Abstract
The physiologic response to stress is highly dependent on the activation of corticotropin-releasing hormone (CRH) neurons by various neurotransmitters. A particularly rich innervation of hypophysiotropic CRH neurons has been detected by nerve fibers containing the neuropeptide PACAP, a potent activator of the cAMP-protein kinase A (PKA) system. Intracerebroventricular (icv) injections of PACAP also elevate steady-state CRH mRNA levels in the paraventricular nucleus (PVN), but it is not known whether PACAP effects can be associated with acute stress responses. Likewise, in cell culture studies, pharmacologic activation of the PKA system has stimulated CRH gene promoter activity through an identified cAMP response element (CRE); however, a direct link between PACAP and CRH promoter activity has not been established. In our present study, icv injection of 150 or 300 pmol PACAP resulted in robust phosphorylation of the transcription factor CREB in the majority of PVN CRH neurons at 15 to 30 min post-injection and induced nuclear Fos labeling at 90 min. Simultaneously, plasma corticosterone concentrations were elevated in PACAP-injected animals, and significant increases were observed in face washing, body grooming, rearing and wet-dog shakes behaviors. We investigated the effect of PACAP on human CRH promoter activity in αT3-1 cells, a PACAP-receptor expressing cell line. Cells were transiently transfected with a chloramphenicol acetyltransferase (CAT) reporter vector containing region −663/+124 of the human CRH gene promoter then treated for with PACAP (100 nM) or with the adenylate cyclase activating agent, forskolin (2.5 μM). Both PACAP and forskolin significantly increased wild-type hCRH promoter activity relative to vehicle controls. The PACAP response was abolished in the CRE-mutant construct. Pretreatment of transfected cells with the PKA blocker, H-89, completely prevented both PACAP- and forskolin-induced increases in CRH promoter activity. Furthermore, CREB overexpression strongly enhanced PACAP-mediated stimulation of hCRH promoter activity, an effect which was also lost with mutation of the CRE. Thus, we demonstrate that icv PACAP administration to rats under non-stressed handling conditions leads to cellular, hormonal and behavioral responses recapitulating manifestations of the acute stress response. Both in vivo and in vitro data point to the importance of PACAP-mediated activation of the cAMP/PKA signaling pathway for stimulation of CRH gene transcription, likely via the CRE.
Keywords: Pituitary adenylate cyclase-activating polypeptide, Corticotropin-releasing hormone, Phosphorylated CREB
1. Introduction
Parvocellular corticotropin-releasing hormone (CRH) neurons in the hypothalamic paraventricular nucleus (PVN) provide a major common pathway for endocrine, autonomic and behavioral responses to stress [25]. In response to stressful stimuli, these neurons secrete CRH (and arginine vasopressin, a supplementary secretagogue) into the hypophysial portal system to stimulate pituitary adrenocorticotropic hormone (ACTH) secretion, which in turn increases the biosynthesis and release of glucocorticoids from the adrenal cortex.
The characterization of neural inputs to parvocellular CRH neurons has suggested roles for catecholamines, glutamate, GABA and neuropeptides in the integration of signals influencing CRH neuronal function in the PVN [26]. A particularly robust innervation to CRH neurons is provided by nerve fibers containing the neuropeptide, pituitary adenylate cyclase-activating polypeptide (PACAP) [38]. Extrahypothalamic brain regions, such as the extended amygdala and lower brainstem that may contribute to the augmentation of the stress response, have also been identified as sites of PACAPergic innervation [34–36,55]. The physiologic relevance of the hypothalamic PACAP–CRH interaction has been supported by the finding that intracerebroventricular (icv) injection of PACAP increases steady-state CRH mRNA levels in the PVN, as evaluated at 4 h post-injection. This effect is blocked by co-administration of a selective PACAP receptor antagonist [20]. Conversely, administration of the PACAP antagonist alone downregulates steady state CRH mRNA levels, suggesting a tonic regulatory role for PACAP on hypophysiotropic CRH neurons [20]. Nevertheless, it has not been determined whether PACAP effects can be linked to acute stress responses in the rat brain.
PACAP is a member of the secretin/glucagon/vasoactive intestinal peptide (VIP) family and exists as either a 38-amino acid peptide (PACAP38) or N-terminally truncated form, PACAP27, of which PACAP38 is the predominant form in the brain [4,42,43]. PACAP acts via binding to G-protein-coupled receptors termed PAC1, VPAC1 and VPAC2 according to their relative affinity for PACAP and VIP [24]. Although originally described as an activator of the cAMP-dependent protein kinase A (PKA) system, PACAP also may stimulate phospholipase C and calcium signaling pathways [50,51,64]. Within the brain, the highest concentration of PACAP and the PACAP selective PAC1 receptors are present in the hypothalamus, including the parvocellular and magnocellular subdivisions of the PVN, which are critical for mediating neuroendocrine responses [29,47,63].
Both in vitro and in vivo experiments have defined the PKA system as a critical modulator of CRH biosynthesis and secretion. Pharmacologic activation of the cAMP/PKA system stimulates CRH gene expression in cultured primary hypothalamic cells as well as a wide variety of heterologous cell lines [1,66,78]. Similarly, local microinjection of the cAMP analogue, 8-Br-cAMP, into the PVN increases endogenous CRH mRNA levels [28]. This cAMP response is mediated, at least in part, via binding of the transcription factor CREB (cyclic AMP response element binding protein) to a classical cyclic AMP response element (CRE) located at position −221 position in the human gene [16,61]. Mutation of the hCRH-CRE prevents CREB binding and substantially blunts PKA responsiveness of the CRH gene promoter. Of note, an increase in the activated form of CREB, phosphorylated CREB (PCREB), coincides with an increase in de novo synthesized CRH transcripts in the PVN following acute stress [32]. Thus, the summary of these findings strongly suggests a link between the PKA system, CREB phosphorylation, and the activation of CRH neurons.
The aim of the present study was to advance the hypothesis that PACAP is a physiologically important regulator of the central limb of the HPA axis using both in vivo and in vitro approaches. We first investigated whether icv administration of PACAP under stress-free handling conditions would result in activation of the CRH neurons of the PVN and whether this treatment would mimic stress-like behavioral and corticosterone responses. Second, we used an immortalized cell culture system to characterize the molecular mechanisms by which PACAP regulates CRH gene transcription with a focus on the PKA system.
2. Materials and methods
2.1. In vivo experiments
2.1.1. Animals
These experiments were performed on adult male Sprague–Dawley rats (n=52, Taconic Farms, Germantown, New York) weighing 210–230 g at the beginning of the study. The animals were housed individually in cages under standard environmental conditions (light between 06:00 and 18:00 h; temperature, 22 ± 1 °C; rat chow and water available ad libitum). All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committees at the Tufts-New England Medical Center Boston, MA and University of South Florida College of Medicine, Tampa, FL.
2.2. Intracerebroventricular (icv) PACAP infusions
Ten days prior to experimentation, a 22-gauge stainless steel guide cannula (Plastic One, Roanoke, VA) was placed into the right lateral cerebral ventricle under stereotaxic control (coordinates from bregma, anteroposterior, 0.8; lateral, 1.3; and ventral, 3.6) through a burr hole in the skull. The cannula was secured to the skull with three stainless steel screws and dental cement and temporarily occluded with a dummy cannula. To eliminate non-specific stress, rats were acclimated by mock injections consisting of removal of the dummy cannula and connection to an empty cannula connector daily for 1 week prior to experimentation. In addition, 2 days before the experiments, all rats were jugular vein-cannulated to ensure rapid and stress-free anesthesia at the termination of the experiment [32,37].
Rats were randomly assigned to experimental groups and injected icv with 150 or 300 pmol doses of PACAP (PACAP38, American Peptide Company, Sunnyvale, CA) in 6 μl of artificial cerebrospinal fluid (aCSF) [28] containing 0.05% pyrogen-free bovine serum albumin (BSA), while animals in control groups were administered 6 μl of aCSF-BSA only. All icv injections were made in freely moving animals through a 28-gauge needle that extended 1 mm below the guide cannula, connected by polyethylene tubing to a 500 μl syringe over 2 min by a microprocessor controlled infusion pump (Bee Electronic Minipump; Bioanalytical Systems, West Lafayette, IN).
2.3. Observation of behavior
Beginning at 1 min post-injection, the first 10-min periods of the survival time for rats in PACAP-injected and control groups were videotaped for subsequent analysis of behavior. Frequencies of stereotypic face washing, body grooming, rearing and wet dog shakes were scored using the 15-s sampling method [18]. The videotaped 10-min post-injection period is divided into 15-s time intervals, and the observer determines whether a specific behavior occurred during each 15-s interval; thus, the maximum possible score is 40 for the 10-min observation period.
2.4. Perfusion and tissue processing
At 15, 30 or 90 min post-injection time points, 0.7 ml blood was drawn passively through the jugular catheter from each rat without handling the animal. Immediately following blood sampling, rats were anesthetized through the jugular catheter with pentobarbital (50 mg/kg) to attain complete anesthesia within 1 min. Next, rats were perfused transcardially with 10 ml of heparinized saline for 10–20 s followed by a mixture of 1% acrolein and 3% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 10 min. The brains were dissected, and hypothalamic blocks cut out using a coronal rat brain matrix and transferred into 20% sucrose in 0.01 M phosphate-buffered saline (PBS) at 4 °C for 2 days. Blocks were then rapidly frozen with dry ice and sectioned with a cryostat. Series of 25-μm-thick sections of the hypothalamus were collected into six vials to obtain sets of sections at regularly spaced intervals (150 μm) for each vial. The position of the cannula track protruding into the lateral ventricle was verified histologically for each brain. Animals with incorrectly placed injection sites were excluded from further data analysis.
2.5. Immunohistochemical detection of PCREB and Fos-immunoreactivity
Immunolabeling for the phosphorylated form of CREB, PCREB, was performed using previously established immunoperoxidase methods in order to determine the extent to which cells within the PVN express PACAP-induced phosphorylation of CREB [37,59]. Briefly, sections were treated sequentially with 1% sodium borohydride in deionized water for 30 min followed by 0.5% H2O2 in PBS for 10 min and 0.5% Triton-X in PBS for 1 h. Sections were incubated in the rabbit polyclonal primary antiserum against phosphorylated CREB128-141 (1:12,000; courtesy of Dr. Marc R. Montminy, Salk Institute, La Jolla, CA) or in Fos rabbit primary antiserum (Ab-5; 1:60,000; Oncogene Research Products, San Diego, CA) for 3 days at 4 °C. After thorough rinsing in PBS, sections were incubated in biotinylated goat anti-rabbit IgG (1:400; Vector Labs, Burlingame, CA). All antibody dilutions were made in 2% normal horse serum in PBS containing 0.08% sodium azide and 0.2% Kodak Photo-Flo. The sections were then washed three times in PBS and incubated in avidin–biotin–peroxidase complex (ABC Elite Kit, Vector Labs; 1:100 in PBS). After three washes in PBS and a rinse in 0.05 M Tris buffer (pH 7.8), the color reaction was developed in 0.025% diaminobenzidine (DAB) containing 0.06% nickel ammonium sulfate and 0.0027% H2O2 for 8 min to yield a dark purple/gray labeling in the cell nucleus. A rinse in Tris buffer was used to stop the reaction. Sets of sections were then mounted onto slides, dehydrated in graded series of ethanol followed by three changes of Histosol (National Diagnostics, Atlanta, GA) and coverslipped from DPX mountant. Specificity of the PCREB antiserum has been established by our laboratory and others, using preabsorption with synthetic peptide antigen [23,32,37]. The specificity of the Fos antiserum has been characterized by the manufacturer as well as previous studies [14,15]. This antiserum recognizes both cellular and viral forms of Fos but does not cross react with Jun.
2.6. Double-labeling immunohistochemistry of PCREB- or Fos-positive nuclei and CRH neurons in the PVN
Sets of free-floating PCREB or Fos-labeled sections were rinsed in Tris-buffered saline (TBS) and then incubated in rabbit antiserum raised against rat CRH (Peninsula Laboratories, San Carlos, CA, USA) at a dilution of 1:20,000 for 2 days at 4 °C. After washing in TBS, sections were incubated in donkey anti-rabbit IgG (1:400, Jackson ImmunoResearch) and the ABC Elite Complex (1:100). The immunolabeling was visualized by 0.025% DAB and 0.0036% H2O2 for 10 min in Tris buffer alone to yield a brown cytoplasmic labeling. After development, sections were rinsed in Tris buffer, mounted onto slides, air-dried and dehydrated with alcohol and xylene then coverslipped.
2.7. Semi-quantitative analysis of PCREB-labeled nuclei and CRH immunoreactive perikarya in the PVN
Double-labeled sections containing PCREB and CRH immunoreactivities were used for quantitative analysis. CRH immunostaining was confined exclusively to perikarya and proximal dendrites, developed with a light-brown chromogen, allowing the determination whether the nucleus contained the previously developed dark-purple label for PCREB. Only cells with intense or medium density nuclear labeling and distinct cytoplasmic reaction product were counted as double labeled. The subdivisions of the PVN were identified based on the rat brain atlas of Paxinos and Watson [53]. Percentages of CRH neurons containing PCREB-labeled nuclei were determined from three mid-level sections of the PVN for each animal.
2.8. Corticosterone assay
Plasma corticosterone concentrations were measured by a radioimmunoassay system as described previously [27]. Plasma samples diluted 1:100 in assay buffer (PBS containing 0.1% gelatin and 0.04% sodium azide, pH 7.4) were heat-denatured at 70 °C for 30 min, then samples of CS standard (Sigma) were incubated overnight at 4 °C with [125I]-labeled corticosterone and a rabbit anti-corticosterone serum (diluted at 1:17,500; ICN Pharmaceuticals). Bound and unbound tracers were separated using a 4-h incubation with sheep anti-rabbit second antibody (diluted at 1:120; in 0.05 M sodium phosphate-EDTA buffer, pH 7.2) followed by the addition of 1 ml of 10% polyethylene glycol (Carbowax 8000, Fisher Scientific, Fairlawn, NJ) in PBS, centrifugation and decanting of supernatants. Intra- and interassay coefficients of variation were 9.0% and 10.1%, respectively.
2.9. In vitro experiments
2.9.1. Reagents for transfection studies
PACAP (PACAP38) and the adenylate cyclase-activating agent, forskolin, were purchased from Peninsula Laboratories, Inc. (Belmont, CA). The PKA-specific inhibitor H-89 was obtained from LC Laboratories (Woburn, MA).
2.10. Plasmids used in transfection studies
The reporter constructs used in this study contain the human CRH gene promoter sequences from −663 to +124 bp present as either the wild type (CRH-CAT) or with a deletion mutation in a previously described cAMP response element at position −221 relative to the transcriptional start site (ΔCRE-CAT) (gift from Dr. Audrey Seasholtz, University of Michigan, Ann Arbor, MI) [22]. In a subset of experiments, cells were cotransfected with an expression vector for CREB, which contains 1123 bp of the wild-type CREB cDNA in the RcRSV expression vector (Invitrogen, San Diego, CA; CREB construct donated by Dr. Richard Goodman, Oregon Health Sciences University, Portland, Oregon).
2.11. Cell culture and transfection
Mouse gonadotrope-derived αT3-1 cells (gift from Dr. Pam Mellon, University of California, San Diego, CA) were cultured in DMEM supplemented with 10% fetal calf serum, penicillin (50 IU/mL), streptomycin (50 IU/mL) and 2 mmol/l l-glutamine at 37 °C and 5% CO2. Approximately 24 h before transfection, αT3-1 cells were split and plated in 35 mm wells at 40–50% confluence (2 × 105 cells/well). Cells were transfected with the CRH reporter constructs (1 μg/well) using Lipofectamine (Life Technologies, Gaithersburg, MD). To correct for differences in transfection efficiency, cells were cotransfected with a Rous sarcoma virus (RSV)-β-galactosidase plasmid (0.3 μg/well). In a subset of experiments, cells received the RcRSV-CREB expression vector or an equal amount of the empty RcRSV vector (0.25 μg/well).
Prior to harvesting, cells were treated with forskolin (2.5 μM), PACAP (100 nM) or vehicle for 12–14 h. In order to investigate the signaling pathway utilized by PACAP in this system, cells were pretreated with 10 μM of the PKA-inhibitor H89 for 1 h-before the addition of either PACAP or forskolin. Cells were harvested 48 h after transfection, and cell extracts were analyzed for both CAT activity by ELISA (Roche Molecular Biochemicals, Mannheim, Germany) and β-galactosidase activity [13]. CAT activity was normalized to the level of β-galactosidase activity, and results calculated as fold-change relative to expression in vehicle-treated control wells. Data are shown as mean ± SEM of at least 3 independent experiments with each point tested in triplicate.
2.12. Statistical analysis
Statistical significance was determined by analysis of variance (ANOVA) followed by post hoc comparison using the Student Newman–Keul ’s test (parametric data) or Dunn’s Method (non-parametric data) (SigmaStat Statistical Software, Package Version 2.0 (SPSS, Inc., Chicago, IL). Differences were considered to be significant at P < 0.05.
3. Results
3.1. Dose–response and time-course characteristics of icv PACAP-induced PCREB immunoreactivity in the PVN
The phosphorylation of CREB, as detected by immunolabeling for PCREB, was first surveyed at the 30-min post-injection time point. In control (aCSF vehicle-treated) animals, PCREB labeling was restricted to neuronal cell nuclei primarily residing in the magnocellular regions of the PVN with only a few nuclei exhibiting PCREB immunoreactivity in the parvocellular regions of the PVN (Fig. 1A). This pattern corresponds to the distribution of PCREB observed in earlier studies of non-stressed animals [32,37]. Icv infusion of PACAP resulted in the robust induction of nuclear PCREB immunoreactivity in all regions in the PVN including its periventricular, ventral and medial parvocellular subdivisions (Figs. 1B,C). Icv PACAP at the 300 pmol dose resulted in a more pronounced increase of PCREB labeling along the ventricular wall and also in the ventral parvocellular subdivision in the PVN (Fig. 1B) compared to the 150 pmol dose, which induced a rather evenly distributed pattern of activation in all PVN subdivisions (Fig. 1C).
Fig. 1.
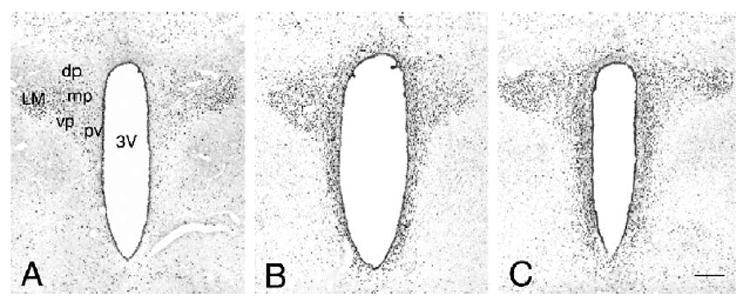
General pattern of nuclear PCREB immunolabeling in the PVN at 30 min following icv PACAP injection. (A) Control aCSF-injected PVN; (B) PACAP 150 pmol C PACAP 300 pmol. 3V = third cerebral ventricle; LM lateral magnocellular subdivision; dp, dorsal; mp, medial; vp, ventral; pv, periventricular parvocellular subdivisions of PVN. Scale bar, 200 μm.
We next performed a time-course analysis of PACAP-induced phosphorylation of CREB in the PVN. At 15 min post-injection, a remarkable increase in the numbers of PCREB-positive nuclei was observed in icv-injected animals at both the 150 and 300 pmol dose as compared to controls (Figs. 2A–C). As described above, at 30 min post-injection, elevations in the number of PCREB labeled nuclei were still evident in the PVN, in both the 150 and the 300 pmol PACAP-treated animal groups (Figs. 2D–F). At 90 min post-injection, icv PACAP-induced PCREB immunoreactivity was diminishing but still elevated compared to the corresponding vehicle-injected control animals (Figs. 2G–I).
Fig. 2.
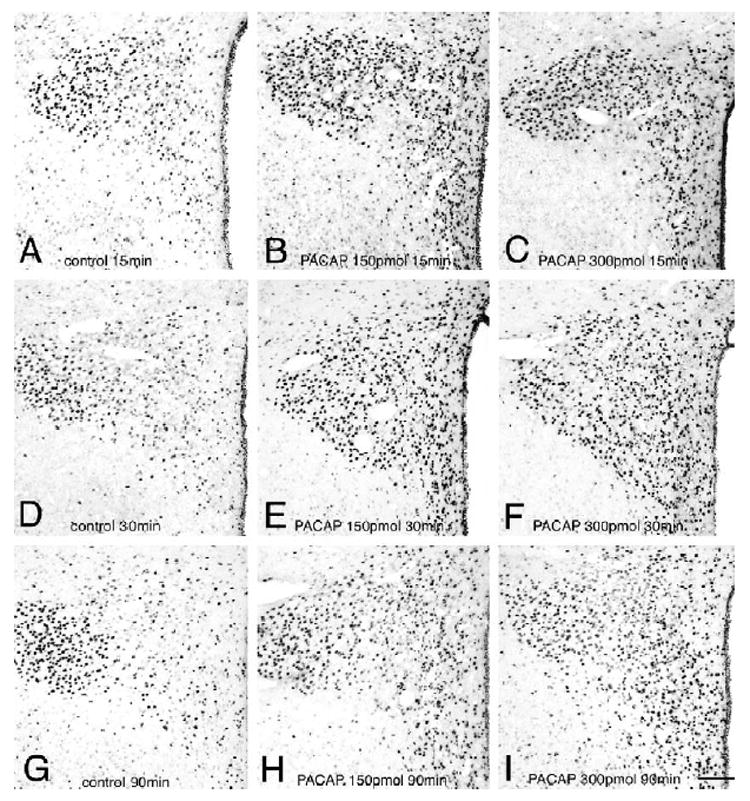
Time course of PCREB induction in the PVN following icv injection of PACAP. (A) Control 15 min; (B) PACAP 150 pmol 15 min; (C) PACAP 300 pmol 15 min; (D) control 30 min; (E) PACAP 150 pmol 30 min; (F) PACAP 300 pmol 30 min; (G) control 90 min; (H) PACAP 150 pmol 90 min; (I) PACAP 300 pmol 90 min. Scale bar, 100 μm.
3.2. Dual labeling of PCREB and CRH in medial parvocellular neurons of the PVN — effect of icv PACAP injection
Fig. 3 shows representative photomicrographs taken from the medial parvocellular subdivision of the PVN demonstrating a marked increase in nuclear PCREB immunolabeling in CRH positive neurons following icv PACAP injection. Quantification of the immunohistochemical findings is summarized in Fig. 4. Double PCREB-CRH immunolabeling in the PVN showed that only a small percentage of CRH neurons contained nuclear PCREB label in the vehicle-injected groups (controls, Fig. 4). Dramatic elevations in the percentage of PCREB containing CRH neurons were observed at 15 min post-injection in both the 150 and 300 pmol PACAP-injected groups (~86%; Fig. 4, top panel) as compared to corresponding controls (20%). At 30 min post-injection, the percentage of CRH neurons with nuclear PCREB labeling remained elevated above the corresponding control at both PACAP doses (47% and 85% in 150 pmol and 300 pmol groups, respectively, vs. 23% in the control group; Fig. 4, middle panel). Ninety minutes following icv infusion, the percentage of CRH neurons containing PCREB remained low in the controls (11%), was moderately increased in the 150 pmol PACAP infused group (35%) and persistently elevated in the 300 pmol PACAP infused animals (60%; Fig. 4, bottom panel).
Fig. 3.
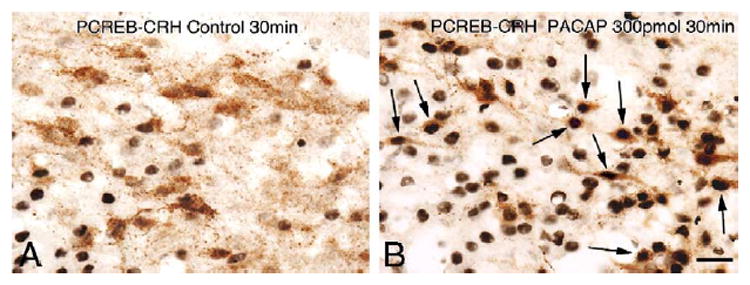
Representative images of PCREB-CRH dual immunolabeling at 30 min post-injection time point. High-power magnification light microscopic images show simultaneous immunolabeling for CRH neurons (brown cytoplasm) and PCREB positive cell nuclei (black nuclear label) in the medial parvocellular subdivision of the PVN. (A) Control and (B) 300 pmol PACAP-treated animals. High numbers of CRH positive neurons contain PCREB-labeled nuclei after icv infusion of PACAP (examples indicated by arrows). Scale bar, 20 μm.
Fig. 4.
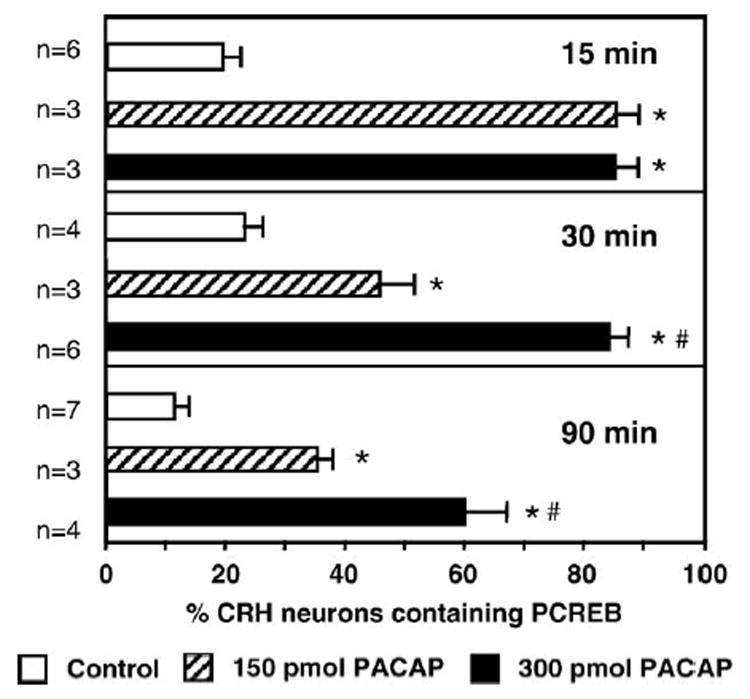
Percentages of PCREB-CRH double labeled neurons in the PVN following icv aCSF vehicle injection (Control) or PACAP (150 or 300 pmol) at 15, 30 and 90 min post-injection. Note robust elevations in the proportion of CRH neurons containing nuclear PCREB following icv PACAP injection. *P < 0.01 versus control; #P < 0.01 versus 150 pmol. n = numbers of animals in experimental groups.
3.3. Induction of nuclear Fos immunoreactivity in the PVN following icv PACAP
At both 30 and 90 min post injection time intervals, Fos-labeled nuclei were only sporadically present in aCSF-injected control animals (Figs. 5A,B). Modest elevations in Fos immunoreactivity were observed at 30 min following 300 pmol PACAP injection in the periventricular area, and the magnocellular division of the PVN and scattered Fos positive nuclei were detected in the medial parvocellular subdivision (Fig. 5C). In contrast, at 90 min following the 300 pmol icv PACAP injection, a significantly more intense and widespread induction of Fos was observed in the PVN, including the medial parvocellular subdivision as well as the magnocellular areas (Fig. 5D). The effect of 150 pmol PACAP on Fos labeling was similar but appeared less intense (data not shown). Double labeling immunohistochemistry for nuclear Fos and cytoplasmic CRH was then performed in PVN sections of animals with the 90-min post injection survival period. In aCSF-injected controls, double labeling was negligible (Fig. 6A) but in 300 pmol PACAP-injected animals numerous CRH neurons expressed nuclear Fos immunoreactivity (Fig. 6B).
Fig. 5.
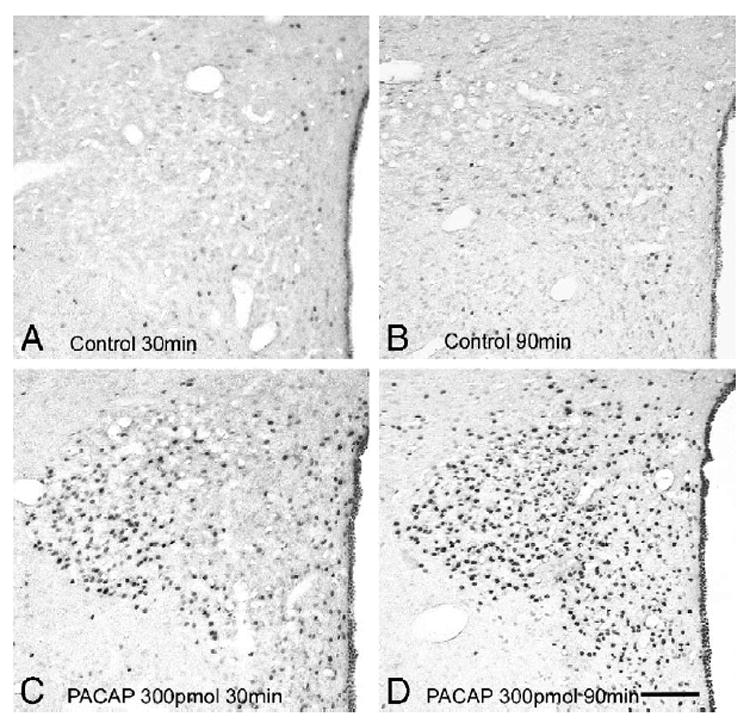
Nuclear Fos-immunoreactivity following icv PACAP injection in the PVN. (A) Control at 30 min; (B) control at 90 min; (C) PACAP 300 pmol at 30 min; (D) PACAP 300 pmol at 90 min. Scale bar in panel (D), 100 μm.
Fig. 6.
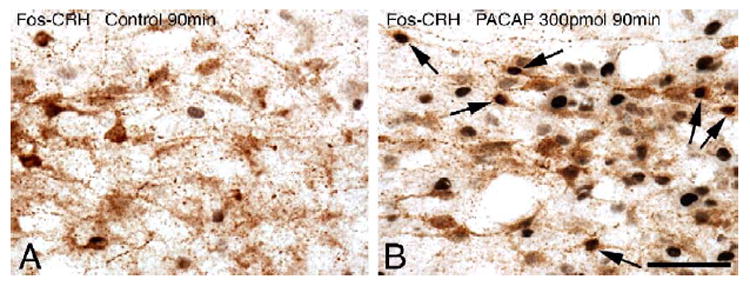
Simultaneous Fos and CRH immunolabeling in the medial parvocellular subdivision of the PVN. (A) Control at 90 min. (B) PACAP 300 pmol at 90 min. Note the presence of several double-labeled neurons 90 min following PACAP injection, containing black nuclear Fos and brown cytoplasmic CRH immunoreactivity (some examples indicated by arrows). Scale bar, 50 μm.
3.4. Hormonal and behavioral activation following icv PACAP administration
Plasma corticosterone levels were measured in controls and in 300 pmol PACAP-injected groups. Icv PACAP injection resulted in significant elevations in plasma corticosterone (control, 0.99 ± 0.61 μg/dl; PACAP 15–30 min, 227.68 ± 13.29 μg/dl). At 90 min post-injection in the icv PACAP-treated group, corticosterone levels fell to 10.22 ± 1.74 μg/dl but were still significantly above controls (P < 0.01).
Quantitative behavioral scores evaluated from the first 10-min period of the survival time in the aCSF and the 150 or 300 pmol PACAP-injected groups are shown in Fig. 7. The most striking of these behavioral manifestations was intense, repetitive face washing and head grooming, typically seen within 5 min of the icv PACAP injection. As the grooming response often exhibited a typical cephalocaudal progression [8], body grooming scores also became highly elevated in the icv 150 and 300 pmol PACAP-injected groups. In addition, the occurrence wet dog shakes also increased significantly in the PACAP-injected groups. Rearing behavior scores were significantly higher in experimental groups injected with the lower dose of PACAP as compared to controls (150 pmol PACAP, 14.0 ± 1.6; aCSF 4.75 ± 1.7, P < 0.05). There was also a tendency for increased rearing scores in the 300 pmol PACAP-injected animals (7.86 ± 2.2), which did not reach statistical significance. In general, animals in the 300 pmol PACAP-injected groups appeared less active than those injected with the 150 pmol dose of the peptide and exhibited a posture that resembled freezing behavior, mainly during the first half of the 10-min behavioral observation peried.
Fig. 7.
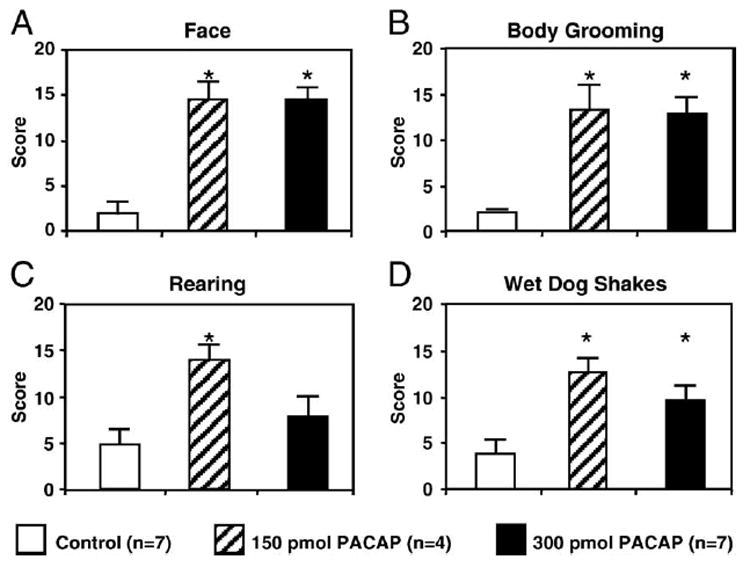
Quantitative scores of icv PACAP-induced behavioral manifestations during the first 10-min period following aCSF vehicle (Control) and 150 or 300 pmol doses of PACAP (PACAP150, PACAP300). Face = face washing and grooming. Asterisks indicate significant changes relative to control (in A, P < 0001; B, P < 0.005; C, P < 0.005; D, P < 0.001). n = numbers of animals in experimental groups.
3.5. PACAP increases hCRH promoter activity via the PKA pathway in vitro
Having demonstrated that PACAP increases PCREB levels in CRH neurons, we wished to more carefully define the functional link between PACAP, the PKA/CREB system and CRH gene transcription. Primary PVN neuronal cells are not amenable for these studies as they are limiting in number and difficult to transfect. Furthermore, to the best of our knowledge, no CRH-expressing hypothalamic neuronal cell lines were available at the time these experiments were performed. Therefore, we utilized a well-defined pituitary cell line, αT3-1, for these studies. This cell line expresses the short and hop forms of the PACAP-specific PAC1 receptor, a pattern which corresponds to the receptor splice variants found abundantly in the brain [57,79]. Furthermore, these cells have been used extensively in the study of PACAP-mediated effects on gene expression [11,56,60,77].
αT3-1 cells were transfected with an RSV-driven CAT reporter construct containing −663 to +124 of the human CRH gene promoter. Treatment with forskolin significantly increased CAT activity 3.6-fold, consistent with previously reported work in other cell lines [22,66]. Similarly, treatment with PACAP-induced hCRH gene promoter activity by 4.7-fold, demonstrating the ability of PACAP to stimulate CRH gene expression.
We next investigated the intracellular signaling pathway(s) through which PACAP regulates CRH promoter activity. Transfected αT3-1 cells were pretreated with the PKA-specific inhibitor H-89 (10 μM) for 1 h prior to stimulation with PACAP or forskolin (Fig. 8A). The addition of H-89 significantly blunted both PACAP- and forskolin-induced increases in hCRH gene promoter activity to the level of the control response. In contrast, pharmacologic blockade of the protein kinase C system did not alter the PACAP response (data not shown).
Fig. 8.
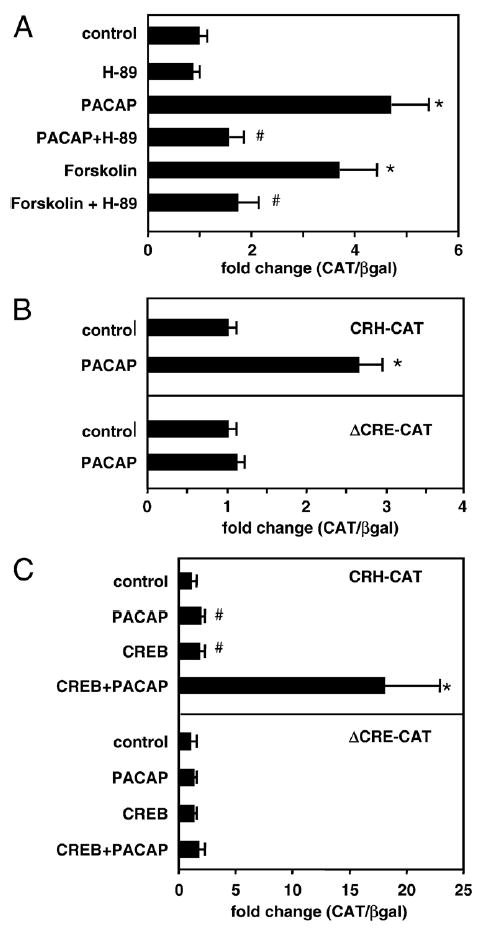
(A) PACAP stimulates hCRH gene promoter activity via the PKA system. αT3-1 gonadotrope cells were transiently transfected with a construct containing region −663/+124 of the human CRH gene promoter linked to a CAT reporter vector. Cells were cotransfected with an RSV-β-galactosidase expression vector. Cells were treated with PACAP38 (100 nM), forskolin (2.5 mM) or vehicle for 12–14 h prior to harvest. Where indicated, cells were pretreated for 1 h with the PKA specific inhibitor, H-89 (10 mM). CAT activity was normalized to β-galactosidase activity and promoter activity expressed as fold-change relative to expression in vehicle-treated control wells. Results are shown as the mean ± SEM. *P < 0.005 versus control; #P < 0.02 versus forskolin or PACAP alone. (B) Loss of PACAP effect with mutation of the cAMP response element (CRE) in the hCRH gene promoter. αT3-1 cells were transiently transfected with a CAT reporter vector containing region 663/+124 of the hCRH gene promoter present as the wild-type sequence (CRH-CAT, upper panel) or with deletion of the previously identified CRE at position −221 (ΔCRE-CAT, lower panel). Cells were treated with vehicle or PACAP (100 nM × 12–14 h) starting 36 h after transfection. *P < 0.005 versus control. (C) CREB augments PACAP-induced activation of the CRH gene. αT3-1 cells were transiently transfected with a CAT reporter vector containing region −663/+124 of the hCRH gene promoter present as the wild-type sequence (CRH-CAT, upper panel) or with deletion of the previously identified CRE (ΔCRE-CAT, lower panel). Cells were cotransfected with an RSV-driven expression vector encoding CREB or with the empty expression vector. Cells were treated with PACAP (100 nM × 12–14 h), and results calculated relative to vehicle treated wells that received the empty expression vector. Results are shown as the mean ± SEM. *P < 0.005 versus control.
3.6. Loss of PACAP effect with mutation of the CRE in the hCRH gene promoter
A consensus cyclic AMP responsive element (CRE) has been identified 221 base pairs upstream from the human CRH gene transcriptional start site [66]. To test the importance of this CRE site for PACAP signaling, both the wild-type (CRH-CAT) and the CRE mutated (ΔCRE-CAT) form of the hCRH vectors were transiently transfected into αT3-1 cells. As shown in Fig. 8B, the PACAP response was eliminated in the CRE-mutated construct, demonstrating that PACAP-mediated regulation of the hCRH gene is critically dependent on an intact CRE site.
3.7. Overexpression of CREB augments PACAP-stimulated hCRH promoter activity
The importance of the CRE suggested that the CRE binding protein, CREB, may mediate the downstream effects of PACAP on the CRH gene. To investigate this possibility, CREB was overexpressed in αT3-1 cells in the presence of the wild-type CRH-CAT construct (Fig. 8C, upper panel). The addition of CREB alone exerted modest, albeit statistically significant, stimulatory effects on CRH promoter activity. In contrast, CREB overexpression markedly augmented PACAP responsiveness in this gene. To test whether CREB-PACAP synergy was functionally dependent on the CRE, we cotransfected cells with CREB and the ΔCRE-CRH construct followed by treatment with vehicle or PACAP. As shown in Fig. 8C (lower panel), deletion of the CRE eliminated the ability of the hCRH promoter to respond to PACAP, CREB or PACAP and CREB together.
4. Discussion
PACAP was originally isolated from ovine hypothalamic tissue based on its ability to stimulate adenylate cyclase activity and intracellular Ca2+ in anterior pituitary, glial and neuronal cells [3,42,43]. Converging lines of evidence have suggested that PACAP may serve as a neurotransmitter or neuromodulator as well as a protective substance for the survival of injured or developing neurons [2,65,73,74].
Central injections of PACAP in rodents elicit a number of responses such as hyperthermia and reversal of reserpine-induced hypothermia, elevations in plasma vasopressin and arterial blood pressure levels, inhibition of food intake and enhancement of motor activity and rearing [10,41,45,46]. However, the physiologic context for the effects of PACAP is poorly understood. It has been speculated that direct or indirect activation of the sympathetic outflow or acetylcholine, dopamine and norepinephrine systems may mediate some of these actions [41]. Nevertheless, effects of PACAP are also similar to the behavioral activation seen following icv CRH administration [67]. A recent pharmacologic study in the chicken brain has also suggested an important role of CRH receptors in the anorexic and neuroendocrine effects of icv-injected PACAP [68]. Therefore, we hypothesized that an important common element in previous findings is that PACAP administration leads to autonomic and behavioral changes that accompany increases in general arousal, alertness and physiologic adaptation to metabolic, physical or neurogenic stressors. Based on our previous chemical neuroanatomical observations, PACAP may contribute to the stress response through dense innervation between PACAP containing nerve fibers and CRH neurons of the hypothalamus [38].
The present study investigated whether icv PACAP administration would activate the HPA axis, CRH neurons of the rat PVN and induce stress-related behaviors. We observed a rapid, robust induction of PCREB in the PVN and periventricular area of the hypothalamus following icv PACAP injection, with particularly intense PCREB labeling in the medial parvocellular subdivision of the PVN, the seat of hypophysiotropic CRH neurons. Several studies have identified PKA-dependent phosphorylation of the transcription factor, CREB, at Ser-133 [23] as a useful indicator of hormonal or drug-induced neuronal activation [21,31,62]. While unphosphorylated CREB can bind to gene promoter regions, PCREB is the active form, which stimulates gene transcription [23]. Physiologic acute stress likewise has been shown to induce CREB phosphorylation in parvocellular CRH neurons in parallel with activation of the HPA axis [32,37]. We also evaluated the phosphorylation of CREB specifically in CRH neurons of the PVN and found that maximal induction of PCREB after icv PACAP treatment was observed at 15 min post-injection time with approximately 90% of parvocellular CRH neurons expressing activated CREB. Concomitantly, plasma corticosterone levels were dramatically elevated in PACAP-injected animals as compared to vehicle-injected controls. As reported earlier, the rapid and transient nature of the stress-induced elevation in both PCREB and the primary CRH transcript (hnRNA) appears to preclude the involvement of inducible transcription factors that require intervening de novo protein synthesis [32]. Indeed, blockade of protein synthesis by cycloheximide treatment 30 min prior to ether stress did not alter the upregulation of CRH hnRNA and PCREB in the PVN but completely abolished the Fos response in CRH neurons and partially inhibited elevation of vasopressin hnRNA in the same group of parvocellular neurons [33]. This is consistent with the notion that phosphorylation of a constitutive nuclear ingredient like CREB may be a molecular trigger mechanism for rapid transcriptional initiation of the CRH gene. It is also likely that PACAP activates CRH neurons by phosphorylation of CREB which may contribute to the release of stored CRH from nerve terminals at the median eminence [9,75,76] followed by rapid initiation of de novo CRH synthesis, presumably to replenish these stores, not unlike the effects of stress as discussed in previous studies [32].
In contrast, activation of the vasopressin gene requires the synthesis of additional factors such as Fos (the product of the immediate-early gene c-fos), explaining the time-course differences between the activation of these two ACTH secretagogues [32,33]. The time course of the PACAP-induced PCREB response and elevated corticosterone levels is consistent with the rapid response, primarily driven by the activation of CRH neurons, as observed following ether and restraint stress [19,32,37]. Likewise, the comparatively delayed activation of nuclear Fos in response to icv PACAP administration in our study is similar to an earlier report linking upregulation of c-fos to PACAP-induced vasopressin gene expression in the PVN and the supraoptic nucleus [48].
Post-injection behavioral manifestations evoked by icv PACAP appear to be complex. First, the substantial elevations in face and body grooming as observed in PACAP-injected animals at both 150 and 300 pmol doses are consistent with the activation of the PVN and its vicinity, as chemical or electrical stimulation of this area produces distinct grooming responses in rats [44,58,71]. Self-grooming in rats is associated with stress as it is frequently observed in the post stress time period. It is presumed to serve as a mechanism of dearousal from the highly activated state induced by stress [72]. Indeed, most recent behavioral investigations from our laboratory have demonstrated grooming as a dominant manifestation following local microinjections of PACAP directly into the PVN, an effect which was further augmented by the presence of stress [49].
On the other hand, it can be assumed that PACAP, like other peptides injected via the icv route, may readily reach brain regions bearing their specific receptors [6], such that other behavioral manifestations found in the present study may be a result of a more global action of PACAP on the brain. Wet dog shakes, for example, may be induced by glutamatergic, noradrenergic, serotonergic and tachykinin-related mechanisms, most of which are augmented by upregulation of the cAMP/PKA pathway [5,17,54,70]. It is therefore plausible that PACAP, a potent activator of adenylate cyclase through PAC1 receptors, may stimulate a number of these neurotransmitter systems.
To complement our in vivo findings, we utilized an in vitro cell culture model system in order to provide a more detailed analysis of the intracellular mechanisms involved in PACAP-mediated stimulation of CRH neurons. In initial reports, PACAP effects were shown to be mediated via the cAMP/PKA system. Subsequent studies have demonstrated the ability of the various PACAP receptors to modulate gene expression via the protein kinase C, Ca2+ and ras/raf pathways [50,51,64]. Of note, in addition to the PKA response, CRH mRNA levels and secretion can be increased by activation of the PKC system, as demonstrated in both primary hypothalamic cells and in a hepatoma cell line which expresses endogenous CRH [16,52].
Our present results demonstrate, for the first time, the ability of PACAP to stimulate hCRH gene promoter activity and confirm prior reports that the adenylate-cyclase activating agent, forskolin, can increase CRH gene expression. Furthermore, pharmacological inactivation of the cAMP/ PKA pathway with H-89 was shown to nearly eliminate both PACAP and forskolin-induced increases in CRH gene promoter activity. In contrast, inhibition of the PKC system was ineffective at altering the PACAP response. Thus, our data suggest that PACAP stimulates hCRH gene expression largely, if not exclusively, through the cAMP/PKA pathway.
PACAP-stimulated transcription is mediated via cAMP response elements (CREs) in a variety of neuroendocrine genes including the prolactin, proopiomelanocortin (POMC) and chromogranin A genes as evaluated in somatolactotrope, corticotrope and pheochromocytoma cell lines [7,12,69]. The hCRH gene has been shown to have a functional CRE domain at position −221 upstream of the transcriptional start site. Mutation of this CRE eliminated the ability of PACAP to stimulate hCRH gene promoter activity, suggesting that an intact CRE is essential for PACAP responsiveness in this gene. Furthermore, over-expression of the CRE binding protein, CREB, had significant but modest effects on hCRH gene expression, presumably due to low levels of phosphorylation in the unstimulated state. In contrast, CREB overexpression strongly enhanced PACAP-mediated stimulation of hCRH promoter activity, an effect that was lost with mutation of the CRE. Thus, these data provide a functional link between the PACAP-induced increase in activated CREB observed in vivo and stimulation of CRH gene expression.
In addition to the CRE, the hCRH gene promoter contains a negative glucocorticoid response element (nGRE) as well as a CDXRE, or homeobox response element [30,39,40]. Interestingly, while glucocorticoids inhibit hCRH promoter activity via the nGRE, the CRE and CDXRE regions are both stimulated in the presence of this steroid. The nGRE region, which contains an AP-1 site in addition to a glucocorticoid receptor binding site, also confers transcriptional activation by cAMP and overex-pressed c-jun or c-fos AP-1 nuclear proteins [39]. Conversely, the CRE has been shown to bind both AP-1 proteins in addition to CREB. In the corticotrope cell line, AtT20, cAMP also activates the CDXRE site. Thus, the functional characteristics and interactions between these three response elements have proven to be complex.
In our in vitro studies, PACAP responsiveness was eliminated in a construct containing an isolated mutation of the hCRH-CRE site, despite the presence of intact nGRE and CDXRE sites. While we have not definitively ruled out a contribution of these additional sites in native CRH neurons, our model system strongly suggests that these sites are not sufficient to provide a response to this physiologic ligand. In the present in vivo studies, we also observed the widespread appearance of Fos protein immunoreactivity in the nuclei of parvocellular CRH neurons at the late post-injection time point (90 min) but not at 15 and 30 min. On the CRH gene promoter, elevated Fos levels could potentially act at either the AP-1 site or the CRE. Therefore, we postulate that delayed expression of this AP-1 protein may indicate a change in neuronal activity leading to long-term plastic changes, which should become a target of further investigation.
In summary, icv PACAP administration to rats under non-stressed handling conditions leads to cellular, hormonal and behavioral responses recapitulating several manifestations of the acute stress response. Both in vivo and in vitro data point to the importance of PACAP-mediated activation of the cAMP/PKA signaling pathway for stimulation of CRH gene transcription, likely via the CRE. Together with the previous anatomical observation that CRH neurons are densely innervated by PACAP fibers, we further suggest that the endogenous PACAPergic system may underlie physiological stress-induced activation of the HPA axis. Specifically, we propose a model in which stress induces secretion of PACAP in the PVN, thereby stimulating CRH gene expression via activation of the cAMP/PKA system.
Acknowledgments
This work was supported by NIH Grant R01 HD38089 (to L.M.H.), NIH Grant R01 MH63320 (to G.L.) and National Science Foundation Grant IBN 0080793 (to G.L.). We thank Dr. Qinheng Huang for his help with the corticosterone radioimmunoassay.
References
- 1.Adler GK, Smas CM, Fiandaca M, Frim DM, Majzoub JA. Regulated expression of the human corticotropin releasing hormone gene by cyclic AMP. Mol Cell Endocrinol. 1990:70. 165–174. doi: 10.1016/0303-7207(90)90156-3. [DOI] [PubMed] [Google Scholar]
- 2.Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- 3.Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995;16:53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- 4.Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- 5.Ary M, Cox B, Lomax P. Dopaminergic mechanisms in precipitated withdrawal in morphine-dependent rats. J Pharmacol Exp Ther. 1977;200:271–276. [PubMed] [Google Scholar]
- 6.Bittencourt JC, Sawchenko PE. Do centrally administered neuro-peptides access cognate receptors? an analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutillier AL, Monnier D, Koch B, Loeffler JP. Pituitary adenyl cyclase-activating peptide: a hypophysiotropic factor that stimulates proopiomelanocortin gene transcription, and proopiomelanocortin-derived peptide secretion in corticotropic cells. Neuroendocrinology. 1994;60:493–502. doi: 10.1159/000126786. [DOI] [PubMed] [Google Scholar]
- 8.Bressers WM, Kruk MR, Van Erp AM, Willekens-Bramer DC, Haccou P, Meelis E. Time structure of self-grooming in the rat: self-facilitation and effects of hypothalamic stimulation and neuropeptides. Behav Neurosci. 1995;109:955–964. doi: 10.1037//0735-7044.109.5.955. [DOI] [PubMed] [Google Scholar]
- 9.Bruhn TO, Plotsky PM, Vale WW. Effect of paraventricular lesions on corticotropin-releasing factor (CRF)-like immunoreactivity in the stalk-median eminence: studies on the adrenocorticotropin response to ether stress and exogenous CRF. Endocrinology. 1984;114:57–62. doi: 10.1210/endo-114-1-57. [DOI] [PubMed] [Google Scholar]
- 10.Chance WT, Thompson H, Thomas I, Fischer JE. Anorectic and neurochemical effects of pituitary adenylate cyclase activating polypeptide in rats. Peptides. 1995;16:1511–1516. doi: 10.1016/0196-9781(95)02048-9. [DOI] [PubMed] [Google Scholar]
- 11.Cheng KW, Leung PC. Human gonadotropin-releasing hormone receptor gene transcription: up-regulation by 3′,5′-cyclic adenosine monophosphate/protein kinase A pathway. Mol Cell Endocrinol. 2001;181:15–26. doi: 10.1016/s0303-7207(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 12.Coleman DT, Chen X, Sassaroli M, Bancroft C. Pituitary adenylate cyclase-activating polypeptide regulates prolactin promoter activity via a protein kinase A-mediated pathway that is independent of the transcriptional pathway employed by thyrotropin-releasing hormone. Endocrinology. 1996;137:1276–1285. doi: 10.1210/endo.137.4.8625900. [DOI] [PubMed] [Google Scholar]
- 13.Edlund T, Walker M, Barr P, Rutter W. Cell specific expression of the rat insulin gene: evidence for a role of two distinct 5′ flanking elements. Science. 1985;230:912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- 14.Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci U S A. 1998;95:741–746. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 16.Emanuel RL, Girard DM, Thull DL, Majzoub JA. Second messengers involved in the regulation of corticotropin-releasing hormone mRNA and peptide in cultured rat fetal hypothalamic primary cultures. Endocrinology. 1990;126:3016–3021. doi: 10.1210/endo-126-6-3016. [DOI] [PubMed] [Google Scholar]
- 17.Fone KC, Johnson JV, Bennett GW, Marsden CA. Involvement of 5-HT2 receptors in the behaviours produced by intrathecal administration of selected 5-HT agonists and the TRH analogue (CG 3509) to rats. Br J Pharmacol. 1989;96:599–608. doi: 10.1111/j.1476-5381.1989.tb11858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gispen WH, Wiegant VM, Greven HM, de Wied D. The induction of excessive grooming in the rat by intraventricular application of peptides derived from ACTH: structure–activity studies. Life Sci. 1975;17:645–652. doi: 10.1016/0024-3205(75)90103-4. [DOI] [PubMed] [Google Scholar]
- 19.Grimee R, Wulfert E. Acute stress in rats produces a rapid and sustained increase in prostacyclin production in aortic tissue: dependence on corticosterone. Life Sci. 1995;57:69–81. doi: 10.1016/0024-3205(95)00244-z. [DOI] [PubMed] [Google Scholar]
- 20.Grinevich V, Fournier A, Pelletier G. Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on corticotropin-releasing hormone (CRH) gene expression in the rat hypothalamic paraventricular nucleus. Brain Res. 1997;773:190–196. doi: 10.1016/s0006-8993(97)01011-1. [DOI] [PubMed] [Google Scholar]
- 21.Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guardiola-Diaz HM, Boswell C, Seasholtz AF. The cAMP-responsive element in the corticotropin-releasing hormone gene mediates transcriptional regulation by depolarization. J Biol Chem. 1994;269:14784–14791. [PubMed] [Google Scholar]
- 23.Hagiwara M, Brindle P, Harootunian A, Armstrong R, Rivier J, Vale W, Tsien R, Montminy MR. Coupling of hormonal stimulation and transcription via the cyclic AMP- responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol Cell Biol. 1993;13:4852–4859. doi: 10.1128/mcb.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- 25.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 26.Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16:381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- 27.Huang QH, Entwistle ML, Alvaro JD, Duman RS, Hruby VJ, Tatro JB. Antipyretic role of endogenous melanocortins mediated by central melanocortin receptors during endotoxin-induced fever. J Neurosci. 1997;17:3343–3351. doi: 10.1523/JNEUROSCI.17-09-03343.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoi K, Horiba N, Tozawa F, Sakai Y, Sakai K, Abe K, Demura H, Suda T. Major role of 3′,5′-cyclic adenosine monophosphate-dependent protein kinase A pathway in corticotropin-releasing factor gene expression in the rat hypothalamus in vivo. Endocrinology. 1996;137:2389–2396. doi: 10.1210/endo.137.6.8641191. [DOI] [PubMed] [Google Scholar]
- 29.Jaworski DM, Proctor MD. Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Dev Brain Res. 2000;120:27–39. doi: 10.1016/s0165-3806(99)00192-3. [DOI] [PubMed] [Google Scholar]
- 30.King BR, Smith R, Nicholson RC. Novel glucocorticoid and cAMP interactions on the CRH gene promoter. Mol Cell Endocrinol. 2002;194:19–28. doi: 10.1016/s0303-7207(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 31.Kopp M, Meissl H, Korf HW. The pituitary adenylate cyclase-activating polypeptide-induced phosphorylation of the transcription factor CREB (cAMP response element binding protein) in the rat suprachiasmatic nucleus is inhibited by melatonin. Neurosci Lett. 1997;227:145–148. doi: 10.1016/s0304-3940(97)00312-1. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacs KJ, Arias C, Sawchenko PE. Protein synthesis blockade differentially affects the stress-induced transcriptional activation of neuropeptide genes in parvocellular neurosecretory neurons. Mol Brain Res. 1998;54:85–91. doi: 10.1016/s0169-328x(97)00324-0. [DOI] [PubMed] [Google Scholar]
- 34.Kozicz T, Arimura A. Dopamine- and cyclic AMP-regulated phosphoprotein-immunoreactive neurons activated by acute stress are innervated by fiber terminals immunopositive for pituitary adenylate cyclase-activating polypeptide in the extended amygdala in the rat. Regul Pept. 2002;109:63–70. doi: 10.1016/s0167-0115(02)00188-x. [DOI] [PubMed] [Google Scholar]
- 35.Kozicz T, Vigh S, Arimura A. Axon terminals containing PACAP-and VIP-immunoreactivity form synapses with CRF-immunoreactive neurons in the dorsolateral division of the bed nucleus of the stria terminalis in the rat. Brain Res. 1997;767:109–119. doi: 10.1016/s0006-8993(97)00737-3. [DOI] [PubMed] [Google Scholar]
- 36.Legradi G, Shioda S, Arimura A. Pituitary adenylate cyclase-activating polypeptide-like immunoreactivity in autonomic regulatory areas of the rat medulla oblongata. Neurosci Lett. 1994;176:193–196. doi: 10.1016/0304-3940(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 37.Legradi G, Holzer D, Kapcala LP, Lechan RM. Glucocorticoids inhibit stress-induced phosphorylation of CREB in corticotropin-releasing hormone neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1997;66:86–97. doi: 10.1159/000127224. [DOI] [PubMed] [Google Scholar]
- 38.Legradi G, Hannibal J, Lechan RM. Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neurosci Lett. 1998;246:145–148. doi: 10.1016/s0304-3940(98)00255-9. [DOI] [PubMed] [Google Scholar]
- 39.Malkoski SP, Dorin RI. Composite glucocorticoid regulation at a functionally defined negative glucocorticoid response element of the human corticotropin-releasing hormone gene. Mol Endocrinol. 1999;13:1629–1644. doi: 10.1210/mend.13.10.0351. [DOI] [PubMed] [Google Scholar]
- 40.Malkoski SP, Handanos CM, Dorin RI. Localization of a negative glucocorticoid response element of the human corticotropin releasing hormone gene. Mol Cell Endocrinol. 1997;127:189–199. doi: 10.1016/s0303-7207(96)04004-x. [DOI] [PubMed] [Google Scholar]
- 41.Masuo Y, Noguchi J, Morita S, Matsumoto Y. Effects of intra-cerebroventricular administration of pituitary adenylate cyclase-activating polypeptide (PACAP) on the motor activity and reserpine-induced hypothermia in murines. Brain Res. 1995;700:219–226. doi: 10.1016/0006-8993(95)00978-y. [DOI] [PubMed] [Google Scholar]
- 42.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 43.Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38) Biochem Biophys Res Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- 44.Monnikes H, Heymann-Monnikes I, Tache Y. CRF in the paraventricular nucleus of the hypothalamus induces dose-related behavioral profile in rats. Brain Res. 1992;574:70–76. doi: 10.1016/0006-8993(92)90801-f. [DOI] [PubMed] [Google Scholar]
- 45.Morley JE, Horowitz M, Morley PMK, Flood JF. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides. 1992;13:1133–1135. doi: 10.1016/0196-9781(92)90019-y. [DOI] [PubMed] [Google Scholar]
- 46.Murase T, Kondo K, Otake K, Oiso Y. Pituitary adenylate cyclase-activating polypeptide stimulates arginine vasopressin release in conscious rats. Neuroendocrinology. 1993;57:1092–1096. doi: 10.1159/000126475. [DOI] [PubMed] [Google Scholar]
- 47.Nomura M, Ueta Y, Serino R, Kabashima N, Shibuya I, Yamashita H. PACAP type I receptor gene expression in the para-ventricular and supraoptic nuclei of rats. NeuroReport. 1996;8:67–70. doi: 10.1097/00001756-199612200-00014. [DOI] [PubMed] [Google Scholar]
- 48.Nomura M, Ueta Y, Serino R, Yamamoto Y, Shibuya I, Yamashita H. Effects of centrally administered pituitary adenylate cyclase-activating polypeptide on c-fos gene expression and hetero-nuclear RNA for vasopressin in rat paraventricular and supraoptic nuclei. Neuroendocrinology. 1999;69:167–180. doi: 10.1159/000054416. [DOI] [PubMed] [Google Scholar]
- 49.Norrholm SD, Das M, Legradi G. Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN) Regul Pept. 2005;128:33–41. doi: 10.1016/j.regpep.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osipenko ON, Barrie AP, Allen JM, Gurney AM. Pituitary adenylyl cyclase-activating peptide activates multiple intracellular signaling pathways to regulate ion channels in PC12 cells. J Biol Chem. 2000;275:16626–16631. doi: 10.1074/jbc.M909636199. [DOI] [PubMed] [Google Scholar]
- 51.Pardi D, Margiotta JF. Pituitary adenylate cyclase-activating polypeptide activates a phospholipase C-dependent signal pathway in chick ciliary ganglion neurons that selectively inhibits alpha7-containing nicotinic receptors. J Neurosci. 1999;19:6327–6337. doi: 10.1523/JNEUROSCI.19-15-06327.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parkes DG, Yamamoto GY, Vaughan JM, Vale WW. Characterization and regulation of corticotropin-releasing factor in the human hepatoma NPLC-KC cell line. Neuroendocrinology. 1993;57:663–669. doi: 10.1159/000126423. [DOI] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. 2. Academic Press; 1986. The Rat Brain in Stereotaxic Coordinates. [DOI] [PubMed] [Google Scholar]
- 54.Picard P, Regoli D, Couture R. Cardiovascular and behavioural effects of centrally administered tachykinins in the rat: characterization of receptors with selective antagonists. Br J Pharmacol. 1994;112:240–249. doi: 10.1111/j.1476-5381.1994.tb13058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piggins HD, Stamp JA, Burns J, Rusak B, Semba K. Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. J Comp Neurol. 1996;376:278–294. doi: 10.1002/(SICI)1096-9861(19961209)376:2<278::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 56.Pincas H, Laverriere JN, Counis R. Pituitary adenylate cyclase-activating polypeptide and cyclic adenosine 3′,5′-monophosphate stimulate the promoter activity of the rat gonadotropin-releasing hormone receptor gene via a bipartite response element in gonadotrope-derived cells. J Biol Chem. 2001;276:23562–23571. doi: 10.1074/jbc.M100563200. [DOI] [PubMed] [Google Scholar]
- 57.Rawlings SR, Hezareh M. Pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP/vasoactive intestinal polypeptide receptors: actions on the anterior pituitary gland. Endocr Rev. 1996;17:4–29. doi: 10.1210/edrv-17-1-4. [DOI] [PubMed] [Google Scholar]
- 58.Roeling TA, van Erp AM, Meelis W, Kruk MR, Veening JG. Behavioural effects of NMDA injected into the hypothalamic paraventricular nucleus of the rat. Brain Res. 1991;550:220–224. doi: 10.1016/0006-8993(91)91321-q. [DOI] [PubMed] [Google Scholar]
- 59.Sarkar S, Legradi G, Lechan RM. Intracerebroventricular administration of alpha-melanocyte stimulating hormone increases phosphorylation of CREB in TRH- and CRH-producing neurons of the hypothalamic paraventricular nucleus. Brain Res. 2002;945:50–59. doi: 10.1016/s0006-8993(02)02619-7. [DOI] [PubMed] [Google Scholar]
- 60.Schomerus E, Poch A, Bunting R, Mason WT, McArdle CA. Effects of pituitary adenylate cyclase-activating polypeptide in the pituitary: activation of two signal transduction pathways in the gonadotrope-derived alpha T3-1 cell line. Endocrinology. 1994;134:315–323. doi: 10.1210/endo.134.1.7903932. [DOI] [PubMed] [Google Scholar]
- 61.Seasholtz AF, Thompson RC, Douglass JO. Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Mol Endocrinol. 1988;2:1311–1319. doi: 10.1210/mend-2-12-1311. [DOI] [PubMed] [Google Scholar]
- 62.Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shioda S, Shuto Y, Somogyvari-Vigh A, Legradi G, Onda H, Coy DH, Nakajo S, Arimura A. Localization and gene expression of the receptor for pituitary adenylate cyclase-activating polypeptide in the rat brain. Neurosci Res. 1997;28:345–354. doi: 10.1016/s0168-0102(97)00065-5. [DOI] [PubMed] [Google Scholar]
- 64.Shioda S, Yada T, Nakajo S, Nakai Y, Arimura A. PACAP increases cytosolic calcium in vasopressin neurons: synergism with noradrenaline. Ann N Y Acad Sci. 1998;865:427–430. doi: 10.1111/j.1749-6632.1998.tb11209.x. [DOI] [PubMed] [Google Scholar]
- 65.Somogyvari-Vigh A, Reglodi D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr Pharm Des. 2004;10:2861–2889. doi: 10.2174/1381612043383548. [DOI] [PubMed] [Google Scholar]
- 66.Spengler D, Rupprecht R, Van LP, Holsboer F. Identification and characterization of a 3′,5′-cyclic adenosine monophosphate-responsive element in the human corticotropin-releasing hormone gene promoter. Mol Endocrinol. 1992;6:1931–1941. doi: 10.1210/mend.6.11.1480179. [DOI] [PubMed] [Google Scholar]
- 67.Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- 68.Tachibana T, Saito ES, Takahashi H, Saito S, Tomonaga S, Boswell T, Furuse M. Anorexigenic effects of pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide in the chick brain are mediated by corticotrophin-releasing factor. Regul Pept. 2004;120:99–105. doi: 10.1016/j.regpep.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 69.Taupenot L, Mahata SK, Wu H, O’Connor DT. Peptidergic activation of transcription and secretion in chromaffin cells. Cis and trans signaling determinants of pituitary adenylyl cyclase-activating polypeptide (PACAP) J Clin Invest. 1998;101:863–876. doi: 10.1172/JCI1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tschope C, Picard P, Culmann J, Prat A, Itoi K, Regoli D, Unger T, Couture R. Use of selective antagonists to dissociate the central cardiovascular and behavioural effects of tachykinins on NK1 and NK2 receptors in the rat. Br J Pharmacol. 1992;107:750–755. doi: 10.1111/j.1476-5381.1992.tb14518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Erp AM, Kruk MR, Willekens-Bramer DC, Bressers WM, Roeling TA, Veening JG, Spruyt BM. Grooming induced by intrahypothalamic injection of ACTH in the rat: comparison with grooming induced by intrahypothalamic electrical stimulation and i.c.v. injection of ACTH. Brain Res. 1991;538:203–210. doi: 10.1016/0006-8993(91)90431-t. [DOI] [PubMed] [Google Scholar]
- 72.van Erp AM, Kruk MR, Willekens-Bramer DC, Fermont PC, Nijsen MJ. PVH lesions do not inhibit stressor-induced grooming in the rat. Physiol Behav. 1995;57:887–892. doi: 10.1016/0031-9384(94)00335-3. [DOI] [PubMed] [Google Scholar]
- 73.Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- 74.Waschek JA. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev Neurosci. 2002;24:14–23. doi: 10.1159/000064942. [DOI] [PubMed] [Google Scholar]
- 75.Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40:573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- 76.Whitnall MH, Mezey E, Gainer H. Co-localization of corticotropin-releasing factor and vasopressin in median eminence neurosecretory vesicles. Nature. 1985;317:248–250. doi: 10.1038/317248a0. [DOI] [PubMed] [Google Scholar]
- 77.Winters SJ, Dalkin AC, Tsujii T. Evidence that pituitary adenylate cyclase activating polypeptide suppresses follicle-stimulating hormone-beta messenger ribonucleic acid levels by stimulating follistatin gene transcription. Endocrinology. 1997;138:4324–4329. doi: 10.1210/endo.138.10.5441. [DOI] [PubMed] [Google Scholar]
- 78.Wolfl S, Martinez C, Majzoub JA. Inducible binding of cyclic adenosine 3′,5′-monophosphate (cAMP)-responsive element binding protein (CREB) to a cAMP-responsive promoter in vivo. Mol Endocrinol. 1999;13:659–669. doi: 10.1210/mend.13.5.0282. [DOI] [PubMed] [Google Scholar]
- 79.Zhou CJ, Kikuyama S, Shibanuma M, Hirabayashi T, Nakajo S, Arimura A, Shioda S. Cellular distribution of the splice variants of the receptor for pituitary adenylate cyclase-activating polypeptide (PAC(1)-R) in the rat brain by in situ RT-PCR. Mol Brain Res. 2000;75:150–158. doi: 10.1016/s0169-328x(99)00300-9. [DOI] [PubMed] [Google Scholar]


