Abstract
MuB, an ATP-dependent DNA-binding protein, is critical for the selection of target sites on the host chromosome during the phage Mu transposition. We developed a multichannel fluidic system to study the MuB–DNA interaction dynamics at the single DNA molecule level by total internal reflection fluorescence microscopy. We analyzed the distribution of MuB along DNA during the assembly and disassembly of MuB polymers on immobilized DNA molecules. The results reveal the absence of a significant correlation of MuB polymer distribution between the assembly and disassembly phases. These observations argue against a model in which MuB polymers on DNA represent a mixture of higher and lower affinity forms, with higher affinity forms being the first to appear and the last to disappear. Instead, assembly and disassembly of MuB polymers involve independent stochastic events. Additionally, we demonstrate that MuB disassembles from the polymer ends at a higher rate than from internal regions of the polymer and MuA stimulates MuB disassembly both at the polymer ends and internally.
Keywords: phage Mu, recombination, DNA-binding protein, biomolecular patterning
Phage Mu transposition is one of the most extensively exploited model systems for studying transpositional DNA recombination (1, 2). The reaction pathway is closely related to DNA integration of HIV-1 and other retroviruses (3) and VDJ recombination (4) in the development of the immune system. The phage Mu transposon integrates into the bacterial host chromosome at many sites with limited target sequence specificity. A puzzling aspect of Mu transposition is how the Mu DNA manages to distinguish itself from the target so as to avoid self-destructive autointegration into itself. Although Mu exhibits only limited target sequence specificity, DNA regions close to the Mu end sequence are poor targets for Mu transposition. This finding and related phenomena exhibited by many other transposons is called “transposition target immunity.” Mu target immunity involves the interplay between two phage-encoded proteins, MuA and MuB, and the formation of MuB distribution patterns along DNA molecules that define the regions preferred as target sites (5–7).
The transposase MuA binds to the multiple copies of binding sequences located at the two Mu DNA ends, synapses the two ends, and forms a stable tetramer to which the two ends are bound. The resulting transposition-competent complex is called a “transpososome.” However, the complex does not promote efficient transposition to a new target site in the absence of MuB, an ATP-dependent DNA-binding protein. MuB activates Mu transpososomes for DNA strand transfer, the pair of transesterification reactions that insert the Mu DNA into target DNA. Thus, MuB-bound DNA acts as an efficient target for Mu transposon (8). Meanwhile, MuA stimulates the MuB ATPase and accelerates MuB dissociation from DNA. In the presence of a Mu end sequence on the DNA, MuA binds to this sequence and stimulates MuB dissociation from the neighboring DNA at a higher rate than it does from distant DNA sites, making the region near the Mu end a poor target. Because the chemical steps of Mu insertion into a new target site are slow compared with the establishment of the MuB distribution pattern by MuA and Mu end DNA sequence, the transposition target site distribution is primarily determined by the MuB–DNA association/dissociation dynamics and the impact of MuA on this process.
We used total internal reflection fluorescence (TIRF) microscopy with surface-immobilized phage λ-DNA to visually monitor the association/dissociation dynamics of fluorescently labeled MuB under various conditions (7, 9–11). At saturating concentrations of MuB in the presence of ATP, λ-DNA was evenly coated with MuB. When the complex was washed with a protein-free buffer, MuB dissociated from some DNA regions faster than others, leaving behind islands of lingering MuB clusters. The distribution of these clusters differed from molecule to molecule and between repeated dissociation cycles on the same DNA molecule. However, when averaged over a large number of molecules, the clusters occurred more frequently at A/T-rich DNA sequences. A qualitatively similar distribution of MuB clusters was observed in the process of MuB assembly on naked DNA, as well as under steady-state conditions with subsaturating concentrations of MuB.
One possible mechanism behind these observations may be that MuB can bind DNA in two distinct states, a weakly bound form and a stably bound form. The initial weakly bound complex undergoes a conformational change into a more stably bound form, and this transition occurs more frequently at A/T-rich DNA sequences. At lower MuB concentrations, the MuB clusters on DNA mostly represent the stable complexes, and at higher MuB concentrations, DNA regions between these stable clusters are filled with unstable complexes. During slow assembly or under steady-state conditions with limiting MuB concentrations, perhaps the stable complexes are mainly observed because the unstable complexes only form at higher MuB concentrations. During disassembly, the weakly bound MuB dissociates more quickly, revealing the stable polymer clusters. The fact that MuB dissociation from individual DNA molecules fits a single-exponential decay profile (11) does not necessarily indicate a uniform state of the DNA-bound MuB if other complexities are taken into account, such as the size heterogeneity of the polymeric complexes.
An alternative model proposes that the observed MuB distribution patterns during assembly and disassembly result from independent stochastic processes. If the former possibility is true, the MuB cluster distribution on each DNA molecule during the assembly and disassembly phases within one cycle should be correlated even if the two observation periods are separated by a short interval of MuB saturation to fill the gaps with the hypothetical unstable complexes. In contrast, the latter model would predict a lack of correlation.
We developed a laminar boundary-steering flow-cell system to answer the question. The experimental results suggest that it is not necessary to postulate two different states of the DNA-bound MuB clusters. Rather, independent stochastic processes can adequately explain the MuB distribution patterns observed during the assembly and disassembly of the MuB–DNA complexes.
Previous experiments indicated that the MuB clusters observed represent a polymeric form of MuB assembled on DNA, and MuB dissociation predominantly takes place at the polymer ends (9). The preferential dissociation of MuB from ends is easily understandable because MuB polymerization must involve cooperative interactions among neighboring monomers, and such stabilizing interactions are partially missing at the polymer ends. We confirmed the previous observation and further examined whether MuA stimulation of MuB disassembly from DNA occurs at the ends of the MuB polymers or in the middle. The results are consistent with the notion that MuB disassembly from both the polymer ends and the middle is stimulated by MuA.
Results
Assembly and Disassembly of the MuB Target Complex.
We used EGFP–MuB to study the assembly/disassembly dynamics of the MuB–target DNA complex in real time by fluorescence microscopy. We devised a system that allows continuous monitoring of individual targets by tethering λ-DNA molecules that are biotinylated at one end to a fused silica surface of a PEG-coated flow cell. The PEG surface included a small fraction of PEG molecules possessing end-modified biotin, thereby allowing specific immobilization of a biotinylated DNA molecule while minimizing nonspecific adsorption of protein to the underlying surface (12). Buffer flow was used to horizontally extend the λ-DNA molecules, confining the target complexes within the evanescent field of the TIRF microscope. Fig. 1 illustrates our experimental setup for studying individual transposition-targeting complexes.
Fig. 1.
A TIRF microscopy system for real-time observation of MuB–target DNA complexes and the laminar boundary-steering flow cell used.
To monitor both the assembly and disassembly phases of the same set of individual target molecules, we performed a continuous wash cycle on the surface-immobilized DNA molecules, proceeding from assembly at a low-MuB concentration (50 nM EGFP–MuB, 2 mM ATP) through a quick saturation with a high-MuB concentration (500 nM EGFP–MuB, 2 mM ATP) to disassembly with buffer without MuB. To effectively switch between buffers, we designed a laminar boundary-steering flow cell with three inlets for the assembly buffer, saturation buffer, and disassembly buffer, respectively. Buffer flow to each inlet was controlled independently by separate syringe pumps. Because the flow cell operates within the laminar flow regime, the three buffer streams are separated by laminar boundaries where only limited local mixing takes place by diffusion provided the boundaries are maintained by constant buffer flow. Fluidic boundary shift was achieved by switching the pump flow rates to make one of the inlets the dominant channel that covers the central portion of the flow cell where observations are made, while maintaining the combined flow rate by reducing without stopping the flows for other channels.
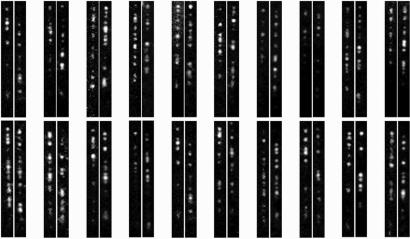
Supporting information (SI) Movie 1 demonstrates the seamless transition from assembly through saturation to disassembly using this flow-cell system. Typically, 1 min of assembly was followed by 30 sec of saturation and >15 min of disassembly to ensure removal of the residual MuB from DNA before subsequent cycles. Images of individual target molecules were collected during the assembly and disassembly phases in each cycle. For the assembly, time lapses of 0.1-sec exposure and 1 frame per sec were taken, whereas for the disassembly, time lapses of 1-sec exposure and 1 frame per min were taken. A shorter exposure time and a faster frame rate were chosen for the assembly phase because of the relatively fast assembly rate at the protein concentration used and the presence of higher fluorescence background because of the EGFP–MuB in solution. Fig. 2 shows representative assembly/disassembly image pairs of individual target molecules. All images were background subtracted, and the contrast was independently adjusted to make pattern comparison easier.
Fig. 2.
Twenty representative fluorescence image pairs of individual MuB target molecules during the assembly and disassembly phases. (Right) The pattern during assembly. (Left) The pattern during disassembly.
MuB Distribution Pattern During the Assembly and Disassembly Phases.
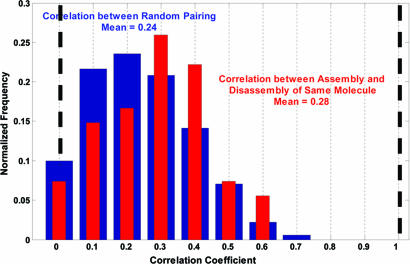
Examination of the MuB distribution patterns formed during assembly and disassembly reveals a weak correlation (Fig. 2). This finding is expected based on the previously reported A/T-rich sequence preference of the MuB cluster distribution (11). We wanted to determine whether the distribution patterns formed during one cycle of assembly and disassembly on the same target DNA molecule correlate significantly above this general background. To answer this question, we took advantage of a statistical correlation analysis using MATLAB software.
Twenty each image pattern as an array, we first calculated the correlation coefficient of all random pairs among the 108 fluorescence patterns of 54 individual target molecules. The normalized distribution of this correlation coefficient is shown in Fig. 3 by the black histogram, yielding a mean value of 0.24. This distribution represents the background correlation that arises from preferential protein binding to certain target sequences. We then calculated the correlation coefficient of the assembly and disassembly patterns during one cycle on the same target molecules and plotted the normalized distribution as a red histogram in Fig. 3. This correlation coefficient has a mean value of 0.28, differing only slightly from the background correlation. Although the difference appears statistically significant, it is far less than expected if the stable clusters established during the assembly phase remained stable through the saturation phase and the rest of the DNA was mainly filled with a weakly bound state of MuB as the concentration was raised.
Fig. 3.
Statistical correlation analysis of the MuB–DNA cluster patterns formed in the assembly and disassembly phases. The blue bars show the distribution of the correlation coefficients among random pairs of all the patterns analyzed, and the red bars show the distribution among matched pairs on the same target molecules before and after brief saturation with EGFP–MuB.
MuB Dissociates from DNA Mainly, but Not Exclusively, at Polymer Ends Both in the Presence and Absence of MuA.
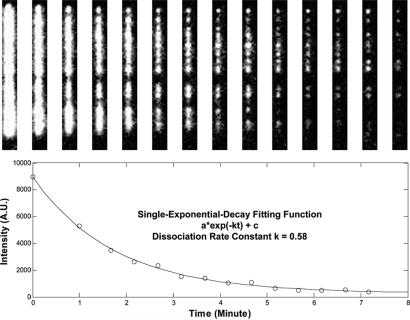
To further explore the dynamic details of MuB disassembly from target DNA and MuA stimulation of this process, we preassembled the target complexes at low EGFP–MuB concentrations (50–100 nM) and subsequently tracked the disassembly of the fluorescent MuB polymers under various buffer conditions. We quantified the process by measuring the apparent dissociation rate constant for individual DNA molecules and building up the rate constant distribution. Fig. 4 displays how we obtained the dissociation rate constant for individual target molecules. A series of time-lapsed fluorescence images for a single target molecule is shown in Fig. 4 Upper. Fig. 4 Lower shows a MuB dissociation curve, where each data point corresponds to the averaged pixel intensity of the isolated image region shown in Fig. 4 Upper, from which the background intensity measured at a nearby DNA-free region was subtracted. The apparent dissociation rate constant, which is an average for the MuB molecules bound to each individual DNA, was extracted by fitting the curve to a single-exponential decay function.
Fig. 4.
Measurement of the MuB dissociation rate constant from single target molecules. (Upper) Time-lapsed fluorescence images of an individual target molecule. (Lower) Corresponding MuB dissociation curve based on the fluorescence intensity in Upper. The line is a single-exponential decay fit used to estimate the apparent dissociation rate constant.
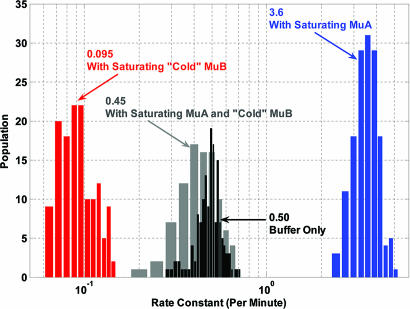
Fig. 5 summarizes the distributions of the observed dissociation rate constant under the four experimental conditions tested. The first scenario is measuring the dissociation of fluorescent MuB from DNA in plain buffer. The mean value of the dissociation rate constants among target DNA molecules was 0.50 min−1 with a half distribution width of 0.074 min−1. The apparent dissociation rate constant observed is consistent with previous reports (9, 11). Because the DNA-bound MuB exists in polymers of heterogeneous sizes, the dissociation curve should not necessarily fit to a single-exponential decay. However, the observed curves fit well to single-exponential decays. We ascribe this result to a combination of factors, such as the polymer size heterogeneity, a significant contribution of dissociation from the middle of polymers, and a possible rate difference in dissociation from different regions of the DNA. The relatively broad distribution of the apparent first-order dissociation rate constant among DNA molecules may in part reflect the difference in size/location distribution of MuB polymers among DNA molecules at time 0.
Fig. 5.
Rate constant histograms of MuB target complex disassembly in plain buffer (black), buffer containing saturating MuA (blue), buffer containing saturating unlabeled MuB (red), and buffer containing saturating unlabeled MuB and MuA (gray).
The second experimental scenario measures the dissociation of fluorescent MuB from DNA in a buffer containing a saturating concentration of MuA (400 nM). The distribution of the dissociation rate constant had a mean value of 3.6 min−1 and a half distribution width of 0.55 min−1. The rate constant increased by 7-fold, compared with that in plain buffer, indicating a dramatically accelerated dissociation of fluorescent MuB in the presence of MuA. Here we used a MuA mutant (MuA248–663), with the DNA-interacting domain truncated because the wild-type MuA at close to saturating concentration condensed λ-DNA and rendered the measurement difficult. The MuA mutant solved the DNA condensation problem without affecting the catalytic activity of MuA on MuB disassembly under the experimental conditions, judged from experiments carried out at lower MuA concentrations (data not shown). We also performed a negative control experiment by using a mutant of MuA, with both the DNA- and MuB-interacting domains deleted (MuA248–605), and found no acceleration of MuB dissociation (data not shown).
The third scenario measures the dissociation of fluorescent MuB from DNA in a buffer containing a saturating concentration of unlabeled MuB (200 nM). The even distribution of EGFP–MuB along λ-DNA at saturating protein concentrations suggests that MuB forms helical polymers along the DNA axis without significant DNA compaction/elongation or off-axial polymerization (11). This model is supported by a preliminary EM examination of the MuB polymers indicating helical filaments (S. Ramon-Maiquès, personal communication). Thus, when a saturating concentration of unlabeled MuB is added to the EGFP–MuB DNA complexes, naked DNA between the EGFP–MuB polymers would be quickly filled by the unlabeled MuB polymers that cap the ends of the fluorescent MuB polymers, effectively eliminating the polymer ends. Thus, the exchange rate observed under these conditions should reflect the off-rate from the middle of the MuB polymers. We confirmed that the unlabeled MuB concentration used was saturating; measurements in buffers containing higher concentrations (400 and 500 nM) of unlabeled MuB gave similar results (data not shown). The measured distribution of the dissociation rate constant has a mean value of 0.095 min−1 and a half distribution width of 0.021 min−1. The dissociation slows down by 4-fold, compared with that in plain buffer.
The fourth scenario is measuring the disassembly of fluorescent MuB polymers in a buffer containing a saturating concentration of unlabeled MuB (200 nM) and a saturating concentration of unlabeled MuA (400 nM). The distribution of the MuB dissociation rate constant among DNA molecules had a mean value of 0.45 min−1 and a half distribution width of 0.10 min−1. The dissociation rate constant increases by almost 5-fold, compared with that for the third scenario. MuA accelerated the dissociation of fluorescently labeled MuB from DNA in the presence of saturating unlabeled MuB. However, the MuA-stimulated dissociation is slower by 8-fold, compared with that in the absence of unlabeled MuB.
Discussion
MuB Target-Site Distribution.
Previously we observed that, in the presence of ATP, polymeric MuB clusters were formed on DNA at nonsaturating MuB concentrations. The distribution of these clusters correlated with the location of A/T-rich sequences in the DNA and also coincided with the transposition hot spots (11). When MuB was added to naked DNA molecules at relatively low concentrations, such as 50 nM, small MuB oligomers initiated the complex assembly on the DNA, and additional MuB molecules joined the initial complex to form larger polymers. The distribution of MuB polymers at early times during assembly was distinct from molecule to molecule, indicating the stochastic nature of the process. However, when averaged over a large number of molecules, the A/T-rich sequence bias emerged. The distribution pattern of DNA-bound MuB evolved relatively slowly, taking many minutes. With time, some of the smaller polymers grew even smaller and disappeared, whereas others grew larger; after a longer incubation, such as 30 min, the MuB distribution pattern of individual DNA molecules became alike (9).
When DNA molecules were exposed to higher concentrations of MuB, the entire DNA appeared to be coated evenly with bound MuB. When the MuB-saturated DNA was washed with buffer without the protein, MuB seemed to dissociate from some DNA locations faster than others, leaving behind slower dissociating MuB polymers, the distribution of which resembled that observed in the assembly experiments (11).
If two types of protein–DNA complexes are formed on the MuB-saturated DNA, one more stable than the other, it would be reasonable to assume that the stable complexes correspond to the MuB polymer clusters observed in the assembly experiments at lower concentrations of MuB. Perhaps formation of the stable MuB polymers on DNA takes place in two steps, with initial weak binding followed by a stochastic conformational change to the stably bound form that is stimulated by the A/T-rich sequence of DNA. Because the MuB distribution pattern changed slowly during assembly, it would be reasonable to assume that, based on the model described above, the stable MuB polymer distribution during disassembly would correlate with that during the assembly even if a saturating concentration of MuB was introduced to the DNA for a brief period between the assembly and disassembly phases. The high concentration of MuB would fill the gaps between the preexisting stable polymers with the less stable complexes and a limited numbers of new stable polymers.
The experiments reported here were designed to test the above mentioned model, and the results seem to argue against the model. We found an insignificant level of correlation between the MuB polymer distribution patterns during the assembly and disassembly phases above what could be explained by the DNA sequence preference for MuB binding.
An alternative and simpler model can explain the observations. Two independent stochastic events, both influenced by the MuB–DNA sequence affinity, are involved in the formation of the MuB distribution pattern during the assembly and disassembly reactions. During the assembly reaction, stochastic nucleation of MuB polymers formed on target DNA, rather than a subsequent conformational transition, determines the early MuB polymer distribution. During disassembly, nucleation of MuB-less holes formed on the target DNA determines the distribution of the remaining MuB polymers. Therefore, this model predicts the absence of significant correlation of the MuB cluster pattern between the assembly and disassembly phases as we observed.
It is easy to understand that the nucleation of the MuB complex assembly involving a small number of MuB protomers is more probable at higher affinity DNA sequences. During disassembly, hydrolysis of the bound ATP by MuB is tightly coupled to the dissociation of MuB protomers from DNA (9–11). Thus, dissociation of a MuB protomer from the middle of MuB-saturated DNA to initiate the disassembly process also must require ATP hydrolysis. This process appears to take place with higher probability at lower affinity DNA sequences, which implies that ATP hydrolysis by the DNA-bound MuB protomers takes place at a higher rate at low-affinity DNA sequences. This notion is not difficult to understand if one assumes synergistic stabilization of the basal tertiary complex by the presence of both bound DNA and ATP with respect to the transition state for hydrolysis. It has been shown previously that DNA binding by MuB reduces the ATP turnover rate and enhances the apparent ATP-binding affinity (13).
The pair-wise correlation coefficient on the same molecule before and after the brief MuB saturation was slightly higher than that among all combinations of random pairs. This difference could be explained by factors such as slightly less than complete saturation of the entire DNA that separated the two observation phases. Additional factors, which are currently unknown but could have influenced this observation, include imperfections at the MuB polymer junctions as they fuse at the saturating MuB concentration. Such defects, which could arise from either an imperfect phase match along the DNA at the polymer junctions or polymer orientation mismatch if the MuB polymer on DNA does not have a twofold symmetry, could act as the initiation point for MuB polymer disassembly. Therefore, we conclude that the MuB polymer distribution during the assembly and disassembly is not intrinsically correlated beyond the background correlation, which is principally because of sequence preference of MuB binding. Our current observations do not warrant postulation of two different conformational states for the DNA-bound MuB polymers.
MuB Target Complex Disassembly.
Fig. 5 summarizes the distributions of the disassembly rate constant for MuB target complex under various conditions. First, we observe a 5-fold decrease in the apparent dissociation rate constant when we compare the rate constant distribution in buffer containing saturating unlabeled MuB (red histogram) with the rate constant distribution in plain buffer without MuB (black histogram). The unlabeled MuB acts as a polymer end-capper and significantly slows down the disassembly of the fluorescent MuB target complexes, confirming previously reported observations (9). However, even higher concentrations of unlabeled MuB do not completely block the exchange of labeled MuB by unlabeled molecules. These results indicate that disassembly proceeds mainly from the ends of the polymeric MuB–DNA complexes, but the slow exchange of labeled MuB with unlabeled protein at saturating concentrations of unlabeled protein could most easily be explained by a significant rate of MuB dissociation from the middle of the polymer.
Second, we note that MuA stimulates MuB disassembly by 7-fold when we compare the rate constant distribution in buffer containing saturating MuA (blue histogram) with that in plain buffer (black histogram). Similarly, MuA stimulates labeled MuB dissociation close to 5-fold in the presence of unlabeled MuB (compare red histogram to gray histogram). These results suggest that MuA can stimulate MuB dissociation from both the MuB polymer ends and the middle of the polymers. MuA monomers interacting with the MuB molecules in the middle of the DNA-bound polymers, which would be more numerous than those at the polymer ends, can stimulate the generation of new MuB polymer ends from which other nearby MuA molecules could continue to dissociate MuB.
Methods
TIRF Microscopy.
The TIRF microscope used for this study has been previously described (9, 11). Briefly, a 488-nm diode-pumped, solid-state laser (Coherent, Santa Clara, CA) was focused through a fused silica prism (J. R. Cumberland, Marlow Heights, MD) onto a flow cell containing the immobilized target complexes. Fluorescence images were collected through a Plan Apo N.A. 1.4 objective lens (×100; Nikon, Mellville, NY) and captured with a CCD (Cascade 512; Roper Scientific, Trenton, NJ; Photometrics, Tucson, AZ) and Metamorph imaging software (Molecular Devices, Dowington, PA).
The flow cell was assembled by using a fused silica microscope slide with drilled inlet/outlet ports (Esco Products, Oakridge, NJ), a glass coverslip (Fisher Scientific Co., Pittsburgh, PA), and a spacer made from 25-μm-thick acrylic transfer tape (3M, St. Paul, MN). The flow-channel pattern was printed on the transfer tape by a laser cutter. The tubing was secured to the inlet/outlet ports by using Nanoport fixtures (Upchurch Scientific, Oak Harbor, WA). Microscope slides were cleaned before assembly by soaking overnight in a sulfuric acid cleaning solution (Nochromix; Sigma–Aldrich, St. Louis, MO), followed by extensive rinsing with milli-Q water, nitrogen drying, and low-power plasma cleaning in the presence of argon and oxygen (Plasma Etcher; South Bay Technology Inc., San Clemente, CA). Assembled flow cells were oven-baked for 2 h at ≈150°C for adhesive curing.
Before use, the following surface treatment was used to minimize nonspecific adsorption of proteins and immobilize biotinylated DNA molecules. Flow cells were first incubated for 1 h at room temperature with 1% (vol/vol) solution of 3-aminopropyltriethoxysilane and then 3 h with a PEG solution containing 20% (wt/wt) M-PEG-SPA Mr 5000, and 0.2% (wt/wt) biotin-PEG-SPA Mr 5000 (Nektar Therapeutics, Huntsville, AL). The flow cells were then rinsed with TE buffer containing 1 mg/ml neutravidin (Pierce Chemical, Rockford, IL). Finally, the flow cells were incubated for 30 min in buffer containing 25 mM Tris (pH 8.0), 150 mM NaCl, 10 mM MgCl2, 10% glycerol, oxygen scavenger agents for reduction of photobleaching (2 mM DTT, 6% glucose, 0.1 mg/ml glucose oxidase, 0.025 mg/ml catalase), and 2 mg/ml casein to ensure blocking of the exposed glass/fused silica surfaces (buffer-A).
Target DNA was prepared by ligating a biotinylated oligo to the right end of λ-DNA (9, 11). We immobilized target DNA molecules on the PEG-coated surface with biotin–neutravidin interaction by incubating the flow cell with 25 pM biotinylated DNA solution (9, 11). The construction and characterization of EGFP–MuB, a fusion of EGFP to the N terminus of MuB, have been reported previously (10). Target complexes were assembled by flowing in buffer A, which contained 2 mM ATP and 50 nM EGFP–MuB (9, 11).
Supplementary Material
Acknowledgments
We thank Dr. Eric C. Greene for guidance with many of the experimental techniques. This work was supported by the National Institutes of Health Intramural Research Program and the Intramural Aids Targeted Antiviral Program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706564104/DC1.
References
- 1.Mizuuchi K, Craigie R. Annu Rev Genet. 1986;20:385–429. doi: 10.1146/annurev.ge.20.120186.002125. [DOI] [PubMed] [Google Scholar]
- 2.Craig N, Craigie R, Gellert M, Lambowitz A. Washington, DC: Am Soc Microbiol; 2001. [Google Scholar]
- 3.Engelman A, Mizuuchi K, Craigie R. Cell. 1991;67:1211–1221. doi: 10.1016/0092-8674(91)90297-c. [DOI] [PubMed] [Google Scholar]
- 4.van Gent DC, Mizuuchi K, Gellert M. Science. 1996;271:1592–1594. doi: 10.1126/science.271.5255.1592. [DOI] [PubMed] [Google Scholar]
- 5.Adzuma K, Mizuuchi K. Cell. 1988;53:257–266. doi: 10.1016/0092-8674(88)90387-x. [DOI] [PubMed] [Google Scholar]
- 6.Adzuma K, Mizuuchi K. Cell. 1989;57:41–47. doi: 10.1016/0092-8674(89)90170-0. [DOI] [PubMed] [Google Scholar]
- 7.Greene EC, Mizuuchi K. Mol Cell. 2002;10:1367–1378. doi: 10.1016/s1097-2765(02)00733-5. [DOI] [PubMed] [Google Scholar]
- 8.Baker TA, Mizuuchi M, Mizuuchi K. Cell. 1991;65:1003–1013. doi: 10.1016/0092-8674(91)90552-a. [DOI] [PubMed] [Google Scholar]
- 9.Greene EC, Mizuuchi K. J Biol Chem. 2004;279:16736–16743. doi: 10.1074/jbc.M311883200. [DOI] [PubMed] [Google Scholar]
- 10.Greene EC, Mizuuchi K. EMBO J. 2002;21:1477–1486. doi: 10.1093/emboj/21.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene EC, Mizuuchi K. Mol Cell. 2002;9:1079–1089. doi: 10.1016/s1097-2765(02)00514-2. [DOI] [PubMed] [Google Scholar]
- 12.Rasnik I, Myong S, Cheng W, Lohman TM, Ha T. J Mol Biol. 2004;336:395–408. doi: 10.1016/j.jmb.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Adzuma K, Mizuuchi K. J Biol Chem. 1991;266:6159–6167. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.