Abstract
Reduced accumulation of the GTP-binding protein Giα subunit CPG-1, due either to hypovirus infection or transgenic cosuppression, correlates with virulence attenuation of the chestnut blight fungus, Cryphonectria parasitica. The role of G protein-mediated signal transduction in fungal virulence was further examined by targeted disruption of the gene cpg-1, encoding CPG-1, and a second Gα gene, cpg-2, encoding the subunit CPG-2. Disruption of cpg-1 resulted in a set of phenotypic changes similar to, but more severe than, those associated with hypovirus infection. Changes included a marked reduction in fungal growth rate and loss of virulence, asexual sporulation, female fertility, and transcriptional induction of the gene lac-1, encoding the enzyme laccase. In contrast, cpg-2 disruption resulted in only slight reductions in growth rate and asexual sporulation and no significant reduction in virulence, female fertility, or lac-1 mRNA inducibility. These results provide definitive confirmation of previous correlative evidence that suggested a requirement of CPG-1-linked signaling for a number of fungal processes, including virulence and reproduction, while demonstrating that a second Gα, CPG-2, is dispensable for these processes. They also significantly strengthen support for the apparent linkage between hypovirus-mediated disruption of G protein signal transduction and attenuation of fungal virulence.
Keywords: fungal pathogenesis, G protein signal transduction, hypovirus, chestnut blight
Fungi, like bacteria, plants, and animals, can serve as hosts for virus infection. Indeed, mycoviruses are known to be widely distributed throughout the Fungi (1, 2). However, mycovirus infections are distinguished by the universal absence of an extracellular route of infection and by a general lack of symptom expression by the infected host (3, 4). Mycoviruses within the genus Hypovirus of the family Hypoviridae provide an interesting exception to the latter general observation, in that infection of the natural host Cryphonectria parasitica, the pathogen responsible for the demise of the American chestnut tree, results in attenuation of fungal virulence (hypovirulence) and several associated stable phenotypic changes (see refs. 5 and 6).
In this regard, a long-standing attraction of the hypovirus/C. parasitica experimental system has been the anticipation that novel insights into the nature and regulation of fungal pathogenesis would derive from an understanding of the mechanism whereby a resident virus attenuates fungal virulence. Recent correlative evidence linking virus-mediated disruption of G protein signaling processes and hypovirulence are beginning to validate this prediction. Specifically, Choi et al. (7) recently reported that infection of C. parasitica with the prototypic hypovirus CHV1-713 resulted in significantly reduced accumulation of a GTP-binding protein α subunit of the Gi family designated CPG-1, which is encoded by the gene cpg-1. The same authors showed that transgenic cosuppression of CPG-1 accumulation in the absence of virus infection also resulted in attenuation of fungal virulence. Wang and Nuss (8) subsequently reported that both CPG-1 cosuppression and hypovirus infection prevented cellulose-dependent induction of C. parasitica-encoded cellulases (including a cloned cellobiohydrolase I), enzymes with potential for degrading host plant cell wall components during the fungal infection process. Using the technique of differential mRNA display, Chen et al. (9) provided evidence for an extensive and stable alteration in the pattern of fungal gene expression as a result of hypovirus infection and showed that the majority of these changes could be attributed to disruption of the CPG-1 signaling pathway. Moreover, these authors reported that both hypovirus infection and CPG-1 transgenic cosuppression result in significantly elevated cAMP levels, consistent with the prediction that CPG-1, like mammalian Giα subunits, may function to negatively regulate adenyl cyclase. Combined, these results suggested an important role for G protein-regulated cAMP accumulation in fungal pathogenesis and provided additional support for the proposal that one major mechanism underlying virus-mediated hypovirulence involves disruption of cellular G protein-linked signal transduction.
Targeted gene disruption provides a powerful technique for rigorously examining the requirement of specific fungal gene products for complex in vivo functions (e.g., refs. 10 and 11). Application of this technique to C. parasitica Gα subunits definitively confirmed that CPG-1 is required for a number of complex and important fungal processes, including asexual sporulation and virulence; roles not previously reported for Gα-linked signaling in a pathogenic fungus. Moreover, the absence of significant phenotypic changes resulting from cpg-2 disruption established distinct roles for the two encoded Gα subunits in C. parasitica virulence, morphology, and reproduction. The relevance of these results to mechanisms underlying hypovirus-mediated alteration of fungal gene expression and phenotype is discussed.
MATERIALS AND METHODS
Fungal Strains and Growth Conditions.
C. parasitica strains EP155 (ATCC 38755, mating type A) and EP146 (ATCC 64671, mating type a) (12) were maintained on potato dextrose agar (PDA; Difco) as described previously (13). For growth studies, colonies were cultured on Petri dishes containing 28 ml of PDA. The dishes were sealed with Parafilm and incubated at room temperature (≈23°C) for 7 days. Liquid cultures were grown in 25 ml of complete medium (14) after inoculation with four 3 × 3 mm mycelial plugs taken from the edge of 5-day-old cultures on PDA plates. To test for laccase activity, colonies were grown on tannic acid-containing medium [0.5% tannic acid (Sigma), 1.5% Difco malt extract, and 2% Difco agar, adjusted to pH 4.5 with NaOH] (15) at room temperature for 4 days. For lac-1 transcript induction with cycloheximide (Sigma), liquid cultures were inoculated and maintained as described (16).
Nucleic Acid Preparation and Analysis.
Total nucleic acids were prepared from C. parasitica mycelium (1–2 g) grown on cellophane-covered PDA plates as described previously (16). Total nucleic acids (10 μg) was used for Southern hybridization analysis after digestion with appropriate restriction enzymes (17). C. parasitica RNAs were prepared as described (16) with minor modifications (18). Total RNA (10–15 μg) was separated on a formaldehyde/1.4% agarose gel, transferred to a Hybond-N nylon membrane (Amersham), and subjected to Northern hybridization analysis as described (17). PCR analyses were performed with 100 ng of total nucleic acid and TaqPlus DNA polymerase (Stratagene) for 30 cycles with the following parameters: 94°C, 30 sec; 55°C, 30 sec; and 68°C, 2 min.
Transformation-Mediated Gene Disruption.
The cpg-1 disruption vector was constructed in a basic pUC18 plasmid using several indirect cloning steps. A cpg-1 genomic clone was modified by replacement of a 958-bp SalI/SphI DNA fragment that extended upstream from a SphI site located 81 nt 3′ of the cpg-1 ATG translation initiation codon with a 2.3-kb SalI/SphI DNA fragment, consisting of the Escherichia coli hygromycin B phosphotransferase gene (hph) flanked by the Aspergillus nidulans trpC promoter and terminator derived from plasmid pDH25 (ref. 19; see Fig. 1A). A 7.1-kb HindIII/KpnI fragment containing the disrupted cpg-1 gene sequence and flanked at each end with 3 vector-derived restriction sites, was isolated from the disruption vector after restriction digestion and used to transform EP155 spheroplasts as previously described (20).
Figure 1.
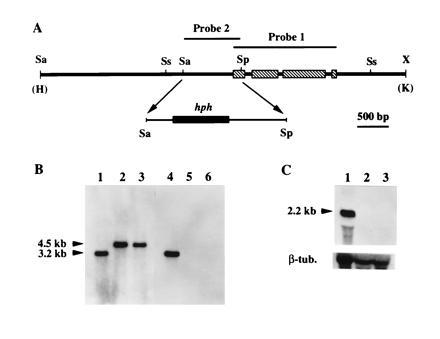
Disruption of cpg-1. (A) Map of the 7.1-kb HindIII/KpnI fragment derived from the cpg-1 disruption construct and used to transform C. parasitica spheroplasts. A 958-bp SalI/SphI fragment that contained 81 nt from the cpg-1 coding domain and the rest from the promoter region was replaced by a 2.3-kb DNA fragment containing the E. coli hph gene flanked by the A. nidulans trpC promoter and terminator (19). Probes used for Southern blot analyses are indicated above the map. The restriction endonuclease cleavage sites are: Sa, SalI; Sp, SphI; Ss, SstI; and X, XbaI. The HindIII (H) and KpnI (K) sites were from the cloning vector pUC18. (B) Results of Southern blot analyses for the cpg-1 disruptants. Total nucleic acid (10 μg) from the wild-type strain EP155 (lanes 1 and 4), and from cpg-1 disruptants G1-1 (lanes 2 and 5) and G1-15 (lanes 3 and 6) were digested with SstI, separated on a 0.8% agarose gel, and transferred to a Hybond-N membrane. DNA in lanes 1–3 was probed with a [32P]cDNA copy of the cpg-1 gene, indicated as probe 1 in A. In lanes 4–6, the blot was stripped and reprobed with a 32P-labeled 958-bp SalI/SphI fragment, indicated as probe 2 in A. The sizes of the expected hybridization bands are indicated at the left. (C) Results of Northern hybridization analyses of cpg-1 transcript accumulation in the wild-type strain EP155 (lane 1) and in cpg-1 disruption mutants G1-1 (lane 2) and G1-15 (lane 3). Total RNA (10 μg) was loaded in each lane, separated in a formaldehyde/1.4% agarose gel, and transferred to a Hybond-N membrane. The blot was hybridized with a [32P]cDNA copy of the cpg-1 gene (probe 1). The size of the transcript was indicated at the left. After autoradiography, the blot was stripped and rehybridized with a C. parasitica β-tubulin gene cDNA probe (16).
The cpg-2 disruption vector was constructed by replacing a 897-bp BamHI/SalI fragment of the cpg-2 coding region, which contained exons 2, 3, 4, and two-thirds of exon 5, with a 1.4-kb BamHI/SalI fragment from plasmid pDH25 (19) containing the E. coli hph gene and the A. nidulans trpC promoter, but lacking the trpC terminator region (see Fig. 2B). The 4.6-kb SphI fragment, which contained the disrupted cpg-2 gene sequence, was then gel-isolated from the cloning vector pUC18 after digestion with SphI and used to transform EP155 spheroplasts.
Figure 2.

Disruption of cpg-2. (A) Map of the 4.6-kb SphI fragment derived from the cpg-2 disruption construct and used to transform C. parasitica spheroplasts. A 897-bp BamHI/SphI fragment that contained exons 2, 3, 4, and two-thirds of exon 5 was replaced by a 1.4-kb fragment containing the E. coli hph gene and the A. nidulans trpC promoter (19). Probes used for Southern blot analyses are indicated above the map. The restriction endonulease cleavage sites are: B, BamHI; H, HindIII; Sa, SalI; Sp, SphI. (B) Results of Southern blot analyses for the cpg-2 disruptants. Total nucleic acid (10 μg) from the wild-type strain EP155 (lanes 1 and 4) and cpg-2 disruptants G2-37 (lanes 2 and 5) and G2-39 (lanes 3 and 6) was digested with SphI, separated on a 0.8% agarose gel and transferred to a Hybond-N membrane. DNA in lanes 1–3 was probed with a 32P-labeled 1.3-kb HindIII/SalI DNA fragment, indicated as probe 1 in A. In lanes 4–6, the blot was stripped and reprobed with a 32P-labeled 584-bp EcoRI/SalI fragment, indicated as probe 2 in A. The sizes of the expected hybridization bands are indicated at the left. (C) Results of Northern hybridization analyses of cpg-2 transcript accumulation in the wild-type strain EP155 (lane 1) and in cpg-2 disruption mutants G2-37 (lane 2) and G2-39 (lane 3). Total RNA (15 μg) was loaded in each lane, separated in a formaldehyde/1.4% agarose gel, and transferred to a Hybond-N membrane. The blot was hybridized with the 32P-labeled 584-bp EcoRI/SalI fragment (probe 2 in A). The size of the transcript is indicated at the left. After autoradiography, the blot was stripped and rehybridized with a C. parasitica β-tubulin gene cDNA probe (16).
Each hygromycin-resistant transformant was cloned by single conidial isolation. Prospective disruption mutants that exhibited consistent phenotypic changes were further characterized by PCR and Southern and Northern blot analyses.
Phenotypic Characterization of the cpg-1 and cpg-2 Disruption Mutants.
The effects of cpg-1 and cpg-2 disruptions on the growth of C. parasitica were evaluated on PDA plates by measuring the diameters of the colonies. To determine growth in liquid cultures, mycelia were collected by filtration through two layers of miracloth (Calbiochem), and mycelial masses were determined after drying at 65°C overnight.
Asexual sporulation was quantified after growth of cultures on PDA plates for 3 weeks as described by Hillman et al. (13). Mating was performed on autoclaved twigs of American chestnut tree embedded in 2% water agar as described by Anagnostakis (21). Strain EP155 and the cpg-1, cpg-2 disruption mutants (mating type A) were tested as female parents in mating experiments in which conidia collected from strain EP146 (mating type a) served as spermatia. Virulence assays were performed on dormant American chestnut tree stems as previously described (22, 23).
Intracellular cAMP levels were determined as reported (9) with a 125I-labeled cAMP scintillation proximity assay (SPA) system (Amersham) following minor modifications. Fresh mycelia (0.4 g) grown on PDA/cellophane plates sealed with Parafilm for 7 days were extracted in 2 ml of standard extraction buffer (9). Protein content of the extracts were determined by the Bradford assay (24) using the Bio-Rad protein assay kit with bovine serum albumin as standard.
RESULTS
Disruption of cpg-1 and cpg-2.
The cpg-1 and cpg-2 disruption vectors were constructed by replacing a portion of each cloned gene (7) with a cassette composed of the E. coli hygromycin phosphotransferase gene (hph) flanked by the A. nidulans trpC promoter and terminator (Figs. 1A and 2A). For cpg-1 disruption, this involved replacement of a 958-bp SalI/SphI fragment that extended upstream from a position 81 nt 3′ of the CPG-1 translation initiation codon (Fig. 1A). Of 150 single-spored hygromycin-resistant C. parasitica colonies transformed with a 7.1-kb HindIII/KpnI fragment containing the modified cpg-1 gene, 23 showed a similar set of phenotypic changes, which included severe growth reduction. Results of preliminary PCR analysis of the putative disruptants were consistent with cpg-1 disruption (data not shown). Two disruptants, designated G1-1 and G1-15, were chosen for detailed characterization, which included Southern and Northern blot analyses as shown in Fig. 1 B and C. Hybridization of SstI-digested genomic DNAs, isolated from wild-type strain EP155, and the two disruptants, with a probe consisting of a cDNA copy of the entire cpg-1 coding region (probe 1 shown in Fig. 1A) revealed a single hybridizing band in each case, but of different sizes for the wild-type and disruptant DNAs. The 3.2-kb band observed for EP155 DNA was replaced by a 4.5-kb band for G1-1 and G1-15 DNAs, consistent with the length expected for a double cross-over event during homologous recombination at the cpg-1 locus with the isolated disruption DNA fragment. To confirm the replacement of the endogenous cpg-1 by the disrupted copy, the blot was stripped and reprobed with the deleted 958-bp SalI/SphI fragment (probe 2 shown in Fig. 1A). As expected, this probe hybridized only to the 3.2-kb band in EP155 DNA, whereas the 4.5-kb bands previously detected in lanes containing DNAs from G1-1 and G1-15 were absent. Similarly, Northern blot analysis revealed a 2.2-kb transcript present in RNA prepared from strain EP155, but absent in RNAs isolated from the two disruptants (Fig. 1C).
A truncated hygromycin resistance cassette (lacking the trpC terminator) was used to replace a 897-bp BamHI/SalI fragment of the cpg-2 coding domain in the construction of the cpg-2 disruption vector (Fig. 2A). Transformation of C. parasitica spheroplasts with a 4.6-kb SphI fragment containing this modified cpg-2 gene resulted in 71 hygromycin-resistant single-spored colonies, 19 of which exhibited a similar set of subtle morphological differences in comparison to the untransformed control. Two of these transformants, designated G2-37 and G2-39, were further characterized by PCR and Southern and Northern blots analyses as described above. Fig. 2B shows the results of Southern blot analysis of SphI-digested genomic DNAs from strains EP155, G2-37, and G2-39, probed with a 1.3-kb HindIII/SalI fragment containing part of the cpg-2 promoter region and exons 1–5 (probe 1 shown in Fig. 2A). The 4.1-kb hybridization band observed for EP155 DNA was replaced by a larger band of 4.6-kb for the DNA preparations from both disruptants. Again, this size difference is predicted for double cross-over homologous recombination events at the cpg-2 locus involving the transforming disruption DNA fragment. Reprobing of the stripped blot with a 584-bp EcoRI/SalI fragment spanning part of exon 2 through part of exon 5 (probe 2 shown in Fig. 2A) further confirmed deletion of the expected portion of the cpg-2 gene coding region. Northern blot analysis confirmed the absence of the cpg-2 transcript in both disruptants, detected as a 2.8-kb band in RNA extracted from strain EP155 (Fig. 2C).
Phenotypic Characterization of cpg-1 and cpg-2 Disruptants.
As shown in Fig. 3, disruptions of cpg-1 and cpg-2 had quite different effects on C. parasitica growth and colony morphology. Growth rates of cpg-1 disruptants G1-1 and G1-15, measured as either colony diameter on PDA or as dry mass in liquid complete medium, was reduced by 65–70%, relative to that of strain EP155. In contrast, cpg-2 disruption marginally affected growth rate, causing a 10–15% reduction in colony diameter and mycelial mass. Additional parameters chosen for comparison in the initial round of characterization included a number of traits that have previously been shown to be affected by hypovirus infection: production of orange pigment, conidiation, female fertility, laccase production, and virulence (see refs. 5 and 6). As is observed upon infection with hypovirus CHV1-713, cpg-1 disruption greatly reduced orange pigmentation and conidiation (Fig. 3). For example, neither G1-1 nor G1-15 produced asexual spores, even after 8 weeks of growth on PDA plates under standard laboratory bench conditions (13). However, no reduction in orange pigmentation was observed for cpg-2 disruptants (Fig. 3) and conidiation was minimally affected relative to strain EP155 (9.2 × 108 versus 4.3 × 109).
Figure 3.
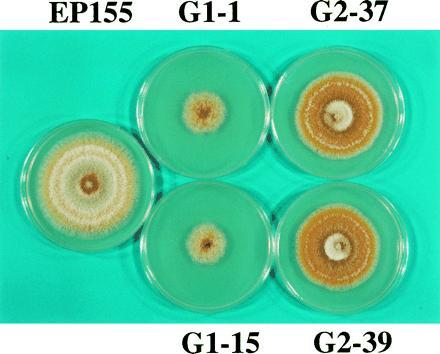
Colony morphology of wild-type strain EP155, cpg-1 disruption mutants G1-1 and G1-15 and cpg-2 disruption mutants G2-37 and G2-39. When grown on PDA, disruptants G1-1 and G1-15 grew much more slowly than control strain EP155, whereas G2-37 and G2-39 had only a slightly reduced growth rate. Orange pigmentation was also reduced for cpg-1 disruptants G1-1 and G1-15, but the cpg-2 disruptants G2-37 and G2-39 had slightly increased orange pigmentation in comparison to EP155.
CHV1-713-infected strains will not function as a female parent in a sexual cross (25). To test female fertility of the cpg-1 and cpg-2 disruptants (mating type A), sexual crosses were performed using conidia from strain EP146 (mating type a) as spermatia. Perithecia formed ≈5 weeks after the initiation of mating for the wild-type strain EP155 and the cpg-2 disruptants G2-37 and G2-39. However, cpg-1 disruptants G1-1 and G1-15 failed to produce any perithecia during an extended period exceeding several additional months. Interestingly, cpg-2 disruptants G2-37 and G2-39 appeared to yield 3 to 4 times more perithecia than did strain EP155 in these crosses (data not shown). Ascospore progeny of cpg-2 disruptants were viable and exhibited the expected segregation ratio for hygromycin resistance and brown pigmentation [from the male parent strain EP146 (26)] (data not shown).
Hypovirus infection of C. parasitica has been reported to affect transcript accumulation of specific host genes (8, 9, 16, 27, 28, 29, 30). In this regard, the effect of hypovirus infection on the expression of lac-1, which encodes the phenoloxidase laccase, has been characterized in considerable detail (16, 27, 30, 31, 32, 33). Conveniently, the lac-1-encoded laccase activity produces an intense brown color when C. parasitica is cultured on solid medium containing tannic acid (15). As shown in Fig. 4A, wild-type strain EP155 and cpg-2 disruptants G2-37 and G2-39 produced significant levels of brown color, while the color reaction was faint to nondetectable on plates containing the cpg-1 disruptants G1-1 and G1-15. Lac-1 transcript accumulation can also be superinduced by treatment with low levels of the protein synthesis inhibitor cycloheximide (16), and this induction is prevented by hypovirus CHV1-713 infection (16). As indicated in Fig. 4B, lac-1 transcripts accumulated to high levels in cycloheximide-treated strain EP155 and in both cpg-2 disruptants, but failed to accumulate in cycloheximide-treated cpg-1 disruptants G1-1 and G1-15.
Figure 4.

Effect of cpg-1 and cpg-2 disruption on laccase activity (A) and cycloheximide-mediated induction of lac-1 transcript accumulation (B). (A) Colonies were grown on tannic acid-containing medium [0.5% tannic acid, 1.5% malt extract, and 2% agar, pH 4.5 (15)] at ≈23°C for 4 days. The level of brown color correlated with the level of laccase activity produced by each strain. (B) Northern hybridization analysis of lac-1 transcript accumulation after induction with 3 μM cycloheximide. lac-1 induction was performed essentially as previously reported by Choi et al. (16). Ten micrograms of total RNA was loaded in each lane, separated in a formaldehyde/1.4% agarose gel, and transferred to a Hybond-N membrane. The blot was hybridized with a 32P-labeled lac-1 specific probe consisting of a 850-bp EcoRI/SalI fragment encompassing exon 10 and most of exon 11 (16). After autoradiography, the blot was stripped and rehybridized with a C. parasitica β-tubulin gene cDNA probe.
Reduced CPG-1 accumulation has previously been shown to correlate with reduced fungal virulence (7). The disruption of cpg-1 and cpg-2 provided an opportunity to test the requirement of two different Gα subunits in C. parasitica virulence expression. As indicated in Table 1, cpg-1 disruption completely abolished C. parasitica virulence on American chestnut tree stems. Disruptants G1-1 and G1-15 failed to initiate any canker formation during the course of the study (30 days after inoculation). However, viable mycelia of both cpg-1 disruptants could be readily recovered from the inoculation sites during the entire period (data not shown). In contrast, the two cpg-2 disruptants, G2-37 and G2-39, produced cankers similar in size to the wild-type strain EP155, indicating that cpg-2 is dispensable for C. parasitica virulence expression.
Table 1.
Mean canker areas induced on dormant stems of American chestnut by a virulent strain (EP155), two cpg-1 disruption mutants (G1-1 and G1-15), and two cpg-2 disruption mutants (G2-37 and G2-39) of C. parasitica
| Strain or mutant | Mean canker area, cm2 |
|---|---|
| EP155 | 22.36 ± 2.97 |
| G1-1 | 0 ± 0 |
| G1-15 | 0 ± 0 |
| G2-37 | 18.22 ± 2.75 |
| G2-39 | 16.61 ± 4.19 |
Mean (±SD) canker areas of six replicates were determined 14 days after inoculation.
Chen et al. (9) recently reported that cAMP levels increased under conditions in which CPG-1 accumulation was reduced, consistent with the prediction that CPG-1, like mammalian Giα subunits, may function to negatively regulate adenyl cyclase. Compatible with those results, the intracellular cAMP levels in cpg-1 disruptants, G1-1 and G1-15, were found to be ≈2.5-fold greater than that measured for wild-type strain EP155. In contrast, disruption of cpg-2 caused an apparent reduction in intracellular cAMP levels to about one-half of the EP155 value (Table 2). This result suggests the possibility that cpg-2, like mammalian Gsα subunits (34), may function as a positive regulator of adenyl cyclase.
Table 2.
Intracellular cAMP accumulation in a virulent strain (EP155), two cpg-1 disruption mutants (G1-1 and G1-15), and two cpg-2 disruption mutants (G2-37 and G2-39) of C. parasitica
| Strain or mutant | cAMP, pmol/mg |
|---|---|
| EP155 | 6.77 ± 0.50 |
| G1-1 | 15.16 ± 1.02 |
| G1-15 | 15.38 ± 3.91 |
| G2-37 | 2.67 ± 0.23 |
| G2-39 | 3.22 ± 0.10 |
Values represent the mean ± SD of three replicates.
DISCUSSION
Signal transduction events that drive complex functions, ranging from pathogenesis and morphological development to reproduction, remain relatively ill-defined for filamentous fungi. In addition to cpg-1 and cpg-2 of C. parasitica, genes encoding Gα subunit have been cloned from the following fungal pathogens: Pneumocystis carinii (35), Candida albicans (36), and Cryptococcus neoformans (37). Suggested roles for Gα-linked signaling in these pathogenic fungi have been limited to an undefined involvement in mating (36, 37). In contrast, recent studies examining CPG-1 accumulation in virus-free and hypovirus-infected C. parasitica strains have provided correlative evidence suggesting a role for this single Gα subunit in multiple fungal processes (7, 8, 9). The results of targeted gene disruption for cpg-1 described in this report definitively confirm that CPG-1 is required for the diverse functions of virulence, optimal hyphal growth, orange pigment production, conidiation, and sexual reproduction. In contrast, the same technique revealed a second C. parasitica Gα subunit, CPG-2, to be dispensable for these processes, indicating quite distinct regulatory roles for these two Gα subunits.
The fact that the cpg-1 disruption vector contained a deletion of only 81 bp of the cpg-1 coding domain and 877 bp of upstream flanking DNA raises the possibility that the phenotypic changes exhibited by the resulting disruptants may not be attributable solely to cpg-1 disruption. However, this possibility appears to be ruled out by the results of subsequent complementation studies. While ectopic integration of a cpg-1 genomic clone, that contained the cpg-1 coding domain flanked by 1219 bp of upstream DNA and 601 bp of downstream DNA, completely rescued the cpg-1 disruption phenotype, the same clone that contained a variety of specific point mutations designed to alter CPG-1 activity (e.g., GTPase-deficient mutations R178C and Q204L) failed to restore the wild-type phenotype (S.G. and D.L.N., unpublished results). Furthermore, different point mutations within the cpg-1 coding domain caused distinct nonrescuing phenotypes. Thus, the ability to rescue the cpg-1 disruption phenotype is directly dependent on the introduction of a functional cpg-1 coding domain.
The finding that transgenic cpg-1 disruption and hypovirus infection both result in a similar set of phenotypic changes also significantly strengthens the proposal that disruption of G protein-linked signal transduction constitutes one major mechanism underlying hypovirus-mediated hypovirulence. There are, however, also interesting differences in the severity of phenotypic changes resulting from hypovirus CHV1-713 infection and transgenic cpg-1 disruption in the same C. parasitica strain (EP155), especially with respect to virulence and growth on synthetic medium. Although significantly reduced in virulence, CHV1-713-infected strains are able to colonize and form superficial necrotic cankers on dormant chestnut tree tissue (e.g., ref. 22). In contrast, cpg-1 disruptants fail to cause any significant necrosis on the same tissue, an apparent complete loss of virulence. The effect of CHV1-713 infection on C. parasitica growth has been reported to range from moderate (16) to none (27). As indicated in Fig. 3, cpg-1 disruption reduces growth rates by 65–70%. Quantitative differences for conidiation, female infertility and orange pigment production were not apparent over the limited range of culture conditions used in this study but may emerge when the different strains are exposed to additional environmental conditions that are known to influence pigmentation and conidiation—e.g., high light intensity (13). Since cpg-1 disruption completely eliminates CPG-1 accumulation, while reduction in CPG-1 accumulation is likely to be less than complete as a result of CHV1-713 infection, it is quite probable that differences in the level of CPG-1 accumulation are responsible for the observed differences in severity of phenotypic changes.
Also of interest, in light of previous studies on lac-1 transcriptional regulation, is the apparent requirement of CPG-1 for induction of laccase production and lac-1 mRNA accumulation (Fig. 4 A and B). Induction of lac-1 transcript accumulation was reported to be mediated by a Ca2+, inositol trisphosphate (IP3), calmodulin-dependent positive pathway and to be prevented by a cyclosporin-sensitive, negative regulatory pathway that requires ongoing protein synthesis (16, 32, 33). Comparative studies of the effect of several pharmacological agents and hypovirus infection on lac-1 transcript accumulation led to the conclusion that virus-mediated reduction in lac-1 transcription results from perturbation of the positive regulatory pathway. Although direct linkage between CPG-1 and the IP3/Ca2+ pathway governing lac-1 transcription has not yet been established, other IP3 and calcium signaling systems have been linked experimentally to a varieties of receptor-coupled G protein signal transduction pathways, including pertussis toxin-sensitive G proteins of the Giα class, in a variety of biological systems (38, 39, 40). In this regard, Liu and Simon (41) recently provided direct evidence for Giα-mediated regulation of an inositol phospholipid-specific phospholipase C activity through an activated cAMP-dependent protein kinase. Thus, one could imagine a similar mechanism to explain hypovirus-mediated suppression of lac-1 transcriptional induction as follows. Hypovirus-mediated reduction in CPG-1 accumulation results in elevated cAMP levels (9), activating a cAMP-dependent protein kinase, which subsequently inhibits phospholipase C activity and, consequently, generation of IP3 and Ca2+ mobilization required for induction of lac-1 transcription. In this context, the diverse nature of the multiple individual processes altered by cpg-1 disruption (e.g., conidiation, sexual mating, orange pigment production, lac-1 induction, and virulence) suggests that CPG-1 functions near the apex of an interconnected network of signaling cascades. Future efforts to dissect, extend, and connect CPG-1-regulated, convergent and divergent signaling pathways will benefit significantly from the recent identification of a battery of C. parasitica genes that are similarly altered in expression as a result of either CHV1-713 infection or of CPG-1 transgenic cosuppression in the absence of virus infection (9).
The finding that cpg-1 disruptants contain elevated cAMP levels (Table 2) is consistent with a recent report linking reduced CPG-1 accumulation and elevated cAMP levels in CHV1-713-infected and CPG-1-cosuppressed strains (9) and supports the prediction that CPG-1 functions as a negative regulator of adenyl cyclase. Roles for cAMP-mediated signaling in infection-related morphogenesis have been implicated for the phytopathogenic fungi Magnaporthe grisea (42, 43) and Ustilago maydis (44). Infection by C. parasitica is not associated with the production of specialized structures, such as the appressorium produced by M. grisea or with dimorphic switching as observed for U. maydis. Rather, the role for G protein-regulated cAMP modulation in C. parasitica infection is currently envisioned as a key sensoring mechanism that mediates responses to dynamic extracellular cues throughout the infection process. The availability of cpg-1 null mutants for complementation studies with appropriately mutated CPG-1 constructs and of cloned, cAMP-responsive C. parasitica genes (9) provides new opportunities for detailed mechanistic studies of signal transduction processes that drive C. parasitica pathogenic interactions.
While the gene disruption studies reported here definitively establish a requirement of CPG-1 for a number of important fungal functions and differentiate the roles of the two C. parasitica Gα subunits identified to date, many additional questions remain unresolved. Issues of central focus in future studies will include the identity of hypovirus-encoded proteins responsible for reduced CPG-1 accumulation, the role of other G protein subunits in the regulation of key fungal processes and the range of environmental cues that are sensed by, and functions that are regulated through, CPG-1-linked signaling pathways.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: PDA, potato dextrose agar; IP3, inositol trisphosphate.
References
- 1.Buck K W. In: Fungal Virology. Buck K W, editor. Boca Raton, FL: CRC; 1986. pp. 1–84. [Google Scholar]
- 2.Lemke P A. Viruses and Plasmids in Fungi. New York: Dekker; 1979. p. 653. [Google Scholar]
- 3.Wickner R B. FASEB J. 1989;3:2257–2265. doi: 10.1096/fasebj.3.11.2550303. [DOI] [PubMed] [Google Scholar]
- 4.Nuss D L, Koltin Y. Annu Rev Phytopathol. 1990;28:37–58. doi: 10.1146/annurev.py.28.090190.000345. [DOI] [PubMed] [Google Scholar]
- 5.Anagnostakis S L. Science. 1982;215:466–471. doi: 10.1126/science.215.4532.466. [DOI] [PubMed] [Google Scholar]
- 6.Nuss D L. Microbiol Rev. 1992;56:561–576. doi: 10.1128/mr.56.4.561-576.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi G H, Chen B, Nuss D L. Proc Natl Acad Sci USA. 1995;92:305–309. doi: 10.1073/pnas.92.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang P, Nuss D L. Proc Natl Acad Sci USA. 1995;92:11529–11533. doi: 10.1073/pnas.92.25.11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen B, Gao S, Choi G H, Nuss D L. Proc Natl Acad Sci USA. 1996;93:7996–8000. doi: 10.1073/pnas.93.15.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stahl D J, Schäfer W. Plant Cell. 1992;4:621–629. doi: 10.1105/tpc.4.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott-Craig J S, Panaccione D G, Cervone F, Walton J D. Plant Cell. 1990;2:1191–1200. doi: 10.1105/tpc.2.12.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anagnostakis S L. Mycologia. 1983;75:777–800. [Google Scholar]
- 13.Hillman B I, Shapira R, Nuss D L. Phytopathology. 1990;80:950–956. [Google Scholar]
- 14.Puhalla J E, Anagnostakis S L. Phytopathology. 1971;61:169–173. [Google Scholar]
- 15.Rigling D, Heiniger U, Hohl H R. Phytopathology. 1989;79:219–223. [Google Scholar]
- 16.Choi G H, Larson T G, Nuss D L. Mol Plant–Microbe Interact. 1992;5:119–128. doi: 10.1094/mpmi-5-119. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 18.Gao S, Choi G H, Shain L, Nuss D L. Appl Environ Microbiol. 1996;62:1984–1990. doi: 10.1128/aem.62.6.1984-1990.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cullen D, Leong S A, Wilson L J, Henner D J. Gene. 1987;57:21–26. doi: 10.1016/0378-1119(87)90172-7. [DOI] [PubMed] [Google Scholar]
- 20.Churchill A C L, Ciufetti L M, Hansen D R, Van Etten H D, Van Alfen N K. Curr Genet. 1990;17:25–31. [Google Scholar]
- 21.Anagnostakis S L. Phytopathology. 1984;74:561–565. [Google Scholar]
- 22.Choi G H, Nuss D L. Science. 1992;257:800–803. doi: 10.1126/science.1496400. [DOI] [PubMed] [Google Scholar]
- 23.Jaynes R A, Elliston J F. Phytopathology. 1980;70:453–456. [Google Scholar]
- 24.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Anagnostakis S L. In: The Ecology and Physiology of the Fungal Mycelium. Jennings D H, Rayner A D M, editors. Cambridge, U.K.: Cambridge Univ. Press; 1984. pp. 353–366. [Google Scholar]
- 26.Chen B, Choi G H, Nuss D L. EMBO J. 1993;12:2991–2998. doi: 10.1002/j.1460-2075.1993.tb05967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigling D, Van Alfen N K. J Bacteriol. 1991;173:8000–8003. doi: 10.1128/jb.173.24.8000-8003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell W A, Van Alfen N K. Mol Cell Biol. 1987;7:3688–3693. doi: 10.1128/mcb.7.10.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Churchill A C L, Kazmierczak P, Kim D, Van Alfen N K. Mol Cell Biol. 1993;13:7782–7792. doi: 10.1128/mcb.13.12.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazmierczak P, Pfeiffer P, Zhang L, Van Alfen N K. J Virol. 1996;70:1137–1142. doi: 10.1128/jvi.70.2.1137-1142.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson T G, Choi G H, Nuss D L. EMBO J. 1992;11:4539–4548. doi: 10.1002/j.1460-2075.1992.tb05555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larson T G, Nuss D L. Proc Natl Acad Sci USA. 1993;90:148–152. doi: 10.1073/pnas.90.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larson T G, Nuss D L. EMBO J. 1994;13:5616–5623. doi: 10.1002/j.1460-2075.1994.tb06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilman A G. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 35.Smulian A G, Ryan M, Staben C, Cushion M T. Infect Immun. 1996;64:691–701. doi: 10.1128/iai.64.3.691-701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadhu C, Hoekstra D, McEachern M J, Reed S I, Hicks J B. Mol Cell Biol. 1992;12:1977–1985. doi: 10.1128/mcb.12.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolkacheva T, McNamara P, Piekarz E, Courchesne W. Infect Immun. 1994;62:2849–2856. doi: 10.1128/iai.62.7.2849-2856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clapham D E. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 39.Berridge M J. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 40.Gollasch M, Kleuss C, Hescheler J, Wittig B, Schultz G. Proc Natl Acad Sci USA. 1993;90:6265–6269. doi: 10.1073/pnas.90.13.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M, Simon M I. Nature (London) 1996;382:83–87. doi: 10.1038/382083a0. [DOI] [PubMed] [Google Scholar]
- 42.Lee Y-H, Dean R A. Plant Cell. 1993;5:693–700. doi: 10.1105/tpc.5.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell T K, Dean R A. Plant Cell. 1995;7:1869–1878. doi: 10.1105/tpc.7.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gold S, Duncan G, Barret K, Kronstad J. Genes Dev. 1994;8:2805–2816. doi: 10.1101/gad.8.23.2805. [DOI] [PubMed] [Google Scholar]


