Abstract
PURPOSE
Primary intraocular lymphoma (PIOL) is a subtype of central nervous system lymphoma. Although this lymphoma is rare, its incidence has tripled in the past 15 years. Currently, the only available model is a murine metastatic malignant lymphoma that occurs after intraperitoneal inoculation of Rev-2-T-6 lymphoma cells into newborn syngeneic mice. The current study was conducted to develop a new experimental model for PIOL.
METHODS
Rev-2-T-6 cells (0.5 × 105 or 1.0 × 105) were inoculated into the vitreous of adult BALB/c mice. Mice were monitored clinically every other day and under fundoscopic examination weekly. They were euthanatized on weeks 3, 5, 6, 7, or 8, after inoculation. All eyes were processed for histology. Immunohistochemistry was performed with an antibody (p14) specific for Rev-2-T-6 cells. Cytokine mRNA expression (IL-2, -4, -6, -10, and IFN-γ and CC chemokine receptor-1 [CCR1]) was assayed in the lymphoma cells by microdissection and RT-PCR. IL-10 and -6 levels in the vitreous were measured by ELISA.
RESULTS
Within 2 to 4 weeks, tumor cells from the vitreous migrate through the retina and gather between the RPE cell and retina. Rarely (>2 months after inoculation), Rev-2-T-6 cells may break through the RPE and infiltrate the choroid and sclera. Tumor localization was confirmed by immunohistochemistry. The intraocular lymphoma cells produce high levels of IL-10, IFN-γ, and CCR1 transcripts. A high level of IL-10 was detected in the vitreous inoculated with Rev-2-T-6 cells.
CONCLUSIONS
The data suggest that RPE cells constitute a barrier to the spread of intraocular lymphoma. Intravitreal injection of Rev-2-T-6 cells is a novel model of PIOL in immunecompetent hosts that will aid in understanding the molecular mechanisms of the disease.
Primary intraocular lymphoma (PIOL) is a subtype of primary central nervous system [CNS] lymphoma (PCNSL) that initially presents in the eye, with or without simultaneous CNS involvement.1,2 PCNSL is an extranodal lymphoma and represents <1% of all brain tumors.2 Although PIOL is a rare tumor, its incidence has reportedly tripled in the past 15 years. To date, the only available experimental model for the infiltration of malignant lymphoma to the eye and brain is one that uses young immune-competent BALB/c mice.3,4 In that model, intraperitoneal inoculation of Rev-2-T-6 cells (derived from S49 mouse T-cell lymphoma cells) into syngeneic 6- to 10-day postnatal mice results in specific homing of these cells to the brain and eyes in 60% of inoculated mice. Histologic and statistical analyses have demonstrated that once within the brain, Rev-2-T-6 cells migrate along the optic nerve sheath into the eye, thus infiltrating the uvea (the choroid, ciliary body, and iris) and the anterior chamber. The infiltration of the orbit that also takes place in that model is independent of the brain– optic nerve–anterior chamber route.4 Rev-2-T-6 cells very rarely cross the RPE into the retina.3 Herein, we report a new model of PIOL that develops after intravitreal inoculation of Rev-2-T-6 cells into adult immune-competent syngeneic mice. This novel model is a complementary extension of the previous model.3 It mimics human PIOL and is therefore useful and practical for the study of this devastating disease.
METHODS
The studies conformed to the principles for laboratory animal research outlined by the Animal Welfare Act (NIH/DHHS) and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the National Eye Institute Animal Care and Use Committee.
Cells
Rev-2-T-6 cells were derived from substrate-adherent, nontumorigenic (immunogenic) variants of the S49 mouse T-cell lymphoma, as described previously.3 They produce progressive, solid abdominal tumors after intraperitoneal inoculation into mature BALB/c mice. These cells demonstrate cell–cell adhesion, growing in suspension culture as cell aggregates. Rev-2-T-6 cells were maintained in DMEM (Biological Industries, Beit Haemek, Israel) supplemented with 10% horse serum, 50 U/mL penicillin, and 50 μg/mL streptomycin.
Mice
Naive BALB/c mice, 6 to 8 weeks old, were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were provided food and water ad libitum and kept on a 12-hour light–dark cycle.
Intravitreal Inoculation
Mice were anesthetized with an intraperitoneal injection of a combination of 44 mg/kg ketamine and 10 mg/kg xylazine. Proparacaine ophthalmic solution was administered topically to the left eye. Under a dissecting microscope, 0.5 × 105 or 1 × 105 Rev-2-T-6 cells in 3 μL PBS were injected through the pars plana into the vitreous of the dilated left eye with a 32-gauge needle attached to a syringe (Hamilton, Reno, NV). The injection was performed by using an aseptic technique throughout. After the injection, erythromycin ophthalmic ointment was applied. Control mice were injected intravitreally with 3 μL PBS only.
Clinical and Pathologic Evaluation
Fundoscopic examination was performed weekly on each mouse. Animals were euthanatized, and their eyes were harvested between 3 to 9 weeks after inoculation. The collected eyes were either snap frozen or fixed in 10% formalin. The formalin-fixed tissues were embedded in paraffin. Frozen serial sections of the eyes were incubated with a polyclonal antibody against p14 with immunoperoxidase staining. p14 is the leader peptide of the mouse mammary tumor virus (MMTV) Env-precursor5 and was used as a specific marker for in situ visualization of Rev-2-T-6 cells. It is expressed in all S49 cell variants as well as in other lymphomas that harbor this virus. Paraffin-embedded eyes were sectioned and stained with hematoxylin and eosin.
ELISA
The eyes injected with Rev-2-T-6 cells or PBS were harvested for IL-10 and -6 assays with ELISA (Pierce Endogen, Cambridge, MA). The assay was performed on only those eyes that showed clinical evidence of intraocular tumor (18 days after inoculation). The control eyes were harvested at the same time (18 days after injection of PBS).
Microdissection and RT-PCR
Lymphoma cells were selected and captured from the eyes manually or by laser capture microdissection (PixCell IIe microscope; Arcturus, Mountain View, CA). RNA was extracted from the microdissected lymphoma cells (PicoPure RNA isolation kit; Arcturus) and used for cDNA synthesis. A reverse transcriptase system (Supercript II RNase H; Invitrogen, Carlsbad, CA) and random primers (Promega, Madison, WI) were used. PCR amplification was performed with the following primer sequences: IL-2, sense, 5′-TTC AAG CTC CAC TTC AAG CTC TAC AGC GGA AG-3′, antisense, 5′-GAC AGA AGG CTA TCC ATC TCC TCA GAA AGT CC-3′; IL-4, sense, 5′-CCA GCT AGT TGT CAT CCT GCT CTT CTT TCT CG-3′, antisense, 5′-CAG TGA TGT GGA CTT GGA CTC ATT CAT GGT GC-3′; IL-6, sense, 5′-TTC CTC TCT GCA AGA GAC T-3′, antisense, 5′-TGT ATC TCT CTG AAG GAC T-3′; IL-10, sense, 5′-ATG CAG GAC TTT AAG GGT TAC TTG GGT T-3′, antisense, 5′-ATT TCG GAG AGA GGT ACA AAC GAG GTT T-3′; IFN-gamma, sense, 5′-CTT CCT CAT GGC TGT TTC-3′, antisense, 5′-CCA GTT CCT CCA GAT ATC-3′; CCR1 (CC chemokine receptor-1), sense, 5′-TAC AAG TTG AAA GAC GAC TGG-3′, antisense, 5′-ATG AGG GCT ACA GGT ACG G-3′; and 18S, sense, 5′-AGG AAT TGA CGG AAG GGC AC-3′, anti-sense, 5′-GGA CAT CTA AGG GCA TCA CA-3′.
Primers were labeled with 32P before PCR. Reactions were conducted (PTC-200 DNA Engine; MJ Research, Waltham, MA) for 40 cycles with an annealing temperature of 56°C for IL-2 and IL-6, 57°C for IL-4 and IL-10, 58°C for CCR1, and 59°C for IFN-gamma and 18S; a denaturing temperature of 94°C; and an extension temperature of 72°C. PCR products were separated on polyacrylamide gel, and radioactive bands were detected (PhosphorImager Storm 860 with Image-Quant software; Molecular Dynamics, Sunnyvale, CA).
RESULTS
Induction of Dose-Dependent PIOL with Intravitreal Rev-2-T-6 Cell Inoculation
Sixteen mice were used for intravitreal inoculation: Eight were injected with 1.0 × 105 Rev-2-T-6 cells and another eight with 0.5 × 105 Rev-2-T-6 cells. Vitreous haze and focal retinal lesions appeared in 3 of 16 mice (2/8 of those that received 1.0 × 105 cells and 1/8 of those that received 0.5 × 105 cells) 1 week after inoculation. By the third week, marked vitreous opacities were observed clinically in seven of eight mice that received 1.0 × 105 and in four of eight mice that received 0.5 × 105 Rev-2-T-6 cells. By the fourth week, the anterior chamber was also infiltrated in seven of seven mice receiving 1.0 × 105 and six of seven mice receiving 0.5 × 105 Rev-2-T-6 cells (Fig. 1). The opacities were persistent and progressive and even obscured the view of the posterior pole in a few animals until the seventh week after inoculation, when the mice were euthanatized. No clinical manifestations were identified in any of the mice other than those in the inoculated eye.
FIGURE 1.
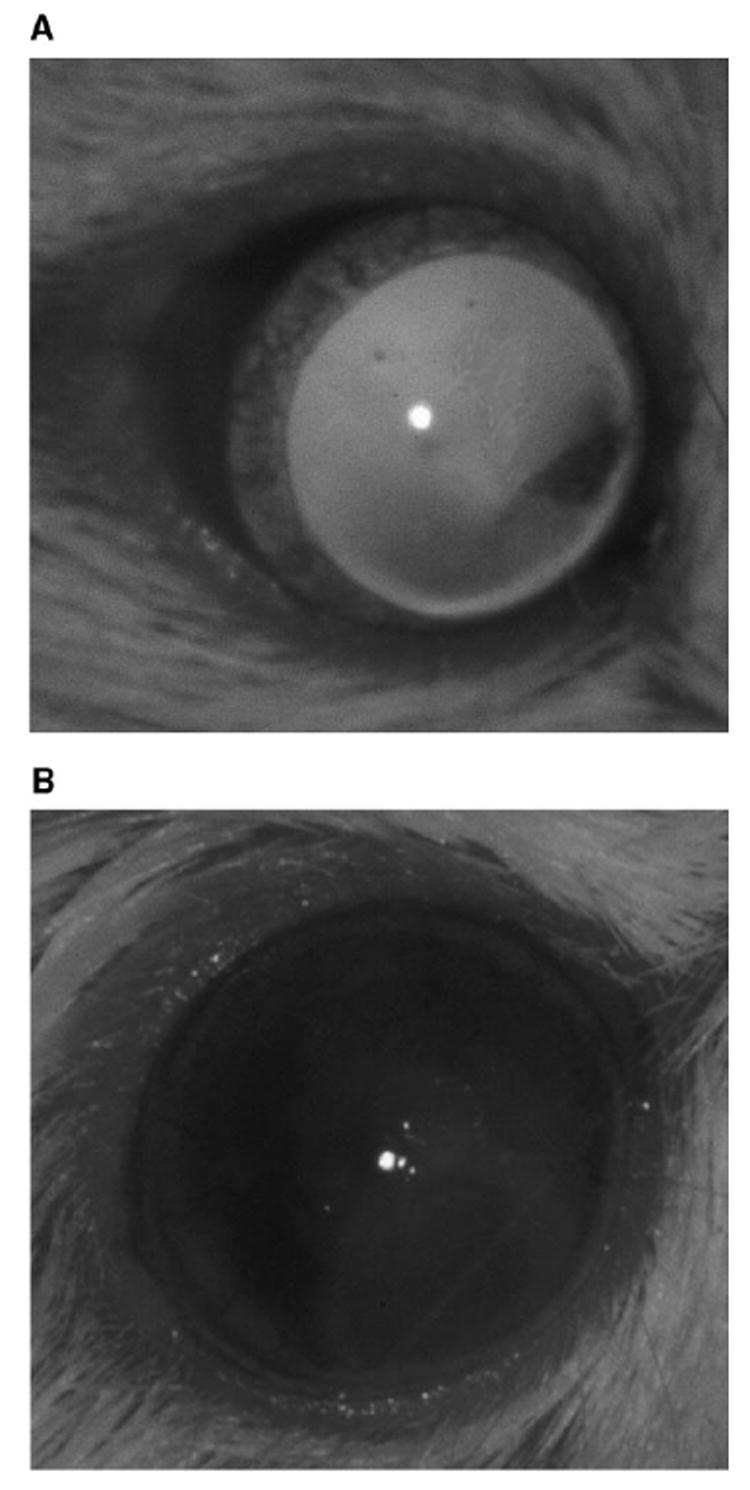
Clinical photographs showing pseudohypopyon (lymphoma infiltration in the anterior chamber) and cloudy cornea in (B) an eye injected with Rev-2-T-6 cells (OS) compared with (A) the contralateral normal eye (OD).
Experiments with intravitreal injection of 1.0 × 105 Rev-2-T-7 cells were repeated three times, and results were reproducible with the development of intraocular lymphoma inside the eye. The incidence of intraocular tumor was up to 100% in the inoculated eyes.
RPE Cell: A Barrier to Lymphoma Invasion
Intraocular Rev-2-T-6 cells were observed mainly in the vitreous and subretinal space above the RPE cells, as demonstrated histologically. These cells were characterized by large nuclei, prominent nucleoli, and mitotic figures. Of note, most tumor cells accumulated between the RPE and neuroretina (Fig. 2). Clumps or individual Rev-2-T-6 cells were present in the vitreous, particularly in the early period after inoculation; fewer isolated Rev-2-T-6 cells were visible in this compartment during a later period after inoculation. Focal choroidal invasion in which Rev-2-T-6 cells penetrated through the RPE was identified in only two eyes 8 weeks after inoculation. The Rev-2-T-6 cells were specifically identifiable through the characteristics of their large size and atypical morphology and by immunoperoxidase staining with a specific polyclonal antibody against Rev-2-T-6 cells (Fig. 3). No cross-reaction with other ocular resident cells was detected. There was little inflammatory cellular infiltration (small lymphocytes) among the tumor cells; however, perivascular lymphocytic infiltration was noted in the retina. No tumor cells were observed within the retinal vessels or Schlemm’s canal.
FIGURE 2.
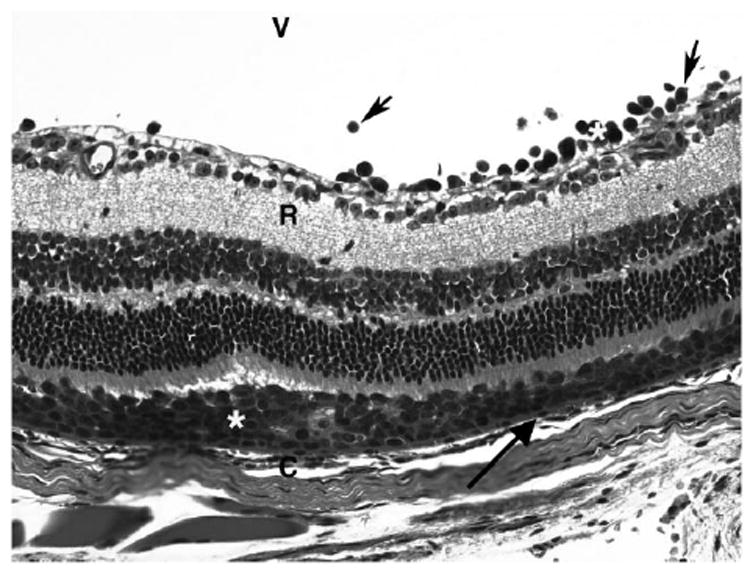
Photomicrograph showing large Rev-2-T-6 lymphoma cells (*) localized in the subretinal space above the choroid (C) between the RPE (arrow) and retina (R). Some lymphoma cells (arrowhead) are also visible in the vitreous. Hematoxylin and eosin. Original magnification, ×400.
FIGURE 3.
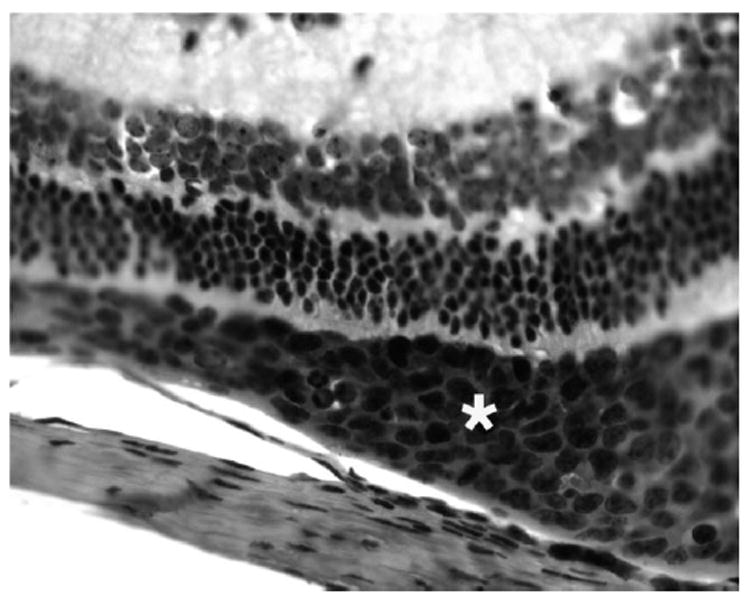
Photomicrograph showing Rev-2-T-6 cells stained positively for p14 (*) in the subretinal space. Immunoperoxidase staining. Original magnification, ×400.
Neither tumor metastasis nor other lesions developed in the contralateral eye, the brain, or systemic organs in any of the inoculated mice.
Expression of IL-10, IFN-γ, and CCR1 Messengers on Ocular Rev-2-T-6 Cells
Because vitreal cytokine levels in human PIOL are helpful in the diagnosis of the disease, it was of interest to evaluate the cytokine profile of ocular Rev-2-T-6 cells, even though they are of T-cell origin. To that effect, we applied laser capture microdissection followed by RT-PCR. We analyzed the transcripts of Th1 cytokines (IL-2 and IFN-γ), Th2 cytokines (IL-4 and -10), the proinflammatory cytokine (IL-6), and CCR1. IFN-γ, IL-10, and CCR1 transcripts were detected in both Rev-2-T-6 cells growing in culture and in the eyes, whereas IL-4 mRNA was barely detected only in Rev-2-T-6 cells growing in the eyes (Fig. 4). However, IL-2 and -6 mRNAs were not detected in Rev-2-T-6 cells. Thus, their ability to produce both Th-1 (IFN-γ) and Th-2 (IL-10 and -4) cytokines is not impaired after intravitreal inoculation.
FIGURE 4.
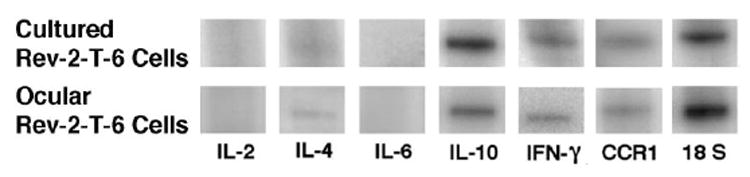
RT-PCR products showing expression of IL-10, IFN-γ, and CCR1 mRNA in Rev-2-T-6 cells, both in culture and in the eye.
Vitreal IL-10 Level in Eyes Inoculated with Rev-2-T-6 Cells
The IL-10 and -6 levels in the vitreous of eyes inoculated with Rev-2-T-6 were 691 and 481 pg/mL, respectively. The IL-10 to -6 ratio was 1.44. In contrast, the IL-10 and -6 levels in the vitreous of eyes injected with PBS were below detectable levels.
DISCUSSION
The model presented in this work of direct inoculation of Rev-2-T-6 cells into the eyes of adult immunocompetent mice closely resembles human PIOL. PIOL is an aggressive disease with a 5-year survival rate of <25%.6 The median age of onset of PCNSL/PIOL in immunocompetent patients is the late 50s and 60s, with a reported range of 15 to 85 years for PIOL.2,6 PIOL commonly masquerades as a chronic uveitis.1,7 The characteristic presenting sign in these patients is orange-yellowish subretinal infiltrates with an overlying retinal pigment epithelial (RPE) detachment. These subretinal infiltrates are deposits of lymphoma cells located in the subretinal space between the RPE and Bruch’s membrane.1,7 The PIOL cells infiltrate the retina, optic nerve, and vitreous, they seldom invade the choroid and sclera.
Clinically, intravitreal injection of Rev-2-T-6 cells results in cells and haze in the vitreous, subretinal infiltration, and a cytokine profile that resembles human PIOL. Although most PIOL is diffuse, large B-cell non-Hodgkin’s lymphoma, T-cell PIOL has been recently reported.6,8-10 To date, no intraocular B-cell lymphoma model exists in animals. Furthermore, the location of tumor cells in the vitreous and the subretinal space above the choroid is the classic clinical and pathologic characterization of PIOL.7,11 It should be emphasized that the localization of Rev-2-T-6 cells between the retina and RPE (and not in the choroid) certainly highlights the barrier function of the RPE and provides a unique in vivo model for the study of the role that the RPE plays in the spread of lymphoma. Based on these data, it is tempting to speculate that PIOL begins in the eye, since the RPE is such an effective barrier to passage of syngeneic lymphoma cells.
Before this work, the only available experimental model for ocular lymphoma involved systemic intraperitoneal inoculation of Rev-2-T-6 cells into newborn immunocompetent mice.3,4 In that model, the Rev-2-T-6 cells spread from the brain along the optic nerve sheath to the eye, infiltrating the choroid, ciliary body, and iris.4 The tumor cells can also spread from the optic nerve head into the vitreous (15%) and from the anterior uvea into the anterior chamber. Rev-2-T-6 cells seldom invade the retina (3%), because they reside in the choroid beneath the RPE.3 Thus, the RPE seems also to play a role in limiting retinal infiltration by Rev-2-T-6 cells through the choroids.
Other intraocular tumor models have been described. For example, in 1981, Niederkorn et al.12 reported the growing of DBA/2 mastocytoma P91 cells in the ocular anterior chamber of histoincompatible mice. In 1995, Grossniklaus et al.13 developed a model of ocular melanoma metastases to the lung and liver after intraocular inoculation of adult C57BL6 mice with B16F10 melanoma cells. Tumors with high-grade vascular patterns were more likely to metastasize than tumors with low-grade patterns. The melanoma metastasizes by invasion through tumor vascular channels.14 In contrast, the experimental model presented herein does not have any vasculature, and the tumor cells are confined to the intraocular compartments.
IL-10, IFN-γ, IL-4, and CCR1, but not IL-2 or IL-6, transcripts are detected in intraocular Rev-2-T-6 cells. Both IL-10 and -6 proteins are detected only in the vitreous of the mice inoculated with Rev-2-T-6 cells. Of note, the level of IL-10 is higher than the level of IL-6 (ratio of 1.44) in the vitreous, which makes it similar to the observation in human PIOL. IL-6 levels in the vitreous of eyes inoculated with Rev-2-T-6 cells most probably resulted from either local inflammatory cells or from ocular resident cells, such as ciliary epithelial cells. Vitreal IL-6 is produced at high levels by inflammatory cells in uveitis. Indeed, we and other investigators have also measured high vitreal levels of IL-10 but not -6 in patients with PIOL.15,16 Abundant IL-10 mRNA is also detected in human PIOL cells.17 Intraocular lymphoma is strongly associated with an increased IL-10 to IL-6 ratio (greater than 1.0). Of the 35 patients with B-cell lymphoma and 64 patients with inflammation, the 1.0 cutoff rule held in 74.7% (sensitivity 74.3%, specificity 75.0%).15
T-cell clones from early-stage human cutaneous T-cell lymphoma have shown both Th-1 and -2 cytokine profiles.18 Elevated serum levels of IL-10 and IFN-γ, especially IL-10, have been reported in patients with either B- or T-cell lymphomas.19 These high serum levels of IL-10 suggest that a compensatory anti-inflammatory process may give rise to an immunosuppressive state and a poor clinical outcome.19 IL-10 seems to be a more direct marker of tumor activity in PIOL. Therefore, the present model will be valuable for the evaluation of targeting IL-10 expression on Rev-2-T-6 cells as a possible therapeutic strategy for human PIOL.
Because primary intraocular B-cell lymphoma expresses Bcell chemokine receptors,20,21 it is interesting to investigate whether Rev-2-T-6 cells express the T-cell chemokine receptor CCR1 in an analogous manner. Indeed, this is the case, as Rev-2-T-6 cells express CCR1 both in vitro and in vivo (Fig. 4). One may speculate that the T-cell chemokine receptor may play a role in the homing of tumor cells to certain sites within the eye.
As stated earlier, Rev-2-T-6 cells are one of a variety of S49 derived cell lines raised in our laboratory. These include, among others, the T-25 highly tumorigenic cells (median survival of 14–16 days) as well as the T-25-Adh nontumorigenic, immunogenic cells.22 Priming of mature syngeneic mice with T-25-Adh cells immunizes the host for practically a lifetime against a challenge with T-25 cells. This new experimental model of PIOL will enable us to compare the lymphoma– host interactions after intraocular administration of cells such as T-25 and T-25-Adh.
In summary, intraocular inoculation of Rev-2-T-6 cells is a novel model for human PIOL. Although this model is not a B-cell lymphoma, there are many features that mimic human PIOL, such as clinical presentation, histologic findings, and cytokine expression pattern. This reproducible model will aid in understanding the molecular and immunologic mechanisms of ocular lymphoma and the role of RPE cells as a putative barrier for tumor invasion, and it will better enable us to evaluate various novel treatments of this devastating and increasingly prevalent disease in humans.
Acknowledgments
The authors thank Michael M. Gottesman (National Cancer Institute) for support, encouragement, and helpful discussions.
Footnotes
Disclosure: C.-C. Chan, None; M. Fischette, None; D. Shen, None; S.P. Mahesh, None; R.B. Nussenblatt, None; J. Hochman, None
References
- 1.Chan CC, Buggage RR, Nussenblatt RB. Intraocular lymphoma. Curr Opin Ophthalmol. 2002;13:411–418. doi: 10.1097/00055735-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Hormigo A, DeAngelis LM. Primary intraocular lymphoma: clinical features, diagnosis and treatment. Clin Lymphoma. 2003;4:22–29. doi: 10.3816/clm.2003.n.010. [DOI] [PubMed] [Google Scholar]
- 3.Assaf N, Hasson T, Hoch-Marchaim H, et al. An experimental model for infiltration of malignant lymphoma to the eye and brain. Virchows Arch. 1997;431:459–467. doi: 10.1007/s004280050124. [DOI] [PubMed] [Google Scholar]
- 4.Hochman J, Assaf N, Deckert-Schluter M, Wiestler OD, Pe’er J. Entry routes of malignant lymphoma into the brain and eyes in a mouse model. Cancer Res. 2001;61:5242–5247. [PubMed] [Google Scholar]
- 5.Hoch-Marchaim H, Weiss AM, Bar-Sinai A, Fromer M, Adermann K, Hochman J. The leader peptide of MMTV Env precursor localizes to the nucleoli in MMTV-derived T cell lymphomas and interacts with nucleolar protein B23. Virology. 2003;313:22–32. doi: 10.1016/s0042-6822(03)00236-8. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman PM, McKelvie P, Hall AJ, Stawell RJ, Santamaria JD. Intraocular lymphoma: a series of 14 patients with clinicopathological features and treatment outcomes. Eye. 2003;17:513–521. doi: 10.1038/sj.eye.6700378. [DOI] [PubMed] [Google Scholar]
- 7.Read RW, Zamir E, Rao NA. Neoplastic masquerade syndromes. Surv Ophthalmol. 2002;47:81–124. doi: 10.1016/s0039-6257(01)00305-8. [DOI] [PubMed] [Google Scholar]
- 8.Gijtenbeek JM, Rosenblum MK, DeAngelis LM. Primary central nervous system T-cell lymphoma. Neurology. 2001;57:716–718. doi: 10.1212/wnl.57.4.716. [DOI] [PubMed] [Google Scholar]
- 9.Lobo A, Larkin G, Clark BJ, Towler HM, Lightman S. Pseudohypopyon as the presenting feature in B-cell and T-cell intraocular lymphoma. Clin Exp Ophthalmol. 2003;31:155–158. doi: 10.1046/j.1442-9071.2003.00624.x. [DOI] [PubMed] [Google Scholar]
- 10.White VA, Gascoyne RD, Paton KE. Use of the polymerase chain reaction to detect B- and T-cell gene rearrangements in vitreous specimens from patients with intraocular lymphoma. Arch Ophthalmol. 1999;117:761–765. doi: 10.1001/archopht.117.6.761. [DOI] [PubMed] [Google Scholar]
- 11.Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc. 2003;101:275–292. [PMC free article] [PubMed] [Google Scholar]
- 12.Niederkorn JY, Shadduck JA, Streilein JW. Immunogenetic basis for immunologic privilege in the anterior chamber of the eye. Immunogenetics. 1981;13:227–236. doi: 10.1007/BF00350789. [DOI] [PubMed] [Google Scholar]
- 13.Grossniklaus HE, Barron BC, Wilson MW. Murine model of anterior and posterior ocular melanoma. Curr Eye Res. 1995;14:399–404. doi: 10.3109/02713689508999938. [DOI] [PubMed] [Google Scholar]
- 14.Grossniklaus HE. Tumor vascularity and hematogenous metastasis in experimental murine intraocular melanoma. Trans Am Ophthalmol Soc. 1998;96:721–52. [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf LA, Reed GF, Buggage RR, Nussenblatt RB, Chan CC. Vitreous cytokine levels. Ophthalmology. 2003;110:1671–1672. doi: 10.1016/S0161-6420(03)00811-X. [DOI] [PubMed] [Google Scholar]
- 16.Merle-Beral H, Davi F, Cassoux N, et al. Biological diagnosis of primary intraocular lymphoma. Br J Haematol. 2004;124:469–473. doi: 10.1046/j.1365-2141.2003.04800.x. [DOI] [PubMed] [Google Scholar]
- 17.Chan CC, Shen DF. Newer methodologies in immunohistochemistry and diagnosis. In: BenEzra D, editor. Uveitis Update. Basel Switzerland: Karger; 1999. pp. 1–13. [DOI] [PubMed] [Google Scholar]
- 18.Harwix S, Zachmann K, Neumann C. T-cell clones from early-stage cutaneous T-cell lymphoma show no polarized Th-1 or Th-2 cytokine profile. Arch Dermatol Res. 2000;292:1–8. doi: 10.1007/pl00007454. [DOI] [PubMed] [Google Scholar]
- 19.Ohno T, Ueda Y, Nagai K, et al. The serum cytokine profiles of lymphoma-associated hemophagocytic syndrome: a comparative analysis of B-cell and T-cell/natural killer cell lymphomas. Int J Hematol. 2003;77:286–294. doi: 10.1007/BF02983788. [DOI] [PubMed] [Google Scholar]
- 20.Chan CC, Shen D, Hackett JJ, Buggage RR, Tuaillon N. Expression of chemokine receptors, CXCR4 and CXCR5, and chemokines, BLC and SDF-1, in the eyes of patients with primary intraocular lymphoma. Ophthalmology. 2003;110:421–426. doi: 10.1016/S0161-6420(02)01737-2. [DOI] [PubMed] [Google Scholar]
- 21.Smith JR, Braziel RM, Paoletti S, Lipp M, Uguccioni M, Rosenbaum JT. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101:815–821. doi: 10.1182/blood-2002-05-1576. [DOI] [PubMed] [Google Scholar]
- 22.Braun E, Rorman E, Lueders KK, Bar-Sinai A, Hochman J. Differential expression of intracisternal A-particle transcripts in immunogenic versus tumorigenic S49 murine lymphoma cells. Virology. 2000;277:136–146. doi: 10.1006/viro.2000.0568. [DOI] [PubMed] [Google Scholar]


