Abstract
The physiological mechanism through which food restriction (FR) enhances the biobehavioral actions of psychostimulants is unknown but may involve the gut peptide ghrelin. Plasma levels of ghrelin are increased by FR and reduced by eating. Moreover, systemically administered ghrelin crosses into the brain and is known to augment the locomotor-stimulating effects of cocaine [COC: Wellman et al, 2004]. This study sought to determine whether pretreatment with ghrelin (5 nmol) would enhance the rewarding properties of COC (0.0, 0.312, 0.625, or 1.25 mg/kg i.p.) as measured by conditioned place preference (CPP). Adult male Sprague-Dawley rats were given free access to both sides of a CPP chamber to determine initial side preference. The rats were then confined for 30 min to either their preferred side or non-preferred side on 8 consecutive days. When rats were confined to the least preferred side, each was injected with 0.5 ml (i.p.) of either ghrelin (5 nmol) or saline 1 h before the conditioning trial and then injected (i.p.) with one of the COC doses immediately prior to the conditioning trial. On alternate days, rats were injected with vehicle one hour before and again immediately before the conditioning trial. Place preference scores were computed as the differences in time (min) spent on the least preferred side of the chamber for the pre-test and the post conditioning test, covaried by the initial degree of preference (% time spent on the black side during the pre-test). These analyses indicated a significant interaction between ghrelin pretreatment and COC dose on changes in preference scores. Significantly higher place preference scores were noted for rats treated with either 0.312 or 0.625 mg/kg COC doses, but only when these COC doses were preceded by administration of 5 nmol ghrelin. In contrast, saline pretreated rats exhibited significant CPP at the 1.25 mg/kg COC dose, but the ghrelin pretreated group did not. These results provide partial support for the contention that ghrelin pretreatment can augment the rewarding effects of sub-threshold doses of COC in a CPP procedure. Moreover, these findings are consistent with the view that ghrelin may play a role in the capacity of FR to augment psychostimulant action.
Keywords: Reward, Psychostimulant, Food restriction
Introduction
Food intake and drug taking share common features and may involve overlapping pathways [1,2]. Ingestion of highly palatable foods or of psychostimulant drugs can trigger dopamine overflow in brain reinforcement circuits [3], whereas food deprivation or food restriction (FR) can increase the rate of acquisition of learned responses for many reinforcers, including food as well as the psychostimulant drugs cocaine or amphetamine [4–6]. A link between caloric homeostasis and psychostimulant action is further supported by evidence that FR, in rats, can augment the capacity of psychostimulants to enhance locomotion and to induce conditioned place preference (CPP) [7]. FR also augments the rewarding effects of electrical stimulation of the lateral hypothalamus (LH) [8,9]. The latter has been used as a model system to explore mechanisms through which FR might alter psychostimulant function.
Signals related to the acute availability of metabolic fuels (e.g., glucose, free fatty acids) are unlikely to wholly account for FR-associated changes in psychostimulant action inasmuch as short-term glucoprivation or lipoprivation does not alter LH self-stimulation [8,10,11]. Prolonged negative energy balance results in increased expression of NPY in the hypothalamus; however, administration of NPY does not alter LH self-stimulation [8]. Although FR can be viewed as a stressor, acute modulation of corticosterone availability does not reverse the capacity of FR to sensitize LH self-stimulation [12]. These studies collectively suggest that FR may act through as yet unidentified feeding relevant system(s) to modulate psychostimulant reactivity.
Ghrelin is a 28 amino acid peptide secreted peripherally by the gut that functions as an orexigenic stimulus [13–18]. Human plasma ghrelin levels are at a nadir after a meal and then peak prior to the next meal [13]. Plasma ghrelin levels increase during periods of FR, and decrease after food intake [14]. Investigations into the effects of peripheral ghrelin injection showed that exogenous ghrelin administration can yield plasma concentrations similar to those induced by fasting. Specifically, low doses of ghrelin (1 nmol ip) increased plasma ghrelin levels in rats from 1.2 pmol/ml (basal value) to 3.4 pmol/ml, which was sustained from 15–30 min post injection. These ghrelin-induced elevated plasma levels were shown to be similar to plasma ghrelin levels measured after a 24-hr fast (3 pmol/ml). [19] Additionally, a dose of 2.4 umole/kg induced very high levels of circulating ghrelin (about 25 ng/ml) versus a control value (about 2ng/ml).[20] In Siberian hamsters, a 3mg/kg dose of ghrelin stimulated plasma levels to a range similar to 24–36 h of fasting.[21]
Ghrelin enhances food intake when administered either peripherally or centrally [10,17,18 Friedman, Kamegai, Kojima] and augments feeding-associated behaviors such as hoarding and foraging [21]. Studies of peripheral feeding responses showed that doses of 1, 3, and 10 nmol injections of ghrelin all produced increased feeding over vehicle in as little as 15 min post-injection and for up to 4 h, but with no significant differences in feeding behavior between high and low doses. [19, 22] Similar results were found in siberian hamsters in that doses of 3, 30, and 200 mg/kg ghrelin all stimulated food intake to a similar degree. [21] Thus, although peripheral ghrelin injection increased plasma concentrations to fasting levels and induced food intake for hours after injection, there does not appear to be a clear dose response profile for exogenous ghrelin administration.
Furthermore, systemic ghrelin is passively transported across the blood-brain barrier [23] and ghrelin receptors have been located on brain dopamine neurons [24–26]. Changes in peripheral ghrelin levels occasioned by FR could result in changes in dopamine signaling in brain reinforcement systems. Consistent with this view, we reported that peripheral ghrelin (5 nmol, i.p.) administration (in non food deprived rats) enhanced COC-induced (2.5, 5.0, 10.0 mg/kg) hyperlocomotion as compared to rats pretreated with saline [27]. The present study determined the potential of ghrelin to alter the rewarding properties of COC as measured in a CPP procedure [28,29]. Briefly, rats were allowed free access to both sides of a CPP chamber to determine initial side preference. The rats were then confined for 30 min to either their preferred side or non-preferred side on 8 consecutive days. On days in which the rats were confined to the least preferred side, each was injected with 0.5 ml (i.p.) of either ghrelin (5 nmol) or saline 1 h before the conditioning trial and then injected with one of four COC doses (0, 0.312, 0.625, or 1.25 mg/kg: IP) immediately prior to the conditioning trial. On alternate days, rats were injected with vehicle one hour before and again immediately before the conditioning trial. The expectation was that ghrelin alone would not produce a change in place preference, but that rats treated with COC after ghrelin pretreatment would show increased place preference for sub-threshold doses of COC relative to rats treated with COC only.
Method
Animals
The subjects were 64 experimentally naïve male Sprague-Dawley rats (Harlan, Houston, TX) weighing approximately 275–300g at the beginning of the experiment. Animals were housed individually in hanging polycarbonate rodent cages with food and water ad libitum. The colony room was maintained at 23.0±1 °C under a 12 h/12 h illumination schedule. Experimental procedures and treatments were approved by the Texas A&M University Laboratory Animal Care Committee.
Drugs
Acylated rat ghrelin (Product 55-0-03, American Peptide, Sunnyvale, CA) was dissolved in physiological (0.9% w/v) saline in a 5 nmol concentration which was administered i.p. in a fixed volume of 0.5 ml. This dose was chosen due to previous literature citing that a dose in this range would elevate plasma ghrelin to fasting levels that would be maintained for more than 1 h. [19, 22] COC HCl was kindly provided by Dr. Kevin Gormley of the Basic Research Division of NIDA. COC was dissolved in 0.9% w/v saline vehicle in concentrations such that doses were 0.312, 0.625, and 1.25 mg/ml. All COC injections were administered i.p in volumes of 1 ml/kg body weight.
Apparatus
Place conditioning and testing were conducted in eight 20 × 60 × 20-cm wooden shuttle boxes with wooden tilt floors. A microswitch was mounted on the wall under the floor at each end of the box and served to detect movement of the tilting floor. The boxes were interfaced with an IBM compatible microcomputer and a BASIC computer program was used to continuously record the number of times and duration the switch was activated through a tilt of the floor by a rat. Half of each box had white walls with a smooth white floor and the other half had black walls and floor with a black wire mesh floor covering. During conditioning sessions, the black and white compartments were separated by appropriately painted, removable partitions. The test (pre and post) session partitions were identical to the conditioning session partitions, except for a semicircular passageway (dia = 12.7 cm) cut out of the bottom edge of the partition to allow free access between compartments. Previous research showed that a natural preference for the black side occurs [cf. 29], therefore, a 40-W light was positioned 50 cm above the black side of the chamber to adjust for this natural preference. These lamps provided the only illumination in the testing room. A 40-dB white noise generator was positioned in the testing room to mask any outside noise. Following each conditioning and test session, the apparatus was cleaned with a mild soap solution and dried with towels.
Procedures
Animals were allowed at least 5 days of home cage acclimation after arrival to the facility. On Day 1 of behavioral training, animals were transferred from the colony to the testing room for 30 min for the purposes of habituating them to transportation, sound, and illumination of the testing room. They were not placed into the apparatus during the first day.
Day 2 was a pre-test to determine the initial preferences for the black or white compartments. On this day, the rats were transported to the testing room and placed into the chambers. The rats received no injection and had free access to both sides of the chambers (i.e. the test access partitions were placed between compartments). Time spent in each compartment was recorded over a 15 min period via the microswitch/computer system. The most preferred side was defined as the side (black or white) in which an animal spent the most time during this pre-test.
Days 3–10 were training days in which rats received an injection of a COC dose paired with the least preferred side (as determined by their Day 2 pre-test score), and, on alternating days, received saline injections paired with the most preferred side. For training days, the full partition was always in place within the chambers such that a rat was confined to one side of the chamber. Order of presentation was randomized such that half the animals in each squad were placed into their least preferred side on the first training day; the other half of the rats were placed into their most preferred side. On days when rats were placed into the least preferred side of the chamber, they were given 0.5 ml of ghrelin or saline 1 h prior to being placed in the chamber. Food was removed immediately after pretreatment injections to prevent the rats from eating, which in turn might decrease circulating ghrelin levels [13]. After this one hour period, the rats were transported to the testing room, given an i.p. injection of COC (0.0, 0.312, 0.625, or 1.25 mg/kg), and placed into the appropriate side of the chamber for 30 min. On the days when saline was paired with the preferred side, they were given a 0.5 ml injection of saline 1 h prior to transport to the testing room. Upon entry to the testing room, they were given an injection of saline (1.0 ml/kg, i.p.) and placed into their most preferred side of the chamber for 30 min. All animals received 4 pairings of the same COC dose with the least preferred side of the chamber after pre-exposure to ghrelin or saline, and, alternately, all animals received 4 training sessions in which saline was paired with the most preferred side of the chamber after a pretreatment injection of saline.
Day 11 was the post-training test day. Procedures for this day were identical to those of the Day 2 pre-test session. No injections were administered and animals had free access to both sides of the chamber for 15 minutes.
Statistical Analysis
The overall design of the study was a 4 (0.0, 0.312, 0.625, or 1.25 mg/kg COC dose) × 2 (0.0 or 5 nmol ghrelin) factorial. Data from 5 animals were excluded due to equipment malfunction during at least one of the test sessions. Additionally, data from 7 animals were excluded as outliers (scores more than 2 SD from the mean). Animals were run in 3 sets of 4 squads each, such that n = 10–12 for each condition. Analyses were run on the difference score as determined by the difference in time spent (min) on Day 2 (pre-training) with time spent (min) on Day 11 (post-training). These difference scores were subjected to an analysis of covariance using the percent of time spent on the black side of the chamber during the pre-test (out of 15 min) as the covariate. The latter was used because of variability in the magnitude of initial preferences of the rats on Day 2 for the preferred side.
Results
The overall analyses indicated a significant interaction between ghrelin pretreatment and COC dose [F (3, 75) = 2.74, p = .049]. Planned comparisons were made between animals pretreated with ghrelin and animals pretreated with saline for each COC dose. As expected, pretreatment with ghrelin alone (0.0 mg/kg COC) did not induce significant place conditioning (or place aversion) relative to animals pretreated with saline and then treated with 0.0 mg/kg COC dose [t (19) = 1.25, p = .227]. As seen in Figure 1, rats pretreated with ghrelin showed progressively augmented place preference scores, relative to saline-pretreated rats, for both the 0.312 mg/kg COC dose [t (19) = −7.3, p = .0001] and the 0.625 mg/kg COC dose [t (18) = −11.757, p = .0001]. However, this trend reversed at the 1.25 mg/kg COC dose, such that saline pretreated animals exhibited significantly higher conditioning scores than did the ghrelin pretreated group [t (17) = 23.618, p = .0001].
Figure 1.
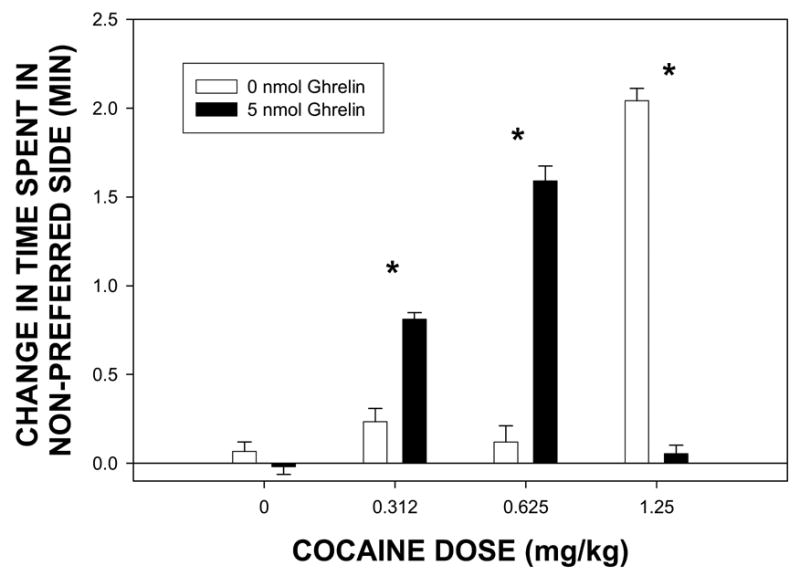
Mean group preference scores for rats pretreated with either 0 (open bars) or 5 nmol ghrelin (black bars) one hour prior to conditioning trials and then treated with either 0, 0.312, 0.625, or 1.25 mg/kg (i.p.) COC just prior to conditioning trials. The preference scores represent the difference in time spent on the least preferred side of the chamber during the postconditioning test less the time spent during the preconditioning test (adjusted for initial percent time spent in the black chamber on the preconditioning test ). The lines above each bar represent the standard error of the mean for that group condition. A * denotes a significant difference (p < 0.05) between the 0 nmol and 5 nmol ghrelin groups at a particular dose level of COC.
Discussion
The major findings of the present experiment are that systemic administration of 5 nmol ghrelin augmented the place conditioning induced by low doses (0.312 or 0.625 mg/kg) of COC, but not the 1.25 mg/kg COC dose. The facilitatory effect of 5 nmol ghrelin on place conditioning scores induced by 0.312 mg/kg or by 0.625 mg/kg COC suggests that ghrelin can decrease the threshold dose at which COC produces reinforcement. The observed relationship for the interaction of ghrelin and cocaine resembled an inverted U in that place preference scores of the 0 nmol pretreatment-1.25 mg/kg COC treatment group showed significant increases, whereas that of the 5 nmol ghrelin pretreatment-1.25 mg/kg COC treatment group returned to baseline. In contrast, Bell and colleagues [7] reported that prolonged FR (resulting in 80% of normal body weight) augmented COC-induced CPP at the highest COC doses tested (5.0 and 10.0 mg/kg) but not at a lower COC dose (2.5 mg/kg). Our use of the 5 nmol ghrelin injection was sufficient, based on the literature, to transiently induce plasma levels similar to 24–36 h deprivation [19, 22]. Such a transient increase in ghrelin levels may not completely mimic the changes the changes in plasma ghrelin levels that occur with prolonged food restriction (i.e. 80% body weight). To date no literature could be found stating the differences between the behavioral responses elicited by plasma levels produced by single-injection and plasma levels exhibited in response to a prolonged food restriction regimen leading to low body weight. Human plasma ghrelin levels are elevated in prolonged fasting states (such as anorexia nervosa) and show an endogenous secretory pattern [30]. Additionally, Drazen and colleagues showed that rat circadian secretory patterns were altered such that increases corresponded to the learned onset of meals. [31] Therefore, a clear understanding between the FR effect on CPP and our present results will require additional study into the potential differences between ghrelin oscillations under fasting versus food restriction.
Furthermore, the reversal of COC induced CPP seen in the current study at the 1.25 mg/kg COC dose is unlikely to reflect an aversive effect of 5 nmol ghrelin, perhaps related to stress [32], inasmuch as ghrelin alone did not alter place conditioning scores in the present study (i.e. at the 0 mg/kg COC dose) nor has there been a report in the literature of ghrelin-induced conditioned taste or food aversion. Food deprivation, per se, augments CPP induced by eating [33]. Ghrelin could alter the rate of degradation of cocaine, but such studies of the impact of ghrelin on cocaine pharmacokinetics (or vice versa) are unknown. Indeed, the literature on cocaine-ghrelin interactions has not developed to the point such that the present interactions can be easily understood in mechanistic terms. However, we note that similar patterns of results (i.e. facilitation of CPP at lower doses, but lack of effect of higher treatment doses) have been reported in the literature for other treatments including NPY [34] and 8-OH-DPAT [35].
The impetus for the present study was an assessment of the view that FR might alter psychostimulant function via increased activity of ghrelin pathways which in turn might modulate dopamine pathways. As mentioned above, systemic ghrelin levels are altered by food restriction/refeeding [13, 14]. Systemic ghrelin is passively transported across the blood-brain barrier [23] and ghrelin receptors are located on neurons [24] within the arcuate hypothalamus, the hippocampus [24–26] as well as the ventral tegmental area and substantia nigra [25, 26]. Although Brunetti et al., [36] reported that bath application of ghrelin was without effect on hypothalamic dopamine release, Jerlhag et al., [37] reported that third ventricular injections of ghrelin stimulate locomotion and increase DA overflow within the nucleus accumbens of mice. The localization of ghrelin receptors on ascending dopamine neurons of the VTA produced dopamine activation in the nucleus accumbens [38], suggesting that this neuropeptide is positioned so as to mediate the modulation of reinforcement circuits by FR. In support of this view, exogenous administration of ghrelin can augment the locomotor-stimulating effects of cocaine [27] and can augment the capacity of lower doses of cocaine to induce CPP [the present results]. Whether ghrelin exerts effects on LH self-stimulation thresholds that parallel those of FR remains to be determined and it will be important to assess the effects of FR on psychostimulant action in ghrelin KO mice [39].
Acknowledgments
Portions of this project were supported by NIDA 1R21DA017230-01A2 to PJW
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–68. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–60. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 3.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–41. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 4.Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205:319–21. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- 5.Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug Alcohol Depend. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- 6.Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–64. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- 7.Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Pychopharmacology. 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- 8.Cabeza de Vaca S, Holiman S, Carr KD. A search for the metabolic signal that sensitizes lateral hypothalamic self-stimulation in food-restricted rats. Physiol Behav. 1998;64:251–260. doi: 10.1016/s0031-9384(98)00050-x. [DOI] [PubMed] [Google Scholar]
- 9.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–8. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- 10.Friedman MI, Tordoff MG. Fatty acid oxidation and glucose utilization interact to control food intake in rats. Am J Physiol. 1986;251:R840–R845. doi: 10.1152/ajpregu.1986.251.5.R840. [DOI] [PubMed] [Google Scholar]
- 11.Carr KD. Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochem Res. 1996;21:1455–1467. doi: 10.1007/BF02532386. [DOI] [PubMed] [Google Scholar]
- 12.Abrahamsen GC, Carr KD. Effects of corticosteroid synthesis inhibitors on the sensitization of reward by food restriction. Brain Res. 1996;726:39–48. [PubMed] [Google Scholar]
- 13.Cummings DE, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–19. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 14.Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- 15.Hosada H, Kojima M, Kangawa K. Ghrelin and the regulation of food intake and energy balance. Molecular Interventions. 2002;2:494–503. doi: 10.1124/mi.2.8.494. [DOI] [PubMed] [Google Scholar]
- 16.Rindi G, Torsello A, Locatelli V, Solcia E. Ghrelin expression and actions: A novel peptide for an old cell type of the diffuse endocrine system. Experimental Biology & Medicine. 2004;229:1007–16. doi: 10.1177/153537020422901004. [DOI] [PubMed] [Google Scholar]
- 17.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, and endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4794–4800. doi: 10.1210/endo.141.12.7920. [DOI] [PubMed] [Google Scholar]
- 18.Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 19.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141(11):4325–8. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 20.Tschopp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 21.Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2004;288:716–721. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- 22.Ruter J, Kobelt P, Tebbe JJ, Avsar Y, Veh R, Wang L, Klapp BF, Weidenmann B, Tache Y, Monnikes H. Intraperitoneal injection of ghrelin induces Fos expression in the paraventricular nucleus of the hypothalamus in rats. Brain Res. 2003;991:26–33. doi: 10.1016/j.brainres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–7. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 24.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 25.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–9. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 26.Naleid AM, Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–9. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Wellman PJ, Davis KW, Nation JR. Augmentation of cocaine hyperactivity in rats by systemic ghrelin. Regulatory Peptides. 2005;125:151–154. doi: 10.1016/j.regpep.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 29.Miller DK, Nation JR. Chronic cadmium exposure attenuates the conditioned reinforcing properties of morphine and fentanyl. Brain Res. 1997;776:162–169. doi: 10.1016/s0006-8993(97)01013-5. [DOI] [PubMed] [Google Scholar]
- 30.Natalucci G, Reidl S, Gleiss A, Zidek T, Frisch H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinology. 2005;152:845–850. doi: 10.1530/eje.1.01919. [DOI] [PubMed] [Google Scholar]
- 31.Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- 32.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M, Katsuura G, Makino S, Fujino MA, Kasuga M. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology. 2001;74:143–147. doi: 10.1159/000054680. [DOI] [PubMed] [Google Scholar]
- 33.Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by delta 9-tetrahydro cannabinol: comparison with cocaine, morphine, and food reward. Life Sci. 1995;56:2073–80. doi: 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- 34.Brown CM, Coscina DV, Fletcher PJ. The rewarding properties of neuropeptide Y in perifornical hypothalamus vs. nucleus accumbens. Peptides. 2000;21:1279–1287. doi: 10.1016/s0196-9781(00)00270-9. [DOI] [PubMed] [Google Scholar]
- 35.Fletcher PJ, Ming ZH, Higgins GA. Conditioned place preference induced by microinjection of 8-OH-DPAT into the dorsal or median raphe nucleus. Psychopharmacology (Berl) 1993;113:31–6. doi: 10.1007/BF02244330. [DOI] [PubMed] [Google Scholar]
- 36.Brunetti L, Recinella L, Orlando G, Michelotto B, Di Nisio C, Vacca M. Effects of ghrelin and amylin on dopamine, norepinephrine and serotonin release in the hypothalamus. Eur J Pharmacol. 2002;454:189–192. doi: 10.1016/s0014-2999(02)02552-9. [DOI] [PubMed] [Google Scholar]
- 37.Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 38.Abizaid A, Liu Z, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschopp MH, Gao X, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116 doi: 10.1172/JCI29867. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Smet B, Depoortere I, Moechars D, Swennen Q, Moreaux B, Cryns K, Tack J, Buyse J, Coulie B, Peeters TL. Energy homeostasis and gastric emptying in ghrelin knockout mice. J Pharmacol Exp Ther. 2006;316:431–9. doi: 10.1124/jpet.105.091504. [DOI] [PubMed] [Google Scholar]


