Abstract
The reason why some bird species live in family groups is an important question of evolutionary biology that remains unanswered. Families arise when young delay the onset of independent reproduction and remain with their parents beyond independence. Explanations for why individuals forgo independent reproduction have hitherto focused on dispersal constraints, such as the absence of high-quality breeding openings. However, while constraints successfully explain within-population dispersal decisions, they fail as an ultimate explanation for variation in family formation across species. Most family-living species are long-lived and recent life-history studies demonstrated that a delayed onset of reproduction can be adaptive in long-lived species. Hence, delayed dispersal and reproduction might be an adaptive life-history decision rather than ‘the best of a bad job’. Here, we attempt to provide a predictive framework for the evolution of families by integrating life-history theory into family formation theory. We suggest that longevity favours a delayed onset of reproduction and gives parents the opportunity of a prolonged investment in offspring, an option which is not available for short-lived species. Yet, parents should only prolong their investment in offspring if this increases offspring survival and outweighs the fitness cost that parents incur, which is only possible under ecological conditions, such as a predictable access to resources. We therefore propose that both life-history and ecological factors play a role in determining the evolution of family living across species, yet we suggest different mechanisms than those proposed by previous models.
Keywords: deferred reproduction, delayed dispersal, kin sociality, group living, parental nepotism, survival
1. Introduction
Understanding what factors select for the cohesion of families is an important question of evolutionary biology that remains unanswered. Family living occurs in a wide range of taxa and sets the scene for cooperative kin societies and complex social behaviours, such as cooperative breeding (Brown 1987; Cockburn 1998). In birds and other animals, families usually form when mature offspring forgo dispersal and independent reproduction and remain with their parents (Brown 1987; Emlen 1994). Since prompt dispersal and independent reproduction are viewed as the optimal strategy to maximize lifetime reproductive success, research has previously focused on identifying constraints on independent reproduction. Several studies have shown that a shortage of high-quality breeding openings or other ecological constraints (EC) limit offspring dispersal and independent breeding (reviewed in Hatchwell & Komdeur 2000). However, this explanation lacks predictive power because offspring in most species face some sort of constraint but disperse promptly after independence (Hatchwell & Komdeur 2000; Cockburn 2003).
Phylogenetic analyses revealed two important characteristics of family living. First, family living has a strong phylogenetic component, being unevenly distributed between families (or between genera in families that present both cooperative and pair breeders), and is the ancestral state in several lineages (Cockburn 1996; Arnold & Owens 1998; Cockburn 2003). Second, family living occurs more frequently among long-lived bird species (Arnold & Owens 1998). This latter finding represents an important link to life-history theory that has previously been recognised (Brown 1987; Pruett-Jones & Lewis 1990; Emlen 1991; Hatchwell & Komdeur 2000), but has not been carefully considered in studies addressing the evolution of family living (but see Russell 1989; Ekman & Rosander 1992; Arnold & Owens 1998; Ekman et al. 2001a).
Life-history theory predicts that long-lived species should benefit from a delayed onset of reproduction (Goodman 1974; Stearns 1992; Charlesworth 1994), and this has been supported by studies that demonstrated a positive effect of delayed onset of reproduction on lifetime reproductive success in long-lived bird species (Stacey & Ligon 1987; Ekman et al. 1999; Krüger 2005). Furthermore, longevity not only gives the option to offspring of delaying the onset of reproduction, but it also reduces the cost to parents of a prolonged investment in offspring (Ekman & Rosander 1992). Extended parental investment is an important factor that has been suggested to facilitate family formation (Brown 1987; Ekman et al. 2001a; Ekman 2006). Integrating life-history theory into family formation theory could therefore provide important insights to explain the distribution of families among species.
Here, we attempt to stimulate research into the evolution of family living by providing a new testable framework. We will focus on factors that select for family cohesion and thus will not address the hypotheses associated with cooperative breeding, because in most species, cooperative breeding is a consequence of family cohesion and families may exist in the absence of cooperative breeding (Ekman et al. 2001a; Ekman 2006). We argue that delayed onset of independent reproduction in long-lived species might be an adaptive life-history strategy to maximize individual fitness, instead of being a ‘best-of-a-bad-job’ strategy. In addition, only parents in long-lived species can afford a prolonged investment in offspring, although this additional investment is only possible when parents have a predictable access to resources. An extended parental investment enhances the fitness of offspring that remain on the parental territory. Consequently, a delayed onset of reproduction combined with increased parental investment can select for the maintenance of families.
2. Prevailing views: ecological constraints and life-history hypotheses
Delayed dispersal and family formation have been widely regarded as a direct response to EC on independent reproduction (Koenig & Pitelka 1981; Emlen 1982; Koenig et al. 1992; table 1). Individuals fail to disperse and reproduce after reaching independence because some constraint, such as lack of suitable territory or shortage of mates, prevents them from doing so (Emlen 1982; Hatchwell & Komdeur 2000). Intraspecific studies where individuals dispersed to breed if vacancies were experimentally created provided compelling support for the EC hypothesis (e.g. Seychelles warblers Acrocephalus sechellensis (Komdeur 1992), superb fairy-wrens Malurus cyaneus (Pruett-Jones & Lewis 1990); see also Hatchwell & Komdeur 2000 for a review).
Table 1.
Main hypothesis put forward to explain the evolution of delayed independent reproduction (DR) and dispersal.
| ecological constraint; Emlen (1982) | |
| hypothesis | DR is as a response to constraints on dispersal options. Habitat saturation or environmental variation leads to the lack of high-quality openings and offspring delay dispersal |
| strong point | manipulation of constraints explains dispersal patterns within some species |
| weak points | most species face some sort of constraints and do not delay dispersal; does not explain interspecific variation |
| life-history; Arnold & Owens (1998) | |
| hypothesis | DR has a phylogenetic component and is more frequent in long-lived species since they occupy their territories for longer, leading to habitat saturation and preventing younger individuals from obtaining breeding positions |
| strong point | partly explains the phylogenetic pattern of DR and attempts to reconcile cooperation with its life-history correlates |
| weak points | the majority of long-lived species are not cooperative; in practice proposes the same mechanism as the EC hypothesis and hence has the same weak points |
| prolonged investment: parental nepotism; Brown (1987); Ekman et al. (2001a) | |
| hypothesis | offspring gain direct fitness benefits from a prolonged association with their parents due to nepotistic parental behaviour that improve offspring fitness |
| strong point | explains why offspring should prefer to wait for the onset of reproduction at home and hence provides the basis to understand family formation. brings together parental investment and life-history characteristics |
| weak points | neglects the role of life-history characteristics on reproductive decisions of offspring; does not entirely explain interspecific variation |
However, this model has been less successful when comparing the effect of EC in different populations (Stacey & Bock 1978; Rathburn & Montgomery 2003; Carmen 2004). Additionally, it remains unclear why in some species, such as fairy-wrens, individuals faced with constraints on reproduction remain at home with their parents, whereas in other species, like northern-temperate Parus tits, individuals always leave, even when faced with strong constraints (e.g. Ekman 1989). Or why can delayed dispersal and family living be found in the presence of suitable breeding openings or in the absence of dispersal constraints (e.g. Emlen 1990; Macedo & Bianchi 1997; Baglione et al. 2002; Covas et al. 2004; Baglione et al. 2005). The failure in explaining this variation between species suggests that EC have a predictive power on dispersal decisions within populations, but are not able to explain why species evolve into solitary or family living and poses a challenge to the EC hypothesis that has not been sufficiently acknowledged (but see Hatchwell & Komdeur 2000; Ekman et al. 2001a; Cockburn 2003).
Attempts to provide an explanation that could account for the interspecific variation in family formation prompted comparative analyses, which indicated that living in family groups is more common in species with high survival and low fecundity (Russell 1989; Cockburn 1996; Arnold & Owens 1998). This idea was formalized in the ‘life-history (LH) hypothesis’ (Russell 1989; Arnold & Owens 1998), which proposes that the evolution of families is a two-step process: low annual mortality predisposes avian lineages to cooperative breeding through a slow territory turnover, then ecological characteristics, such as being sedentary, further lead to an overcrowded population and reduced breeding openings (Arnold & Owens 1998). Recognizing the relationship between life histories and family living was an important step, since it helped understanding the patchy phylogenetic distribution of families (Cockburn 1996; Arnold & Owens 1998; Hatchwell & Komdeur 2000) that could not be reconciled with any ecological features (Ford et al. 1988; du Plessis et al. 1995; Arnold & Owens 1999).
However, there are several problems with the current formulation of the LH hypothesis (see also Ekman et al. 2001a; Cockburn 2003). First, habitat saturation occurs whenever recruitment of individuals into a given population exceeds mortality (Kokko & Lundberg 2001) as, for example, in northern-temperate Parus species, which have high fecundity and low survival and nonetheless show prompt dispersal (Ekman 1989; Cramp & Perrins 1993). Second, although the majority of family-living species are long-lived, the majority of long-lived species does not live in families (Cockburn 2003). Third, the mechanism proposed by the LH hypothesis is still an ‘EC’ mechanism: independent breeding is limited since high longevity of territory owners, instead of some characteristic of the species' ecology, is expected to slow down the territory turnover (Russell 1989; Arnold & Owens 1998). Hence, the mechanism proposed by the LH hypothesis cannot account for the formation of families in the absence of habitat saturation (e.g. Baglione et al. 2005), or explain why some species with obvious constraints on reproduction disperse to float or breed in lower quality habitats (e.g. Carmen 2004).
3. The role of life histories revisited
Given that longevity is a prevailing characteristic of species that exhibit delayed dispersal and family living, understanding the link between these two factors remains an important question and one that might shed light on the distribution of family living across species. The key is to determine which characteristics associated with longevity might predispose species to delay dispersal. We argue that the positive relationship between high survival and the age at first reproduction observed across species coupled with an extended parental investment that only long-lived species can afford might explain the link between life-history characteristics and the prevalence of family living.
(a) Age of first breeding in a life-history context
Animal life histories are represented over a continuum that ranges from slow life histories typified by low fecundity, slow development and high survival, to high fecundity, fast development and low survival (Roff 1992; Stearns 1992). The optimal onset of reproduction is also linked to lifespan: in short-lived species, reproduction is expected to start early in life (Stearns 1992; Charlesworth 1994) and offspring should disperse quickly to be able to find a breeding vacancy (Nilsson 1989).
Long-lived species, on the contrary, might benefit from delaying the onset of reproduction. These species have typically low fecundity and increase their lifetime reproductive success through maximizing the number of breeding events in life (Goodman 1974; Clutton-Brock 1988; Charlesworth 1994; Barbraud & Weimerskirch 2001; Martin 2002). In these species, individuals should be reluctant to engage in any activities that might decrease their lifespan, since even small reductions in survival can reduce their fitness through a lower number of lifetime breeding attempts (Clutton-Brock 1988). A comparative study of parental risk taking supported this hypothesis that long-lived bird species are more risk adverse than short-lived species (Ghalambor & Martin 2001): individuals from species with low survival prospects incurred higher risks in order to reduce the risks to their young, while individuals from species with higher survival prospects responded by reducing the risks to themselves.
Breeding activity incurs several costs (Williams 1966; Stearns 1992), including decreased survival prospects (Golet et al. 1998; Visser & Lessells 2001), lower future reproductive investment (Young 1996; Hanssen et al. 2005) or decreased health state (Ardia et al. 2003; Hanssen et al. 2005). Importantly, reproductive costs can be higher for young breeders, since they are less experienced, poorer competitors and less likely to settle in good quality territories (Ens et al. 1995; Ekman et al. 2001b). As a result, they might experience increased mortality (Pyle et al. 1997; Tavecchia et al. 2001; Orell & Belda 2002) and lower reproductive success (Green 2001; Krüger 2005) when compared with individuals that start breeding later in life.
Hence, an early onset of reproduction might pose a substantial fitness cost to long-lived species, particularly under poor breeding conditions. This prediction has been supported by several studies on long-lived species that found higher lifetime reproductive success for individuals with delayed reproduction than for individuals that started breeding early in life, both in family-living species (acorn woodpeckers Melanerpes formicivorus, Stacey & Ligon 1987; Seychelles warblers, Komdeur 1992; Siberian jays Perisoreus infaustus, Ekman et al. 1999) and a species with prompt juvenile dispersal (goshawk Accipiter gentiles, Krüger 2005). Therefore, contrary to what is proposed by the EC hypothesis or LH, empirical studies suggest that the delayed onset of reproduction in long-lived species, and thus most family-living species, might in fact be a selected trait and not only a best-of-a-bad-job response to dispersal constraints. It should also be noticed that the delayed onset of reproduction in family-living birds is unlikely to be caused by delayed sexual maturity, since physiological studies demonstrated that retained 1-year-old offspring are sexually mature (Vleck et al. 1991; Schoech et al. 1996).
Whenever a suitable breeding opening emerges, individuals have to weight the benefits of taking the opening, which is dependent on environmental factors (e.g. territory quality, population density, mate quality, mate availability) and intrinsic factors (own phenotypic quality, sex or age). For example, in a population of superb fairy-wrens (Pruett-Jones & Lewis 1990), the sex ratio was biased towards males and young males did not disperse into vacant territories from which the females had been removed. However, they dispersed promptly when the females were reintroduced into those territories. Hence, both the species' life history and the breeding conditions found should influence dispersal decisions. Where key resources are scarce or unpredictable in occurrence, individuals should be more opportunistic than where conditions are more stable and predictable.
While delaying reproduction might often be advantageous, this alone does not explain delayed dispersal, since young can wait for a high-quality breeding opening away from the natal territory and, in fact, the majority of species does appear to do so (Koenig et al. 1999; Cockburn 2003). So why delay dispersal and associate with the parents?
(b) Slow life histories and prolonged parental investment
Staying on the parental territory gives the benefits of remaining in a familiar territory and of a prolonged association with the parents, which entails important direct fitness benefits for offspring (table 2; see also review in Ekman 2006). In particular, parents provide nepotistic access to resources and predator protection to their offspring that they withhold from unrelated immigrants (Ekman & Griesser 2002; Griesser 2003; Griesser & Ekman 2004, 2005). These benefits have been shown to result in a fitness gain to philopatric offspring through substantially improved survival (Griesser et al. 2006). Moreover, philopatric offspring in many species help to raise younger siblings and may thereby gain both direct and indirect (kin selected) fitness benefits (Brown 1987; Emlen 1991; Griffin & West 2003). However, indirect benefits of alloparental care can only occur if offspring delay dispersal until the next reproductive event (Ekman 2006), and thus per se cannot select for the formation of families (Ekman et al. 2001a). In spite of the incentive to remain in the natal territory provided by the direct benefits gained through associating with the parents, individuals in most species disperse after independence. Why do so many species disperse and float?
Table 2.
Benefits for offspring gained through delaying dispersal in birds.
| selection level of benefit | category of benefit | specific benefit | species | reference |
|---|---|---|---|---|
| direct benefit from delaying dispersal | access to high-quality breeding territory | neighbour territoryparental territory | Siberian jay Mexican jay | Brown & Brown (1984),Ekman et al. (1999) |
| direct benefits given by parents | access to resources | food | western CrowMexican jay | Verbeek & Butler (1981),Barkan et al. (1986) |
| Siberian jay | Ekman et al. (1994) | |||
| tufted titmouse | Pravosudova et al. (2000) | |||
| western bluebird | Dickinson & McGowan (2005) | |||
| future mates | various species | Cockburn (1998) | ||
| protection from competitors | intraspecific aggression | Berwick's swan | Scott (1980),Black & Owen (1987) | |
| barnacle goose | ||||
| protection from predators | nepotistic alarm calling | Siberian jay | Griesser & Ekman (2004) | |
| nepotistic vigilance | Siberian jay | Griesser (2003) | ||
| nepotistic mobbing of predators | Siberian jay | Griesser & Ekman (2005) | ||
| direct benefits through activities of offspring | alloparental care | improved parental skills | Seychelles warbler | Komdeur (1996) |
| improved attractiveness to mate | white-fronted bee-eater | Wrege & Emlen (1994),Zack & Rabenold (1989) | ||
| stripe-backed wren | ||||
| consequences of delayed dispersal | improved survival | western bluebird | Kraaijeveld & Dickinson (2001),Griesser et al. (2006) | |
| Siberian jay | ||||
| indirect benefitsa | alloparental care | raising siblings | various species | Cockburn (1998) |
Although in about 50% of all cases no indirect fitness benefit to helper was possible to measure.
A slow life-history influences reproductive decisions of not only the offspring but also the parents. Work by Ekman and colleagues indicates that only in species with high survival parents can afford to invest in prolonged parental care after offspring independence without increasing their own mortality disproportionably (Ekman & Rosander 1992; Ekman et al. 2001a; Ekman 2005, unpublished data). A recent model that analysed natal dispersal under different life-history scenarios indicated that the crucial factor for the evolution of delayed dispersal is the ratio of parent versus offspring survival (Ekman unpublished data). Parents should invest into extended parental care only when the expected increment in offspring survival compensates for the increase in parental investment. Hence, there is a potential conflict between parent and offspring over investment: for the offspring, it is advantageous to delay dispersal at a lower survival gain, but the parents require a larger offspring survival gain due to incongruent fitness interests. Thus, when the increment in offspring survival does not reach the necessary threshold, delayed dispersal is unlikely to evolve (see also Kokko & Lundberg 2001 for a discussion on the consequences of differential survival of philopatric offspring and floaters). This could be the case for species with either very low or, particularly, very high juvenile survival. Hence, it could explain why delayed dispersal is absent in long-lived species such as storks and herons (Cramp & Simmons 1977), where survival of dispersing juveniles may be very high in any case and thus juveniles would not benefit significantly from an extended parental investment.
For parents, the cost/benefit ratio of retaining young will change with offspring age. Parental investment in early offspring life has a higher value than investment later on, since juvenile mortality can be expected to decrease through time as, for example, young become more efficient foragers (Desrochers 1992) or less susceptible to predators (Martin 1995). In addition, the onset of a new reproductive event influences parental investment strategies and may lead to the eviction of philopatric offspring by their parents ahead of the breeding season, as reported in the Texas green jay Cyanocorax yncas, grey jay Perisoreus canadensis or superb fairy-wren M. cyaneus (Gayou 1986; Strickland & Ouellet 1993; Mulder 1995). Older offspring can also represent a reproductive competitor (Griffin & West 2002; Ridley & Sutherland 2002; West et al. 2002), which can explain why parents become less tolerant towards philopatric offspring over time, as reported in the Siberian jay (Ekman et al. 1994).
In spite of the potential advantages associated with waiting for the initiation of breeding activity on the natal territory close to the parents, in many species with low adult mortality and deferred breeding, the offspring disperse directly after independence. Hence, life history alone cannot account for the distribution of family living across species. What other factors might differ among slow life-history species with delayed dispersal and those with prompt dispersal?
4. The role of ecology and demography revisited
Several studies have looked for ecological correlates of family living, but failed to find common factors (Ford et al. 1988; du Plessis et al. 1995; Arnold & Owens 1999; see also review in Hatchwell & Komdeur 2000), suggesting that ecological models have limited potential to explain the occurrence of family living (Hatchwell & Komdeur 2000; Cockburn 2003). The only aspect that has been repeatedly linked to family living in both observational and theoretical studies is year-round territoriality (Russell 1989; Arnold & Owens 1999; Kokko & Lundberg 2001; Ekman 2006). This suggests that a predictable and exclusive access to resources to share with the offspring is a key ecological characteristic that makes extended care affordable to parents (see also Ekman et al. 2001a; Ekman 2006). Hence, environmental characteristics that cause individuals to abandon their territory after the breeding season are likely to disrupt family cohesion. This process is illustrated by carrion crows Corvus corone populations in Italy and Spain (Baglione et al. 2005). Spanish crows live mostly in family groups, whereas only pair breeding is known in Italy. The Spanish population inhabits an area where suitable habitat remains unoccupied, and where competition and variation in habitat quality are low (Baglione et al. 2002). In contrast, the non-philopatric Italian population inhabits a highly variable and competitive environment. The key difference behind these dissimilarities in kin structure between populations seems to be the distribution of resources outside the breeding season. Year-round territoriality is possible in Spain, whereas the patchy and temporally variable distribution of food in intensive agricultural areas in Italy forces the crows to move continually outside the breeding season (Baglione et al. 2005).
Despite the traditional emphasis on year-round residency to promote the evolution of delayed dispersal and family living, examples from migratory Bewick's swans Cygnus columbianus, barnacle geese Branta leucopsis and common cranes Grus grus support the suggestion that predictability in access to resources might be the crucial factor. In all the three species, offspring migrate together with their parents to the wintering grounds, where parents defend winter territories and give offspring exclusive access to feeding resources and protection from intraspecific competitors (Scott 1980; Black & Owen 1987; Alonso et al. 2004). If parents cannot provide significant benefits to offspring through resource sharing or other forms of protection, then offspring benefit less from a prolonged association with their parents and are likely to disperse after independence. This mechanism could explain why family living is ostensibly missing from long-lived groups, such as seabirds. The almost complete absence of family living in seabirds with the exception of some skuas Stercorarius spp. (Hemmings 1994) is particularly challenging, because, in addition to a slow life history, many seabirds are highly philopatric and offspring often return to breed within few metres of their hatching site. However, if there are no advantages in maintaining family cohesion after young gain independence and leave the colonies, then the family bond is broken.
5. A pathway towards family living
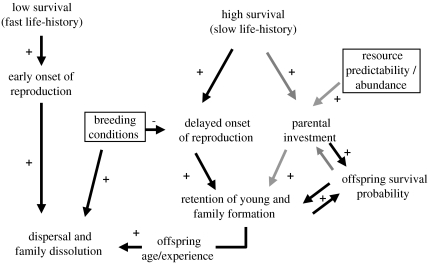
In this paper we have attempted to identify the shortcomings of previous frameworks and bring together studies on life history and family living to provide a new framework to understand variation in family formation across bird species. We propose that family formation across species might evolve as follows (figure 1): life-history strategies play a role through favouring a delayed onset of reproduction in long-lived species, i.e. individuals in these species should prefer to breed when they are more experienced and can have access to better territories (or wait for better breeding conditions). Simultaneously, only long-lived species can afford prolonged parental investment. Moreover, any prolonged investment should be guided by changes in survival prospects of both parents and offspring. Parents should only invest in prolonged care to their offspring if this investment substantially improves offspring survival and discontinue investing when this improvement is no longer significant (e.g. when offspring acquire better skills). Also, parents should only invest in offspring beyond independence if they have a predictable access to resources and in the absence of within-family competition for resources. Hence, similarly to the LH hypothesis (Arnold & Owens 1998, 1999), we suggest that the evolution of family living results from a combination of life-history predisposition and ecological facilitation. However, the processes proposed here differ from those suggested by the LH hypothesis: slow life histories predispose clades to family living, given the intrinsic tendency to delay the onset of independent reproduction and the possibility for parents of a prolonged investment in offspring. In addition, ecological characteristics determine whether or not parents can afford to share resources or provide other forms of protection to their offspring. Finally, within species or populations, the frequency and quality of breeding openings should influence individual decisions on independent breeding, i.e. in agreement with EC hypothesis.
Figure 1.
Proposed pathway leading to family formation in birds. The effects of life history and environmental factors on parental decisions are illustrated by the grey arrows, while the effects on offspring decisions are shown by the black arrows. The environmental factors are shown in boxes. The directions of the relationships between traits or factors are illustrated by ‘plus’ for positive correlations and ‘minus’ for negative correlations. Species with high survival tend to start breeding later in life and can invest more in offspring. These two factors combined can cause offspring to delay dispersal leading to the formation of families. Environmental factors such as good breeding conditions will promote dispersal. Resource availability allows parents to invest more in offspring without incurring strong costs. However, parents should only prolong investment in offspring if this increases offspring survival prospects substantially without compromising their own survival, and thus there should not be parental investment when offspring survival is too low or too high.
However, the proposed framework does not exclude the possibility that family associations might arise through different pathways. For example, cooperatively breeding long-tailed tits (Aegithalos caudatus) are short-lived and individuals always attempt to breed in their first year of life, as predicted by life-history theory (Hatchwell et al. 1999). Interestingly, in this species, family cohesion is reached through a different pathway. Owing to high nest predation, population density is low, reducing kin competition and giving offspring the option to delay dispersal and remain associated with their parents throughout their first winter. Then, they disperse and attempt to reproduce independently, but return to the parental territory to help their relatives if their own breeding attempt fails. In this species, family living seems unrelated to life-history characteristics and appears instead linked to environmental factors that allow the family structure to be kept after the breeding season and to their nesting in close spatial association.
6. Testing the adaptive delayed dispersal hypothesis
Our framework emphasizes the need to address the evolution of family living at an interspecific level. While some questions can only be addressed at the intraspecific level (see below), more interspecific studies are needed to compare how different species respond to variation in different factors. Below we develop some ideas on how to test the framework proposed (see also figure 1).
One of the basic issues of our hypothesis is that delayed reproduction and dispersal in long-lived species might be adaptive and not just a result of constraints. In other words, it might be a better strategy for offspring to remain at home than to disperse and breed under suboptimal conditions. Thus, a key prediction of our model is that the delayed onset of reproduction is only a beneficial strategy in long-lived species but not in short-lived ones. This is an important point since it could explain the link between longevity and propensity for sociality. We therefore predict that longer-lived species are more likely to avoid breeding in suboptimal conditions to avoid incurring a higher reproductive cost and compromising their lifetime reproductive success. In contrast, short-lived species cannot afford to postpone the onset of reproduction and thus should breed under poorer conditions, since they have fewer chances of additional breeding opportunities during their short lives. This hypothesis can be tested by manipulating breeding conditions to create less favourable conditions, or territories, in species with contrasting survival prospects. Similar mechanisms should influence dispersal decisions within species and individuals are expected to choose dispersal strategies according to an interaction of individual factors (e.g. age, sex, phenotypic quality) and external factors that affect fitness (e.g. quality of the breeding opening, population density, mate quality). Hence, within species, we expect EC to play a role in limiting independent breeding. However, the two levels of analysis should not be confounded.
We also expect parental investment strategies in offspring and parent/offspring survival ratio to influence investment and hence offspring dispersal decisions. This can be tested through comparative studies by analysing parental and offspring survival in relation to dispersal and reproductive decisions of offspring. These studies can be field based or literature based, as better quality data from long-term studies become available. In some systems it should also be possible to manipulate (improve) parental or offspring survival prospects to provide an experimental test of this hypothesis, although it might be difficult to achieve this without manipulating resource levels, and hence it could be complicated to distinguish the effects of the two.
Resource levels and predictability (after the breeding season) and low within-family competition are also crucial factors to allow family living. Studies that manipulated resources to modify dispersal behaviour have already been conducted successfully (e.g. Dickinson & McGowan 2005; Baglione et al. 2006) and provided support to the idea that an experimental change in available resources affects the offspring dispersal decisions. At this point, a decisive experimental test would be to cause offspring in a non-social species (from a family social clade) to delay dispersal and form family groups through continuous food supplementation or improvement of other crucial resource. Such a change of behaviour, if obtained in a situation where nearby breeding vacancies remained available, would be in striking contrast to what is expected under EC hypotheses.
Previous comparative analyses have failed to find an effect of ‘ecology’ on family formation among species (Cockburn 2003). However, we propose that ecological factors which affect the survival of individuals and the predictability of access to resources might reveal significant associations. This could be investigated by manipulating and comparing different populations or through broader literature-based comparative analyses.
Finally, when offspring decide to postpone independent reproduction and the parents tolerate the presence of young, remaining in a family group should be the best strategy, in terms of lifetime reproductive success, for offspring to follow until becoming breeders. Tests of this hypothesis might only be possible intraspecifically, where fitness of offspring with contrasting strategies is compared. Good evidence in support of this hypothesis is already available from field studies (Stacey & Ligon 1987; Ekman et al. 1999, 2001b; Griesser et al. 2006), but more studies in other systems comparing lifetime reproductive success of individuals with delayed versus prompt reproduction are needed to substantiate or reject this hypothesis.
More generally, studies on family living have normally focused on costs and benefits of delayed dispersal and helping behaviour in cooperative breeders. We suggest that we should look to alternative systems in order to progress. Novel and important insights might now be achieved by studying non-family-living species in family-living clades (e.g. Green & Cockburn 2001) or species from non-family-living clades that have cooperative behaviours. In addition, the overwhelming majority of studies have focused on what happens during the breeding season, but to reproduce individuals must survive the non-breeding season, and thus the factors affecting the survival of individuals outside the breeding season should be crucial. Finally, it would also be important to understand why delayed dispersal and family living are not found in many species (both short- and long-lived) living in saturated habitats. In fact, most situations where kin associations should be found but are not might provide the best opportunities to falsify hypotheses and obtain novel insights into the evolution of family living.
Acknowledgments
We thank Morné du Plessis, Corinne Eising, Ben Hatchwell, Penn Lloyd, Mandy Ridley, Andy Russell, Stuart Sharp, Rob Simmons, Andrew Taylor and three anonymous referees for their comments that greatly improved previous versions of the manuscript. Jan Ekman kindly gave us access to unpublished material. This work has been financed by the Portuguese Science and Technology Foundation (R.C.) and the Swiss National Research Foundation (M.G.).
References
- Alonso J.C, Bautista L.M, Alonso J.A. Family based territoriality versus flocking in wintering common carnes Grus grus. J. Avian Biol. 2004;35:425–433. doi:10.1111/j.0908-8857.2004.03290.x [Google Scholar]
- Ardia D.R, Schat K.A, Winkler D.W. Reproductive effort reduces long-term immune function in breeding tree swallows (Tachycineta bicolor) Proc. R. Soc. B. 2003;270:1679–1683. doi: 10.1098/rspb.2003.2424. doi:10.1098/rspb.2003.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K.E, Owens I.P. Co-operative breeding in birds: a comparative test of the life-history hypothesis. Proc. R. Soc. B. 1998;265:739–745. doi:10.1098/rspb.1998.0355 [Google Scholar]
- Arnold K.E, Owens I.P.F. Cooperative breeding in birds: the role of ecology. Behav. Ecol. 1999;10:465–471. doi:10.1093/beheco/10.5.465 [Google Scholar]
- Baglione V, Canestrari D, Marcos J.M. Cooperatively breeding groups of the carrion crow Corvus corone corone in northern Spain. Auk. 2002;119:790–799. doi:10.1642/0004-8038(2002)119[0790:CBGOCC]2.0.CO;2 [Google Scholar]
- Baglione V, Marcos J.M, Canestrari D, Griesser M, Andreotti G, Bardini C, Bogliani G. Does year-round territoriality rather than habitat saturation explain delayed natal dispersal and cooperative breeding in the carrion crow? J. Anim. Ecol. 2005;74:842–851. doi:10.1111/j.1365-2656.2005.00983.x [Google Scholar]
- Baglione V, Canestrari D, Marcos J.M, Ekman J. Experimentally increased food resources in the natal territory promote offspring philopatry and helping in cooperatively breeding carrion crows. Proc. R. Soc. B. 2006;273:1529–1535. doi: 10.1098/rspb.2006.3481. doi:10.1098/rspb.2006.3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbraud C, Weimerskirch H. Emperor penguins and climate change. Nature. 2001;411:183–186. doi: 10.1038/35075554. doi:10.1038/35075554 [DOI] [PubMed] [Google Scholar]
- Barkan C.P.L, Craig J.L, Strahl S.D, Stewart A.M, Brown J.L. Social dominance in communal Mexican jays Aphelocoma ultramarina. Anim. Behav. 1986;34:175–187. doi:10.1016/0003-3472(86)90021-7 [Google Scholar]
- Black J.M, Owen M. Determinant factors of social rank in goose flocks: acquisition of social rank in young geese. Behaviour. 1987;102:129–146. [Google Scholar]
- Brown J.L. Princeton University Press; Princeton, NJ: 1987. Helping and communal breeding in birds. [Google Scholar]
- Brown J.L, Brown E.R. Parental facilitation: parent–offspring relations in communally breeding birds. Behav. Ecol. Sociobiol. 1984;14:203–209. doi:10.1007/BF00299620 [Google Scholar]
- Carmen W.J. Behavioral ecology of the California scrub-jay (Aphelocoma californica): a non-cooperative breeder with close cooperative relatives. Stud. Avian Biol. 2004:28. [Google Scholar]
- Charlesworth B. Cambridge University Press; Cambridge, UK: 1994. Evolution in age-structured populations. [Google Scholar]
- Clutton-Brock T.H. University of Chicago Press; Chicago, IL: 1988. Reproductive success. [Google Scholar]
- Cockburn A. Why do so many Australian birds cooperate: social evolution in the Corvida? In: Floyd R.B, Sheppard A.W, Barro P.J.D, editors. Frontiers of population ecology. CSIRO; East Melbourne, Australia: 1996. pp. 451–472. [Google Scholar]
- Cockburn A. Evolution of helping behaviour in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 1998;29:141–177. doi:10.1146/annurev.ecolsys.29.1.141 [Google Scholar]
- Cockburn A. Cooperative breeding in Oscine passerines: does sociality inhibit speciation? Proc. R. Soc. B. 2003;270:2207–2214. doi: 10.1098/rspb.2003.2503. doi:10.1098/rspb.2003.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covas R, Doutrelant C, du Plessis M.A. Experimental evidence of a link between breeding conditions and the decision to breed or to help in a colonial cooperative breeder. Proc. R. Soc. B. 2004;271:827–832. doi: 10.1098/rspb.2003.2652. doi:10.1098/rspb.2003.2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramp S, Perrins C.M. Oxford University Press; Oxford, UK: 1993. Handbook of the birds of Europe, the Middle East and North Africa. [Google Scholar]
- Cramp S, Simmons K.E. Oxford University Press; Oxford, UK: 1977. Handbook of the birds of Europe, the Middle East and North Africa. [Google Scholar]
- Desrochers A. Age and foraging success in European blackbirds: variation among and within individuals. Anim. Behav. 1992;43:885–894. doi:10.1016/0003-3472(92)90002-Q [Google Scholar]
- Dickinson J.L, McGowan A. Winter resource wealth drives delayed dispersal and family-group living in western bluebirds. Proc. R. Soc. B. 2005;272:2423–2428. doi: 10.1098/rspb.2005.3269. doi:10.1098/rspb.2005.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis M.A, Siegfried W.R, Armstrong A.J. Ecological and life-history correlates of co-operative breeding in South-African birds. Oecologia. 1995;102:180–188. doi: 10.1007/BF00333250. doi:10.1007/BF00333250 [DOI] [PubMed] [Google Scholar]
- Ekman J. Ecology of non-breeding social systems of Parus. Wils. Bull. 1989;101:263–288. [Google Scholar]
- Ekman J. Family cohesion among birds. J. Avian Biol. 2006;37:289–298. doi:10.1111/j.2006.0908-8857.03666.x [Google Scholar]
- Ekman J, Griesser M. Why offspring delay dispersal: experimental evidence for a role of parental tolerance. Proc. R. Soc. B. 2002;269:1709–1713. doi: 10.1098/rspb.2002.2082. doi:10.1098/rspb.2002.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman J, Rosander B. Survival enhancement through food sharing: a means for parental control of natal dispersal. Theor. Popul. Biol. 1992;42:117–129. doi: 10.1016/0040-5809(92)90008-h. doi:10.1016/0040-5809(92)90008-H [DOI] [PubMed] [Google Scholar]
- Ekman J, Sklepkovych B, Tegelström H. Offspring retention in the Siberian jay (Perisoreus infaustus): the prolonged brood care hypothesis. Behav. Ecol. 1994;5:245–253. doi:10.1093/beheco/5.3.245 [Google Scholar]
- Ekman J, Bylin A, Tegelström H. Increased lifetime reproductive success for Siberian jay (Perisoreus infaustus) males with delayed dispersal. Proc. R. Soc. B. 1999;266:911–915. doi:10.1098/rspb.1999.0723 [Google Scholar]
- Ekman J, Baglione V, Eggers S, Griesser M. Delayed dispersal: living under the reign of nepotistic parents. Auk. 2001a;118:1–10. doi:10.1642/0004-8038(2001)118[0001:DDLUTR]2.0.CO;2 [Google Scholar]
- Ekman J, Eggers S, Griesser M, Tegelström H. Queuing for preferred territories: delayed dispersal of Siberian jays. J. Anim. Ecol. 2001b;70:317–324. doi:10.1046/j.1365-2656.2001.00490.x [Google Scholar]
- Emlen S.T. The evolution of helping. I. An ecological constraints model. Am. Nat. 1982;119:29–39. doi:10.1086/283888 [Google Scholar]
- Emlen S.T. White-fronted bee-eaters: helping in a colonially nesting species. In: Stacey P.B, Koenig W.D, editors. Cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 1990. pp. 489–526. [Google Scholar]
- Emlen S.T. The evolution of cooperative breeding in birds and mammals. In: Krebs J.R, Davies N.B, editors. Behavioural ecology: an evolutionary approach. Blackwell Scientific Publications; Oxford, UK: 1991. pp. 301–337. [Google Scholar]
- Emlen S.T. Benefits, constraints and the evolution of the family. Trends Ecol. Evol. 1994;9:282–285. doi: 10.1016/0169-5347(94)90030-2. doi:10.1016/0169-5347(94)90030-2 [DOI] [PubMed] [Google Scholar]
- Ens B.F, Weissing F.J, Drent R.H. The despotic distribution and deferred maturity: two sides of the same coin. Am. Nat. 1995;146:625–650. doi:10.1086/285818 [Google Scholar]
- Ford H.A, Bell H, Nias R, Noske R. The relationship between ecology and the incidence of cooperative breeding in Australian birds. Behav. Ecol. Sociobiol. 1988;22:239–249. doi:10.1007/BF00299838 [Google Scholar]
- Gayou D. The social system of the Texas green jay. Auk. 1986;103:540–547. [Google Scholar]
- Ghalambor C.K, Martin T.E. Fecundity–survival trade-offs and parental risk-taking in birds. Science. 2001;292:494–497. [Google Scholar]
- Golet G.H, Irons D.B, Estes J.A. Survival costs of chick rearing in black-legged kittiwakes. J. Anim. Ecol. 1998;67:827–841. doi:10.1046/j.1365-2656.1998.00233.x [Google Scholar]
- Goodman D. Natural selection and a cost ceiling on reproductive effort. Am. Nat. 1974;108:247–268. doi:10.1086/282906 [Google Scholar]
- Green D.J. The influence of age on reproductive performance in the brown thornbill. J. Avian Biol. 2001;32:6–14. doi:10.1034/j.1600-048X.2001.320102.x [Google Scholar]
- Green D.J, Cockburn A. Post-fledging care, philopatry and recruitment in brown thornbills. J. Anim. Ecol. 2001;70:505–514. doi:10.1046/j.1365-2656.2001.00503.x [Google Scholar]
- Griesser M. Nepotistic vigilance behavior in Siberian jay parents. Behav. Ecol. 2003;14:246–250. doi:10.1093/beheco/14.2.246 [Google Scholar]
- Griesser M, Ekman J. Nepotistic alarm calling in the Siberian jay (Perisoreus infaustus) Anim. Behav. 2004;67:933–939. doi:10.1016/j.anbehav.2003.09.005 [Google Scholar]
- Griesser M, Ekman J. Nepotistic mobbing in the Siberian jay, Perisoreus infaustus. Anim. Behav. 2005;69:345–352. doi:10.1016/j.anbehav.2004.05.013 [Google Scholar]
- Griesser M, Nystrand M, Ekman J. Reduced mortality selects for family cohesion in a social species. Proc. R. Soc. B. 2006;273:1881–1886. doi: 10.1098/rspb.2006.3527. doi:10.1098/rspb.2006.3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin A.S, West S.A. Kin selection: fact and fiction. Trends Ecol. Evol. 2002;17:15–21. doi:10.1016/S0169-5347(01)02355-2 [Google Scholar]
- Griffin A.S, West S.A. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science. 2003;302:634–636. doi: 10.1126/science.1089402. doi:10.1126/science.1089402 [DOI] [PubMed] [Google Scholar]
- Hanssen S.A, Hasselquist D, Folstad I, Erikstad K.E. Cost of reproduction in a long-lived bird: incubation effort reduces immune function and future reproduction. Proc. R. Soc. B. 2005;272:1039–1046. doi: 10.1098/rspb.2005.3057. doi:10.1098/rspb.2005.3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchwell B.J, Komdeur J. Ecological constraints, life history traits and the evolution of cooperative breeding. Anim. Behav. 2000;59:1079–1086. doi: 10.1006/anbe.2000.1394. doi:10.1006/anbe.2000.1394 [DOI] [PubMed] [Google Scholar]
- Hatchwell B.J, Russell A.F, Fowlie M.K, Ross D.J. Reproductive success and nest site selection in a cooperative breeder: the effect of experience and a direct benefit of helping. Auk. 1999;116:355–363. [Google Scholar]
- Hemmings A.D. Cooperative breeding in the skuas (Stercorariidae)—history, distribution and incidence. J. R. Soc. New Zeal. 1994;24:245–260. [Google Scholar]
- Koenig W.D, Pitelka F.A. Ecological factors and kin selection in the evolution of cooperative breeding in birds. In: Alexander R.D, Tinkle D.W, editors. Natural selection and social behaviour. Chiron Press; New York, NY: 1981. pp. 261–280. [Google Scholar]
- Koenig W.D, Pitelka F.A, Carmen W.J, Mumme R.L, Stanback M.T. The evolution of delayed dispersal in co-operative breeders. Q. Rev. Biol. 1992;67:111–150. doi: 10.1086/417552. doi:10.1086/417552 [DOI] [PubMed] [Google Scholar]
- Koenig W.D, Stanback M.T, Haydock J. Demographic consequences of incest avoidance in the cooperatively breeding acorn woodpecker. Anim. Behav. 1999;57:1287–1293. doi: 10.1006/anbe.1999.1093. doi:10.1006/anbe.1999.1093 [DOI] [PubMed] [Google Scholar]
- Kokko H, Lundberg A. Dispersal, migration and offspring retention in saturated habitats. Am. Nat. 2001;157:188–202. doi: 10.1086/318632. doi:10.1086/318632 [DOI] [PubMed] [Google Scholar]
- Komdeur J. Importance of habitat saturation and territory quality for evolution of cooperative breeding in the Seychelles warbler. Nature. 1992;358:492–495. doi:10.1038/358493a0 [Google Scholar]
- Komdeur J. Influence of helping and breeding experience on reproductive performance in the Seychelles warbler: a translocation experiment. Behav. Ecol. 1996;7:326–333. doi:10.1093/beheco/7.4.417 [Google Scholar]
- Kraaijeveld K, Dickinson J.L. Family-based winter territoriality in western bluebirds (Sialia mexicana): the structure and dynamics of winter groups. Anim. Behav. 2001;61:109–117. doi: 10.1006/anbe.2000.1591. doi:10.1006/anbe.2000.1591 [DOI] [PubMed] [Google Scholar]
- Krüger O. Age at first breeding and fitness in goshwaks Accipter gentilis. J. Anim. Ecol. 2005;74:266–273. doi:10.1111/j.1365-2656.2005.00920.x [Google Scholar]
- Macedo R.H, Bianchi C.A. Communal breeding in the tropical guira cuckoos Guira guira: sociality in the absence of a saturated habitat. J. Avian Biol. 1997;28:207–215. [Google Scholar]
- Martin K. Patterns and mechanisms for age-dependent reproduction and survival in birds. Am. Zool. 1995;35:340–348. [Google Scholar]
- Martin T.E. A new view for avian life history evolution tested on an incubation paradox. Proc. R. Soc. B. 2002;269:309–316. doi: 10.1098/rspb.2001.1879. doi:10.1098/rspb.2001.1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder R.A. Natal and breeding dispersal in a cooperative extra-group-mating bird. J. Avian Biol. 1995;26:234–240. [Google Scholar]
- Nilsson J.-A. Causes and consequences of natal dispersal in the marsh tit Parus palustris. J. Anim. Ecol. 1989;58:619–636. doi:10.2307/4852 [Google Scholar]
- Orell M, Belda E.J. Delayed cost of reproduction and senescence in the willow tit Parus montanus. J. Anim. Ecol. 2002;71:55–64. doi:10.1046/j.0021-8790.2001.00575.x [Google Scholar]
- Pravosudova E.V, Grubb T.C, Parker P.G. The influence of kinship on nutritional conditions and aggression levels in winter social groups of tufted titmouse. Condor. 2000;103:821–828. doi:10.1650/0010-5422(2001)103[0821:TIOKON]2.0.CO;2 [Google Scholar]
- Pruett-Jones S, Lewis M.J. Sex ratio and habitat limitation promote delayed dispersal in superb fairy-wrens. Nature. 1990;348:541–542. doi:10.1038/348541a0 [Google Scholar]
- Pyle P, Nur N, Sydeman W.J, Emslie S.D. Cost of reproduction and the evolution of deferred breeding in the western gull. Behav. Ecol. 1997;8:140–147. doi:10.1093/beheco/8.2.140 [Google Scholar]
- Rathburn M.K, Montgomery R. Breeding biology and social structure of white-winged fairy-wrens (Malurus leucopterus): comparison between island and mainland species having different plumage phenotypes. Emu. 2003;103:295–306. doi:10.1071/MU03011 [Google Scholar]
- Ridley J, Sutherland W.J. Kin competition within groups: the offspring depreciation hypothesis. Proc. R. Soc. B. 2002;269:2559–2564. doi: 10.1098/rspb.2002.2208. doi:10.1098/rspb.2002.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff D.A. Chapman and Hall; New York, NY: 1992. The evolution of life histories. [Google Scholar]
- Russell E. Co-operative breeding—a Gondwanan perspective. Emu. 1989;89:61–62. [Google Scholar]
- Schoech S.J, Mumme R.L, Wingfield J.C. Prolactin and helping behaviour in the cooperatively breeding Florida scrub jay, Aphelocoma c. coerulescens. Anim. Behav. 1996;52:445–456. doi:10.1006/anbe.1996.0189 [Google Scholar]
- Scott D.K. Functional aspects of prolonged parental care in Bewick's swans. Anim. Behav. 1980;28:938–952. doi:10.1016/S0003-3472(80)80156-4 [Google Scholar]
- Stacey P.B, Bock C.E. Social plasticity in the acorn woodpecker. Science. 1978;202:1298–1300. doi: 10.1126/science.202.4374.1298. doi:10.1126/science.202.4374.1298 [DOI] [PubMed] [Google Scholar]
- Stacey P.B, Ligon J.D. Territory quality and dispersal options in the acorn woodpecker, and a challenge to the habitat-saturation model of cooperative breeding. Am. Nat. 1987;130:654–676. doi:10.1086/284737 [Google Scholar]
- Stearns C.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Strickland, D. & Ouellet, H. 1993 Gray jay. In The birds of North America, vol. 40 (eds A. Poole, P. Stettenheim & F. Gill). Philadelphia, PA: The Natural Academy of Sciences; Washington, DC: The American Ornithologists' Union.
- Tavecchia G, Pradel R, Boy V, Johnson A.R, Cézilly F. Sex- and age-related variation in survival and cost of first reproduction in greater flamingos. Ecology. 2001;82:165–174. doi:10.2307/2680094 [Google Scholar]
- Verbeek N.A.M, Butler W.R. Cooperative breeding of the northwestern crow, Corvus caurinus, in British Columbia, Canada. Ibis. 1981;123:183–189. [Google Scholar]
- Visser M.E, Lessells C.M. The costs of egg production and incubation in great tits (Parus major) Proc. R. Soc. B. 2001;268:1271–1277. doi: 10.1098/rspb.2001.1661. doi:10.1098/rspb.2001.1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleck C.M, Mays N.A, Dawson J.W, Goldsmith A.R. Hormonal correlates of parental and helping behaviour in cooperatively breeding Harris' hawks (Parabuteo unicinctus) Auk. 1991;108:638–648. [Google Scholar]
- West S.A, Pen I, Griffin A.S. Cooperation and competition between relatives. Science. 2002;296:72–75. doi: 10.1126/science.1065507. doi:10.1126/science.1065507 [DOI] [PubMed] [Google Scholar]
- Williams G.C. Natural selection, the cost of reproduction, and a refinement of Lack's principle. Am. Nat. 1966;100:687–690. doi:10.1086/282461 [Google Scholar]
- Wrege P.H, Emlen S.T. Family structure influences mate choice in white-fronted bee-eaters. Behav. Ecol. Sociobiol. 1994;35:185–191. [Google Scholar]
- Young B.E. An experimental analysis of small clutch size in tropical house wrens. Ecology. 1996;77:472–488. doi:10.2307/2265623 [Google Scholar]
- Zack S, Rabenold K.N. Assessment, age and proximity in dispersal contests among cooperative wrens—field experiments. Anim. Behav. 1989;38:235–247. doi:10.1016/S0003-3472(89)80086-7 [Google Scholar]