Abstract
Summary
Several synthetic histidine‐lysine (HK) polymers have been screened for their efficacy as carriers of nucleic acids in vitro. One branched HK polymer, H2K4b (and its derivatives), has been particularly effective as an in vitro carrier of plasmids. In this study, we investigated whether various salt conditions during formation of the H2K4b/plasmid DNA polyplex affected transfection. We compared the transfection ability of H2K4b polyplexes prepared under three conditions: 1) water, 2) water and then Opti‐MEM (or 300 mM NaCl), or 3) Opti‐MEM (or 150 mM NaCl). The milieu in which the H2K4b polyplexes were prepared significantly affected in vitro transfection, and conditions that resulted in highest to lowest transfection levels were as follows: water and then Opti‐MEM > Opti‐MEM (or 150 mM NaCl)>> water. Several biophysical properties (size and shape of polyplex, surface charge, stability) were examined for their correlation with the level of transfection by the HK carrier. Strikingly, electron micrographs showed that HK polyplexes, first formed in water and then in salt, had a needle‐like morphology with a mean length of 170 nm and a width of 53 nm; these needle‐like polyplexes were observed intracellularly and absorbed to the cell surface, which was in marked contrast to the spherical HK polyplexes formed in water or in Opti‐MEM. Notably, these needle‐like HK polyplexes entered the cell through clathrin‐mediated endocytosis, in contrast to spherical polyplexes, which entered primarily through non clathrin‐mediated endocytosis.
Keywords: polymer, branched, peptide, histidine, lysine, transfection
Introduction
Several laboratories have used cationic liposomes as in vitro and in vivo carriers of plasmids (Zhu et al, 1993; Lesoon‐Wood, 1995; Xu et al, 1997). Because we found that cationic liposomes were not particularly effective carriers in vivo (Xu et al, 1997), we are currently developing histidine‐lysine (HK) peptides as nucleic acid carriers. With HK peptides, lysines serve as a core for branched points and to electrostatically bind to the negatively charged DNA phosphates, while histidines buffer the acidic endosomes and aid in release of DNA from the endosomes. We have determined that specific forms of these peptides were effective carriers of plasmids in vitro (Chen et al, 2001; Leng and Mixson, 2005). The increase in transfection efficiency activity has been found to depend on HK polymer structure including number of branches, the sequence within the arms, and whether the peptides are used alone or in combination with cationic liposomes. Liposomes in combination with linear HK peptides were initially required for adequate transfection in vitro; later, with the synthesis of branched HK peptides, we established that a polymer referred to as H2K4b is active as a single transfection agent and can adequately transfect cells in vitro. Nevertheless, we have not yet determined the optimal transfection formulation and conditions for HK polyplexes (e.g., size of polyplex, salt conditions in which polyplex was formed) and the endocytic pathways by which these polyplexes enter the cell.
Numerous in vitro studies have examined the relationship between size/aggregation of the non‐viral complexes and their transfection activity, but conclusions concerning this relationship have been variable. Thus, several studies have demonstrated that small compact polyplexes are less effective than larger, less compact polyplexes in transfecting cells (Ogris et al, 1998; Lucius et al, 2001; Dalluge et al, 2002; Haberland et al, 2005), but other reports showed that small compact polyplexes formed first in high salt conditions before dilution effectively transfect cells in vitro and in vivo (Liu et al, 2001). Moreover, studies with polyamidoamine dendrimers showed no clear correlation between the size of polyplex and transfection efficiency (Tang et al, 1996; Tang and Szoka, 1997). It is also unclear from these studies whether the presence of a ligand in a polyplex affects the relationship between size and transfection activity. The lack of consensus between the size of non‐viral complexes and transfection activity found in these studies may be due in part to different carriers, different media in which the polyplexes are formed, and different transfected cell lines.
Nevertheless, changes in salt concentration during formation of the polyplex may influence its charge, size, and release of DNA from the polymer (Ferkol et al, 1996; Ogris et al, 1998; Liu et al, 2001). Given these findings, we investigated whether various salt conditions during formation of the HK:DNA polyplex affect transfection activity and efficiency. In this study, we showed that ionic conditions of the aqueous solutions used to form HK polyplexes can result in different particle morphologies and that one condition favoring formation of a needle‐like morphology was the most effective in transfecting several cell lines.
II. Materials and methods
A. Materials
MDA‐MB‐435, C6, LN229, and Chinese Hamster Ovary (CHO) cells were purchased from American Type Culture Collection (Manassas, VA); human microvascular endothelial cells (HMVEC) were obtained from Cambrex (Walkersville, MD). Media (Dulbecco’s Modified Essential Medium (DMEM); Opti‐MEM Reduced Serum Media (Opti‐MEM); fetal calf serum), ethidium bromide, and the β‐galactosidase staining kit were purchased from Invitrogen (Carlsbad, Ca). Opti‐MEM is an isotonic media that includes Eagle’s minimal essential media supplemented with HEPES (13 mM), vitamins, trace elements, glutamine, and low amounts of protein (insulin and transferrin:total protein‐15 µg/ml). The endocytyic pathway inhibitors (polyclonal clathrin antibody, filipin, and cytochalasin D) were purchased from Sigma (St. Louis, MO). Phosphate‐buffered saline (PBS) and sodium chloride were obtained from Biosource (Rockville, MD) and Sigma, respectively. The passive lysis buffer (1x) and luciferase assay reagent were obtained from Promega Corp (Madison, WI.). The BCA protein assay kit was purchased from Pierce Chemical Co. (Rockford, IL).
B. Cell lines
MDA‐MB‐435 (human breast cancer cell line), C6 (rat glioma line), LN229 (human glioma) and CHO cells were maintained in DMEM/10% fetal calf serum. Human microvascular endothelial cells (HMVEC) were grown in EGM‐2 Bullet Kit medium.
C. Polymers and liposomes
The biopolymer facility at the University of Maryland synthesized the H2K4b polymer. The structure/sequence of this branched HK polymer is as follows:
where R=[KHKHHKHHKHHKHHKHHKHK]). The polymer (H2K4b) has 4 terminal branches and a molecular weight of 10,816. The polymer was then purified by HPLC.
D. Transfection protocol
Initially, 1 × 105 cells were plated in 24‐well plates in 500 µl of DMEM with 10% serum; after 24 h, the cells reached approximately 70% confluency. To prepare polyplexes for transfection, the branched H2K4b peptide (4 µg) in complex with a reporter‐containing plasmid (1 µg) were formed in various media: 1) in Opti‐MEM (50 µl) for 45 min (Opti‐MEM); 2) in water (50 µl) for 45 min (water); 3) in water (50 µl) for 30 min, followed by Opti‐MEM (50 µl) for 15 min (water/Opti‐MEM); 4) in water (50 µl) for 30 min, followed by NaCl solutions (50 µl; range, 100 to 600 mM) (water/salt) ; 5) in water (50 µl) for 30 min, followed by water (50 µl) for 15 min (water/water); and 6) in 300 mM NaCl (50 µl) followed by water (50 µl) (NaCl/water); the designation for the manner in which the HK polymers were formed are in parentheses. The complexes were added to medium (500 µl of DMEM with 10% serum) containing cells in 24 multi‐well plates. After approximately 24 h (between 20 to 26 h), the appropriate reporter assay was then performed. The data for in vitro transfection experiments are represented by the mean and standard deviation of four experiments.
E. Luciferase reporter assay
The cells were washed once with PBS, and then lysed with 100 µl of 1× passive lysis buffer. Protein concentration was measured by using the BCA protein assay kit. Luciferase activity in the lysate was measured after addition of the luciferase reagent with the direct current Turner 20/20 luminometer (Turner Design, Sunnyvale, CA). Duplicate measurements were performed for each concentration and three separate experiments were conducted. Note that, although the direct current Turner luminometer and the more commonly used photon luminometers have similar sensitivities, the reported RLU values with the Turner luminometer are approximately 1/1000th the reported RLU values with a photon luminometer.
F. β‐Galactosidase staining
MDA‐MB‐435 cells transfected with the β‐gal gene were stained with the β‐galactosidase staining kit as directed by the manufacturer. In brief, the growth medium was removed from the transfected cells and the cells were rinsed once with PBS. The cells were fixed for 10 min at room temperature, rinsed twice with 1× PBS, and then incubated with X‐Gal staining solution for 6 h at 37°C.
G. Measurement of particle size/zeta potential of peptide—DNA polyplexes
The branched HK peptide (40 µg) was mixed with the plasmid (PCI‐Luc, 10 µg) and prepared as described under transfection conditions (N/P ratio, ≈10/1; total volume: 100 µl). After 45 min, the polyplex that formed was diluted to a volume of 1.25 ml. The particle size of various polyplexes was determined by measurement of dynamic light scattering (DLS) at a 90 degree angle on an N4 Submicron Particle Size Analyzer (Beckman Coulter, Hialeah, FL). The particle size is reported as the average size obtained from unimodal analysis carried out with software provided by the instrument manufacturer. Zeta potential was measured with a Delsa 440 SX instrument (Coulter). Each data point represents the mean ± S.D. of three measurements.
H. Atomic force microscopy
Morphology of polymer/plasmid complexes was analyzed by atomic force microscopy (AFM) essentially as described by Chan et al. (Chan et al, 2000). Briefly, H2K4b and plasmid DNA polyplexes were formed under various conditions as described above, 4 µl of the polyplex were added to freshly cleaved mica, and allowed to air‐dry for 15 minutes. Tapping mode AFM in air, by using magnetic oscillation of the cantilever, was then carried out using a Pico Plus AFM (Molecular Imaging Corporation, Phoenix, AZ), scanning with a type II silicon MAClever cantilever (225 mm).
I. Transmission electron microscopy
Four hours after transfection with HK polyplexes, the MDA‐MB‐435 cells were washed with PBS, fixed with 4% formaldehyde/1% glutaraldehyde at room temperature overnight, and then rinsed 3 times with 0.1 M sodium cacodylate/0.2 M sucrose buffer. After fixation, the cells were incubated in osmium tetroxide (1%) for 1 h, dehydrated in a series of ethanol washes, and then embedded in epoxy. When the epoxy had polymerized at 65°C for 24 h, sections were then cut onto carbon‐coated copper grid and viewed with a transmission electron microscope (Jeol 1200).
J. Inhibition of endocytosis
MDA‐MB‐435 (1 × 105 cells) were placed in 24‐well plates 24 h before transfection. After the cells were pretreated with a polyclonal clathrin antibody (1:125 and 1:250 vol/vol. dilution in cell culture medium), filipin (2.0 µg/ml), or cytochalasin D (2 µg/ml and 5.0 µg/ml) for 30 min, the medium was changed to fresh DMEM/10% serum. As discussed previously, HK polyplexes were then prepared and added to each well and luciferase activity was measured approximately 24 h later.
III. Results
A. Sequential addition of water and then salt during HK polyplex formation increases transfection in MDA‐MB‐435 cells
We examined the effects of varying salt concentrations during the formation of HK polyplexes in a sequential manner on their subsequent transfection activity. These preliminary transfection experiments were performed with MDA‐MB‐435 cells. When polyplexes were formed only in water with no later addition of a cell culture medium containing salt, luciferase activity was lowest (Figure 1). In comparison, preparation of polyplexes only in Opti‐MEM or in NaCl (150 mM) gave similar and intermediate luciferase activities (Figure 1). In our prior studies, polyplexes were formed in Opti‐MEM (50 µl). Notably, HK polyplex first formed in water (50 µl) with subsequent addition of Opti‐MEM (50 µl) gave the highest luciferase activity (Figure 1). Substituting Opti‐MEM with NaCl in the sequential reaction reduced transfection by approximately 20%, suggesting that NaCl was not the only factor for enhancement of transfection. Furthermore, sequential additions of the same medium during polyplex formation (as a control to mirror the water/salt condition) showed comparable activity to that obtained by using medium without sequential additions (e.g., Opti‐MEM/Opti‐MEM (100 µl) vs. Opti‐MEM (50 µl); water/water (100 µl) vs. water (50 µl); Figure 1 and Figure 2).
Figure 1. Varying conditions during HK polyplex formation affects transfection in MDA‐MB‐435 cells.
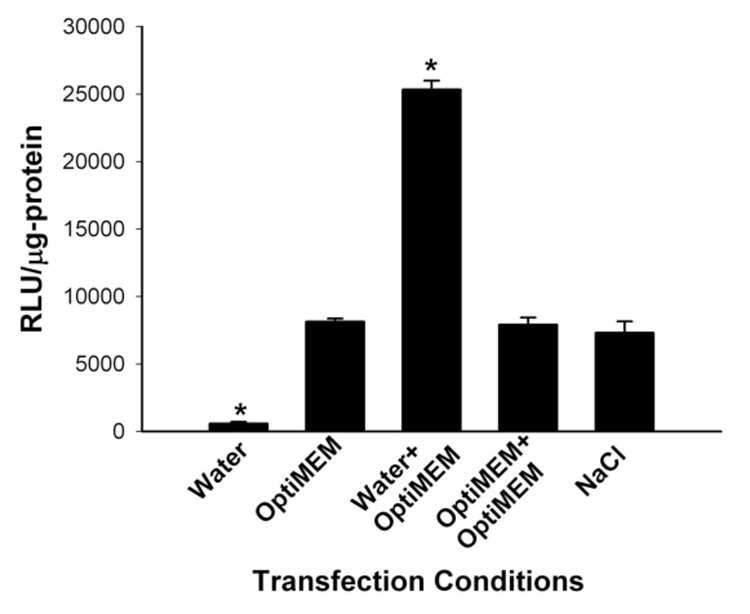
When the polyplex was formed only in water with no later addition of medium containing salt, luciferase activity was lowest. Preparation of polyplex only in Opti‐MEM (50 µl), Opti‐MEM (50 µl) followed by Opti‐MEM (50 µl), and NaCl (150 mM) gave similar luciferase activities. Compared to these conditions, the formation of the HK polyplex first formed in water and then in Opti‐MEM gave the highest luciferase transfection. *, P<0.05; Water or Water/Opti‐MEM vs. Opti‐MEM; multiple comparisons versus control (Opti‐MEM) Group (Bonferroni t‐test).
Figure 2. Order of addition of media varied during HK polyplex formation.
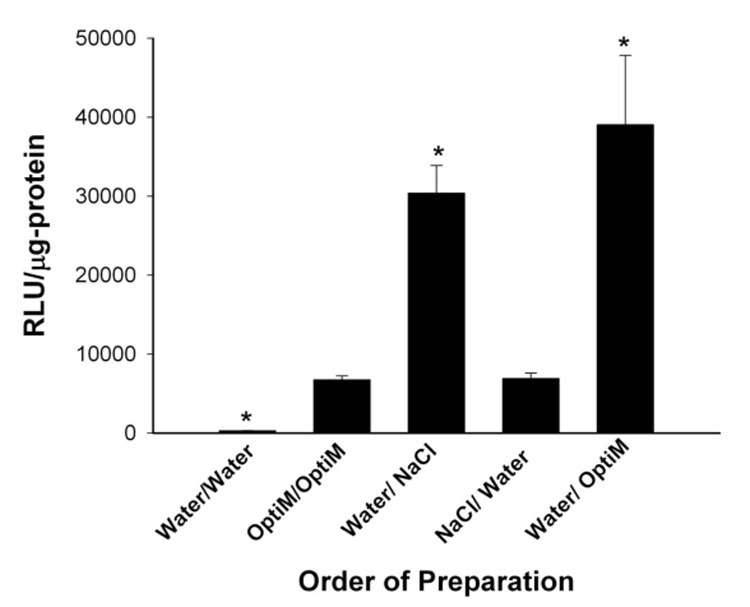
Sequential addition of water followed by NaCl (300 mM) or water followed by Opti‐MEM (OptiM) were conditions during the polyplex formation that gave the highest luciferase activity in MDA‐MB‐435 cells. *, P<0.05; Water vs. Opti‐MEM; Water/Opti‐MEM vs. Opti‐MEM; Water/NaCl vs. Opti‐MEM; multiple comparison test compared to control (Opti‐MEM) group (Bonferonni t‐test).
B. Order of addition of media during HK polyplex formation affects transfection
To determine the role of sodium chloride and importantly its order of addition in augmenting transfection, we substituted NaCl for Opti‐MEM. The sequential addition of water followed by NaCl (300 mM; final NaCl concentration in which polyplex was formed, 150 mM) or water followed by Opti‐MEM were conditions that gave the highest luciferase activity in MDA‐MB‐435 cells. Because the sequential conditions of water/NaCl and water/Opti‐MEM gave similar transfection results, a substantial part of the luciferase increase was due to sodium chloride (Figure 2). Interestingly, there were significant differences in transfection activity when the polyplex was first formed in water followed by addition of NaCl compared to when polyplexes were first formed in NaCl followed by addition of water (Figure 2). Transfection studies with plasmids expressing β‐galactosidase or green fluorescent protein gave results similar to those obtained with the plasmids expressing luciferase (data not shown).
C. Determining the optimal salt concentration after H2K4b polyplex first formed in water
We next examined the effects on transfection when the HK polyplex was first formed in water, followed by solutions containing different concentrations of NaCl. HK polyplexes were first formed in water (50 µl) for 30 min and then NaCl solutions (50 µl) containing a range of concentrations from 100 to 600 mM) were added for 15 min (Figure 3). The complexes in which NaCl concentrations of 200 and 300 mM were added (note that the final NaCl concentration was half the concentration of the added solution) had the highest transfection activity. This also supports the conclusion that the high transfection activity seen with the sequential addition of Opti‐MEM to water appears primarily to be due to its sodium chloride content (Figures 2 and 3).
Figure 3. Effect on transfection with HK polyplex formed by sequential addition of water followed by increasing NaCl concentrations.
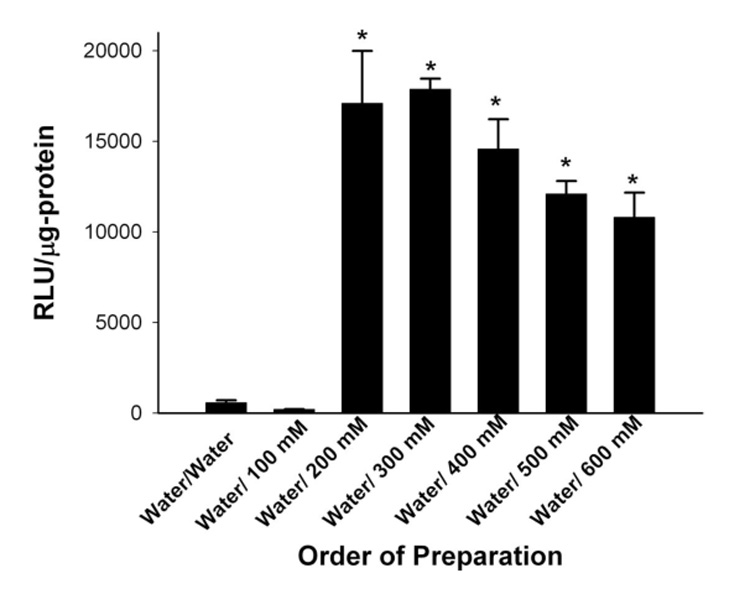
HK polyplex formed first in water for 30 min followed by addition of NaCl solutions varying from 100 for 600 mM for 15 min. Twenty‐four hours after complexes were added to medium, luciferase activity was measured. The complexes in which NaCl concentrations of 200 to 600 mM were added (final concentration of NaCl in which polyplex was formed was one‐half these) had the highest transfection activity. *, P<0.01, water/NaCl ≥ 200 mM vs water; multiple comparison test compared to Control (water) Group (Bonferonni t‐test).
D. Biophysical properties of H2K4b polyplexes: size, charge, and stability
To determine the mechanism by which the sequential addition of sodium chloride during formation of HK complexes may augment transfection, we measured the particle size distribution and surface charge (Table 1). We found that preparation of HK polyplex initially with water followed by addition of NaCl (300 mM) or Opti‐MEM had an intermediate size compared to those polyplexes formed only in water, only in Opti‐MEM, or only in NaCl (150 mM). Dynamic light scattering (DLS) measurements showed that HK polyplexes formed in water were 81±26 nm; moreover, atomic force microscopy confirmed that HK polyplexes were approximately 100 nm in size and that these polyplexes had small and distinct images on the mica, suggesting that these HK polyplexes were compact. In contrast, the HK polyplex formed in water followed by addition of Opti‐MEM had a mean diameter of 761±292 nm, whereas the HK polyplex formed in only Opti‐MEM was even larger with a mean diameter of 1729±559 nm (Table 1). The particle size determined by DLS of HK polyplexes formed under a variety of conditions was corroborated by AFM measurements. Unlike particle size, the effect of salt conditions during formation of the HK polyplex was found to have only minimal effects on the surface charge (Table 1).
Table 1.
Characterization of HK Polyplexes Examined by Various methods
| Conditions | HK Sizea‐N4 (nm) | HK Sizeb‐TEM (nm) | HK Zeta Potentiala (mV) |
|---|---|---|---|
| Water | 81 (±26) | 128 (±35.2) | 25.5 (±0.2) |
| Water/Opti‐MEM | 761 (±292) | 170 (±21.8 ) × 53 (±2.6) | 21.9 (±1.7) |
| Water/ NaCl (300mM) | 1132 (±427) | ---- | 17.5 (±0.6) |
| Opti‐MEM | 1729 (±559) | 225 (±28.2) | 18.8 (±0.75) |
| NaCl (150 mM) | 1633 (±541) | ---- | 17.4 (±0.51) |
The polymer was mixed with plasmid DNA in medium that varied in its salt concentration. After the polyplex formed, the size (diameter or dimensions) of the complex was measured by the N4 Submicron Particle Size Analyzer or HK polyplex absorbed to cell surface by TEM. The cell surface charge (zeta potential) of HK polyplexes was measured with the Delsa 440 SX. For those polyplexes in which NaCl was added, the final concentration was 150 mM.
Data represent mean (±S.D) of 3 measurements
data represent mean (±S.D.) of 20 measurements.
E. Characterization of the HK polyplex associated with cells
In addition to measuring the size and shape of HK polyplexes by DLS and AFM, we also determined the size and shape of the HK polyplexes during transfection of cells. We visualized the polyplexes by electron microscopy (Figure 4). The most striking finding is that the majority of HK polyplexes formed in water and then Opti‐MEM were needle‐like in shape and numerous needle‐like structures were found intracellularly as well as associated with the cell surface (Figure 4C). In contrast, we did not observe any needle‐like structures with HK polyplexes formed only in water or only in Opti‐MEM that were either absorbed to the cell surface or found internally. Although large aggregates of HK polyplexes were observed with polyplexes formed in the sequential water/ OptiMEM method or only in OptiMEM medium, these aggregates were not attached to the cell surface. Polyplexes formed only in water were spherical and few were internalized (Figure 4A). Similarly, polyplexes formed only in Opti‐MEM were spherical to elliptical in shape, and consistent with the higher transfection activity, these polyplexes were more frequently observed within the cell compared to those made only in water (Figure 4B). In contrast to HK polyplexes formed by the sequential water and then OptiMEM method (Figure 4C), there were few polyplexes absorbed to the cell surfaced prepared under other conditions (Figure 4A, Figure 4B). Furthermore, it is unlikely that the needle‐like particles are artifacts in that non‐transfected cells showed no evidence of these structures intracellularly. Notably, other polyplexes not formed with HK but with PEI polyplexes made in various salt conditions were spherical irrespective of transfection conditions, with no evidence of needle‐like polyplexes (data not shown). The sizes of HK polyplexes formed under different conditions are summarized in Table 1.
Figure 4. Transmission electron microscopy of H2K4b polyplexes associated with cells.
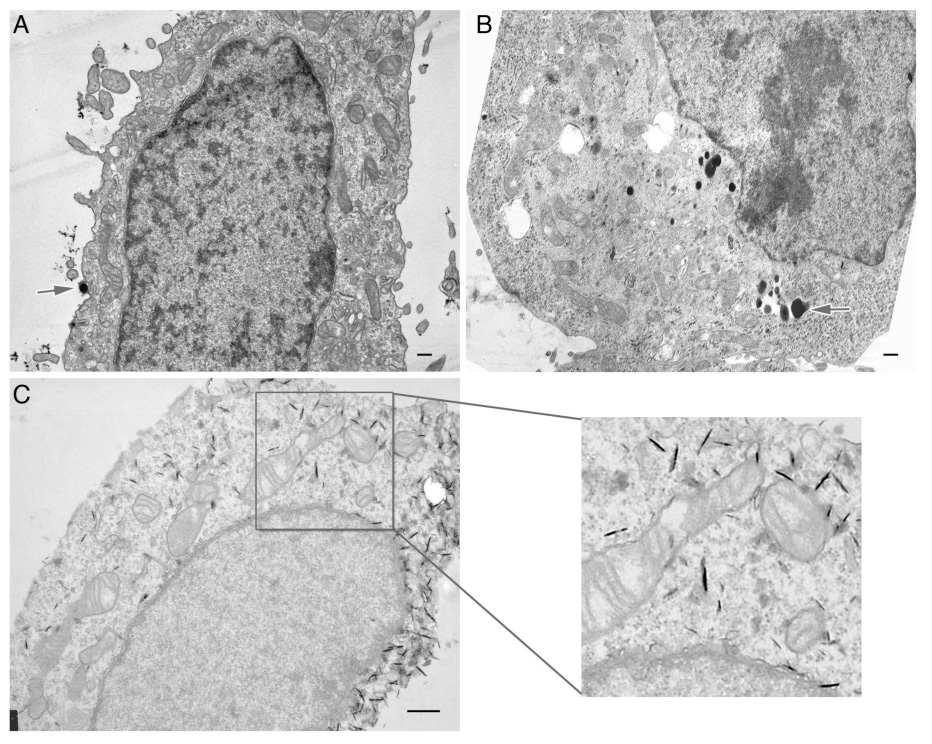
After HK polyplexes were prepared under different conditions, transfected MDA‐MB‐435 cells were prepared as detailed under Materials and Methods. The cells were then viewed with a transmission electron microscope. HK polyplexes formed (A) in water, (B) in OptiMEM, and (C) in water/OptiMEM. The arrows signify HK polyplexes absorb to the cell surface (A) or internalized (B); the needle‐like formations are observed in (C) and in the magnified insert. In contrast to the other conditions in which HK polyplexes were formed, needle‐like formations were only observed with the sequential method. The bar equals 500 nm.
F. Depending on its formation, the H2K4b polyplex enters the cell by different routes
Non‐viral complexes, differing in their size, may enter the cell through different endocytic pathways (Rejman et al, 2006;von Gersdorff et al, 2006). As a result, we examined whether the endocytic pathway is dependent on the manner in which the H2K4b polyplex was formed and found that indeed the polyplexes formed under different conditions entered by a particular pathway. When the polyplex was formed first in water followed later by the addition of Opti‐MEM, the uptake of H2K4b polyplex is primarily by the clathrin‐mediated pathway. Specific blockade of clathrin‐mediated endocytosis by antibodies (the most specific of the inhibitors used in this study) reduced transfection by nearly 90% (Figure 5). Inhibitors of other endocytic pathways only modestly reduced transfection activity by H2K4b polyplexes formed by the sequential water/OptiMEM method. HK polyplexes formed only in water or only in OptiMEM entered the cell by non‐clathrin‐mediated endocytosis. Cytochalasin D, an inhibitor of macropinocytosis, markedly reduced the transfected luciferase levels of HK polyplexes formed only in water, whereas none of the inhibitors (clathrin antibody, cytochalasin D, filipin) significantly reduced the transfection levels of polyplexes formed only in Opti‐MEM.
Figure 5. Selective inhibition of endocytosis pathways.
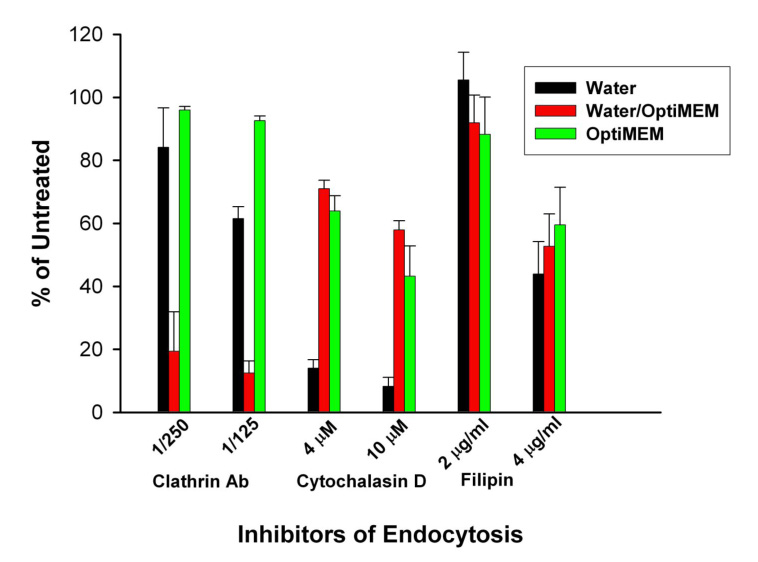
After HK polyplexes were formed under various conditions, the specific endocytic pathway by which polyplexes entered MDA‐MB‐435 cells was examined. Prior to transfection with the polyplexes, the cells were first treated for 30 min with the following endocytic inhibitors: clathrin antibody (1/125 or 1/250 dilution), filipin (2.0 or 4.0 µg/ml), or cytochalasin D (2.0 µg/ml or 5.0 µg/ml).
G. H2K4b polyplexes formed by sequential addition of water followed by Opti‐MEM increased transfection activity in several cell lines
In addition to MDA‐MB‐435 cells, we examined a panel of cells (HMVEC, LN229, CHO, and C6) to determine if the HK polyplex formed by sequential addition of water followed by Opti‐MEM increased transfection activity consistently. All cells tested had increased luciferase activity when the HK polyplex was generated with the sequential water/salt method compared to when the polyplex was formed only in Opti‐MEM (Figure 6).
Figure 6. H2K4b polyplex formed by sequential addition of water followed by Opti‐MEM increased transfection activity in cell lines.
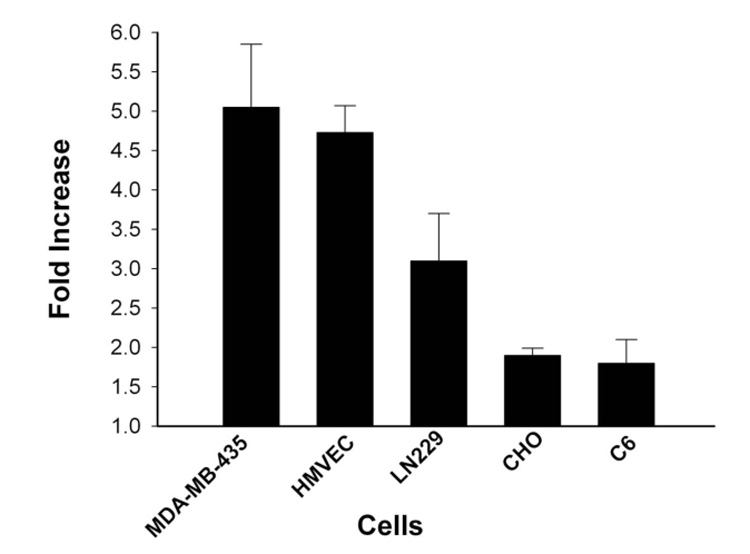
All cells had increased luciferase activity when the H2K4b polyplex was formed under sequential conditions compared to when polyplex was formed only in Opti‐MEM.
IV. Discussion
The objective of this study was to identify critical formulation parameters and biophysical properties of active HK polyplexes that enable transfection. We examined the effects of adding salt containing aqueous solutions during HK polyplex formation on transfection activity and on a variety of biophysical properties of the resulting HK polyplexes.
The results obtained in this study reinforce earlier findings that many common methods used to evaluate polyplex structure/biophysical characteristics require careful evaluation and may not be useful in determining the essential properties of the transfection polyplex. The difficulty with many of these methods or the manner in which these methods are applied is that they tend to examine the average biophysical property of the total population of the polyplex. Unfortunately, the properties of the total population may not specify the precise active species that augments transfection (Ahearn and Malone).
For example, the mean size of the HK polyplexes, formed sequentially in water and then salt, as measured by DLS instrumentation, is 761±292 nm. This polyplex mean size was intermediate to the size to those formed only in water and in Opti‐MEM, yet had the greatest transfection activity of the three types of HK polyplexes suggesting particle size is not necessarily the important parameter. These measurements were obtained with the commonly used unimodal analysis of the N4 submicron particle sizer instrument. Moreover, attempts to define the particle size of the active species more precisely (with the size distribution processor (SDP) analysis of N4) were unsuccessful, because they were not reproducible. Similarly, atomic force microscopy, perhaps due to selection bias, found that most particles were ellipsoid in shape and approximately 1 micron in size. It is clear from the electron micrographs, however, that active polyplexes for transfection are significantly smaller than was indicated by either the N4 or AFM. Furthermore, the broad ellipsoid morphology seen with AFM does not correlate with needle‐like morphology observed with EM. Although osmium tetroxide used for processing samples for EM may affect the shape of the polyplex, we think that it is unlikely to have occurred in this case, because similar needle‐like polyplexes were repeatedly observed in several experiments, both intra‐ and extracellularly. None of the other transfection conditions produced HK polyplexes with needle‐like structures associated with cells. Moreover, electron microscopy showed that PEI polyplexes prepared under a variety of conditions were spherical and did not form needle‐like structures (data not shown). Taken together, our results indicate that the needle‐like HK particles observed with EM are an important aspect of the active HK polyplex transfection and do not appear to be artifacts.
Because the size and shape of the non‐viral complex may affect the endocytic pathways through which these complexes entered (Rejman et al, 2006; von Gersdorff et al, 2006), specific inhibitors of endocytosis were investigated to determine which pathway was used by specific HK polyplexes. We found that the medium in which the HK polyplex was formed influenced the pathway of entry. The needle‐shaped HK polyplex, formed in water and then salt, entered the cell primarily through clathrin‐mediated endocytosis. Notably, clathrin‐mediated endocytosis may accommodate particles up to approximately 200 nm. Thus, our data suggest that the majority of HK polyplexes (by mass) formed sequentially in water and then Opti‐MEM (or in salt) or formed only in Opti‐MEM with mean diameters of 1 and 2 µm, respectively, do not participate directly in the transfection of cells. In most cells, only macrosomes may be sufficiently large to engulf transfection particles of this magnitude, and this pathway does not appear to participate significantly in the uptake of HK polyplexes formed in either Opti‐MEM or sequentially in water/Opti‐MEM solutions. Interestingly, it is the small compact HK polyplexes formed in water only that appear to be taken up by macropinocytosis. Nonetheless, these findings that small HK polyplexes may enter through macropinocytosis are consistent with what other investigators have reported (Khalil et al, 2006).
With HK polyplexes formed by the sequential water/Opti‐MEM method, there were marked differences in sizes between the active transfection species measured by EM and the total population measured by DLS. Because of this discrepancy, conclusions about the active HK species drawn from biophysical tests which determine the properties of the total population of HK polyplexes formed under this condition are questionable. In contrast, HK nanoparticle polyplexes, formed only in water, were the only particles in which all the biophysical tests were consistent. Atomic force microscopy, transmission electron microscopy, and dynamic light scattering (DLS), and ethidium bromide intercalation assays (data not shown) all indicate that these HK polyplexes formed in water are small, contain compact DNA, and are probably the most stable HK polyplexes in serum. Nevertheless, these small compact polyplexes had the lowest transfection in vitro, indicating that release of DNA from these HK polyplexes intracellularly may be decreased and rate‐limiting. Previous studies from our laboratory suggested that it may be necessary for the DNA in the HK polyplexes to be released for endosomal lysis to occur (Chen et al, 2002). Without such a transformation releasing the HK polymer leading to endosomal lysis, the compact HK polyplexes would most likely be degraded by lysosomal enzymes. Consequently, it seems likely that the competing requirements of stability to serum and instability leading to endosomal release may lead to different optimal formulations for in vitro and in vivo applications.
In summary, we established that HK polyplexes formed under a wide variety of aqueous conditions have markedly different transfection efficiencies and biophysical properties. In all cell lines, the HK polyplexes first formed in water, with a Opti‐MEM solution added later, gave the highest transfection. Despite higher transfection in cells by HK polyplexes formed by the sequential water/salt method, there was significant variability in the transfection enhancement among cells; we are examining whether the variability of this enhancement may be due to differences in endocytic pathways of these cell lines. Unlike HK polyplexes formed under other conditions, the polyplexes formed sequentially in water and then Opti‐MEM were needle‐like and entered the cell primarily through clathrin‐mediated endocytosis. To our knowledge, the needle‐like morphology of HK polyplexes formed under certain conditions has not reported previously for other polyplexes and may indicate significant differences in their physiochemical properties.
Acknowledgements
We thank Drs. Martin Woodle and Pamela Talalay for their careful reading and helpful suggestions of this manuscript. This work was supported by the National Institutes of Health (CA096984).
Abbreviations
- β‐gal
β‐galactosidase
- GFP
green fluorescent protein
- H‐K
histidine‐lysine
- Opti‐MEM
Opti‐MEM Reduced Serum Media
- PCI‐luc
PCI‐luciferase
- PBS
phosphate‐buffered saline
References
- Ahearn A, Malone R. Models of cationic liposome mediated transfection. Gene Ther Mol Biol. 1999;4:159–170. [Google Scholar]
- Chan CK, Senden T, Jans DA. Supramolecular structure and nuclear targeting efficiency determine the enhancement of transfection by modified polylysines. Gene Ther. 2000;7:1690–1697. doi: 10.1038/sj.gt.3301275. [DOI] [PubMed] [Google Scholar]
- Chen QR, Zhang L, Luther PW, Mixson AJ. Optimal transfection with the HK polymer depends on its degree of branching and the pH of endocytic vesicles. Nucleic Acids Res. 2002;30:1338–1345. doi: 10.1093/nar/30.6.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QR, Zhang L, Stass SA, Mixson AJ. Branched copolymers of histidine and lysine are efficient carriers of plasmids. Nucleic Acids Res. 2001;29:1334–1340. doi: 10.1093/nar/29.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalluge R, Haberland A, Zaitsev S, Schneider M, Zastrow H, Sukhorukov G, Bottger M. Characterization of structure and mechanism of transfection‐active peptide‐DNA complexes. Biochim Biophys Acta. 2002;1576:45–52. doi: 10.1016/s0167-4781(02)00291-9. [DOI] [PubMed] [Google Scholar]
- Ferkol T, Perales JC, Mularo F, Hanson RW. Receptor‐mediated gene transfer into macrophages. Proc Natl Acad Sci U S A. 1996;93:101–105. doi: 10.1073/pnas.93.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland A, Cartier R, Heuer D, Zaitsev S, Paulke BR, Schafer‐Korting M, Bottger M. Structural aspects of histone H1‐DNA complexes and their relation to transfection efficiency. Biotechnol Appl Biochem. 2005;42:107–117. doi: 10.1042/BA20040155. [DOI] [PubMed] [Google Scholar]
- Khalil IA, Kogure K, Futaki S, Harashima H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J Biol Chem. 2006;281:3544–3551. doi: 10.1074/jbc.M503202200. [DOI] [PubMed] [Google Scholar]
- Leng Q, Mixson AJ. Modified branched peptides with a histidine‐rich tail enhance in vitro gene transfection. Nucleic Acids Res. 2005;33:e40. doi: 10.1093/nar/gni040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesoon‐Wood LA, Kim WH, Kleinman HK, Weintraub BD, Mixson AJ. Systemic gene therapy with p53 reduces growth and metastases of a malignant human breast cancer in nude mice. Hum Gene Ther. 1995;6:395–405. doi: 10.1089/hum.1995.6.4-395. [DOI] [PubMed] [Google Scholar]
- Liu G, Molas M, Grossmann GA, Pasumarthy M, Perales JC, Cooper MJ, Hanson RW. Biological properties of poly‐L‐lysine‐DNA complexes generated by cooperative binding of the polycation. J Biol Chem. 2001;276:34379–34387. doi: 10.1074/jbc.M105250200. [DOI] [PubMed] [Google Scholar]
- Lucius H, Haberland A, Zaitsev S, Dalluge R, Schneider M, Bottger M. Structure of transfection‐active histone H1/DNA complexes. Mol Biol Rep. 2001;28:157–165. doi: 10.1023/a:1015230010230. [DOI] [PubMed] [Google Scholar]
- Ogris M, Steinlein P, Kursa M, Mechtler K, Kircheis R, Wagner E. The size of DNA/transferrin‐PEI complexes is an important factor for gene expression in cultured cells. Gene Ther. 1998;5:1425–1433. doi: 10.1038/sj.gt.3300745. [DOI] [PubMed] [Google Scholar]
- Rejman J, Conese M, Hoekstra D. Gene transfer by means of lipo‐ and polyplexes: role of clathrin and caveolae‐mediated endocytosis. J Liposome Res. 2006;16:237–247. doi: 10.1080/08982100600848819. [DOI] [PubMed] [Google Scholar]
- Tang MX, Redemann CT, Szoka FC., Jr In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug Chem. 1996;7:703–714. doi: 10.1021/bc9600630. [DOI] [PubMed] [Google Scholar]
- Tang MX, Szoka FC. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997;4:823–832. doi: 10.1038/sj.gt.3300454. [DOI] [PubMed] [Google Scholar]
- Von Gersdorff K, Sanders NN, Vandenbroucke R, De Smedt SC, Wagner E, Ogris M. The Internalization Route Resulting in Successful Gene Expression Depends on both Cell Line and Polyethylenimine Polyplex Type. Mol Ther. 2006;14:745–753. doi: 10.1016/j.ymthe.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Xu M, Kumar D, Srinivas S, Detolla LJ, Yu SF, Stass SA, Mixson AJ. Parenteral gene therapy with p53 inhibits human breast tumors in vivo through a bystander mechanism without evidence of toxicity. Hum Gene Ther. 1997;8:177–185. doi: 10.1089/hum.1997.8.2-177. [DOI] [PubMed] [Google Scholar]
- Zhu N, Liggit D, Liu Y, Debs R. Systemic gene expression after intravenous DNA delivery into adult mice. Science. 1993;261:209–211. doi: 10.1126/science.7687073. [DOI] [PubMed] [Google Scholar]


