Abstract
The primary focus of this investigation was to study the relationship between neuroendocrine (NE) differentiation and epidermal growth factor (EGF) because both have been implicated in the progression of prostate cancer. For this purpose, we used gefitinib and trastuzumab, which are inhibitors of EGF receptor (EGFR) and ErbB2, respectively. EGF prevents NE differentiation induced by androgen depletion. This effect is prevented by gefitinib, which blocks the activation of EGFR and ErbB2, stimulation of mitogen-activated protein kinase (MAPK), and cell proliferation induced by EGF. Conversely, trastuzumab does not inhibit the effect of EGF on EGFR phosphorylation, MAPK activity, cell proliferation, and NE differentiation, although it reduces ErbB2 levels specifically, suggesting that ErbB2 is not necessary to inhibit NE differentiation. Prevention of NE differentiation by EGF is mediated by a MAPK-dependent mechanism and requires constitutive Akt activation. The abrogation of the PI3K/Akt pathway changes the role of EGF from inhibitor to inductor of NE differentiation. We show that EGFR tyrosine kinase, MAPK, and PI3K inhibitors inhibit the cell proliferation stimulated by EGF but induce the acquisition of NE phenotype. Altogether, the present data should be borne in mind when designing new clinical schedules for the treatment of prostate cancer, including the use of ErbB receptors and associated signaling pathway inhibitors.
Keywords: EGF, prostate cancer, neuroendocrine differentiation, gefinitib, trastuzumab
Introduction
Prostate cancer is one of the most common malignancies among men in the western world and a major health problem in many industrialized countries. Because the tumor is initially androgen-dependent in the majority of cases, endocrine manipulation is the first-line therapy for metastatic and locally advanced cancers and often leads to remission or stabilization of the disease [1]. However, this period of remission is invariably followed by tumor relapse, and available treatment options are only palliative. Patients with metastatic prostate cancer develop an androgen-refractory phenotype that leads to disease progression and eventual death [2]. Therefore, an understanding of what drives progression to androgen independence is critical. In fact, the prostate is known to be dependent not exclusively on androgens but also on growth factors and neuropeptides secreted by neuroendocrine (NE) cells that maintain normal prostate function and play a role in the development of pathological conditions [3].
NE cells comprise a minor fraction of the total epithelial population but are thought to have a paracrine role in the growth and differentiation of a normal prostate gland [4]. These cells secrete a number of neuropeptides from a large class that includes bombesin, neurotensin, serotonin, calcitonin, thyroid-stimulating hormone, and parathyroid hormone-related peptide, which exhibit a wide range of cellular activities associated with tumor proliferation, transformation, and metastasis [4]. These cells lack nuclear androgen receptors [5] and, thus, represent an androgen-insensitive cell phenotype in the prostate.
Long-term androgen ablation therapy tends to select prostate tumor populations that are enriched in NE cells [6,7]. Those tumors with an increased NE cell population are often more aggressive and have a poorer prognosis [4,8–10]. Therefore, it has been hypothesized that NE cells can lead to the development and growth of androgen-refractory prostate tumors through the secretion of neuropeptides that induce the proliferation of adjacent carcinoma cells in an androgen-depleted condition [11]. This notion is supported by the observation that the proliferation index of proximate cancer cells surrounding NE cells is higher than that of distal cancer cells [12,13]. However, the exact role that NE cells play in the development of androgen-independent prostate carcinomas is a matter of debate, and the molecular mechanism of NE cell enrichment remains an enigma [14]. Some authors have found that the acquisition of these NE characteristics is fully reversible, suggesting that the phenotype of cells within tumors is dynamic and will be determined, in part, by the balance of differentiating and mitogenic factors in the local environment.
One of the most important mitogenic factors presently known to regulate normal prostate function is epidermal growth factor (EGF) [15,16].Our current knowledge on the role of EGF strongly suggests that this growth factor plays a fundamental role in stromal-epithelial interactions during the initiation and progression of prostate cancer. In primary tumors, neoplastic cells express EGF receptor (EGFR), and surrounding stromal cells express transforming growth factor-α, whereas in advanced disease, neoplastic cells coexpress both EGFR and transforming growth factor-α [17]. However, EGF can stimulate androgen-mediated gene transcription in the absence of androgen, suggesting that the androgen signaling pathway may be activated by an androgen-independent mechanism [18].
EGF binds specifically to ErbB1 or EGFR, the prototypical member of the ErbB family of receptors that includes ErbB2 (HER2, neu), ErbB3 (HER3), and ErbB4 (HER4) [19–21]. Ligand binding to a cognate ErbB receptor induces receptor homodimer and heterodimer formation, leading to stimulation of the intrinsic tyrosine kinase activity of the receptor. These activated receptor tyrosine kinase complexes activate, in turn, a number of cytoplasmic signaling pathways, including the mitogen-activated protein kinase (MAPK) and phosphoinositol 3′-kinase (PI3K)/Akt pathways. Although no direct ligand for ErbB2 has been identified, it appears to be the preferred heterodimerization partner of all ErbB proteins and plays an important role in the potentiation of ErbB receptor signaling. In particular, EGFR and ErbB2 are important regulatory elements of the growth and progression of many epithelial-derived tumors, including prostate cancer [16].
The primary focus of this investigation was to study the relationship between NE differentiation and EGF because both have been implicated in the progression of prostate cancer.
In this report, we present evidence that EGF prevents NE differentiation induced by androgen depletion. This effect does not require the presence of ErbB2 and occurs through the action of an extracellular signal-regulated kinase (ERK) 1/2-dependent mechanism. Furthermore, our results suggest that the constitutive activation of Akt detected in these cells supports this effect of EGF because abrogation of PI3K/Akt signaling changes the EGF-induced NE differentiation-inhibitory response toward cell cycle arrest and NE differentiation.
Materials and Methods
Cell Culture
LNCaP human prostate cancer cells were obtained from the American Type Culture Collection (Manassas, VA) and were routinely cultured in RPMI 1640 medium (Invitrogen, San Diego, CA) containing 7% fetal bovine serum (FBS; Invitrogen), 0.25 mg/ml penicillin, 0.25 mg/ml streptomycin, and 2.5 mg/ml fungizone. Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Reagents
EGF was purchased from Sigma (St. Louis, MO), U0126 was purchased from Promega (Madison, WI), LY294002 was purchased from Calbiochem (Darmstadt, Germany), gefitinib was provided by Astra-Zeneca (Alderley Park, Cheshire, UK), and trastuzumab was obtained from Roche Farma (Madrid, Spain). The corresponding antibodies used for these analyses included the following: anti-phospho-ErbB2 (clone PN2A), anti-ErbB2 (clone 3B5), and anti-neurone-specific enolase (NSE) from Lab Vision Corporation Neomarkers (Fremont, CA); anti-phospho-EGFR (Y1068), anti-phospho-Akt, and anti-phospho-p44/42 MAPK (Thr202/Tyr204) from Cell Signaling Technology, Inc. (Denvers, MA); anti-EGFR from BD Transduction Laboratories (San Diego, CA); and anti-actin from Calbiochem.
Western Blot Analysis
Cells were treated as indicated in figure legends. They were then collected, washed twice in cold phosphate-buffered saline (PBS), and solubilized with 50 mM Tris-HCl buffer (pH 7.5) containing 140 mM NaCl, 1 mM EDTA, 0.3 mg/ml soybean trypsin inhibitor, and 0.1 mM phenylmethylsulfonyl fluoride (buffer A) in the presence of 1% Triton X-100 and 0.5 mM sodium orthovanadate. The mixture was gently agitated for 30 minutes at 4°C and then centrifuged at 18,500g for 20 minutes. Soluble proteins (50–90 µg) were resolved through 7.5% sodium dodecyl sulfate polyacrylamide gels, transferred to a nitrocellulose membrane, and immunoblotted with primary antibodies. Immunoreactive proteins were visualized by the ECL immunodetection system(Pierce, Rockford, IL) with horseradish peroxidase-conjugated secondary antibodies and quantified using the Image Scion computer program (Scion Corporation, Frederick, MD).
Immunoprecipitation
Cells were washed and solubilized with 50 mM Tris-HCl buffer (pH 7.5) containing 140 mM NaCl, 1 mM EDTA, 0.3 mg/ml soybean trypsin inhibitor, and 0.1 mM phenylmethylsulfonyl fluoride (buffer A) in the presence of 1% Triton X-100 and 0.5 mM sodium orthovanadate. The mixture was gently agitated for 30 minutes at 4°C and thereafter centrifuged at 18,500g for 20 minutes. Soluble proteins (400–600 µg) were incubated for 2 hours at 4°C with an anti-EGFR protein antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Protein G-Sepharose (Sigma, St. Louis, MO) was then added, and samples were rotated for another hour. Immunoprecipitates were then washed thrice with buffer A and resuspended in Laemmli sample buffer. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane. Western blot analyses were performed as described above.
Flow Cytometric Analysis of ErbB2 Localization
The amount of ErbB2 at the cell surface was determined by flow cytometry using indirect fluorescent staining methods. Briefly, cells were plated onto 60-mm culture plates. After 48 hours, cells were starved in serum-free medium for 24 hours and subsequently treated with trastuzumab. After 24 hours, the cells were harvested with trypsin and EDTA, and then washed with PBS containing 1% bovine serum albumin (PBS-A). In the primary reaction, the cells were incubated at 4°C for 30 minutes with 5 µg/ml trastuzumab or the same amount of human IgG. We used trastuzumab as the primary antibody because it recognizes the extracellular domain of ErbB2. After washing the cells with PBS-A to remove unbound primary antibody, they were incubated with goat anti-human IgG AlexaFluor488 (Invitrogen) at 4°C for 30 minutes. After further washing with PBS-A, the cells were brought up in PBS-A, and flow cytometry was performed using the Becton Dickinson FACScalibur apparatus (Becton Dickinson, Palo Alto, CA). The percentage of cells with specific staining and the intensity of staining were determined using the Becton Dickinson Cell Quest program.
Cell Cycle Analysis
Cells were plated onto 60-mm culture plates. The medium was changed 48 hours later, and treatment was initiated as described in figure legends. After 48 hours, cells were trypsinized, washed thrice with ice-cold PBS, and resuspended in propidium iodide solution. This solution contained 50 µg/ml propidium iodide, 0.5% NP40, and 63 µg/ml RNase A in PBS. After 30 minutes of incubation in the dark on ice, cell cycle distribution was measured with a Becton Dickinson FACS-calibur flow cytometer.
Results
EGF Prevents NE Differentiation in LNCaP Cells
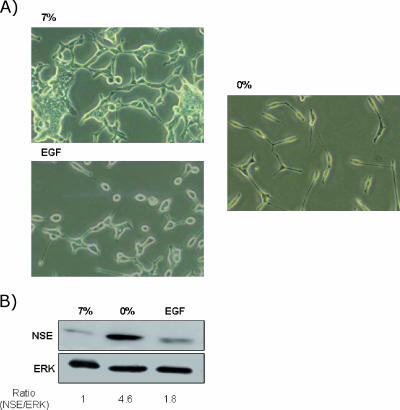
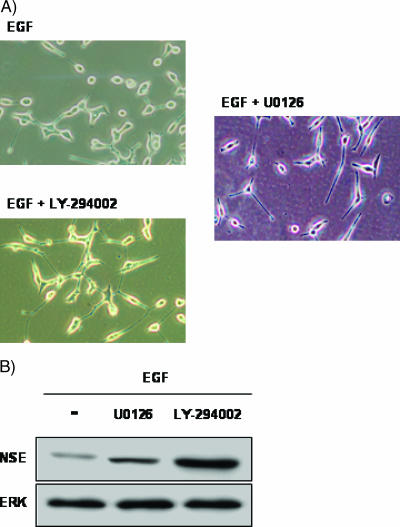
To determine the effect of EGF on the NE differentiation of LNCaP cells induced by long-term androgen deprivation, LNCaP cells were serum-starved and treated with EGF (10-8 M) for 5 days. Photomicrographs (Figure 1) illustrate morphologic changes induced in response to these treatments. Cells cultured without serum exhibited moderate morphologic differentiation, as indicated by the appearance of long and branched neuritic extensions that often possessed growth cone-like structures. All these changes were associated with the NE differentiation process. The formation of neuritic processes was visible during the 2 to 3 days of treatment and then continued to develop, persisting as long as the cells were maintained under differentiating conditions. However, the LNCaP cells treated with EGF in the absence of serum exhibited a rounded morphology with short rarely branched cellular processes, suggesting that EGF blocks the acquisition of NE phenotype through induction by the absence of serum.
Figure 1.
Effect of EGF on cell morphology and NSE expression in LNCaP cells. LNCaP cells were cultured in RPMI with 7% FBS. After 2 days of culture, the medium was removed, and the cells were washed with PBS, cultured in RPMI with 7% FBS (7%) or in the absence of serum (0%), and exposed to EGF (10-8 M) for 4 days. (A) Phase-contrast photomicrographs illustrating morphologic changes in LNCaP cells were taken at x20 magnification 4 days after treatment. (B) NSE expression in LNCaP cells after indicated treatment. ERK was used as an equal-loading marker. The figure is representative of four different experiments.
To evaluate NE differentiation more objectively, expression of NSE was examined by Western blot analysis. This marker has previously been used to assess the extent of NE differentiation in LNCaP cells [22,23]. As shown in Figure 1B, in LNCaP cells grown in serum-free medium, NSE levels were strongly elevated. The level of NSE was only slightly elevated in EGF-treated cells.
These results indicate that EGF prevents the NE differentiation of LNCaP cells induced by serum deprivation.
Effect of Gefitinib and Trastuzumab on EGFR/EGFR Homodimers and EGFR/ErbB2 Heterodimers Induced By EGF in LNCaP Cells
EGF binds to EGFR and promotes the formation of EGFR/EGFR homodimers and EGFR/ErbB2 heterodimers. These dimeric combinations of receptors are believed to produce radically different downstream signals [20]. Because both receptors have been detected in LNCaP cells, we sought to determine which dimers are involved in the effect of EGF on NE differentiation. To this end, we used gefitinib, an EGFR-selective tyrosine kinase inhibitor, and trastuzumab, a monoclonal antibody that blocks ErbB2 function.
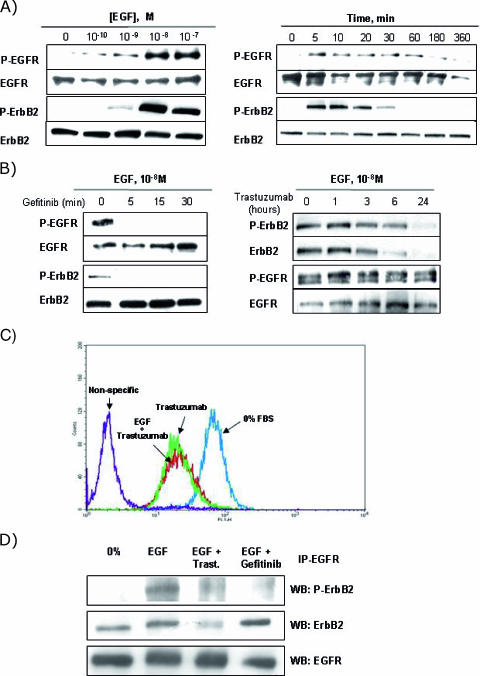
Firstly, we determined whether EGF induced the formation of EGFR/EGFR homodimers and EGFR/ErbB2 heterodimers in LNCaP cells. As shown in Figure 2A, the addition of EGF induced the phosphorylation of EGFR and ErbB2 in a time-dependent and dose-dependent manner. Maximal phosphorylation was reached at the same time (5–10 minutes) of incubation and EGF concentration (10-8 M) for both receptors. As ErbB2 does not have a known ligand, its phosphorylation could be due to transactivation, so EGF would activate EGFR, which in turn would phosphorylate ErbB2 through the EGFR/ErbB2 heterodimers formed. In fact, incubation of LNCaP cells with gefitinib for 5, 15, and 30 minutes before EGF stimulation markedly inhibited EGF-induced EGFR phosphorylation without any accompanying change in EGFR levels (Figure 2B, left). Gefitinib also inhibited EGF-induced ErbB2 phosphorylation, which strongly suggests that ErbB2 is transactivated by EGFR. Importantly, phosphorylation of ErbB2 was reduced in parallel with that of EGFR, illustrating the significance of EGFR in maintaining the activity of ErbB2 and in suggesting the existence of EGFR/ErbB2 heterodimers.
Figure 2.
Effect of gefitinib and trastuzumab on EGF-stimulated EGFR and ErbB2 activation in LNCaP cells. LNCaP cells were cultured in RPMI with 7% FBS for 2 days and then placed in serum-free medium for 24 hours. (A) The cells were then treated for 5 minutes with increasing concentrations of EGF (left) with 10-8 M EGF for the indicated times (right), or (B) were pretreated with 5 µM gefitinib (left) or 25 µg/ml trastuzumab (right) for the indicated times before adding 10-8 M EGF for 5 minutes. Cellular lysates were monitored by immunoblotting using specific antibodies against the phosphorylated and total forms of EGFR and ErbB2, as described in the Materials and Methods section. (C) Effect of trastuzumab on cell surface ErbB2. Serum-starved cells were treated with 25 µg/ml trastuzumab alone or in combination with 10-8 M EGF for 24 hours. ErbB2 levels were determined by FACScan analysis with specific anti-ErbB2 antibody, as described in the Materials and Methods section. The nonspecific area corresponds to staining arising from the omission of the primary antibody. Ordinate: relative cell number; abscissa: log fluorescence. (D) Effect of gefitinib and trastuzumab on EGFR/ErbB2 heterodimer formation induced by EGF. Serum-starved cells were pretreated with 5 µM gefitinib for 30 minutes or with 25 µg/ml trastuzumab for 24 hours before adding 10-8 M EGF for 5 minutes. Protein lysates were quantified, and equal amounts of total protein were then subjected to immunoprecipitation using anti-EGFR antibody. Immunoprecipitates were monitored by Western blot analysis using specific antibodies against the phosphorylated and total forms of ErbB2, as described in the Materials and Methods section. The total EGFR level was used as an equal-loading marker. Each figure is representative of four different experiments.
The pretreatment of LNCaP cells with trastuzumab 1, 3, 6, and 24 hours beforeEGF stimulation decreased EGF-induced ErbB2 phosphorylation (Figure 2B, right). This inhibition was evident after 3 hours of treatment with trastuzumab and was maximal after 24 hours. A decrease in ErbB2 levels with kinetics very similar to that of ErbB2 phosphorylation was also observed, suggesting that the decrease in EGF-induced ErbB2 phosphorylation could be due to downregulation of ErbB2. However, trastuzumab did not modify the phosphorylation or the levels of EGFR, suggesting the existence of EGFR/EGFR homodimers that maintain EGFR phosphorylation.
To confirm whether the downregulation of ERB2 levels also reduced ErbB2 levels in the plasma membrane, we measured the levels of ERB2 expression at the cell surface by flow cytometry. As shown in Figure 2C, trastuzumab alone or in combination with EGF reduced the surface expression of ErbB2 by approximately 70%.
To provide direct additional evidence for EGFR/ErbB2 heterodimers and to confirm the results obtained with inhibitors, we incubated LNCaP cells with EGF alone or in combination with gefitinib and trastuzumab, and then measured the presence and the degree of phosphorylation of ErbB2 in EGFR immunoprecipitates. As shown in Figure 2D, ErbB2 coimmunoprecipitates with EGFR under basal conditions and in the presence of EGF, although ErbB2 is phosphorylated only when LNCaP cells are treated with EGF. This finding indicates that EGF activates previously constituted EGFR/ErbB2 heterodimers. The presence of gefitinib slightly increased the amount of ErbB2 that coimmunoprecipitated with EGFR but completely inhibited its phosphorylation, suggesting the presence of inactive heterodimers. Treatment with trastuzumab reduced ErbB2 phosphorylation in parallel with the formation of a smaller amount of heterodimers.
These findings strongly suggest that EGF induced the activation EGFR/EGFR homodimers and EGFR/ErbB2 heterodimers. The activation of both types of dimer, but not their formation, was inhibited by gefitinib, whereas trastuzumab inhibited the activation and formation of EGFR/ErbB2 heterodimers.
Effect of Gefitinib and Trastuzumab on Changes in LNCaP Cell Morphology Induced By EGF
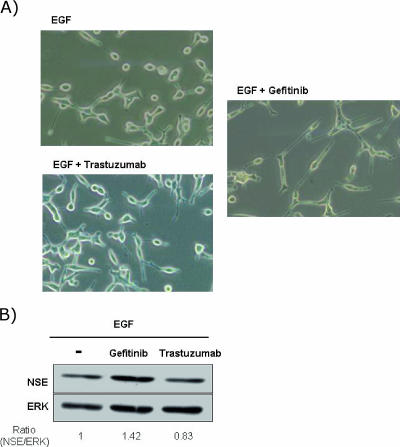
Having established the effect of gefitinib and trastuzumab on EGFR and ErbB2 activation, we examined whether these drugs modified the effect of EGF on LNCaP cell morphology. Figure 3 illustrates that gefitinib, but not trastuzumab, prevents the effect of EGF on LNCaP cell morphology. LNCaP cells treated with EGF in the presence of gefitinib for 5 days displayed a clear NE morphology (Figure 3A) coincident with an increase in NSE expression (Figure 3B), indicating that EGFR tyrosine kinase activity was required for EGF-induced NE differentiation prevention. However, trastuzumab failed to prevent the effect of EGF on LNCaP morphology and NSE expression levels (Figure 3, A and B), which indicates that ErbB2 may not be required.
Figure 3.
Effect of gefitinib and trastuzumab in the presence of EGF on LNCaP cell morphology. LNCaP cells were cultured in RPMI with 7% FBS. After 2 days of culture, the medium was removed, and the cells were washed with PBS, cultured in RPMI in the absence of serum, and exposed to EGF (10-8 M), alone or in combination with gefitinib (5 µM) or trastuzumab (25 µg/ml), for 4 days. (A) Phase-contrast photomicrographs illustrating morphologic changes in LNCaP cells were taken at x20 magnification 4 days after treatment. (B) NSE expression in LNCaP cells after indicated treatment. ERK was used as an equal-loading marker. The figure is representative of six different experiments.
Effect of LY294002 and U0126 on EGF-Dependent Signaling in LNCaP Cells
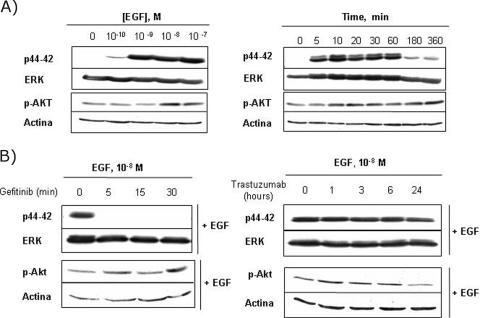
To study the downstream effects of gefitinib and trastuzumab receptors, we analyzed the signaling pathway molecules p42/44 MAPK and Akt, which are activated by ErbB receptors in multiple biologic processes. The activation states of the two enzymes were measured by immunoblotting with antibodies specific for activating phosphorylation sites (see the Materials and Methods section). As expected, MAPK activity increased on treatment with EGF. The activation was maximal after 5 to 10 minutes of EGF exposure and occurred in a dose-dependent fashion (Figure 4A). When LNCaP cells were pretreated with gefitinib, EGF-induced MAPK activation was completely inhibited at all times tested (Figure 4B, left). However, pretreatment with trastuzumab had no effect (Figure 4B, right), even after 24 hours, when the ErbB2 downregulation induced by trastuzumab was maximal (Figure 4B, right).
Figure 4.
Effect of gefitinib and trastuzumab on EGF-stimulated signaling pathways. LNCaP cells were cultured in RPMI with 7% FBS for 2 days and then placed in serum-free medium for 24 hours. (A) The cells were then treated for 5 minutes with increasing concentrations of EGF (left) or with 10-8 M EGF for the indicated times (right). (B) Serum-starved cells were pretreated with gefitinib (5 µM; left) or trastuzumab (25 µg/ml; right) for the indicated times before adding 10-8 M EGF for 5 minutes. Cellular lysates were monitored by immunoblotting using the indicated specific antibodies, as described in the Materials and Methods section. The figure is representative of three different experiments.
There were no significant differences in Akt activity with the different treatments, possibly as a consequence of the constitutively high basal levels of Akt activity observed (Figure 4, A and B). Signaling through the PI3K pathway is required to prevent cell death in LNCaP and also constitutive activation due to the loss of PTEN function previously described in these cells [24].
These results suggest that EGF-induced NE differentiation inhibition could be mediated by ERK1/2 activation and requires Akt activity. To test this hypothesis, we incubated LNCaP cells with EGF, EGF with U0126, or EGF with LY294002, and examined the cell morphology and levels of NSE. As shown in Figure 5, the presence of both inhibitors prevented EGF effects and induced NE differentiation, as demonstrated by changes in cell morphology. NSE levels also increased, suggesting that EGF-induced NE differentiation inhibition requires ERK1/2 and Akt activity.
Figure 5.
Effect of LY294002 and U0126 in the presence of EGF on LNCaP cell morphology. LNCaP cells were cultured in RPMI with 7% FBS; after 2 days of culture, the medium was removed, and the cells were washed with PBS, cultured in serum-free medium, and exposed to EGF (10-8 M), alone or in combination with LY294002 (20 µM) or U0126 (5 µM), for 4 days. (A) Phase-contrast photomicrographs illustrating morphologic changes in LNCaP cells were taken at x20 magnification 4 days after treatment. (B) NSE expression in LNCaP cells after indicated treatment. Actin was used as an equal-loading marker. The figure is representative of six different experiments.
Effect of Gefitinib, Trastuzumab, U0126, and LY294002 on the Presence of EGF in Cell Cycle Progression
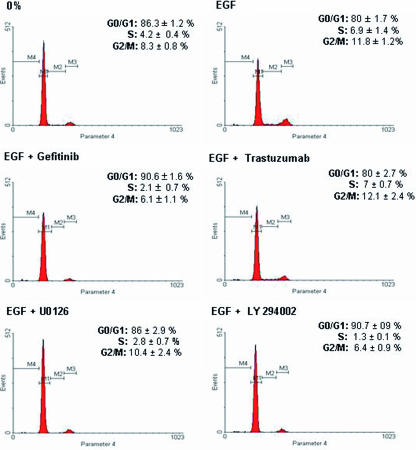
Although one characteristic ascribed to prostatic NE cells is loss of mitogenic activity, it has also been suggested that NE differentiation is not obligatorily coupled to withdrawal from the cell cycle [22]. To determine whether acquisition or loss of the NE phenotype detected with our treatments is associated with changes in mitotic activity, we analyzed the effect of these treatments on cell cycle progression. As shown in Figure 6, stimulation of quiescent LNCaP cells with EGF led to a 65% increase in S-phase fraction. The presence of 5 µM gefitinib, 5 µM U0126, or 20 µM LY294002 prevented the effect of EGF decreasing the percentage of cells in S-phase by 70%, 60%, and 80% respectively, compared with EGF alone. Moreover, gefitinib and LY294002 also decreased the number of cells in G2/M-phase by about 50%. Finally, treatment with trastuzumab did not alter the cell cycle distribution of LNCaP cells incubated with EGF.
Figure 6.
Gefitinib, U0126, and LY294002, but not trastuzumab, in the presence of EGF induces G1 arrest in LNCaP cells. LNCaP cells were cultured in RPMI with 7% FBS; after 2 days of culture, the medium was removed, and the cells were washed with PBS, cultured in RPMI in the absence of serum (0%), and exposed EGF (10-8 M), alone or in combination with gefitinib (5 µM), trastuzumab, (25 µg/ml), U0126 (5 µM), or LY294002 (20 µM), for 2 days. After the desired treatment, cells were trypsinized, and FACS analysis was performed as described in the Materials and Methods section. The cell cycle phase distribution is representative four different experiments with similar findings. Numeric data are presented as the mean ± SEM of these four experiments.
These results clearly demonstrate that these treatmentinducing morphologic changes also block cell cycle progression, showing a direct association between NE differentiation and inhibition of the mitotic activity of LNCaP cells.
Discussion
Cells with NE phenotype increase in number as cancer progresses to an androgen-refractory condition, and they become the prevalent cell type after long-term antiandrogen therapy [7]. In this study, we have demonstrated that EGF, a growth factor implicated in prostatic tumoral progression, prevents NE differentiation induced by hormonal deprivation.
We have used the androgen-responsive prostate tumor cell line LNCaP, which acquires an NE phenotype in response to certain culture manipulations, including prolonged androgen depletion [25], pharmacological elevation of intracellular cAMP [26,27], and exposure of cells to heparin-binding EGFlike growth factor [22] or interleukin-6 [23]. In our case, we chose the transdifferentiation of LNCaP induced by androgen depletion resembling the clinical phenomenon in androgen ablation therapy. This transdifferentiation is characterized by the appearance of long, branched, neurite-like processes; inhibition of mitotic activity; and increased expression of NE markers such as NSE. EGF prevented all these changes: Cells exhibited fewer and shorter processes, lower NSE levels, and renewed mitotic activity, suggesting that cells did not acquire an NE phenotype.
We have demonstrated that in LNCaP cells, EGF activates EGFR and ErbB2 through the formation of EGFR/ErbB2 dimers. This finding is supported by several pieces of evidence. First, ErbB2, a receptor with no known ligand, was phosphorylated by EGF in parallel with EGFR. Second, the inhibition of EGFR tyrosine kinase by gefitinib blocked 100% EGF-stimulated ErbB2 phosphorylation in parallel with that EGFR. Gefitinib also targets the tyrosine kinase domain of ErbB2, although because the IC50 (>3.7 µM) of gefitinib for the inhibition of ErbB2 kinase activity in vitro is at least two orders of magnitude greater that that for the inhibition of EGFR (0.033 µM) [28], it is unlikely that such a mechanism is responsible in this instance. Third, ErbB2 coimmunoprecipitates with EGFR and is activated in the presence of EGF.
Furthermore, we analyzed the specific contributions of both receptors to EGF-mediated NE differentiation inhibition, demonstrating that the presence of ErbB2 is not necessary. Gefitinib was not a useful inhibitor for discriminating between the two receptors because it inhibits the activation of EGFR and ErbB2. However, trastuzumab drastically reduced ErbB2 levels and EGFR/ErbB2 heterodimer formation, but it did not inhibit either EGFR phosphorylation or EGF-dependent signaling, which suggests that EGFR activation was sufficient to prevent NE differentiation and that the absence of ErbB2 can be compensated by EGFR. In fact, the reduction of EGFR/ErB2 heterodimer formation by the use of trastuzumab could promote the liberation of EGFR and EGFR/EGFR homodimerization. These homodimers could activate ERK1/2 and cell proliferation, mediating the inhibition of NE differentiation by EGF in the absence of ErbB2. These results are surprising given the demonstration by Mellinghoff et al. [29] that ErbB2, but not EGFR, is required to impair growth and androgen receptor activity in human prostate cancer cell lines. Moreover, forced overexpression of ErbB2 in prostate cancer cells enhances androgen receptor function and hormoneindependent growth [30,31]. It is possible that the relative contribution of EGFR and ErbB2 to tumoral progression was dependent on the particular repertoire of ErbB receptors and their relative expression levels on different stages of the tumor.
It is interesting to note that we have detected in LNCaP cells EGFR/ErbB2 heterodimers previously constituted in basal conditions that are activated by the addition of EGF. EGF produced by LNCaP cells [32] may be responsible for the formation of the hetorodimers detected. However, the absence of the phosphorylation of EGFR and ErbB2 in basal conditions rules out this possibility. Other authors have also demonstrated the presence of EGFR dimers in the absence of EGF stimulation, which indicates that receptor dimerization and activation are mechanistically distinct and separable events [33,34].
Our data suggest that ERK1/2 is involved in the EGF-mediated inhibition of NE differentiation. In fact, ERKs were activated under culture conditions in which NE differentiation was not observed, and the MEK inhibitor U0126 blocked the effect of the EGF induction of NE differentiation and increased NSE levels. Interestingly, Akt activity was not modified by any of the treatments, but its inhibition blocked the effect of EGF on NE differentiation. The PI3K/Akt pathway is constitutively active in LNCaP cells due to a mutation in the PTEN tumor-suppressor gene, a negative regulator of this pathway [35]. The importance of this pathway for cell survival in the absence of androgens is obvious because treatment with the PI3K inhibitor LY294002 leads to apoptosis. This apoptotic effect can be antagonized by different ligands of EGFR, including EGF [24]. Consistent with these data, we found that EGF rescued LNCaP cells from LY294002-induced apoptosis but was unable to stimulate cell proliferation or to inhibit NE differentiation. These findings suggest that abrogation of PI3K/Akt signaling changed the proliferative role of EGF in LNCaP cells to one of differentiation. The clinical relevance of our findings is indicated by a recent clinicopathological study showing that phosphorylation of Akt is an excellent predictor of poor clinical outcome in prostate cancer [36]; this raises the possibility that targeting the PI3K pathway may prove to be an effective means of chemotherapeutic intervention in vivo. However, the ability of the PI3K/Akt pathway to modulate the effect of EGF must be taken into account. Inhibitors of PI3K/Akt could induce apoptosis and tumor regression, but their effect could be compensated by the presence of EGF stimulating an alternative survival signal. Under these conditions, EGF will not directly stimulate cell proliferation but could increase the number of mitogenic factor-secretor NE cells contributing to tumoral progression.
Results obtained with gefitinib and trastuzumab have been useful not only for characterizing the receptors and intracellular pathways that drive NE differentiation inhibition by EGF but also for examining the mechanisms of action of these inhibitors. In this study, we have shown that gefitinib strongly inhibits EGF-dependent signaling through a double complementary mechanism of action: the inhibition of EGFR and ErbB2 activation, and the formation of inactive EGFR/ErbB2 heterodimers. The formation of inactive EGFR/ErbB2 heterodimers could potentially reduce the population of ErbB2 available to form new secondary dimers with other ErbB receptors present in LNCaP cells [37,38]. This double mechanism may explain the very efficient prevention by gefitinib of EGF-dependent signaling in LNCaP cells. These findings substantiate and extend a number of reports that demonstrate the antiproliferative effects of gefitinib on other prostatic cancer cell lines and on human prostate cancer xenografts, and support the use of gefitinib in the treatment of prostate cancer. Unfortunately, the activity of gefitinib in recent prostate cancer trials has been disappointing [39]. We may speculate on whether the capacity of gefitinib to induce NE differentiation in the presence of EGF was the principal reason for the failure of gefitinib. This inhibitor may be able to suppress cell proliferation stimulated by EGF but in turn induces NE differentiation that could provide proliferative stimuli to surrounding cancer cells.
Trastuzumab, a humanized monoclonal antibody, is an effective treatment for advanced breast cancer with ErbB2 gene amplification but has a poor efficacy in treating hormonerefractory prostate cancer. Although it is not clear how trastuzumab works, stimulation of ErbB2 endocytosis and removal of ErbB2 from the cell surface in overexpressing cells seem to be principal elements of its effectiveness [40]. In this context, our studies have shown that trastuzumab reduced ErbB2 levels and the formation of active EGFR/ErbB2 heterodimers in LNCaP cells that did not overexpress ErbB2 but did not inhibit EGF effects. Thus, the downregulation of ErbB2 induced by trastuzumab was not always associated with its efficacy [41,42]. In our case, the inefficacy of trastuzumab in inhibiting the effect of EGF seems be due to the coexpression of EGFR, which circumvents the absence of ErbB2 produced by trastuzumab. This suggests that the biologic function of ErbB2 in each case and the relative levels of the other ErbB receptors may be more important than the expression level of ErbB2. Another possible explanation for the inefficacy of trastuzumab is the absence of PTEN from LNCaP cells. Nagata et al. [41] have demonstrated that trastuzumab responsiveness depends not only on the downregulation of ErbB2 and the inhibition of ErbB2-related downstream events but also on the status of PTEN. They report that trastuzumab specifically downregulates PI3K signaling through activation of PTEN and that PTEN loss renders ErbB2-overexpressing breast cancer resistant to trastuzumabbased therapy. Therefore, it is pertinent to investigate in LNCaP cells whether the efficacy of trastuzumab could be enhanced with inhibitors of the PI3K pathway. It might be worthwhile to reconsider trastuzumab-based chemotherapy for prostate cancer if the principal reason for the failure of trastuzumab has been inadequate PI3K inhibition because this could be overcome by the addition of a PI3K pathway inhibitor.
In summary, we have demonstrated that, in LNCaP cells, EGF prevents the acquisition of NE phenotype by an ErbB2- independent and ERK1/2-dependent mechanism and requires Akt activation. We have also shown that EGFR tyrosine kinase and PI3K inhibitors can inhibit the cell proliferation stimulated by EGF but induce the acquisition of NE phenotype. Under these conditions, EGF does not directly stimulate cell proliferation but could increase the population of mitogenic factor-secretor NE cells that maintain the growth potential of tumor cells. Such that EGFR, HER-2, and Akt constitute biologic targets for innovative treatment in hormone-refractory prostate cancer [43,44], the present data should be borne in mind when designing new clinical schedules for the treatment of prostate cancer, including the use of ErbB receptors and associated signaling pathway inhibitors.
Footnotes
This work was supported by grants 04077-00 and 01035 from the Consejería de Sanidad de Castilla-La Mancha. R.M.M. and C.A. are recipients of grant PI020964 from the Spanish Health Ministry “Fondo de Investigaciones Sanitarias.”
References
- 1.Huggins C. Endocrine-induced regression of cancers. Cancer Res. 1967;27:1925–1930. [PubMed] [Google Scholar]
- 2.Craft N, Chhor C, Tran C, Belldegrun A, DeKernion J, Witte ON, Said J, Reiter RE, Sawyers CL. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 1999;59:5030–5036. [PubMed] [Google Scholar]
- 3.Evangelou AI, Winter SF, Huss WJ, Bok RA, Greenberg NM. Steroid hormones, polypeptide growth factors, hormone refractory prostate cancer, and the neuroendocrine phenotype. J Cell Biochem. 2004;91:671–683. doi: 10.1002/jcb.10771. [DOI] [PubMed] [Google Scholar]
- 4.Abrahamsson A. Neuroendocrine differentiation in prostatic carcinoma. Prostate. 1999;39:135–148. doi: 10.1002/(sici)1097-0045(19990501)39:2<135::aid-pros9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Bonkhoff H. Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Ann Oncol. 2001;12(Suppl 2):S141–S144. doi: 10.1093/annonc/12.suppl_2.s141. [DOI] [PubMed] [Google Scholar]
- 6.Guate JL, Escaf S, Menendez CL, del Valle M, Vega JA. Neuroendocrine cells in benign prostatic hyperplasia and prostatic carcinoma: effect of hormonal treatment. Urol Int. 1997;59:149–153. doi: 10.1159/000283051. [DOI] [PubMed] [Google Scholar]
- 7.Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004;45:586–592. doi: 10.1016/j.eururo.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 8.Cussenot O, Villette JM, Cochand-Priollet B, Berthon P. Evaluation and clinical value of neuroendocrine differentiation in human prostatic tumors. Prostate Suppl. 1998;8:43–51. [PubMed] [Google Scholar]
- 9.Fixemer T, Remberger K, Bonkhoff H. Apoptosis resistance of neuroendocrine phenotypes in prostatic adenocarcinoma. Prostate. 2002;53:118–123. doi: 10.1002/pros.10133. [DOI] [PubMed] [Google Scholar]
- 10.Grobholz R, Griebe M, Sauer CG, Michel MS, Trojan L, Bleyl U. Influence of neuroendocrine tumor cells on proliferation in prostatic carcinoma. Hum Pathol. 2005;36:562–570. doi: 10.1016/j.humpath.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Vashchenko N, Abrahamsson PA. Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities. Eur Urol. 2005;47:147–155. doi: 10.1016/j.eururo.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Bonkhoff H, Wernert N, Dhom G, Remberger K. Relation of endocrine-paracrine cells to cell proliferation in normal, hyperplastic, and neoplastic human prostate. Prostate. 1991;19:91–98. doi: 10.1002/pros.2990190202. [DOI] [PubMed] [Google Scholar]
- 13.Cockett AT, di Sant'Agnese PA, Gopinath P, Schoen SR, Abrahamsson PA. Relationship of neuroendocrine cells of prostate and serotonin to benign prostatic hyperplasia. Urology. 1993;42:512–519. doi: 10.1016/0090-4295(93)90260-h. [DOI] [PubMed] [Google Scholar]
- 14.Bostwick DG, Qian J, Pacelli A, Zincke H, Blute M, Bergstralh EJ, Slezak JM, Cheng L. Neuroendocrine expression in node positive prostate cancer: correlation with systemic progression and patient survival. J Urol. 2002;168:1204–1211. doi: 10.1016/S0022-5347(05)64626-5. [DOI] [PubMed] [Google Scholar]
- 15.El Sheikh SS, Domin J, Abel P, Stamp G, Lalani el-N. Phosphorylation of both EGFR and ErbB2 is a reliable predictor of prostate cancer cell proliferation in response to EGF. Neoplasia. 2004;6:846–853. doi: 10.1593/neo.04379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratan HL, Gescher A, Steward WP, Mellon JK. ErbB receptors: possible therapeutic targets in prostate cancer? BJU Int. 2003;92:890–895. doi: 10.1111/j.1464-410x.2003.04503.x. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Sarkis A, Reuter V, Cohen D, Netto G, Petrylak D, Lianes P, Fuks Z, Mendelsohn J, Cordon-Cardo C. Changing pattern of expression of the epidermal growth factor receptor and transforming growth factor alpha in the progression of prostatic neoplasms. Clin Cancer Res. 1995;1:545–550. [PubMed] [Google Scholar]
- 18.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:159–167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- 20.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 21.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Adam RM, Freeman MR. Activation of the ERK mitogen-activated protein kinase pathway stimulates neuroendocrine differentiation in LNCaP cells independently of cell cycle withdrawal and STAT3 phosphorylation. Cancer Res. 2002;62:1549–1554. [PubMed] [Google Scholar]
- 23.Deeble PD, Murphy DJ, Parsons SJ, Cox ME. Interleukin-6- and cyclic AMP-mediated signaling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol Cell Biol. 2001;21:8471–8482. doi: 10.1128/MCB.21.24.8471-8482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Adam RM, Santiestevan E, Freeman MR. The phosphatidylinositol 3′-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer Res. 1999;59:2891–2897. [PubMed] [Google Scholar]
- 25.Shen R, Dorai T, Szaboles M, Katz AE, Olsson CA, Buttyan R. Transdifferentiation of cultured human prostate cells to a neuroendocrine cell phenotype in a hormone-depleted medium. Urol Res. 1997;3:67–75. doi: 10.1016/s1078-1439(97)00039-2. [DOI] [PubMed] [Google Scholar]
- 26.Bang YJ, Pirnia F, Fang WG, Kang WK, Sartor O, Whitesell L, Ha MJ, Tsokos M, Sheahan MD, Nguyen P. Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP. Proc Natl Acad Sci USA. 1994;91:5330–5334. doi: 10.1073/pnas.91.12.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox ME, Deeble PD, Lakhani S, Parsons SJ. Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression. Cancer Res. 1999;59:3821–3830. [PubMed] [Google Scholar]
- 28.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61:8887–8895. [PubMed] [Google Scholar]
- 29.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 30.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signalling by HER2-neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 31.Ye S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci USA. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limonta P, Dondi D, Marelli MM, Moretti RM, Negri-Cesi P, Motta M. Growth of the androgen-dependent tumor of the prostate: role of androgens and of locally expressed growth modulatory factors. J Steroid Biochem Mol Biol. 1995;53:401–405. doi: 10.1016/0960-0760(95)00086-f. [DOI] [PubMed] [Google Scholar]
- 33.Johannessen LE, Haugen KE, Østvold AC, Stang E, Madshus IH. Heterodimerization of the epidermal-growth-factor (EGF) receptor and ErbB2 and the affinity of EGF binding are regulated by different mechanisms. Biochem J. 2001;35:87–96. doi: 10.1042/0264-6021:3560087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X, Sharma KD, Takahashi T, Iwamoto R, Mekada E. Ligand-independent dimer formation of epidermal growth factor receptor (EGFR) is a step separable from ligand-induced EGFR signalling. Mol Biol Cell. 2002;13:2547–2557. doi: 10.1091/mbc.01-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapma NJ. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–2723. [PubMed] [Google Scholar]
- 36.Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg PM, Ghosh PM. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–5236. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 37.Gamett DC, Pearson G, Cerione RA, Friedberg I. Secondary dimerization between members of the epidermal growth factor receptor family. J Biol Chem. 1997;272:12052–12056. doi: 10.1074/jbc.272.18.12052. [DOI] [PubMed] [Google Scholar]
- 38.Anido J, Matar P, Albanell J, Guzman M, Rojo F, Arribas J, Averbuch S, Baselga J. ZD1839, a specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, induces the formation of inactive EGFR/HER2 and EGFR/HER3 heterodimers and prevents heregulin signaling in HER2-overexpressing breast cancer cells. Clin Cancer Res. 2003;9:1274–1283. [PubMed] [Google Scholar]
- 39.Canil CM, Moore MJ, Winquist E, Baetz T, Pollak M, Chi KN, Berry S, Ernst DS, Douglas L, Brundage M, et al. Randomized phase II study of two doses of gefitinib in hormone-refractory prostate cancer: a trial of the National Cancer Institute of Canada—Clinical Trials Group. J Clin Oncol. 2005;23:455–460. doi: 10.1200/JCO.2005.02.129. [DOI] [PubMed] [Google Scholar]
- 40.Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin Oncol. 1999;26(Suppl 12):60–70. [PubMed] [Google Scholar]
- 41.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- 43.Baselga J, Rischin D, Ranson M, Calvert H, Raymond E, Kieback DG, Kaye SB, Gianni L, Harris A, Bjork T, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumours types. J Clin Oncol. 2002;20:4292–4302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- 44.Small EJ, Bok R, Reese DM, Sudilovsky D, Frohlich M. Docetaxel, estramustine, plus trastuzumab in patients with metastatic androgen-independent prostate cancer. Semin Oncol. 2001;28:71–76. doi: 10.1016/s0093-7754(01)90159-9. [DOI] [PubMed] [Google Scholar]