population size or reproductive responses. Population size responses included those measured directly, as density, minimum numbers known to be alive, numbers of breeding pairs (as an index of population size), rate of increase or survival; and catch-per-unit effort indices such as the number of animals per area, trapline or transect. Reproductive responses included numbers of juveniles or broods produced, numbers of females with young, nesting success, survival of young and mean recruitment. Per capita measures, such as brood size per hen, number of juveniles per hen, number of broods per pair, number of fledglings/ducklings per pair, number of chicks fledged per pair, number of fawns/100 does, etc., were not included. The studies also had to have been run for long enough (one prey generation or more) for a prey demographic response to be possible. The studies measuring other parameters or using other units than those described were omitted. No authors were contacted to obtain missing data.
Necessary data (sample sizes, means of controls and treatments and their standard deviations/standard errors/confidence limits) were extracted from the text, tables or figures of the articles. In cases where error bars were not symmetrical about means, variances were calculated conservatively using the longest bars provided and, if no variances were given, these were calculated from raw results. Where possible, we used data taken at the end of experiments, but otherwise used an arithmetic mean of responses over the course of the studies. In cyclically fluctuating prey species, such as some small mammals, data were taken for consistency from the peak phase of the cycle. Reproductive responses were taken separately for each year, and an arithmetic mean calculated across the duration of the study to obtain one effect size per study.
Publications were scored also for the type of prey (e.g. rodent, ungulate, marsupial), prey class (bird/mammal), origin of predator (native/introduced), predator class (bird/mammal/both), the method of manipulation (addition/removal), location (mainland/island), habitat, spatial and temporal scales of the experiment (manipulation area and manipulation time), the continent in which the study was conducted and whether it was conducted in an exclosure or open terrain (manipulation type). We also recorded the mean weight of prey and predator species to calculate a predator/prey weight ratio for each study. Predators were considered to be introduced or native based on definitions provided in each study and confirmed using Long (2003). ‘Predator addition’ means either release of predators into experimental areas or attraction of predators (e.g. attraction of raptors with perch sites). ‘Predator removal’ refers to either exclosure experiments or manipulations, where predators were killed or relocated. Two persons (M.N. and P.S.) were responsible for data collection.
The 45 replicated experiments (table S1 in electronic supplementary material) were examined for publication bias using the normal quantile plot method (Wang & Bushman 1998), and no evidence of publication bias was found (figure S1 in electronic supplementary material). This analysis was not possible for unreplicated studies and, therefore, the publication of such studies may have been biased towards large positive effects of predator removal on prey. However, it is rather unlikely that the results, whether significant or not, of very expensive, long-lasting predator manipulation experiments would remain unpublished, strongly reducing the likelihood for the file-drawer problem (Rosenthal 1979) particularly in this meta-analysis.
For each replicated study, we calculated the standardized effect size as Hedges' d using MetaWin v. 2.1 (Rosenberg et al. 2000). There are also other metrics available for this type of primary data (means, variances and sample sizes), such as the log response ratio lnR (Rosenberg et al. 2000), but we chose d because our data were not suitable for use of the response ratio (e.g. in some studies, the control group value was zero; Hedges et al. 1999). Positive values of d indicate that the predator treatment had a positive effect on prey species, zero means that there was no difference between treatment and control, and negative values signify a greater response in controls. For studies that reported the responses of multiple prey species to predator manipulation, we used the mean effect size across all species to retain independence. In one study, predators had been both added and removed; also here a mean effect for the whole study was calculated from the effect sizes of both treatments.
Our first prediction was that introduced predators should have more pronounced effects than native predators on the population sizes and reproductive outputs of their prey. To test this prediction with the 45 replicated experiments, we carried out a categorical summary analysis using the homogeneity statistic, Q, in MetaWin v. 2.1. As with variance in ANOVA, the total heterogeneity QT can be partitioned into QM, the variation explained by the model, and QE, the residual error variance (Rosenberg et al. 2000). Continuous summary analysis (weighted linear regression) was used to determine whether d was affected by the spatial or temporal scale of the studies. We used random effects models and conducted resampling tests with 4999 iterations. Bias-corrected confidence intervals were used to evaluate the probability at 0.05. All tests were two-tailed.
To expand the coverage of research that has evaluated the impacts of predation, we conducted a similar analysis on the unreplicated predator removal experiments. Altogether, 34 unreplicated studies fulfilled the criteria, but two were excluded as they reported earlier stages of experiments that were represented in the analysis by later, more inclusive papers. In two cases, different aspects of the same experiment were reported in separate papers, which were then combined to gain one effect size. One study consisted of six experiments at different locations, which were therefore treated as independent studies in the dataset. Hence, the final dataset has 35 rows (table S2 in electronic supplementary material).
We classified the traits of the unreplicated experimental systems as described previously and defined the effect size as Xe/Xc, where Xe and Xc are the treatment and control prey responses, respectively. A ratio over 1 means that predator manipulation had a positive effect on the prey species, while a ratio up to 1 means that manipulation did not affect the prey species or the effects were negative. These unreplicated data cannot be analysed using typical meta-analysis approaches; therefore, we tested for differences in effect size in the study traits using Student's t-test with the Satterthwaite option for heteroscedastic variances (procedure TTEST, SAS Statistical Package, v. 9.1; SAS Institute, Cary, NC, USA). Effect size was ln transformed to meet the assumptions of normality.
Finally, a generalized linear model was built in order to further test our first and second predictions (i.e. that alien predators would have more impact than native predators on prey populations and that predation impacts would be greater on prey in island ecosystems compared with mainland ecosystems), and to explore possible interactions of the different explanatory variables. Neither MetaWin nor t-test allows the simultaneous analysis of multiple factors, and the sample sizes of replicated and unreplicated experiments alone were too small for such an analysis. Therefore, we pooled population size responses of the replicated and unreplicated experiments using Xe/Xc as the effect size measure. The model was fitted with a negative binomial distribution of the response and a log link function with the negative binomial GLM (glm.nb) procedure in the MASS library of S-Plus (v. 6, Insightful Corporation, Seattle, USA).
The main explanatory variables in the model were origin of predator (native versus alien), type of manipulation (open area versus predator exclosure), predator class (mammal, bird and both) and location (mainland versus island) together with their second-order interactions. Predator/prey weight ratio, manipulation area and duration of manipulation were included as continuous variables. Not all interactions of the classifying variables could be included, since they produced empty cells (singularities): origin of predator×predator class was removed because all introduced predators were mammals; origin of predator×manipulation type was removed because all except one study on introduced predators were conducted in open areas; and location×manipulation type was removed because there was only one study on islands using enclosures. Australia was classified as mainland in the analysis. The step Akaike information criterion (AIC) procedure in the MASS library of S-Plus was used for a stepwise model selection procedure, which selects the model with the lowest AIC value, starting with the global model. Owing to small sample size, AICc was used (Burnham & Anderson 2000). The support for each alternative model was evaluated by calculating: (i) AICc differences Δi, where models with Δi≤2 are considered to have substantial support and (ii) Akaike weights wi, which describe the weight of evidence that model i is the best model from the set of alternative models (Burnham & Anderson 2000).
3. Results
Of the 45 replicated studies included in the analyses (table S1 in electronic supplementary material), 12 studies recorded only reproductive responses and 23 provided only population size responses. Ten studies reported both reproductive and population size effects, but from these only population size effects were used. The manipulated predator species were native in 37 studies (82%) and introduced in 8 studies. Manipulations were carried out primarily on predatory mammals (24 studies) and mammalian and avian predators combined (17 studies), with only four studies on predatory birds alone. The effect of manipulations involved mammalian prey in 27 studies and birds in 18 studies. Mammalian prey were mostly small rodent species (mice and voles; 23 studies), whereas waterfowl was the most studied prey group among birds (10 studies). Most predator manipulations (41 studies) were removals or reductions; three studies attempted to add predators to experimental areas, while one used both predator addition and removal protocols. The experiments lasted from 2.5 months to 9 years (median 25 months) and used areas ranging from 0.13 ha to 77.5 km2 (overall median 2.2 km2; predator exclosures median 0.5 ha and open areas median 3 km2).
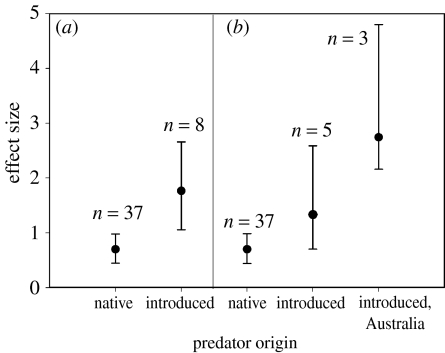
In the replicated experiments, the effects of introduced predators on prey were more than double those of native predators (figure 1a; QM=4.96, d.f.=1, p=0.020). Further partitioning revealed very striking effects of introduced predators in Australia compared with other parts of the world. The mean effect size of prey responding to the removal of introduced predators in Australia (all mammalian responses) was twofold higher than that of prey responding to introduced predators elsewhere and threefold higher when compared with native predators generally (figure 1b; QM=7.07, d.f.=2, p=0.022).
Figure 1.
Mean effect sizes of prey in replicated predator manipulation experiments. (a) Effects of native and introduced predators and (b) effects of native and introduced predators, with the effects of introduced predators divided between Australian experiments and experiments conducted elsewhere. Effect size is calculated as Hedges' d. Bars represent 95% bias-corrected confidence intervals.
There was a significant difference between population size effects and reproductive responses in the experiments, the latter being larger (table 1). Therefore, we reanalysed the data from only those experiments where population size responses were measured. The analysis of population size responses revealed the significant overall difference between alien and native predators again and also a significant difference between native and alien predators outside Australia (table 1). This is probably because most of the studies on reproductive responses had manipulated native predators (table S1 in electronic supplementary material).
Table 1.
Results of homogeneity tests for the 45 replicated experiments in the meta-analysis. (Effect size is calculated as Hedges' d.)
| variable | levels | mean effect size d | lower 95% CL | upper 95% CL | n | QM | d.f. | p |
|---|---|---|---|---|---|---|---|---|
| manipulation type | exclosure | 0.588 | 0.125 | 1.085 | 14 | |||
| open | 0.798 | 0.546 | 1.070 | 30 | 0.47 | 1 | 0.439 | |
| predator origin | native | 0.653 | 0.400 | 0.910 | 23 | |||
| (excluding exclosures) | introduced | 1.996 | 1.224 | 3.046 | 7 | 6.79 | 1 | 0.001 |
| response type | reproduction | 1.203 | 0.838 | 1.701 | 12 | |||
| population size | 0.601 | 0.327 | 0.913 | 33 | 4.04 | 1 | 0.033 | |
| predator origin | native | 0.400 | 0.164 | 0.667 | 26 | |||
| (only population size effects) | introduced | 1.996 | 1.242 | 3.013 | 7 | 9.54 | 1 | 0.001 |
| predator origin | native | 0.400 | 0.157 | 0.672 | 26 | |||
| (only population size effects) | introduced | 1.562 | 0.719 | 2.761 | 4 | |||
| introduced Australia | 2.733 | 2.077 | 4.799 | 3 | 10.89 | 2 | 0.003 |
Experiments in exclosures may be confounded by the ‘fence effect’, whereby the enclosed populations reach high densities when emigration is prevented (Krebs et al. 1969). To explore the influence of this phenomenon on our results, we tested whether there were differences in predation impacts between exclosure and open-area experiments. Interestingly, the effect sizes of prey populations in exclosure experiments were similar to those in open terrain, but removal of exclosure experiments from the analysis increased the difference in effect sizes produced by introduced and native predators (table 1). We also checked for possible biases arising from differences among studies in their duration and spatial extent by regressing effect size on time and area of study (continuous meta-analysis). There was no bias arising from either study duration (slope=0.017, d.f.=1, p=0.99, n=45) or spatial coverage (slope=0.001, d.f.=1, p=0.51, n=45).
Of the 35 unreplicated experiments included in the analyses, 13 removed alien predators (table S2 in electronic supplementary material). Six studies were from islands, 12 were from Australia and New Zealand and 17 were from mainland areas. Twenty-two studies examined the responses of mammalian prey, while the rest investigated birds. Eighteen studies recorded population size responses, and six reported reproductive responses. In 11 studies, both responses were measured but, as in the replicated studies, only population size responses were used. There was no difference between the two responses in the pooled data (t=1.18, d.f.=13.7, p=0.258; reproduction: back-transformed ln(Xe/Xc)=1.681, lower 95% CL=0.881, upper 95% CL=3.210, n=6; population size: back-transformed ln(Xe/Xc)=2.486, lower 95% CL=1.449, upper 95% CL=3.863, n=29).
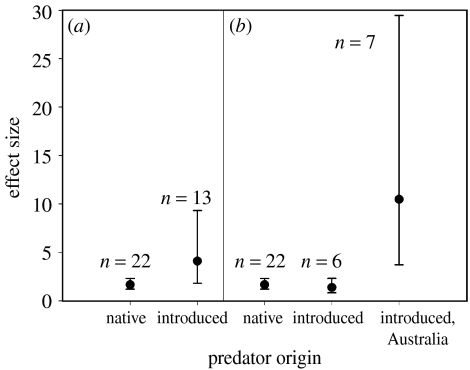
Analyses of the unreplicated studies yielded remarkably similar results to those of the replicated experiments (figure 2). The effects of introduced predators were on average 2.5 times higher than for native predators (figure 2a; t=2.22, d.f.=16.3, p=0.041), but again the difference appeared to be influenced mainly by the large effects in Australian studies (figure 2b). There were no obvious biases in effect size resulting from study duration or spatial coverage (Spearman's rank correlation, rs=0.246, p=0.16, n=35 and rs=0.184, p=0.31, n=33, respectively).
Figure 2.
Mean effect sizes of prey in unreplicated predator manipulation experiments. (a) Effects of native and introduced predators and (b) effects of native and introduced predators, with the effects of introduced predators divided between Australian experiments and experiments conducted elsewhere. Effect size presented as back-transformed ln(Xe/Xc), where Xe and Xc are the treatment and control prey responses, respectively. Bars represent 95% confidence intervals.
In further analyses, a generalized linear model of the pooled data (prey population size effects from replicated (n=32) and unreplicated (n=28) experiments) was built to further test the effects of predator origin and location, and to explore possible interactions among the different explanatory variables. The stepwise model selection procedure combined with AICc differences, and Akaike weights showed substantial support for the three most reduced models which included the variables, predator origin (native versus alien), location (mainland versus island) and their interaction (table 2a). Analysis of deviance on the model including all the three variables showed a highly significant effect of predator origin on effect size (table 2b; deviance=52.63, residual d.f.=58, p<0.0001). Also the interaction between origin and location was slightly significant (table 2b; deviance=3.90, residual d.f.=56, p=0.048), whereas location alone had no obvious impact on effect size (table 2b; deviance=2.16, residual d.f.=57, p=0.14).
Table 2.
Effects of explanatory variables on prey population size responses in a total of 60 replicated and unreplicated predator manipulation experiments. (a) The summary statistics of the stepwise model selection procedure based on AICc values. The main explanatory variables in the model were origin of predator (O; native versus introduced), type of manipulation (Mtype; open area versus predator exclosure), predator class (PC; mammal, bird and both) and location (L; mainland versus island) with their second-order interactions. Predator/prey weight ratio (Pw), manipulation area (Marea) and duration of manipulation (Mtime) were included as continuous variables. k, number of parameters in each model; log (L), value of the maximized log-likelihood function; Δi, AIC difference; wi, Akaike weights. (b) The medians and tenth and ninetieth centiles of the main variables and predator origin×location interaction.
| (a) model | k | log (L) | AICc | Δi | wi |
|---|---|---|---|---|---|
| O | 4 | 211.58 | −414.43 | 0.00 | 0.47 |
| O, L | 6 | 213.52 | −413.45 | 0.98 | 0.29 |
| O, L, O×L | 10 | 218.41 | −412.34 | 2.09 | 0.17 |
| O, L, O×L, Mtime | 11 | 218.78 | −410.06 | 4.37 | 0.05 |
| O, L, O×L, Pw, Mtime | 12 | 218.89 | −407.15 | 7.28 | 0.01 |
| O, L, Mtype, O×L, Pw, Mtime | 14 | 219.90 | −402.48 | 11.95 | <0.01 |
| O, L, Mtype, O×L, Marea, Pw, Mtime | 15 | 219.91 | −398.90 | 15.53 | <0.01 |
| O, L, PC, Mtype, O×L, Marea, Pw, Mtime | 18 | 220.99 | −389.31 | 25.12 | <0.01 |
| O, L, PC, Mtype, O×L, L×PC, Marea, Pw, Mtime | 24 | 225.47 | −368.65 | 45.78 | <0.01 |
| O, L, PC, Mtype, O×L, PC×Mtype, L×PC, Marea, Pw, Mtime | 30 | 229.61 | −335.08 | 79.35 | <0.01 |
| (b) variable | levels | median | tenth centile | ninetieth centile | n |
|---|---|---|---|---|---|
| predator origin | native | 1.519 | 0.783 | 3.771 | 44 |
| introduced | 3.835 | 1.246 | 22.952 | 16 | |
| location | mainland | 1.597 | 0.783 | 12.146 | 44 |
| island | 1.848 | 1.225 | 4.214 | 16 | |
| predator class | mammal | 1.838 | 0.809 | 21.108 | 34 |
| bird | 1.092 | 0.882 | 3.771 | 7 | |
| both | 1.733 | 0.783 | 4.000 | 19 | |
| manipulation type | open | 1.852 | 1.004 | 17.435 | 43 |
| exclosure | 1.073 | 0.445 | 6.132 | 17 | |
| origin×location | native mainland | 1.408 | 0.722 | 4.000 | 36 |
| native island | 1.867 | 1.091 | 3.771 | 8 | |
| introduced mainland | 14.791 | 1.852 | 22.952 | 8 | |
| introduced island | 1.842 | 1.225 | 29.333 | 8 |
4. Discussion
Our results strongly suggest that alien predators have more severe impacts on prey than native predators. However, this interpretation must be considered with regards to the nature of our available dataset. The large overall effect of alien predators on prey appeared to be influenced mainly by the consistently high effect sizes in the results from Australian studies. But overall, there were comparatively few experiments on alien predators available from other areas and none from mainland situations outside Australia. This relative scarcity of studies outside Australia probably derives from the well-documented invasions of alien species to Australia (Rolls 1969; Dickman 1996; Kinnear et al. 2002; Long 2003) and the coincidental acute loss of native mammals, which prompted some of the earliest experimental studies of the impacts of alien terrestrial predators. It is also possible that the lack of extensive data on alien effects in other mainland ecosystems may be linked to the practical and financial difficulties of building large-scale experiments to remove alien species only; in most (61%) experiments included in our dataset, more than one predator species had been removed or substantially reduced.
Despite the skew in availability of research on alien predators, there was evidence that the greater impact of alien predators is not simply restricted to Australia. Outside of Australia, there was also a higher impact of alien predators compared with native predators on prey population responses in the replicated studies (table 1). Furthermore, analyses of the different explanatory variables and their interactions in the broader pooled data from replicated and unreplicated studies revealed that only predator origin (native versus alien) had a highly significant effect on population size responses of prey animals. Although the interaction between predator origin and location (mainland versus island) was also slightly significant, these results together suggest that, in general, alien predators may have more severe impacts on prey than do native predators. One potential cause of the consistently large impact of alien predators on prey in Australia is small sample size bias. Three of the eight replicated studies were from Australia and were ranked second, sixth and seventh in magnitude of effect sizes. The seven unreplicated Australian studies also revealed an identical phenomenon, making a total of 10 studies that met our strict criteria for inclusion in the analysis. These studies were diverse; they spanned the continent, were conducted in environments ranging from temperate forest through to deserts and agricultural areas and involved prey body sizes from small rodents to large marsupials. We surmise that the geographical and faunal characteristics of Australia strongly contribute to especially large alien predator impacts there (discussed in detail below) and, therefore, feel confident that the 10 studies are a good representation of the situation there.
In communities where predators and prey have coexisted for long periods, prey often respond to predatory pressure by developing behaviours or morphologies that reduce the chance of encounters with predators or enhance the chance of escape once detected (Lima & Dill 1990). In contrast, prey in communities with novel alien predators are likely to be predator-naive and to lack specific avoidance behaviours. Such naiveté would facilitate greater hunting efficiency in predators and lead to greater suppressive impacts on naive prey compared with native predators hunting prey with which they have long coexisted. It is possible that the frequent historical biotic interchanges between the contiguous continents of Eurasia, Africa and the Americas cause them to share similar terrestrial predator archetypes, which may render prey less naive towards novel predators introduced from the same continents (Cox & Lima 2006). Recent research shows that Australia has also possessed a rich assemblage of marsupial carnivores from Miocene to recent times (Wroe et al. 2004) and that the native fauna may not be as predator naive as previously thought. However, Australia never had placental carnivores until they were introduced by humans, and it may be that these novel predators use tracking and hunting tactics that differ from those of their marsupial counterparts, to which native prey have little or no defence (Croft & Eisenberg 2006).
Alien predators have long been presumed to have greater impacts in island ecosystems when compared with the mainland ecosystems, for reasons including prey naiveté, yet we found no support for such phenomena. In these analyses, Australia was considered mainland; but despite its large size, Australia's island-like characteristics, such as geographical isolation and diversity of endemic species, may also have contributed to the profound impacts of alien predators there. Many native Australian prey populations are now restricted to small, island-like refugia within large tracts of unsuitable, disturbed land owing to the widespread alteration and fragmentation of habitat, and natural adaptations to stochastic environmental conditions (Morton 1990; Letnic & Dickman 2006). Compared with real island ecosystems, the large land mass of Australia may have provided more room for such refugia, which may possibly have saved some native prey populations from otherwise inevitable extinction. Although the large-scale effects of alien predators may be blunted by such refuge areas, the island-like characteristics of the refugia make them especially vulnerable to alien predators, which could be expected to have very depressive effects on local populations of native prey where predator activity is intense. Indeed, one of the largest observed effect sizes, 4.80, followed the removal of the red fox from rock outcrops containing remnant colonies of rock wallabies (Petrogale lateralis; Kinnear et al. 1998).
The concept of filter effects suggests that remnant species which survive the initial alien invasion are resistant to their ongoing impacts (Pimm et al. 1995). For example, it has recently been reported that extinction events of island birds can be related to the numbers of introduced predator species, but no relationship was found between the numbers of exotic predators and the current risk of extinction for remnant populations of surviving species (Blackburn et al. 2004; but see Blackburn et al. 2005; Didham et al. 2005). Our results challenge this notion and show that once released from the impact of alien predators, remnant bird and mammal populations undergo greater population increases than those experiencing predation from native predators. This indicates that alien predators can impose intense population suppression, which also puts prey at greater inherent risk of extinction from stochastic forces. Our results also contradict suggestions that alien predators merely compensate for native predators which themselves have been lost from the system (de Vos et al. 1956; Long 2003). Instead, the intense impacts may be compounded if additive to other sources of mortality, and could be expected to further destabilize community processes.
Although studies on alien predator effects are dominated by Australian examples, our review, based on 80 published experimental manipulations of predator densities, provides the first explicit support for the prevailing dogma that alien predators can have more detrimental effects on population sizes of prey than native predators. The evidence implicating alien predators in the historic extirpation of prey is mostly correlative with few direct accounts, and the contribution of alien predators to current extinction risks continues to be controversial (Gurevitch & Padilla 2004; Clavero & Garcia-Berthou 2005). Our review reveals an ongoing crisis because alien predators can have significantly greater suppressive impacts than native predators, keeping prey populations further from their predator-free population size, making them more vulnerable to stochastic extinction forces, and thus leading to native biodiversity losses at regional scales. The impact of alien predators is further complicated by habitat loss, fragmentation and the provision of alternative, introduced prey (Burbidge & McKenzie 1989; Saunders et al. 1995; Roemer et al. 2002). We suggest that future work should increase the number of alien predator manipulations in mainland areas and focus on disentangling the relative impact and interactions of alien predators with other factors in causing native animal populations to decline.
Acknowledgments
We thank Roosa Leimu, Esa Lehikoinen, Petri Suorsa and Tapio Eeva for their assistance with the analyses, and Mathew Crowther, Tero Klemola and two anonymous reviewers for their comments on the draft manuscript. The study was supported by the Maj and Tor Nessling Foundation.
Supplementary Material
Possible sources of bias in meta-analysis and a list of references used as data sources for this study
A data table of the replicated experiments
A data table of the unreplicated experiments
A normal quantile plot of the replicated experiments (n=45) for examining publication bias
References
- Blackburn T.M, Cassey P, Duncan R.P, Evans K.L, Gaston K.J. Avian extinction and mammalian introductions on oceanic islands. Science. 2004;305:1955–1958. doi: 10.1126/science.1101617. doi:10.1126/science.1101617 [DOI] [PubMed] [Google Scholar]
- Blackburn T.M, Cassey P, Duncan R.P, Evans K.L, Gaston K.J. Response to comment on “Avian extinction and mammalian introductions on oceanic islands”. Science. 2005;307:1412b. doi: 10.1126/science.1101617. doi:10.1126/science.1107480 [DOI] [PubMed] [Google Scholar]
- Burbidge A.A, McKenzie N.L. Patterns in the modern decline of Western Australia vertebrate fauna—causes and conservation implications. Biol. Conserv. 1989;50:143–198. doi:10.1016/0006-3207(89)90009-8 [Google Scholar]
- Burnham K.P, Anderson D.R. Springer; New York, NY: 2000. Model selection and inference. A practical information-theoretic approach. [Google Scholar]
- Clavero M, Garcia-Berthou E. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 2005;20:110. doi: 10.1016/j.tree.2005.01.003. doi:10.1016/j.tree.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Côté I.M, Sutherland W.J. The effectiveness of removing predators to protect bird populations. Conserv. Biol. 1997;11:395–405. doi:10.1046/j.1523-1739.1997.95410.x [Google Scholar]
- Courchamp F, Chapuis J.-L, Pascal M. Mammal invaders on islands: impact, control and control impact. Biol. Rev. 2003;78:347–383. doi: 10.1017/s1464793102006061. doi:10.1017/S1464793102006061 [DOI] [PubMed] [Google Scholar]
- Cox J.G, Lima S.L. Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends Ecol. Evol. 2006;21:674–680. doi: 10.1016/j.tree.2006.07.011. doi:10.1016/j.tree.2006.07.011 [DOI] [PubMed] [Google Scholar]
- Croft D.B, Eisenberg J.F. Behaviour. In: Armati P.J, Dickman C.R, Hume I.D, editors. Marsupials. Cambridge University Press; Cambridge, UK: 2006. pp. 229–298. [Google Scholar]
- de Vos A, Manville R.H, van Gelder R.G. Introduced mammals and their influence on native biota. Zoologica (N.Y.) 1956;41:163–194. [Google Scholar]
- Diamond J. Overview of recent extinctions. In: Western D, Pearl M, editors. Conservation for the 21st century. Oxford University Press; New York, NY: 1989. pp. 37–41. [Google Scholar]
- Dickman C.R. Impact of exotic generalist predators on the native fauna of Australia. Wildl. Biol. 1996;2:185–195. [Google Scholar]
- Didham R.K, Ewers R.M, Gemmell N.J. Comment on “Avian extinction and mammalian introductions on oceanic islands”. Science. 2005;307:1412a. doi: 10.1126/science.1107333. doi:10.1126/science.1107333 [DOI] [PubMed] [Google Scholar]
- Errington P.L. Factors limiting higher vertebrate populations. Science. 1956;124:304–307. doi: 10.1126/science.124.3216.304. doi:10.1126/science.124.3216.304 [DOI] [PubMed] [Google Scholar]
- Glen A.S, Dickman C.R. Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management. Biol. Rev. 2005;80:387–401. doi: 10.1017/s1464793105006718. doi:10.1017/S1464793105006718 [DOI] [PubMed] [Google Scholar]
- Gurevitch J, Padilla D.K. Are invasive species a major cause of extinctions? Trends Ecol. Evol. 2004;19:470–474. doi: 10.1016/j.tree.2004.07.005. doi:10.1016/j.tree.2004.07.005 [DOI] [PubMed] [Google Scholar]
- Gurevitch J, Morrison J.A, Hedges L.V. The interaction between competition and predation: a meta-analysis of field experiments. Am. Nat. 2000;155:435–453. doi: 10.1086/303337. doi:10.1086/303337 [DOI] [PubMed] [Google Scholar]
- Hedges L.V, Gurevitch J, Curtis P.S. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–1156. doi:10.2307/177062 [Google Scholar]
- Kavanagh R.P. The impact of predation by the powerful owl, Ninox strenua, on a population of the greater glider, Petauroides volans. Aust. J. Ecol. 1988;13:445–450. doi:10.1111/j.1442-9993.1988.tb00992.x [Google Scholar]
- Kinnear J.E, Onus M.L, Summer N.R. Fox control and rock-wallaby population dynamics—II. An update. Wildl. Res. 1998;25:81–88. doi:10.1071/WR96072 [Google Scholar]
- Kinnear J.E, Summer N.R, Onus M.L. The red fox in Australia—an exotic predator turned biocontrol agent. Biol. Conserv. 2002;108:335–359. doi:10.1016/S0006-3207(02)00116-7 [Google Scholar]
- Korpimäki E, Krebs C.J. Predation and population cycles of small mammals—a reassessment of the predation hypothesis. BioScience. 1996;46:754–764. doi:10.2307/1312851 [Google Scholar]
- Korpimäki E, Brown P.R, Jacob J, Pech R.P. The puzzles of population cycles and outbreaks of small mammals solved? BioScience. 2004;54:1071–1079. doi:10.1641/0006-3568(2004)054[1071:TPOPCA]2.0.CO;2 [Google Scholar]
- Krebs C.J, Keller B.L, Tamarin R.H. Microtus population biology—demographic changes in fluctuating populations of M. ochrogaster and M. pennsylvanicus in southern Indiana. Ecology. 1969;50:587–607. doi:10.2307/1936248 [Google Scholar]
- Letnic M, Dickman C.R. Boom means bust: interactions between the El Niño/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia. Biodiv. Conserv. 2006;15:3847–3880. doi:10.1007/s10531-005-0601-2 [Google Scholar]
- Lima S.L, Dill L.M. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 1990;68:619–640. [Google Scholar]
- Long J.L. CSIRO Publishing; Melbourne, Australia: 2003. Introduced mammals of the world. [Google Scholar]
- Morton S.R. The impact of European settlement on the vertebrate animals of arid Australia: a conceptual model. Proc. Ecol. Soc. Aust. 1990;16:201–213. [Google Scholar]
- Newton I. Academic Press; London, UK: 1998. Population limitation in birds. pp. 211–248. [Google Scholar]
- Pimm S.L, Moulton M.P, Justice L.J. Bird extinctions in the central Pacific. In: Lawton J.H, May R.M, editors. Extinction rates. Oxford University Press; Oxford, UK: 1995. pp. 75–87. [Google Scholar]
- Roemer G.W, Donlan C.J, Courchamp F. Golden eagles, feral pigs, and insular carnivores: how exotic species turn native predators into prey. Proc. Natl Acad. Sci. USA. 2002;99:791–796. doi: 10.1073/pnas.012422499. doi:10.1073/pnas.012422499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.C. Angus & Robertson; Sydney, Australia: 1969. They all ran wild: the story of pests on the land in Australia. [Google Scholar]
- Rosenberg M.S, Adams D.C, Gurevitch J. Sinauer Associates, Inc; Massachusetts, MA: 2000. MetaWin. Statistical software for meta-analysis. Version 2. [Google Scholar]
- Rosenthal R. The “File Drawer Problem” and tolerance for null results. Psychol. Bull. 1979;86:638–641. doi:10.1037/0033-2909.86.3.638 [Google Scholar]
- Saunders G, Coman B, Kinnear J, Braysher M. Australian Government Publishing Service; Canberra, Australia: 1995. Managing vertebrate pests: foxes. [Google Scholar]
- Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002;17:170–176. doi:10.1016/S0169-5347(02)02495-3 [Google Scholar]
- Sinclair A.R.E, Pech R.P, Dickman C.R, Hik D, Mahon P, Newsome A.E. Predicting effects of predation on conservation of endangered prey. Conserv. Biol. 1998;12:564–575. doi:10.1046/j.1523-1739.1998.97030.x [Google Scholar]
- Troughton E. Angus and Robertson; Sydney, Australia: 1941. Furred animals of Australia. [Google Scholar]
- Vitousek P.M, Mooney H.A, Lubchenco J, Melillo J.M. Human domination of Earth's ecosystems. Science. 1997;277:494–499. doi:10.1126/science.277.5325.494 [Google Scholar]
- Wang M.C, Bushman B.J. Using the normal quantile plot to explore meta-analytic data sets. Psychol. Methods. 1998;3:46–54. doi:10.1037/1082-989X.3.1.46 [Google Scholar]
- Wood Jones F. Government Printer; Adelaide, Australia: 1925. The mammals of South Australia. [Google Scholar]
- Wroe S, Argot C, Dickman C. On the rarity of big fierce carnivores and primacy of isolation and area: tracking large mammalian carnivore diversity on two isolated continents. Proc. R. Soc. B. 2004;271:1203–1211. doi: 10.1098/rspb.2004.2694. doi:10.1098/rspb.2004.2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Possible sources of bias in meta-analysis and a list of references used as data sources for this study
A data table of the replicated experiments
A data table of the unreplicated experiments
A normal quantile plot of the replicated experiments (n=45) for examining publication bias